Abstract
Oritavancin is a novel lipoglycopeptide with demonstrated effectiveness against complicated skin and skin structure infections (cSSSI) caused by Gram-positive pathogens, including those caused by methicillin-resistant Staphylococcus aureus (MRSA). The pharmacokinetic and pharmacodynamic profile of oritavancin is favorable for single or infrequent dosing. A phase 2, multicenter, randomized, double-blind, parallel, active-comparator study (ClinicalTrials.gov identifier, NCT00514527) of single and infrequent dosing of intravenous (i.v.) oritavancin for the treatment of cSSSI caused by Gram-positive pathogens (wound infections, major abscess, and cellulitis) was undertaken to evaluate the noninferiority of front-loaded dosing regimens compared to a daily-dosing regimen. A total of 302 patients ≥18 years of age were randomized equally to one of three oritavancin treatment groups, receiving either a daily dose (200 mg) administered for 3 to 7 days, a single dose (1,200 mg), or an infrequent dose (800-mg dose, with the option for an additional 400 mg on day 5). The primary efficacy was defined as a clinical response in clinically evaluable (CE) patients assessed at days 21 to 29 (test of cure [TOC]). The cure rates in the CE population were 72.4% (55/76) in the daily-dose group, 81.5% (66/81) in the 1,200-mg-single-dose group, and 77.5% (55/71) in the infrequent-dose group. In patients with MRSA at baseline, the cure rates were 78.3% (18/23), 73.0% (27/37), and 87.0% (20/23) in the daily-, 1,200-mg-single-, and infrequent-dose groups, respectively; however, the study was not powered to assess outcomes in the MRSA subpopulation, and given the heterogeneity of the types of infection and the small sample size, these do not suggest any true differences in efficacy rates for these pathogens. The frequencies of adverse events were similar among treatment groups. The results of this study show that single- and infrequent-dosing schedules of oritavancin were as efficacious as daily administration and had a similar safety profile in treating cSSSI caused by Gram-positive pathogens, including MRSA.
INTRODUCTION
Complicated skin and skin structure infections (cSSSI) (since this study was designed, the indication has been redefined by the 2010 FDA draft guidance as acute bacterial skin and skin structure infections) are primarily caused by Gram-positive bacteria, including Staphylococcus aureus (both methicillin-susceptible and methicillin-resistant strains), Streptococcus pyogenes, and, less frequently, Enterococcus faecalis (43). Complicated skin and skin structure infections involve deeper skin or soft tissue structures and require rapid and intensive antimicrobial intervention to minimize tissue damage and prevent further spread of infection. S. aureus remains the leading etiology, with an increasing prevalence of methicillin-resistant S. aureus (MRSA) being seen in the United States (31). Oritavancin is a novel intravenous (i.v.) lipoglycopeptide with multiple mechanisms of action (10, 12, 30, 39). It has broad in vitro activity against Gram-positive pathogens, including MRSA (3, 5) and S. aureus strains with reduced susceptibility to vancomycin (7). It has also demonstrated in vivo activity in animals against S. aureus, including MRSA (32, 38). Oritavancin exhibits rapid in vitro concentration-dependent bactericidal activity against common skin pathogens (34) and is active intracellularly against pathogens sequestered in neutrophils (1, 33) and macrophages (37).
Available treatment regimens for cSSSI can range from 7 to 14 days for once-daily dosing with daptomycin (22) and telavancin (46) to twice-per-day dosing with linezolid for 10 to 14 days (40) and i.v. vancomycin for 7 to 14 days (8). As the incidence of infections due to resistant pathogens increases, new antimicrobial agents and innovative regimens of current therapies continue to be explored to establish dosing regimens that maximize the benefit of therapy and contain the spread of resistance (48).
The pharmacodynamic (PD) and pharmacokinetic (PK) profiles of oritavancin are unique and suggest that oritavancin could be effective given in a single dose (15, 17, 21, 29). Oritavancin's maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) are linear and dose proportional across administered doses (1 to 1,200 mg) (42). In humans, the mean ± standard deviation (SD) plasma and blister fluid exposure values for oritavancin, measured as AUC0-24 h, resulting from a single dose of 800 mg were 1,111 ± 316 and 208 ± 76.7 μg·h/ml, respectively (24). These values exceeded by 12-fold and 2.4-fold, respectively, the plasma exposure values necessary for a 1-log10 reduction in CFU of S. aureus in neutropenic mouse thigh infection modeling (14). Furthermore, a humanized dosing regimen mimicking a 1,200-mg single dose of oritavancin administered to neutropenic mice with S. aureus thigh infections resulted in a greater rate and extent of bacterial kill than did a regimen simulating 400 mg once daily for 3 days, indicating that a front-loaded dose of oritavancin could provide for faster and more sustained bacterial killing activity than an equivalent cumulative dose administered in a fractionated manner (16, 38).
Oritavancin is not metabolized following i.v. dosing. Instead, it is slowly excreted, unchanged, in both the urine and the feces (terminal half-life = 393 ± 73.5 h), which means that no dosage adjustment is required for age, or for renal or mild to moderate hepatic dysfunction (42).
In two previous phase 3 studies evaluating the efficacy of oritavancin in treating cSSSI when dosed daily for 3 to 7 days (28), oritavancin was noninferior to the comparator (twice-daily vancomycin for 7 to 10 days [14 days for MRSA] followed by oral cephalexin). The SIMPLIFI study was designed to evaluate the noninferiority of two front-loaded treatment regimens (a single 1,200-mg dose and an infrequent dose of 800 mg on day 1, with an optional 400 mg on day 5) to the daily-dose regimen used in the previous phase 3 studies (200 mg administered for 3 to 7 days) for the treatment of cSSSI due to Gram-positive pathogens.
(Results of this study were presented at the 19th European Congress of Clinical Microbiology and Infections Diseases, Helsinki, Finland, May 2009.)
MATERIALS AND METHODS
This was a phase 2, multicenter, randomized, double-blind, parallel, active-comparator controlled study (ClinicalTrials.gov identifier, NCT00514527) designed based on the 1998 FDA guidance to industry on development of drugs for uncomplicated and complicated SSSI (26). Patients were enrolled at 43 sites in 5 countries (Australia, India, Romania, Ukraine, and the United States) between 9 September 2007 and 23 April 2008. The study was approved by site-specific ethics review boards, and all patients signed an approved-informed-consent document.
