Abstract
Ceftaroline (CPT) is a new cephalosporin exhibiting bactericidal activity against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Streptococcus pneumoniae (MDRSP), as well as common Gram-negative pathogens. This study investigated the in vivo efficacy of a 48-hour simulated human dose regimen of CPT compared with ceftriaxone (CRO) against isolates of S. pneumoniae with different susceptibilities to penicillin in a rabbit pneumonia model. Three S. pneumoniae strains were used: CRO-susceptible penicillin-susceptible S. pneumoniae (CRO-S PSSP), CRO-susceptible penicillin-intermediate S. pneumoniae (CRO-S PISP), and CRO-resistant penicillin-resistant S. pneumoniae (CRO-R PRSP). Animals were randomized to the control group (no treatment) (n = 22) or to a group given intravenous (IV) CPT human equivalent (HE) dosage (600 mg/12 h; n = 19) or IV CRO HE dosage (1 g/24 h; n = 19). The total doses needed to achieve the HE dosage were 71 and 82 mg/kg of body weight/24 h for CRO and CPT, respectively. One group of rabbits infected with the CRO-R PRSP strain received intramuscular (IM) administration of CPT (5 or 20 mg/kg twice daily; n = 5 for each). Evaluation of efficacy was based on bacterial counts in the lungs and spleen. For IV CPT and IV CRO, the mean areas under the concentration-time curves from 0 to 24 h (AUC0–24s) were 155 and 938 mg · h/liter, respectively, the maximum concentrations in serum (Cmaxs) were 20 and 158 mg/liter, respectively, and the minimum concentrations in serum (Cmins) were 1.3 and 6 mg/liter, respectively. Both agents effectively treated pulmonary infections caused by CRO-S PSSP or CRO-S PISP with complete bacterial eradication in the lungs and spleen after 2 days of treatment. Against PRSP, CPT demonstrated excellent bactericidal activity, reducing bacterial counts in the lungs and spleen by approximately 8 and 4 log units, respectively (P < 0.001); CRO treatment resulted in a 2-log-unit reduction in the bacterial counts in lungs that did not reach statistical significance. Twice-daily IM CPT (5 mg/kg) reduced the bacterial burden by approximately 6 log units in the lungs and 3 log units in the spleen, and the 20-mg/kg dosage effectively eradicated PRSP infection. These findings further validate the in vivo bactericidal activity of CPT against pneumococci.
Streptococcus pneumoniae is the main causative organism for community-acquired pneumonia worldwide (21, 31). The prevalence of penicillin-resistant S. pneumoniae (PRSP) and multidrug-resistant S. pneumoniae (MDRSP) has increased during the past decade (2, 13, 16), and some pneumococcal isolates have developed high-level resistance to expanded-spectrum cephalosporins (5). In addition, resistance to fluoroquinolones is emerging, and treatment failures for levofloxacin have been reported (12, 14, 17, 32). Previously, we demonstrated that a parC mutation facilitates the enrichment of highly fluoroquinolone-resistant mutants in an in vivo model of pneumococcal pneumonia during treatment with fluoroquinolones administered using a simulated human dosing regimen (8, 9, 10, 15).
Ceftaroline (CPT) is a novel, parenteral, broad-spectrum cephalosporin exhibiting bactericidal activity against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and MDRSP as well as common Gram-negative pathogens (26, 28, 29). Ceftaroline recently received Food and Drug Administration (FDA) approval for use in treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia. CPT was highly active against community-acquired pneumonia bloodstream isolates, including penicillin-intermediate S. pneumoniae (PISP; MIC of 0.12 to 1.0 mg/liter) and penicillin-resistant S. pneumoniae (MIC ≥ 2 mg/liter) strains (25). MIC values for CPT against S. pneumoniae isolates are very low (MIC90 = 0.015 mg/liter for penicillin-susceptible S. pneumoniae [PSSP] [MIC ≤ 0.06 mg/liter] and MIC90 = 0.12 to 0.25 mg/liter for PRSP) (4, 18, 25). In these in vitro studies, CPT MICs were 8 to 16 times lower than those of ceftriaxone (CRO) against PRSP.
The present study was designed to investigate the in vivo efficacy of a 48-hour simulated human dose regimen of CPT (600 mg per 12 h) compared with CRO (1 g per 24 h) against isolates of S. pneumoniae with different susceptibilities to penicillin in a rabbit pneumonia model.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and antibiotics.
Susceptibility breakpoints from January 2008 for S. pneumoniae were used (i.e., penicillin MICs of ≤2, 4, and ≥8 mg/liter for susceptible, intermediate, and resistant S. pneumoniae, respectively, and ceftriaxone MICs ≤1, 2, and ≥4 mg/liter for susceptible, intermediate, and resistant S. pneumoniae, respectively, for patients with nonmeningitis syndromes treated with intravenous [IV] therapy) (6). Three clinically invasive pneumococcal strains with different susceptibilities to penicillin (PEN) and ceftriaxone (CRO), were tested in this study: PSSP (penicillin-susceptible and CRO-susceptible [CRO-S], strain Sp195, serotype 19), PISP (penicillin-intermediate and CRO-S, strain Sp16089, serotype 9V), and PRSP (penicillin-resistant and CRO-resistant [CRO-R], strain Sp1308, serotype 19F).