Selection of participants.
Patients were enrolled in the study if they had a cSSSI, presumed or proven to be caused by a Gram-positive pathogen(s), that met disease diagnostic criteria (listed below and classified by disease state), were ≥18 years of age, and had a body mass index of ≥17 kg/m2 and ≤40 kg/m2. Patients could be inpatient or outpatient, but outpatients were required to receive study drug infusions in a controlled setting. Investigators were responsible for screening patients to ensure that inclusion/exclusion criteria were met.
For the skin and skin structure infection to be classified as complicated, one or more of the following criteria had to be met: (i) infection required significant surgical intervention within 48 h before or after enrollment; (ii) the infection process was suspected or confirmed to involve deeper subcutaneous tissue (soft tissue), excluding fascia and/or muscle layers; or (iii) significant underlying disease was present that complicated the response to treatment, such as diabetes mellitus, bacteremia, cellulitis with an involvement of approximately 3% or more of the total body surface area, corticosteroid therapy (>7.5-mg/day equivalent of prednisone), neutropenia (absolute neutrophil count [ANC], 500/mm3 or less), cirrhosis (Child Pugh class B or C), burn (approximately 10% or more of the total body surface area), radiation therapy (local or systemic), or known immunosuppression (for example, organ transplantation, immunosuppressive therapy, or HIV infection or other immunosuppressive disease). Patients with renal insufficiency could also be included if renal function was stable.
Additional criteria had to be met for each of the three categories of infection (wound infection, major abscess, and cellulitis). Wound infections had to have involved purulent drainage from the wound or ulcer, but not from the organ/space component of the injury, and one or more of the following: systemic manifestations of infection, fever (>38°C), leukocytosis (white blood cell [WBC] count of >10,000/mm3 and/or a differential count showing >10% band forms), localized pain or tenderness, erythema, or localized swelling. Major abscesses had to have involved the following: an acute onset within 7 days prior to enrollment; purulent drainage or purulent aspirate; systemic manifestations of infection with fever or leukocytosis; erythema, induration (≥2 cm in diameter from the peripheral margin of the abscess), or tenderness; and evidence of loculated fluid by physical examination, blind aspiration, or ultrasound which required intervention (e.g., aspiration, incision and drainage, or excision) within 48 h of enrollment. Cellulitis had to have involved the following: an acute onset within 7 days prior to enrollment, pain or tenderness, cutaneous erythema, advancing edema or induration, and a history of fever within 3 days prior to enrollment.
Patients were excluded from the study if they had received any systemic antimicrobial agent with Gram-positive-pathogen coverage for more than 24 h within the 3 days prior to enrollment (unless the Gram-positive pathogen was resistant in vitro to the antimicrobial agent or the patient had failed prior therapy), had a history of severe hypersensitivity reactions to glycopeptides and any of their excipients (patients who had histamine-like infusion reactions to the glycopeptide vancomycin were not excluded), had an anticipated need for more than 10 days of conventional antibiotic therapy, or had an infection predominately caused by aerobic or anaerobic Gram-negative bacilli (e.g., diabetic foot ulcers). Pregnant women or women who were nursing were also excluded from the study, as were patients with underlying conditions that, in the opinion of the investigator, would have precluded the performance of protocol safety and efficacy assessments. The other exclusion criteria were as follows: toxic shock syndrome or toxic-like syndrome; presumed or proven infection caused by Clostridium species; incision wound or abscess that extended into visceral compartments; contiguous bone involvement; ischemic ulcers or wounds associated with arterial insufficiency or gangrene; infection of prosthetic materials that could not be removed as part of the treatment of current infection; infection of the scrotum, perineum, or parianal region; infection of a full-thickness burn wound or burn wound that was ≥20% of the total body surface area; malignant otitis externa; infection possibly containing Vibrio species; or secondary infection of a preexisting skin disease that could interfere with the clinical evaluations of the study. Additionally, patients with poor venous access and those who require monitoring of activated partial thromboplastin time (aPTT) were excluded (as oritavancin interferes with the aPTT test).
Study design.
Patients were randomized through an interactive voice response system at a 1:1:1 ratio to receive either the oritavancin comparator daily dose (200 mg i.v. daily for 3 to 7 days, as determined by the blinded investigator, based on clinical criteria), the oritavancin single dose (1,200 mg oritavancin i.v. on day 1), or the oritavancin infrequent dose (800 mg i.v. on day 1, with an optional 400 mg i.v. on day 5, as determined by the blinded investigator, based on clinical criteria). The investigator decided if further i.v. therapy was required only while remaining blinded to the treatment arm. During randomization, patients were stratified by the following disease categories: wound infection, major abscess, and cellulitis. Patients and investigators were blind to treatment assignment. An i.v. placebo (5% dextrose in water) was given at various time points, depending on the treatment arm, to maintain the blinding.
The primary objective of the study was to determine the clinical response rate of each treatment regimen in the CE and ITT populations at test of cure (TOC), which occurred on days 21 to 29. The secondary objective was to evaluate the safety of each dosing regimen.
During the study, basic clinical assessments were conducted daily from the baseline visit through the end of therapy (day 3 to day 7), at TOC, and at the late-follow-up time point (day 35 to day 42). These included investigator assessments of signs and symptoms of infection, such as fever, pain, tenderness, erythema, induration, edema, purulent drainage, eschar, and devitalized tissue, as well as safety monitoring. Safety monitoring included physical examinations, assessment of adverse events, laboratory tests (including hematology and clinical chemistry tests), electrocardiograms, and assessment of vital signs. At baseline, end of therapy, and TOC, photographic documentation and measurement (in centimeters) of the primary site of infection (minimum and maximum of erythema from edge of wound or abscess cavity) were included in these assessments.