CRO-S PSSP and CRO-S PISP isolates were obtained from the Centre National de Référence des Pneumocoques, Paris, France. PRSP was kindly provided by K. Heilmann (Medical Microbiology Division, University of Iowa College of Medicine). The MICs for CPT, CRO, and PEN for these isolates are listed in Table 1.
TABLE 1.
Ceftaroline and ceftriaxone MICs in vitro against selected isolates of S. pneumoniae
| Strain | Phenotypea | MIC (mg/liter) |
||
|---|---|---|---|---|
| Penicillin | Ceftriaxone | Ceftaroline | ||
| Sp195 | CRO-S PSSP | 0.016 | 0.06 | 0.015 |
| Sp16089 | CRO-S PISP | 4 | 1 | 0.125 |
| Sp1308 | CRO-R PRSP | 8 | 4 | 0.25 |
Abbreviations: CRO-S PSSP, ceftriaxone-susceptible penicillin-susceptible S. pneumoniae; CRO-S PISP, ceftriaxone-susceptible penicillin-intermediate S. pneumoniae; CRO-R PRSP, ceftriaxone-resistant penicillin-resistant S. pneumoniae.
Bacteria were grown in 5% CO2 in brain heart infusion broth (bioMérieux, Marcy l'Etoile, France) or on sheep blood agar plates (bioMérieux). Bacterial stocks were kept at −80°C in brain heart infusion broth supplemented with 15% (vol/vol) glycerol. Stock cultures were replenished every month with isolates recovered from untreated infected rabbits.
For in vitro studies, active ceftaroline (CPT) (lot M599-R1001; Cerexa, Inc., Alameda, CA) and CRO (Sigma-Aldrich, Saint Quentin Fallavier, France) were reconstituted according to the manufacturer's instructions. For rabbit studies, the commercial form of CRO was reconstituted with sterile saline, and the prodrug of CPT (ceftaroline acetate; lot 44027700014) was used to prepare the dosage solution in 1.9% arginine according to the procedure provided by Cerexa, Inc.
In vitro susceptibility testing methods.
Isolates were tested for susceptibility to CPT and CRO by a standard dilution method in agar according to the Comité de l'Antibiogramme de la Société Française de Microbiologie guidelines and interpretive criteria (7).
Time-kill studies.
The bactericidal activities of CPT and CRO were determined by time-kill studies. These studies were performed in triplicate with the antimicrobial agents tested at concentrations equal to 2, 4, and 8 times the MIC (Table 1). Following growth in antibiotic-containing media for 0, 2, 4, and 24 h, viable counts were determined by plating dilutions of the cultures on sheep blood agar. The limit of detection was 1 log10 CFU/ml.
Preparation of the inoculum.
Before each animal experiment, the pneumococcal strain from one frozen aliquot was inoculated into brain heart infusion broth, cultured on agar plates, and incubated for 24 h at 37°C in 5% CO2. Twenty-five to 30 colonies were taken and inoculated into 9 ml of brain heart infusion broth, incubated for 6 h at 37°C, and then cultured on agar plates for 18 h at 37°C in 5% CO2. This culture was diluted in physiologic saline to obtain a final inoculum of 5 × 109 CFU in 0.5 ml of saline. Viable bacterial counts were determined using optical density measurements in reference to a standard curve and then confirmed by using successive dilution cultures and plating on agar.
Animals.
Immunocompetent male New Zealand rabbits (body weight, 2.8 to 3 kg) were used in the present study. Animals were placed in individual cages and nourished ad libitum with drinking water and feed according to current recommendations. The experimental protocol was approved by the local ethics committee for animal experiments.
Experimental pneumonia model.
Production of pneumonia in immunocompetent rabbits and the installation of two central venous catheters (one for infusion drug and one for blood sampling) were performed as previously described (11, 27). Briefly, 24 h after jugular catheterization, bacterial pneumonia was induced by endobronchial challenge of the animals with 0.5 ml of saline containing 5 × 109 CFU of the strain to be tested. Animals were randomly assigned to control group (no treatment) or to an antibiotic regimen 5 h after bacterial challenge. Six to eight animals were used in each group.
Antibiotics (for treated rabbits) or saline water (for control rabbits without treatment) were delivered through the first central venous catheter with infusion rates controlled by a computer-controlled electric pump and at doses that simulated antibiotic kinetics observed in human serum as follows: (i) 1 g of CRO given intravenously once daily (a 1-g dosage was chosen in accordance with the recommendations for the treatment of nonsevere, hospitalized, community-acquired pneumonia [CAP] in several countries [30, 33], 1 to 2 g per day being the dosage recommended for the treatment of severe CAP) and (ii) 600 mg of CPT given IV twice a day. The total doses needed to achieve the human dosage were 71 and 82 mg/kg of body weight/24 h for CRO and CPT, respectively. Antibiotic treatment was continued for 2 days.
Intramuscular (IM) administration of CPT was also studied in the rabbits infected with the CRO-R PRSP strain. The 5-mg/kg CPT (n = 5) and 20-mg/kg CPT (n = 5) doses were evaluated following administration twice daily (every 12 h) by injection into the right thigh of the animal.
Pharmacokinetic analyses.
The parameters needed to simulate the kinetics of CPT and CRO in human serum were initially investigated. After a 1-hour bolus of 10-mg/kg CPT or CRO, iterative blood samples were taken from healthy rabbits to determine spontaneous drug kinetics. Using a microbiological assay to determine serum drug concentrations, the pharmacokinetic (PK) data were compared with those for humans following the normal dosing regimen. A computer-controlled system was used to deliver CPT or CRO in rabbits to mimic the PK parameters observed in healthy humans after 600 mg of CPT administered over 1 h (mean half-life [t1/2] = 2 h and maximum concentration in serum [Cmax] = 18 to 20 mg/liter [19]), or 1 g CRO given over 30 min (mean t1/2 = 6 h and Cmax = 150 mg/liter [20, 24]). Pharmacokinetic data were analyzed using Kinetica software (Innaphase, Philadelphia, PA).
Antimicrobial concentrations in serum.
For each animal, the concentrations of antibiotics in serum were determined from iterative blood samples, obtained through a second catheter. Treated rabbits were bled about 8 to 10 times per 48 h (about 1.5 ml per sample and the total volume of blood samples during the experiment was less than 10% of the total blood volume of the animal). Samples were centrifuged for 10 min at 10,000 × g, and serum was removed. CPT and CRO concentrations were determined in triplicate by a disk plate bioassay method with antibiotic medium II (Difco Laboratories, Detroit, MI) and Bacillus subtilis (for CPT) or Proteus mirabilis (for CRO) as the indicator organisms. The standards were prepared in saline water. The limit of detections were 0.25 mg/liter for CPT and 0.5 mg/liter for CRO. The linearity of the standard curves used for disk plate bioassays was at least 0.98 (coefficient of correlation [r2]). Protein binding of CRO and CPT to serum proteins was also performed by ultrafiltration methods on rabbit plasma at different concentrations (5, 50, and 150 mg/liter). After centrifugation through 20,000-molecular-weight cutoff filters, serum ultrafiltrates were analyzed for CRO or CPT by a bioassay. The amount of antibiotic able to pass through the filter represents the unbound portion of drug in serum. The amount of nonspecific binding was evaluated on protein-free plasma filtrate (50 mg/liter only).
Evaluation of infection.
The rabbits were anesthetized and sacrificed 2 h after the end of the 48-hour antibiotic infusion. The spleen and each pulmonary lobe were weighed and homogenized in 5 ml of sterile saline (MiniMix; Intersciences, France). Bacteria were counted in a sample of this crude homogenate by plating 10-fold dilutions on sheep blood agar and incubating the plates for 24 h at 37°C. Bacterial concentrations in each lobe and in the spleen were determined after adjusting for weight. The threshold value was 1 log10 CFU/g. For statistical comparisons of the difference between the pulmonary bacterial densities, culture-negative lobes were considered to contain 1 log10 CFU/g. For each rabbit, the mean pulmonary pneumococcal concentration was calculated according to each lobar bacterial concentration with lobar weight [e.g., mean concentration = ∑(lobar concentration × lobar weight)/∑(lobar weights)].
Pharmacokinetic-pharmacodynamic analysis.
From the individual pharmacokinetics of each treated animal, the following PK-PD (pharmacodynamic) parameters were calculated: cumulative percentage of a 24-h period that the drug concentration exceeds the MIC (%T>MIC), area under the curve (AUC)/MIC ratio, Cmax/MIC ratio, and AUC that exceeds the MIC (AUC>MIC).
Statistical analysis.
The results were expressed as means ± standard deviations (SDs). Quantitative variables were compared to Mann-Whitney test or analysis of variance and eventually completed by a posthoc analysis using Bonferroni's test. Percentages were compared using the Fisher exact test. The quantitative relationships between antimicrobial efficacy and each of the PK-PD parameters were determined using an Emax model (Hill formula) with SigmaPlot software (version 9.0).
RESULTS
MIC and time-kill curves.