Study drug was administered every 24 h in a controlled setting (i.e., a hospital or outpatient care center). Aztreonam and/or metronidazole could be administered at the discretion of the investigator for polymicrobial infections that included a Gram-negative pathogen(s) and/or anaerobes. Any other topical, oral, or systemic antibiotics with activity against Gram-positive pathogens were prohibited. Standard-of-care cSSSI therapy (including daily debridement and dressing changes) was mandatory. Other interventions for facilitating the care of patients, including surgical or nonsurgical debridement of devitalized tissue, removal of prosthetic material, incision and drainage, suture removal, percutaneous aspiration, packing, dressings, or irrigation, were left to the discretion of the investigator. Treatment interventions for cSSSI planned prior to randomization could occur at any time. If the patient's infection worsened, thereby requiring unanticipated or unplanned interventions ≥48 h after initiation of study drug therapy, then a clinical response of failure was assigned.
Blood and infection site cultures were obtained within 3 days prior to enrollment (often done at the time of enrollment). Baseline cultures obtained after randomization were excluded from microbiological assessment. End-of-therapy, TOC, and late-follow-up cultures (or those done at early relapse) were obtained from the infection site, if clinically indicated. If a patient proved to have bacteremia at baseline, follow-up blood cultures were performed at a minimum at end of therapy and TOC. Specimens were cultured, and pathogens were identified at each investigative site's certified laboratory. All isolated Gram-positive pathogens that were obtained from the infection site at baseline/randomization and up to and including the TOC time point were subcultured and sent to a central laboratory (Covance Clinical Laboratories, Indianapolis, IN) for confirmatory identification and susceptibility testing. Susceptibility was assessed using broth microdilution with 0.002% polysorbate 80 (6, 18, 19). In cases of discrepancy of identity of the isolate between the local and the central laboratory, central-laboratory results were used.
Endpoints.
Clinical response was assessed by the investigator as part of the end-of-therapy, TOC, and late-follow-up procedures by assessing the signs and symptoms of infection. A clinical response of cure, improvement, failure, or indeterminate was assigned at end of therapy and TOC by the investigator based on clinical signs and symptoms. At the late-follow-up time point (day 35 to day 42), the investigator could assign the clinical response of relapse in addition to the cure, improvement, or indeterminate response. Only patients with a clinical response of cure or improvement at TOC were assessed for clinical response at the late-follow-up time point.
Clinical cure in the study protocol included both cure and improvement assessments by the investigator. Investigator-assessed cure was defined at each time point (end of therapy, TOC, and late follow-up) as resolution of purulent drainage, pain, edema, fever, erythema, tenderness, and induration. Serous drainage or aspirate and/or granulation tissue could be present. To be assessed as an “improvement,” patients had to have resolution of purulent drainage and, in the case of cellulitis, cessation of fever and pain. Patients assessed as “improvement” could still have the following symptoms: residual erythema, edema, pain (in the case of wound or abscess), tenderness and/or induration, serous drainage, granulation tissue, eschar, and/or devitalized tissue. However, their primary infection had to have improved such that no further antimicrobial therapy was warranted, as determined by the blinded investigator. If nonstudy systemic antimicrobial therapy was initiated for the primary infection site, the investigator was questioned about the patient's clinical response and the patient was assigned the clinical response of failure. Failure included any of the following: presence of purulent drainage (or aspirate) and/or fever; unanticipated need for abscess drainage, unplanned debridement or increased number of debridements beyond what was initially anticipated at baseline as part of the expected evolution of the infection site, or removal of sutures (for treatment of infection) >48 h after initiation of study therapy; or treatment with a nonstudy systemic antibiotic having activity against a Gram-positive pathogen(s) for the primary infection site or use of topical antibiotics at the site of the primary infection 24 h or more after initiation of study medication therapy. If the investigator was unable to determine a clinical response, the infection was assessed as “indeterminate.” The reasons that a clinical response could not be determined involved either an error in the initial diagnosis of cSSSI or administration of an antibiotic with activity against the causative pathogens for an infection other than the cSSSI. Relapse, which could be determined only at the late-follow-up time point, was defined as a reappearance of signs and symptoms at the primary infection site, or the patient received a nonstudy antibiotic with activity against infections caused by Gram-positive pathogens for the previously treated skin and skin structure infection after a response of cure or improvement at TOC.
Planned analyses.
Analyses were performed on four patient populations: the intent-to-treat (ITT), microbiological intent-to-treat (MITT), clinically evaluable (CE), and microbiologically evaluable (ME) populations. The ITT population included all patients who were randomized to treatment and received any amount of study medication. The MITT population included all ITT patients with a Gram-positive pathogen isolated at baseline. The CE population included ITT patients who met enrollment criteria, received a minimum (≥80%) of the intended study drug dose, and did not have a clinical response of indeterminate. Patients in the ME population were CE patients who had a Gram-positive pathogen isolated at baseline.
To determine the sample size for this study, the clinical response rates at TOC in the CE population were assumed to be 85% for treatment groups. A total of 210 CE patients (70 per treatment group) yielded nearly 80% power to declare noninferiority of oritavancin front-loaded dosing regimens to the daily-dosing regimen within a margin of 15% at the 1-sided alpha level of 0.05. A 15% noninferiority margin was considered justified for a phase 2 clinical trial based on regulatory guidance documents and regulatory feedback at the time of protocol development. Assuming an evaluability rate of 70%, a sample of 300 total patients (100 per treatment group) needed to be enrolled to obtain 210 CE patients.
The primary hypothesis was that oritavancin single or infrequent doses were noninferior to oritavancin daily doses. The primary efficacy endpoint was clinical response (either cure or improvement versus failure) at TOC in the CE population. “Improvement” was included as a clinical response as long as the signs and symptoms of the primary infection improved significantly enough such that no further systemic antimicrobial therapy was required, as determined by the investigator. This approach was in line with the 1998 FDA guidance to industry (26) for developing antimicrobial drugs for treatment of uncomplicated and complicated SSSI but would not apply in a more contemporary study design. It is important to emphasize that any patient who was considered improved could not have received systemic antimicrobial therapy for the primary infection site through the late-follow-up period, 35 to 42 days after the initial diagnosis of cSSSI. The primary efficacy analysis was a comparison of the proportion of CE patients in the daily-dose group with a clinical response of cure (defined as investigator assessment of cure or improvement) and the proportions of patients in the 1,200-mg-single-dose group and the infrequent-dose group with a clinical response of cure. Adjusted estimates for the differences in response rates and the corresponding confidence intervals (CIs) were constructed using the Mantel-Haenszel method stratified by disease category.
All adverse events were evaluated by the investigator for intensity, seriousness, and causal relationship to the use of study medication. An adverse event was any untoward medical occurrence experienced by the patient, including death, intercurrent illness, or change in medical status from baseline. A serious adverse event was defined as any adverse event that resulted in (i) death, (ii) medical or surgical intervention associated with initial or prolonged hospitalization (extending drainage of an abscess was not considered a serious adverse event), (iii) a life-threatening experience, (iv) severe or permanent disability, (v) a significant hazard, contraindication, side effect, or precaution, as determined by the investigator or the Sponsor's medical review, or (vi) an adverse event that was significant for other reasons. All adverse events were categorized as mild (awareness of signs or symptoms which were tolerated with minimum discomfort), moderate (enough discomfort to cause interference but not an inability on the part of the patients to perform their usual level of activity), or severe (enough discomfort to cause an inability on the part of the patients to perform their usual level of activity). All adverse event terms were coded to the Medical Dictionary for Regulatory Activities (MedDRA, version 10.1). Patients were evaluated up to the late-follow-up time point (days 35 to 42) to assess for potentially late adverse events.
Data were tabulated by treatment group. Descriptive statistics for continous variables included number of patients (n), mean, standard deviation (SD), median, and range (minimum and maximum). For categorical data, frequency counts and percentages were presented. Statistical analyses were performed using SAS version 9.1 or higher.
RESULTS
Patients.
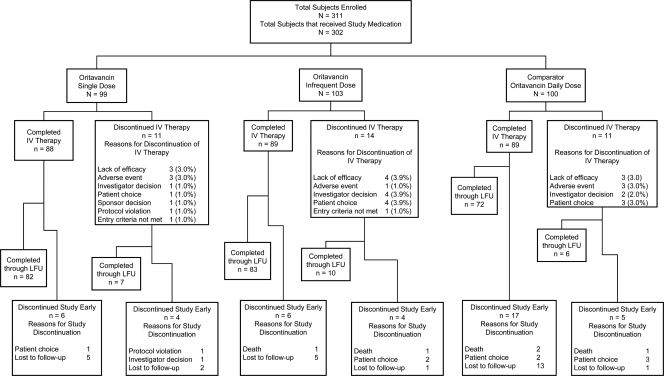
A total of 302 patients were randomized and received study medication (100 in the daily-dose group, 99 in the 1,200-mg-single-dose group, and 103 in the infrequent-dose group), with 228 (75.5%) of these patients being clinically evaluable (Table 1 and Fig. 1). Two patients in the ITT population were potentially unblinded due to human error prior to completion of the study. Both patients were in the 200-mg-daily-dosing group and were assessed as cure at TOC. Due to concerns that site personnel could determine the patients' treatment assignments, which might affect their blinded assessment of efficacy, these two patients were not included in any efficacy populations, although they were included in all safety analyses. Including these patients in the efficacy analyses does not change the statistical significance between the treatment groups. In the ITT population, 89.9% [89/100] of patients in the daily-dose group, 88.9% [88/99] of patients in the 1,200-mg-single-dose group, and 86.4% [89/103] of patients in the infrequent-dose group completed i.v. therapy. In the ITT population, 31.8% (96/302) of patients had wound infections (19 with surgical infections, 47 with trauma, 1 with infected burn, and 29 with skin ulcers), 37.7% (114/302) had major abscesses, and 30.5% (92/302) had cellulitis. Demographics and baseline characteristics were statistically equivalent among the three dosing groups. Patients received the following planned interventions: wound dressing (81.5% [246/302]), debridement (50.3% [152/302]), incision and drainage (33.4% [101/302]), packing and dressing change (12.9% [39/302]), and aspiration (4.6% [14/302]). A few patients (≤5%) received other standard care procedures, such as compresses or grafting. Unplanned interventions, including unplanned debridements ≥48 h after study drug initiation, were investigator-assessed as treatment failure.
Table 1.
Study populations
| Patient population | No. of patients given indicated oritavancin dose(s) (%) |
|||||
|---|---|---|---|---|---|---|
| 200 mg (n = 100) | 1,200 mg (n = 99) | 800 mg |
Total (n = 302) | |||
| All (n = 103) | 800 mg only (n = 34) | 800 and 400 mg (n = 69) | ||||
| Intent to treata | 100 (100) | 99 (100) | 103 (100) | 34 (100) | 69 (100) | 300 (99.3) |
| Clinically evaluable | 76 (76.0) | 81 (81.8) | 71 (68.9) | 23 (67.6) | 48 (69.6) | 228 (75.5) |
| Microbiological intent to treat | 72 (72.0) | 68 (68.7) | 69 (67.0) | 18 (52.9) | 51 (73.9) | 209 (69.2) |
| Microbiologically evaluable | 55 (55.0) | 58 (58.6) | 48 (46.6) | 11 (32.4) | 37 (53.6) | 161 (53.3) |
Two patients in the intent-to-treat population were unblinded prior to completion of the study. These two patients were not included in any efficacy populations but were included in all safety analyses.
Fig. 1.
Overall patient disposition. LFU, late follow-up.
Baseline pathogens and susceptibility.
At least one Gram-positive organism was isolated from the infection site at baseline in 69.2% (209/302) patients in the ITT population. The most commonly isolated pathogen was S. aureus, which was isolated from 87.6% (183/209) of MITT patients. Methicillin-resistant S. aureus was isolated in 49% (103/209) of MITT patients. The other three most common pathogens identified in the MITT population were Streptococcus pyogenes (5.7% [12/209]), Streptococcus agalactiae (3.8% [8/209]), and Enterococcus faecalis (3.8% [8/209]). The range of oritavancin MICs for S. aureus in the MITT population was 0.008 to 0.5 μg/ml. The oritavancin MIC90 for all S. aureus isolates and for the methicillin-susceptible S. aureus (MSSA) and MRSA subsets was 0.12 μg/ml. The values for microbiological parameters, including area under the bactericidal curve, derived from time-kill studies with 87 S. aureus isolates from the CE population and the oritavancin MICs for these same isolates were not significantly different between the cure and failure groups (9). Furthermore, in vitro susceptibilities to oritavancin and vancomycin for S. aureus (n = 181) from patients in the MITT population were not substantially correlated (35).