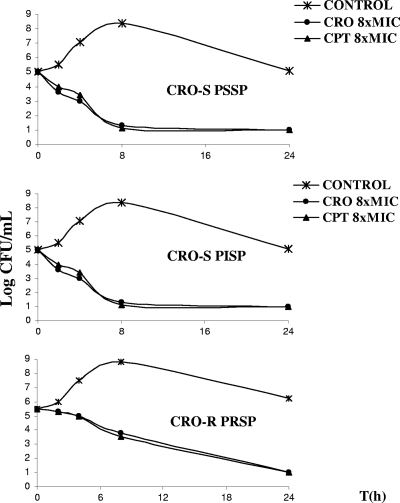
The MICs for CPT, CRO, and PEN for the PSSP, PISP, and PRSP isolates used to induce pneumonia are shown in Table 1. The MICs of CPT were lower than those of CRO. Both CPT and CRO were bactericidal after 24 h of exposure in vitro for all concentrations tested (2, 4, or 8 times the MIC) for each strain (PSSP, PISP, and PRSP) (Fig. 1). For PISP and PRSP strains, the bactericidal effects for CPT and CRO were exerted more slowly than for the PSSP strain.
FIG. 1.
Time-kill curves for ceftriaxone (CRO) and ceftaroline (CPT) (8 times the MIC [8xMIC]) compared with control (no treatment) against penicillin-susceptible Streptococcus pneumoniae (ceftriaxone-susceptible penicillin-susceptible S. pneumoniae [CRO-S PSSP]), penicillin-intermediate S. pneumoniae (ceftriaxone-susceptible penicillin-intermediate S. pneumoniae [CRO-S PISP]), and penicillin-resistant S. pneumoniae (ceftriaxone-resistant penicillin-resistant S. pneumoniae [CRO-R PRSP]) strains. For each graph, the x axis shows log CFU/ml, and the y axis shows time (in hours).
Pharmacokinetic simulation of IV treatments like the human dosing regimen.
Serum drug concentrations obtained after simulated human dosing of rabbits with CRO and CPT are shown in Fig. 2. The corresponding PK parameters (Cmax, minimum drug concentration in serum [Cmin], and area under the concentration-time curve from 0 to 24 h [AUC0–24]) are presented in Table 2. There was good agreement between the human PK parameters and those reproduced in rabbits during these studies.
FIG. 2.
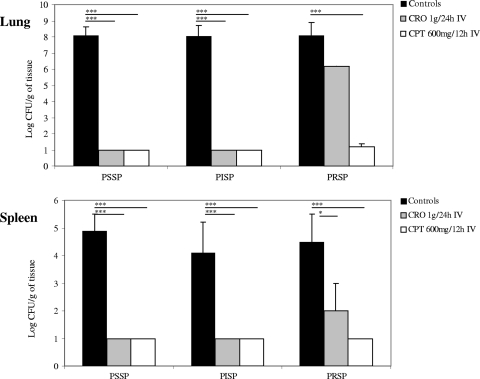
Bacterial content in the lungs and spleen in rabbits after 2 days of treatment with ceftriaxone (CRO) equivalent to 1 g given intravenously (IV) daily or ceftaroline (CPT) equivalent to 600 mg IV twice daily in the pneumonia model. The three strains used in Fig. 1 were used here. Values are means plus standard deviations (SDs) (error bars). The lower limit of detection was 1 log CFU/g. Values that are significantly different are indicated by the horizontal lines and asterisks as follows: *, P < 0.05; ***, P < 0.001. Abbreviations: PSSP, penicillin-susceptible S. pneumoniae, PISP, penicillin-intermediate S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae.
TABLE 2.
Pharmacokinetics of a model simulating the human dosing regimen in rabbitsa
| PK parameterb | Subject | Value for PK parameter(mean ± SD) |
|||
|---|---|---|---|---|---|
| Ceftriaxone IVc | Ceftaroline IV | Ceftaroline IMd |
|||
| 5 mg/kg | 20 mg/kg | ||||
| Cmax (mg/liter) | Rabbit | 158 ± 30 | 20 ± 5 | 2.8 ± 0.2 | 12.2 ± 0.2 |
| Human | 130–200 | 23.5 ± 3.8 | ND | ND | |
| Cmin (mg/liter) | Rabbit | 6 ± 1.7 | 1.3 ± 0.3 | 0 ± 0 | 0 ± 0 |
| Human | 5–7 | 0.46 ± 0.23 | ND | ND | |
| AUC0–24 (mg · h/liter) | Rabbit | 938 ± 80 | 155 ± 14 | 14 ± 2 | 55 ± 2.5 |
| Human | 800–1,000 | 124 ± 22 | ND | ND | |
Pharmacokinetics of a model simulating the human dosing regimen in rabbits using ceftriaxone equivalent to 1 g given intravenously (IV) daily, ceftaroline equivalent to 600 mg given IV twice daily, or ceftaroline at a dose of 5 mg/kg given intramuscularly (IM) or a dose of 20 mg/kg given IM twice daily for 48 h (expressed as the total drug fraction).
PK, pharmacokinetic; Cmax, maximum drug concentration in serum; Cmin, minimum drug concentration in serum; AUC0–24, area under the concentration-time curves from 0 to 24 h.
Human pharmacokinetic data for ceftriaxone are expressed as a range because of the large variation that has been reported (20, 24).
ND, not determined.