Clinical efficacy.
The clinical cure rates at TOC in the CE population were 72.4% (55/76), 81.5% (66/81), and 77.5% (55/71) in the daily-dose, 1,200-mg-single-dose, and infrequent-dose groups, respectively (Table 2). The estimated difference in cure rates (90% CIs) between the single- and daily-dose groups was 8.6% (−2.5, 18.2). The difference in cure rates between the infrequent- and daily-dose groups was 5.2% (−6.8, 15.4). The single-dose and infrequent-dose regimens of oritavancin were noninferior to the daily-dose regimen. For clinically evaluable patients in the infrequent-dose group who received 800 mg on day 1 (67.6% [23/71]) and those who received 800 mg on day 1 plus the optional 400-mg dose on day 5 (69.6% [48/71]), the cure rates were 78.3% (18/23) and 77.1% (37/48), respectively. These were comparable to overall cure rates. The cure rates by disease category were comparable among all treatment groups for patients with wound infections and major abscesses and between the infrequent- and daily-dose groups for patients with cellulitis (Table 2). A statistically higher cure rate (90% CI, 9.2 to 49.1) was seen for patients with cellulitis in the 1,200-mg-single-dose group (87.5% [21/81]) than for patients with cellulitis in the daily-dose group (58.3% [14/76]). Of the cellulitis patients whose outcome was failure, more patients in the daily-dose group had unplanned surgical procedures or interventions (29% [4/14]) than in the single-dose group (0/9) or the infrequent-dose group (7% [1/14]). Unplanned surgical procedures and interventions could include surgical or nonsurgical debridement of devitalized tissue, removal of prosthetic material, incision and drainage, suture removal, percutaneous aspiration, packing, dressings, or irrigation.
Table 2.
Clinical cure rates at test of cure in efficacy populationsa
| Patient population | % of patients cured at indicated oritavancin dose (no. of patients with response/total no. of patients)b |
Estimated difference in % of patients cured at indicated oritavancin doses (90% CI)c |
|||
|---|---|---|---|---|---|
| 200 mg (n = 98)d | 1,200 mg (n = 99) | 800 mg (n = 103) | 1,200 and 200 mg | 800 and 200 mg | |
| Intent to treat | 72.4 (63/87) | 81.8 (72/88) | 78.2 (68/87) | 8.7 (−1.7, 17.8) | 5.1 (−5.8, 14.6) |
| Clinically evaluable | 72.4 (55/76) | 81.5 (66/81) | 77.5 (55/71) | 8.6 (−2.5, 18.2) | 5.2 (−6.8, 15.4) |
| Wound | 65.4 (17/26) | 66.7 (18/27) | 72.0 (18/25) | 1.3 (−20.1, 22.7) | 6.6 (−14.7, 27.9) |
| Major abscess | 92.3 (24/26) | 90.0 (27/30) | 87.5 (21/24) | −2.3 (−14.8, 10.1) | −4.8 (−18.9, 9.2) |
| Cellulitis | 58.3 (14/24) | 87.5 (21/24) | 72.7 (16/22) | 29.2 (9.2, 49.1)e | 14.4 (−8.4, 37.2) |
| Microbiological intent to treat | 68.8 (44/64) | 80.3 (49/61) | 80.6 (50/62) | 10.1 (−2.7, 20.9) | 11.1 (−1.5, 21.7) |
| Microbiologically evaluable | 69.1 (38/55) | 79.3 (46/58) | 81.3 (39/48) | 8.5 (−5.2, 20.0) | 11.0 (−2.9, 22.6) |
“Cure” includes cure and improvement outcomes.
Excludes missing or intermediate patients.
Difference in response rate between patients as determined by using the Mantel-Haenszel method, stratified by disease.
Two patients in the intent-to-treat population were unblinded prior to completion of the study. These two patients are therefore not included in any efficacy analyses. Both patients were in the 200-mg-daily-dosing group and were assessed as cured at TOC. Including these patients in the efficacy analyses does not change the statistical significance among the treatment groups.
After data analysis was complete, it was discovered that one patient randomized to the 1,200-mg-single-dose group actually received 200 mg/day for 6 days. A sensitivity analysis was performed by switching the patient from the single-dose group to the daily-dose group, and the statistical significance remained unchanged in the cellulitis disease category.
The cure rates at TOC were 67.4% (31/46), 78.9% (45/57), and 79.5% (31/39) for patients with S. aureus at baseline and 78.3% (18/23), 73.0% (27/37), and 87.0% (20/23) for patients with MRSA at baseline in the daily-dose, 1,200-mg-single-dose, and infrequent-dose groups, respectively (Table 3). There was no obvious relationship between reduced oritavancin susceptibility (increased MIC) and rate of cure for patients, including patients with S. aureus and MRSA at baseline. At TOC in the ME population, very few isolates had an MIC of >0.12. Relapse rates among CE patients were low. There were no patients (0/45) with relapses in the daily-dose group, and only 1/61 (1.6%) patient in the single-dose-group and 2/54 (3.7%) patients in the infrequent-dose groups. In the ITT population, the mean durations of study medication (oritavancin or placebo) were similar in the daily-, single-, and infrequent-dose groups (5.4, 5.1, and 5.2 days, respectively).
Table 3.
Clinical cure rates at test-of-cure in the microbiologically evaluable population with Gram-positive pathogens at baselinea
| Pathogen | % of patients cured at indicated oritavancin dose (no. of patients cured/total no. of patients) |
||
|---|---|---|---|
| 200 mg | 1,200 mg | 800 mg | |
| Staphylococcus aureus | 67.4 (31/46) | 78.9 (45/57) | 79.5 (31/39) |
| MRSA | 78.3 (18/23) | 73.0 (27/37) | 87.0 (20/23) |
| MSSA | 56.5 (13/23) | 90.9 (20/22) | 68.8 (11/16) |
| Streptococcus pyogenes | 66.7 (4/6) | 100 (1/1) | 100 (2/2) |
| Streptococcus agalactiae | 33.3 (1/3) | 100 (1/1) | 100 (1/1) |
| Enterococcus faecalis | 50.0 (2/4) | 100 (1/1) | 100 (3/3) |
“Cure” includes cure and improvement outcomes.