Following IM administration of CPT at dosages of 5 mg/kg or 20 mg/kg twice daily, the mean Cmaxs ± SDs were 2.8 ± 0.2 and 12.2 ± 0.2 mg/liter, respectively. Additional data on the pharmacokinetic parameters following IM administration are presented in Table 2.
Protein binding.
For ceftaroline, the nonspecific binding at 50 mg/liter was 30%, and the level of protein binding corresponded to a mean of 47% with a free drug percentage of 53%. Conversely, the level of protein binding for ceftriaxone was variable, depending on the concentrations tested (66% for high concentrations in plasma and 95.5% for low concentrations in plasma).
Antimicrobial effects of CPT and CRO using simulated human dosing in a rabbit model of experimental pneumococcal pneumonia.
None of the animals died in the first 48 h after infection regardless of the S. pneumoniae strain used or treatment tested. At the start of therapy, the mean pulmonary bacterial concentration was 8.73 ± 0.54 log10 CFU/g. All rabbits in the control group (no treatment) were septicemic with high bacterial concentrations in the spleen (ranging from 4 to 4.91 log10 CFU/g).
Both CPT and CRO were effective in treating pulmonary infection caused by CRO-S PSSP or CRO-S PISP isolates. Complete bacterial eradication in the lungs and spleen was observed after 2 days of treatment (Fig. 2).
Against PRSP, 2 days of treatment with IV CPT demonstrated excellent bactericidal activity in vivo compared to the controls (no treatment) (P < 0.001). CPT treatment was associated with complete bacterial eradication in the spleen and nearly complete eradication in lungs (Fig. 2). This activity was consistent across all animals tested and correlated with the in vitro activity of CPT. In contrast, after IV CRO treatment, bacterial counts in the lungs of rabbits infected with the same PRSP strain were not significantly different from those of the controls (Fig. 2). Bacterial reductions in the spleen, however, were significant (P < 0.05).
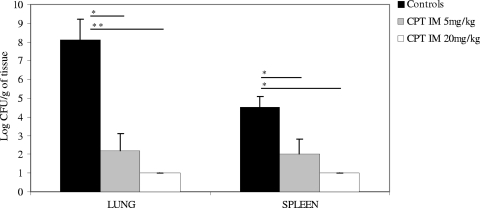
Intramuscular administration of CPT for 2 days at both dosages tested was very effective against PRSP pneumonia, with the highest activity observed in the 20-mg/kg twice-daily group (Fig. 3). Bacterial reductions in the lungs and spleen after CPT treatment were statistically significant compared to the counts for control animals. In the spleen, a 3-log-unit reduction was obtained with the 5-mg/kg twice-daily dosage and complete eradication was achieved with the 20-mg/kg twice-daily dosage of CPT.
FIG. 3.
Bacterial content in the lungs and spleen in rabbits infected with penicillin and ceftriaxone-resistant Streptococcus pneumoniae after 2 days of ceftaroline (CPT) therapy (5 mg/kg or 20 mg/kg IM twice daily) in the pneumonia model. Results are expressed as means plus SDs. The lower limit of detection was 1 log CFU/g. Values that are significantly different are indicated by the horizontal lines and asterisks as follows: *, P < 0.05; **, P < 0.01. IM, intramuscular.
Pharmacodynamic analysis.
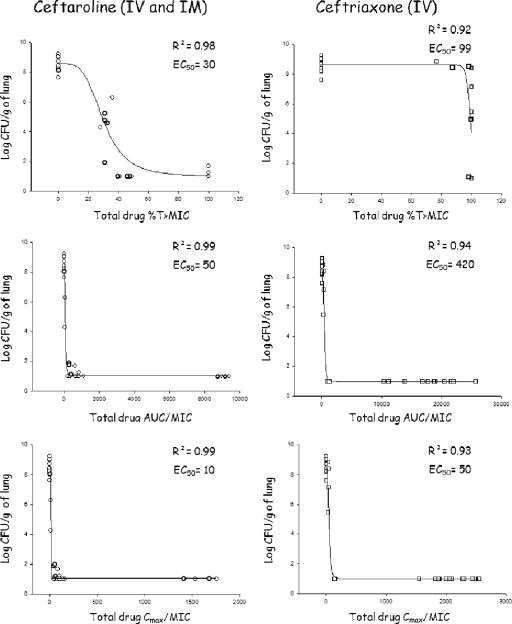
The %T>MIC, AUC/MIC, and Cmax/MIC values for both antibiotics are shown in Fig. 4 (for total drug). As expected for cephalosporin antibiotics, %T>MIC was the PD parameter that best predicted the efficacy of both drugs. A %T>MIC of 40 for CPT (50% effective concentration [EC50] = 30%) was associated with complete eradication of bacterial content in this PRSP rabbit model of pneumonia, whereas a %T>MIC of 100 for CRO (EC50 = 99%) never achieved complete eradication. Efficacy was also correlated with AUC/MIC and Cmax/MIC (r2 = 0.9). The magnitude of different PK/PD indices for free drug were evaluated on the basis of the protein binding values obtained ex vivo. For ceftriaxone, as the percentage of protein binding was quite variable, the PK/PD indices for free drug were difficult to simulate, and there was no Hill slope that could be found significant. Nevertheless, the EC50 for %T>MIC was greater than 40% (r2 = 0.67).