Safety and tolerability.
Overall, safety findings were comparable among the three treatment groups. The most common adverse events were nausea, phlebitis, diarrhea, headache, infusion site extravasation, vomiting, and constipation. A total of 8.3% (25/302) of patients experienced a serious adverse event. The incidence of serious adverse events was higher in the daily-dose group (11% [11/100]) than in the 1,200-mg-single-dose group (7.1% [7/99]) and the infrequent-dose group (6.8% [7/103]). Two patients, both in the 1,200-mg-single-dose group, had a serious adverse event that was investigator assessed as being related to study medication (dyspnea and hypersensitivity). Five patients died during the study (three in the daily-dose group and two in the infrequent-dose group). There were no deaths in the 1,200-mg-single-dose group. The adverse events leading to the deaths were cardiac arrest, cardiopulmonary failure, septic shock, myocardial infarction, and pulmonary embolism. None of the deaths were investigator assessed as being related to study medication, and no patients died during therapy. These five deaths also represented the only discontinuations due to adverse events from study. The most common reason for early discontinuation of study medication was lack of efficacy (3.3% [10/302]).
In the daily-, single-, and infrequent-dose groups, 56 (56%), 55 (55.6%), and 63 (61.2%) of patients experienced an adverse event. The majority of adverse events were considered by the investigator to be mild or moderate in severity (85.7% [48/56], 94.5% [52/55], and 95.2% [60/63] in the daily-, single-, and infrequent-dose groups, respectively), with more than half in each treatment group being mild (51.8% [29/56], 58.2% [32/55], and 60.3% [38/63] in the daily-, single-, and infrequent-dose groups, respectively). The majority of adverse events (58.0% [101/174]) were considered by the investigator to be unrelated to study medication. Of the adverse events that the investigator recorded as related to study medication, nausea (7.3% [22/302]), phlebitis (6.6% [20/302]), and diarrhea (5.3% [16/302]) were the most common. The percentages of patients with phlebitis that were assessed by the investigator as being related to study medication were 3.0% (3/100), 4.0% (4/99), and 8.7% (9/103) in the daily-, single-, and infrequent-dose groups, respectively, and none of the phlebitis events were severe.
There was a numerically higher incidence of blood creatine phosphokinase (CPK) increased from baseline in the 1,200-mg-single-dose group than in the daily- and infrequent-dose groups; however, the differences between treatment groups were not statistically significant. Two of these events (one in the 1,200-mg-single-dose group and one in the infrequent-dose group) were considered related to study drug. A review of all patients with CPK increases showed that patients with increases in CPK had mild, asymptomatic elevations from the normal level or levels that were already slightly elevated at baseline. Most had normalized by the last visit. No evidence of myopathy occurred in any of the patients who experienced CPK increases.
Results of clinical laboratory tests were generally unremarkable. Vital sign data were unremarkable and typical of patients being treated for complicated skin and skin structure infections. There were no clinically significant treatment group differences in these parameter values or any indication of unexpected adverse systemic effects of the treatment.
Post hoc analysis.
The recent draft FDA guidance document Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment (25) recommends the following clinical endpoint for studies in patients with acute bacterial skin and skin structure infections (ABSSSI): “Cessation of the spread of the redness, edema, and/or induration of the lesion or reduction in the size (length, width, and area) of redness, edema, and/or induration at 48 to 72 h after enrollment and resolution (absence) of fever.” The SIMPLIFI data were analyzed post hoc to assess the concordance of these endpoints with the traditional clinical cure endpoint used in the trial. Since the SIMPLIFI study was designed according to the 1998 FDA guidance (26) on trials for the treatment of cSSSI, it is important to note that there are key differences between the actual data collected in this study and the new ABSSSI guidance recommendations. First, the entry criteria for wound infections, abscesses, and cellulitis for this study were different from those the new guidance document, which requires a minimum lesion size of 75 cm2 and accompanying systemic signs of infection, such as lymph node enlargement or a fever of ≥38°C. Second, day 4 (72 to 96 h) was chosen for the analysis, as this was the earliest point postbaseline that lesion data were collected. And third, because these measures were not an endpoint in this study, the use of standard antipyretics and NSAIDS was allowed at any time and may have impacted the fever and lesion data.
Given those inconsistencies, an evaluation of the timing of fever resolution and cessation of lesion spread showed a reduction in both endpoints within a short period of time. The percentages of patients who demonstrated the reduction of lesion size or cessation of lesion spread were 92.3% (72/78), 95.5% (63/66), and 92.7% (76/82) in the daily-, 1,200-mg-single-, and infrequent-dose groups, respectively, as evaluated at the day 4 time point. The total area of these lesions was determined by length times width, in accordance with the 2010 FDA guidance (25). Resolution of fever was also seen at day 4 in all treatment groups. A total of 83 patients (28 in the daily-dose group, 32 in the single-dose group, and 23 in the infrequent-dose group) had a fever (≥38°C) at baseline, whereas at day 4, only 12 patients (2 in the daily-dose group, 3 in the single-dose group, and 7 in the infrequent-dose group) had a fever. A composite endpoint, which combines these two objective measures with no use of rescue antibiotic therapy, was compared to the traditional clinical cure at end of therapy. At day 4, 85% (136/160) of the patients who had signs of cessation of lesion spread and resolution of fever, with no use of rescue antibiotic, also had an assessment of clinical cure or improvement maintained at end of therapy. Therefore, the concordance of resolution of fever, cessation of spread of lesion, and no rescue antibiotic with clinical cure demonstrates that this objective, a composite endpoint, may be in concordance with the endpoint of clinical cure.
As stated above, these data were not collected for the purpose of this endpoint and therefore are not perfectly aligned with the new draft guidance. This analysis was only intended to provide the best comparison possible with the current guidelines to indicate how patients' fever resolution and cessation of lesion spread might respond to oritavancin in the currently ongoing phase 3 trials (SOLO I and SOLO II [A Multicenter, Double-Blind, Randomized Study to Evaluate the Efficacy and Safety of Single-Dose i.v. Oritavancin versus i.v. Vancomycin for the Treatment of Patients with Acute Bacterial Skin and Skin Structure Infection] [ClinicalTrials.gov identifiers, NCT01252719 and NCT01252732]).
DISCUSSION
This clinical study demonstrated that oritavancin given as a single dose of 1,200 mg or an infrequent dose of 800 mg with an optional 400-mg dose on day 5 was noninferior to a 200-mg daily dose for 3 to 7 days for the treatment of patients with cSSSI. All patients in this study had complicated infections involving systemic signs, including a fever and/or an elevated white blood cell count. S. aureus was the most frequently isolated pathogen, with an incidence (87.6% [183/209] of the MITT patients) similar to that in a recently published study of cSSSI (45). Over half (56.3% [103/183]) of the S. aureus strains in this study were MRSA, reflective of the incidence of this pathogen (31). Differences in efficacy rates were seen in ME patients with S. aureus at baseline (in the daily-dose group, 67.4% [31/46]; in the single-dose group, 78.9% [45/57]; and in the infrequent-dose group, 79.5% [31/39]) and in ME patients with MRSA at baseline (in the daily-dose group, 78.3% [18/23]; in the single-dose group, 73.0% [27/37]; and in the infrequent-dose group, 87.0% [20/23]) (Table 3); however, given the heterogeneity of the types of infection and the sample size, these do not suggest any true differences in efficacy rates for these pathogens. The range of efficacy in the microbiological subgroups is consistent with that reported in other studies of cSSSI (2, 23, 44, 47).
Oritavancin pharmacokinetics are well described by a three-compartment model (42). Mean population-predicted pharmacokinetic parameter estimates for patients from the phase 2 and 3 studies of oritavancin yield α, β, and γ half-lives of 2.0, 31.2, and 393 h, respectively (42). Theoretically, oritavancin's pharmacokinetics and pharmacodynamics, combined with its concentration-dependent activity, substantial accumulation, and optimized activity from dose pooling rather than dose fractionation, should enable shorter courses of treatment, with preserved efficacy, than for other anti-MRSA agents that have lower plasma exposure values or shorter half-lives in the central compartment.
The oritavancin AUC/MIC ratio was the PK-PD index that was considered to be of greatest relevance during studies (14, 32, 38, 41) to justify the dose regimens that were tested in SIMPLIFI. Although SIMPLIFI was not designed to evaluate PK-PD relationships and hence did not collect PK data with which to match exposure values to outcomes, phase 3 studies of oritavancin in ABSSSI (SOLO I and SOLO II) will include PK-PD assessments as an objective.
The basis of the oritavancin doses used in this study stems from PK simulations that predicted that a single dose of 1,200 mg, or a dose of 800 mg on day 1 followed by an optional “booster” dose of 400 mg on day 4, 5, or 6, would provide a cumulative exposure value similar to that seen with the daily 200-mg dose administered for 7 days (41). Although cumulative exposure values were similar for these regimens, the shape of the plasma concentration-time curve was different, with higher concentrations lasting for up to 3 days after drug administration of a 1,200-mg single dose or the infrequent-dose regimen.
Front loading of drug exposure may result in the greatest and most rapid bactericidal effect, especially with drugs that show concentration-dependent killing. Azithromycin, a drug that is taken up in macrophages, similar to oritavancin, has been shown to clear Haemophilus influenzae in vivo in gerbil otitis media models more rapidly when a single-dose regimen was administered than when a 2- or 3-dose regimen was administered. These results corresponded to observations in clinical studies that suggest there could be a benefit in minimizing the emergence of resistance with the single dose (27). Oritavancin demonstrates concentration-dependent bacterial killing in vitro (11) and in vivo (17, 29). Hence, dosing regimens that allow for front loading of oritavancin exposure would be expected to be associated with optimal outcome.
In addition to efficacy, the front-loaded dosing regimens also had a safety profile similar to that of the daily-dosing regimen. The incidence of serious adverse events was higher in the daily-dose group than in the two front-loaded regimens, and phlebitis was the most common adverse event related to study drug in all treatment groups. Whereas the 1,200-mg single dose of oritavancin was administered over 2.5 h in 750 ml of D5W (5% dextrose in water) in the SIMPLIFI study, in the current phase 3 oritavancin trials it is administered over 3 h in 1,000 ml of D5W to further reduce the incidence of phlebitis.
Concerns about the emergence of resistance are inherent to all antibiotics. Resistance typically emerges most rapidly to agents with a single mechanism of action following their introduction into clinical practice. Oritavancin's multiple mechanisms of action, which target both the barrier function of the bacterial membrane and the synthesis of the bacterial cell wall (10, 12, 30, 39), may slow the development of mutational resistance. Indeed, decreases in oritavancin susceptibility beyond 2-fold from baseline during therapy have not been encountered in the clinical development program to date, including the SIMPLIFI study.
Broad interpretation of these study results is limited by the small sample size of this study. As a phase 2 study, this study was intended to explore the potential of front-loaded dosing regimens of oritavancin. Furthermore, since completion of this study, new draft guidance on appropriate endpoints and inclusion criteria for the evaluation of antibiotics for the treatment of ABSSI have been provided by the FDA. These study endpoints, while still clinically relevant, do not entirely conform to the current FDA guidance. However, based upon the results from this study and supporting nonclinical work (4, 13), further evaluation of the safety and efficacy of a single 1,200-mg dose is warranted. Two phase 3 trials are currently ongoing to evaluate the efficacy and safety of a single 1,200-mg dose of oritavancin (SOLO I and SOLO II). In compliance with the new FDA guidance (25), both phase 3 studies have an objective primary endpoint at early clinical evaluation (48 to 72 h) that includes cessation of lesion spread, no fever, and no use of rescue antibiotic therapies. A secondary endpoint of clinical cure will be assessed at test of cure.
Summary.
In this study, 302 patients were equally randomized to one of three oritavancin treatment regimens, consisting of a daily dose (200 mg) administered for 3 to 7 days, a single dose (1,200 mg), and an infrequent dose (an 800-mg dose with the option of an additional 400 mg on day 5).