FIG. 4.
Relationship between the total drug levels of ceftaroline and ceftriaxone and the pharmacodynamic parameters %T>MIC, Cmax/MIC, and AUC0–24/MIC for Streptococcus pneumoniae (all strains combined CRO-S PSSP, CRO-S PISP, and CRO-R PRSP) with the residual bacterial concentration in the lungs after 48 h of therapy. Each symbol represents the value for an individual rabbit. Abbreviations: AUC, area under the curve; Cmax, maximum drug concentration in serum; EC50, dose necessary to achieve 50% of the bactericidal effect; IM, intramuscular; IV, intravenous; R2, coefficient of correlation; %T>MIC, cumulative percentage of a 24-h period that the drug concentration exceeds the MIC.
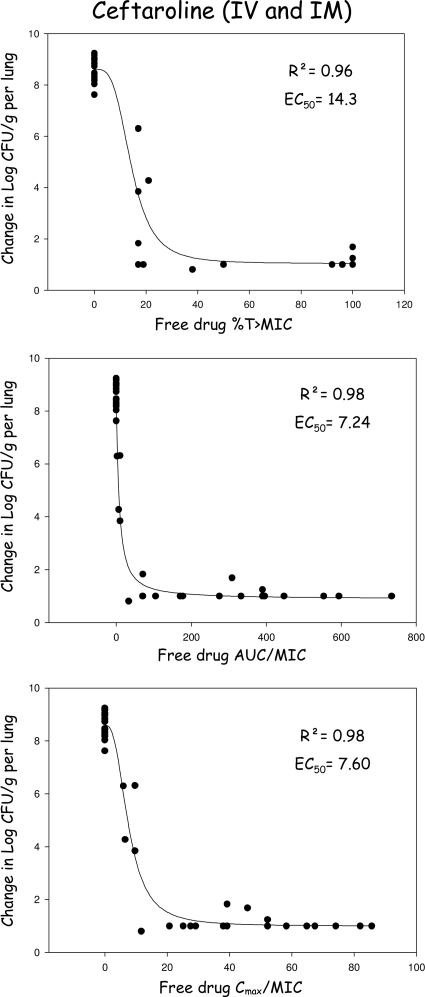
For ceftaroline, the PK/PD indices based on free drug concentrations are shown in Fig. 5. The EC50 for %T>MIC was 14.3, which was significantly lower than that required for ceftriaxone, and complete eradication was obtained for a free %T>MIC of 25.
FIG. 5.
Relationship between the free drug levels of ceftaroline and the pharmacodynamic parameters %T>MIC, Cmax/MIC, and AUC0–-24/MIC for Streptococcus pneumoniae (all strains combined CRO-S PSSP, CRO-S PISP, and CRO-R PRSP) with the residual bacterial concentration in the lungs after 48 h of therapy. Each symbol represents the value for an individual rabbit. AUC, area under the curve; Cmax, maximum serum drug concentration; EC50, dose necessary to achieve 50% of the bactericidal effect; IM, intramuscular; IV, intravenous; R2, coefficient of correlation; %T>MIC, cumulative percentage of a 24-h period that the drug concentration exceeds the MIC.
DISCUSSION
Previous in vitro studies have demonstrated that CPT has excellent broad-spectrum activity, including potent activity against Gram-positive organisms such as MRSA and MDRSP, as well as activity against Gram-negative species, including Haemophilus influenzae, Moraxella catarrhalis, and ceftazidime-sensitive members of the family Enterobacteriaceae (4, 18, 25, 28, 29). The efficacy of CPT in vivo was previously demonstrated against MRSA in a rabbit endocarditis model (22) and against Gram-negative species in mouse thigh and pneumonia models (1).
In the present study employing simulated human dosing in a rabbit model, we showed that both CPT and CRO, when administered IV, were highly effective against pneumonia induced by CRO-S PSSP or CRO-S PISP strains. Against the PRSP strain, however, CRO treatment (equivalent to a human dose of 1 g daily) resulted in a 2-log-unit reduction in bacterial counts in lungs that did not reach a level of statistical significance. Ceftaroline (equivalent to a human dose of 600 mg twice daily) exhibited superior efficacy, reducing bacterial counts in the lungs and spleen by approximately 8 and 4 log units, respectively, and essentially eradicating the infection.
Ceftaroline administered IM at 5 mg/kg or 20 mg/kg twice daily was also highly effective in reducing PRSP bacterial counts in the lungs and spleen. The 5-mg/kg twice-daily dosage reduced the bacterial burden by approximately 6 log units in the lungs and 3 log units in the spleen, and the 20-mg/kg twice-daily dosage effectively eradicated the infection (the difference in CFU between the two dosages of ceftaroline was not significant). These data suggest an excellent profile and bioavailability for CPT following IM administration.
Our findings suggest that infections caused by S. pneumoniae that are currently treated with CRO may be more appropriately treated with CPT given the increasing prevalence of MDRSP (2, 13, 16). These infections include community-acquired pneumonia. Ceftaroline may be an effective alternative to CRO where resistant S. pneumoniae is suspected on the basis of local surveillance and antibiogram data.
A treatment regimen of CPT simulating the 600-mg twice-daily dosage in humans was associated with 95% and 100% eradication of pulmonary and splenic bacterial counts, respectively. Data from the IM administration of CPT allowed us to perform a PD analysis and to model a sigmoid dose-response relationship. The %T>MIC was found to be one of the best PD predictors of efficacy. A %T>MIC of greater than 30 was strongly predictive of bactericidal efficacy of CPT in this model.
Most of the described murine pneumococcal pneumonia efficacy models (3, 23) utilize immunocompromised animals to avoid spontaneous bacterial clearance and may also use drugs that induce renal insufficiency to avoid rapid antibiotic elimination. Advantages of the rabbit model utilized in the present study include the facts that severe disease can be induced in immunocompetent animals and that pharmacokinetics reflective of human dosing can be simulated using computer-driven infusion pumps.
Conclusions.
Ceftaroline, a novel cephalosporin recently approved by the FDA for use in acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia has been demonstrated to be highly active against three strains of S. pneumoniae with various susceptibilities to penicillin (CRO-S PSSP, CRO-S PISP, and CRO-R PRSP) in a rabbit model of pneumonia using IV administration with simulated human dosing. Ceftaroline was also effective when administered by the IM route in the PRSP pneumonia model, suggesting an alternative administration option for certain treatment settings. These findings further validate the in vivo bactericidal activity of CPT against pneumococci. Ceftaroline appears to be a promising antimicrobial for the treatment of pneumococcal pneumonia, including infections caused by penicillin-resistant strains.
ACKNOWLEDGMENTS
We acknowledge Scientific Therapeutics Information, Inc., Springfield, NJ, for providing editorial assistance for the manuscript.
Funding for these studies and for editorial assistance was provided by Forest Laboratories, Inc.
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Andes D., Craig W. A. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appelbaum P. C. 1996. Emerging resistance to antimicrobial agents in gram-positive bacteria. Drugs 51(Suppl. 1):S1–S5 [DOI] [PubMed] [Google Scholar]
- 3. Azoulay-Dupuis E., et al. 1991. Antipneumococcal activity of ciprofloxacin, ofloxacin and temafloxacin in an experimental mouse pneumonia model at various stages of the disease. J. Infect. Dis. 163:319–324 [DOI] [PubMed] [Google Scholar]
- 4. Biek D., Ge Y., Sahm D. F. 2008. Activity of ceftaroline against common respiratory-associated pathogens from a collection of recent US isolates, poster C19. 2008 Am. Thor. Soc. Int. Conf., 16 to 21 May 2008, Toronto, Canada [Google Scholar]
- 5. Centers for Disease Control and Prevention 2007. Active Bacterial Core Surveillance (ABCs) Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2006 Centers for Disease Control and Prevention, Atlanta, GA http://www.cdc.gov/abcs/reports-findings/survreports/spneu06.pdf [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement. CLSI document M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Comité de l'Antibiogramme de la Société Française de Microbiologie 2008. Recommandations 2008, p. 38–40 Société Française de Microbiologie, Paris, France: http://www.sfm-microbiologie.org/UserFiles/file/CASFM/casfm_2008.pdf [Google Scholar]
- 8. Croisier D., et al. 2002. Pharmacodynamics (PKPD) of human-like treatment with moxifloxacin (MFX) and levofloxacin (LEV) on experimental pneumonia due to penicillin-resistant pneumococci (PRSP) with or without parC mutations. Presented at the 42nd Intersci. Conf. Antimicrob. Agents Chemother., 27 to 30 September 2002, San Diego, CA [Google Scholar]
- 9. Croisier D., et al. 2002. Value of the selection index (SI) and mutant selection window (MSW) in the pharmacodynamics (PKPD) of mutants (M): application to fluoroquinolones versus pneumococcal pneumonia (PP). Presented at the 42nd Intersci. Conf. Antimicrob. Agents Chemother., 27 to 30 September 2002, San Diego, CA [Google Scholar]
- 10. Croisier D., et al. 2002. Efficacy and pharmacodynamics of simulated human-like treatment with levofloxacin on experimental pneumonia induced with penicillin-resistant pneumococci with various susceptibilities to fluoroquinolones. J. Antimicrob. Chemother. 50:349–360 [DOI] [PubMed] [Google Scholar]
- 11. Croisier D., et al. 2004. In vivo pharmacodynamic efficacy of gatifloxacin against Streptococcus pneumoniae in an experimental model of pneumonia: impact of the low levels of fluoroquinolone resistance on the enrichment of resistant mutants. J. Antimicrob. Chemother. 54:640–647 [DOI] [PubMed] [Google Scholar]
- 12. Davidson R., et al. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747–750 [DOI] [PubMed] [Google Scholar]
- 13. Draghi D. C., et al. 2006. Geographically-based evaluation of multidrug resistance trends among Streptococcus pneumoniae in the USA: findings of the FAST surveillance initiative (2003-2004). Int. J. Antimicrob. Agents 28:525–531 [DOI] [PubMed] [Google Scholar]
- 14. Empey P. E., et al. 2001. Levofloxacin failure in a patient with pneumococcal pneumonia. Ann. Pharmacother. 35:687–690 [DOI] [PubMed] [Google Scholar]
- 15. Etienne M., et al. 2004. Effect of low-level resistance on subsequent enrichment of fluoroquinolone-resistant Streptococcus pneumoniae in rabbits. J. Infect. Dis. 190:1472–1475 [DOI] [PubMed] [Google Scholar]
- 16. Felmingham D., et al. 2005. The Alexander Project: the benefits from a decade of surveillance. J. Antimicrob. Chemother. 56(Suppl. 2):ii3–ii21 [DOI] [PubMed] [Google Scholar]
- 17. Fishman N. O., et al. 1999. Three levofloxacin treatment failures of pneumococcal respiratory tract infections. Presented at the 39th Intersci. Conf. Antimicrob. Agents Chemother., 26 to 29 September 1999, San Francisco, CA [Google Scholar]
- 18. Ge Y., et al. 2007. In vitro activity of ceftaroline against a collection of recent gram-positive and gram-negative US isolates. Presented at the 47th Intersci. Conf. Antimicrob. Agents Chemother., 17 to 20 September 2007, Chicago, IL [Google Scholar]
- 19. Ge Y., et al. 2006. The pharmacokinetics (PK) and safety of ceftaroline (PPI-0903) in healthy subjects receiving multiple-dose intravenous (IV) infusions. Presented at the 46th Intersci. Conf. Antimicrob. Agents Chemother., 17 to 20 September 2006, San Francisco, CA [Google Scholar]
- 20. Goonetilleke A. K., et al. 1996. A comparative analysis of pharmacokinetics of ceftriaxone in serum and pleural fluid in humans: a study of once daily administration by intramuscular and intravenous routes. J. Antimicrob. Chemother. 38:969–976 [DOI] [PubMed] [Google Scholar]
- 21. Jacobs M. R., et al. 2008. Changes in serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae strains in Cleveland: a quarter century of experience. J. Clin. Microbiol. 46:982–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacqueline C., et al. 2007. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob. Agents Chemother. 51:3397–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leggett J. E., et al. 1990. Comparative dose-effect relations at several dosing intervals for beta-lactam, aminoglycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis model. Scand. J. Infect. Dis. Suppl. 74:179–184 [PubMed] [Google Scholar]
- 24. Meyers B. R., et al. 1983. Crossover study of the pharmacokinetics of ceftriaxone administered intravenously or intramuscularly to healthy volunteers. Antimicrob. Agents Chemother. 24:812–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morrissey L., et al. 2007. Activity of ceftaroline against community-acquired pneumonia (CAP) bloodstream isolates. Presented at the 47th Intersci. Conf. Antimicrob. Agents Chemother., 17 to 20 September 2007, Chicago, IL [Google Scholar]
- 26. Mushtaq S., Warner M., Ge Y., Kaniga K., Livermore D. M. 2007. In vitro activity of ceftaroline (PPI-0903M, T-91825) against bacteria with defined resistance mechanisms and phenotypes. J. Antimicrob. Chemother. 60:300–311 [DOI] [PubMed] [Google Scholar]
- 27. Piroth L., et al. 1999. Development of a new experimental model of penicillin-resistant Streptococcus pneumoniae pneumonia and amoxicillin treatment by reproducing human pharmacokinetics. Antimicrob. Agents Chemother. 43:2484–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sader H. S., Fritsche T. R., Jones R. N. 2008. Antimicrobial activities of ceftaroline and ME1036 tested against clinical strains of community-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 52:1153–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sader H. S., et al. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Société de Pathologie Infectieuse de Langue Française 2000. Révision de la IVe conférence de consensus en thérapeutique anti-infectieuse de la Société de Pathologie Infectieuse de Langue Française. Méd. Mal. Infect. 30:566–580 [Google Scholar]
- 31. Song J. H., et al. 2008. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int. J. Antimicrob. Agents 31:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wortmann G. W., Bennett S. P. 1999. Fatal meningitis due to levofloxacin-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 29:1599–1600 [DOI] [PubMed] [Google Scholar]
- 33. Zervos M., et al. 2004. Comparative efficacies and tolerabilities of intravenous azithromycin plus ceftriaxone and intravenous levofloxacin with step-down oral therapy for hospitalized patients with moderate to severe community acquired pneumonia. Treat. Respir. Med. 3:329–336 [DOI] [PubMed] [Google Scholar]