The adverse events were similar to those reported previously with oritavancin (28, 36), with no unexpected safety concerns. The safety profile was similar to that of other glycopeptides, with the most common medication-related events being phlebitis, diarrhea, and nausea. Of significance, allergic or hypersensitivity reactions that might cause complications due to the long half-life of oritavancin were not seen in this study. The phlebitis rate ranged from 3.0% to 8.7%, with the highest incidence in the infrequent-dose group and the highest incidence of histamine-like reactions in the single-dose group. These events did not lead to a higher discontinuation rate. All phlebitis events were of mild to moderate severity. This rate is lower than the range reported for other glycopeptide i.v. antibiotics; for example, phlebitis has been reported to occur in 13.7% to 23.0% of vancomycin patients (20). Discontinuations due to an adverse event were infrequent in all treatment groups (1.0% to 3.0%), and the numbers of these discontinuations were similar to or lower than those reported for other medications for cSSSI (23, 44). This study demonstrated that these two alternative infrequent-dosing strategies for oritavancin appeared to be safe and tolerable in the intended population.
Conclusions.
Oritavancin has been demonstrated to be clinically effective, safe, and tolerated as a 1,200-mg single dose or as an infrequent-dosing (800-mg dose, with the option for an additional 400 mg on day 5) regimen for the treatment of cSSSI. Single and infrequent doses of oritavancin were as efficacious as daily doses for complicated skin and skin structure infections caused by Gram-positive pathogens, including MRSA. Safety and tolerability were similar among dosing groups. This initial proof-of-concept study along with PK-PD modeling provides evidence that further evaluation of the 1,200-mg-single-dose and infrequent-dose regimens is warranted in the ongoing phase 3 studies. Success with such an approach may offer significant cost savings and increased patient convenience and compliance compared with more-conventional daily-treatment regimens.
ACKNOWLEDGMENTS
We thank Katherine Oneacre for her medical writing contributions, Kathryn Engstrom for her contributions to the data management of this study, and Amy Rosen and Rob Bandy for their assistance with statistical analysis. We also thank Tom Parr, Greg Moeck, and Francis Arhin for their advice on microbiological aspects of the manuscript and for their review of the manuscript and Maureen Fitzpatrick and Steve Billstein for their medical expertise.
We thank the SIMPLIFI Study Team. The members of this team are as follows: from Australia, Enzo Binotto, Damon Eisen, Afif Hadj, and David Looke; from India, V. P. Hathila, Bharat Kalambe, Rama Kant, M. K. Ramesh, Anthony Rozario, P. P. Shilotri, B. Ravinder Reddy, and Manjulata Anchalia; from Romania, Doina Dumitrescu-Ionescu; from Ukraine, Igor Hospodarskyy, Volodymyr Yareshko, Oleksandr Puptyuk, and Antoliy Zaychuk; from the United States of America, Ian Baird, Christopher Bunce, A. C. Charters, Marc Fernandez, Donald Graham, John Prestigiacomo, Luis Jauregui-Peredo, Thomas Sheftel, Arnold Luterman, Purvi Mehra, Judy Stone, Ellis Tobin, Brian Sullivan, Christian Schrock, Stanley Klein, Jose Vazquez, Paul Manos, Kleper De Almeida, Marc Alpert, Oscar De Valle, Robert Eyzaquirre, Richard C. Keech, Soledad Lee, Alan E. Nolasco, and Vladimir Samonte.
This study was supported by Targanta Therapeutics Corporation, now a wholly owned subsidiary of the Medicines Company. T.M., J. M., and M. M. W. were all full-time employees of the Sponsor Company at the time this work was conducted.
Footnotes
Published ahead of print on 2 May 2011.
REFERENCES
- 1. Al-Nawas B., Swantes J., Shah P. M. 2000. In vitro activity of LY333328, a new glycopeptide, against extracellular and intracellular vancomycin-resistant enterococci. Infection 28:214–218 [DOI] [PubMed] [Google Scholar]
- 2. Arbeit R. D., Maki D., Tally F. P., Campanaro E., Eisenstein B. I. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673–1681 [DOI] [PubMed] [Google Scholar]
- 3. Arhin F. F., et al. 2009. Comparative in vitro activity profile of oritavancin against recent gram-positive clinical isolates. Antimicrob. Agents Chemother. 53:4762–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arhin F. F., et al. 2010. A single 1200 mg human equivalent (HEQ) dose of oritavancin—assessment of in vitro and in vivo killing of Staphylococcus aureus strains, abstr. E-1567. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September 2010 [Google Scholar]
- 5. Arhin F. F., Moeck G., Draghi D. C., Pillar C. M., Sahm D. F. 2010. Longitudinal analysis of the in vitro activity profile of oritavancin and comparator glycopeptides against Gram-positive organisms from Europe: 2005–2008. Int. J. Antimicrob. Agents 36:474–476 [DOI] [PubMed] [Google Scholar]
- 6. Arhin F. F., et al. 2008. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob. Agents Chemother. 52:1597–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arhin F. F., Sarmiento I., Parr T. R., Jr., Moeck G. 2009. Comparative in vitro activity of oritavancin against Staphylococcus aureus strains that are resistant, intermediate or heteroresistant to vancomycin. J. Antimicrob. Chemother. 64:868–870 [DOI] [PubMed] [Google Scholar]
- 8. Baxter Healthcare Corporation 2008. Vancomycin injection. Package insert. Baxter Healthcare Corporation, Deerfield, IL [Google Scholar]
- 9. Belley A., et al. 2010. Is there a relationship between oritavancin in vitro microbiological parameters and clinical outcome in a 2007–2008 phase 2 study of complicated skin and skin-structure infections?, abstr. P-934. Twentieth Eur. Congr. Clin. Microbiol. Infect. Dis., Vienna, Austria, 10 to 13 April 2010 [Google Scholar]
- 10. Belley A., et al. 2010. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant enterococci to effect rapid bacterial killing. Antimicrob. Agents Chemother. 54:5369–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belley A., et al. 2008. Assessment by time-kill methodology of the synergistic effects of oritavancin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob. Agents Chemother. 52:3820–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belley A., et al. 2009. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob. Agents Chemother. 53:918–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Belley A., et al. 2010. Evaluation of a single 1200 mg dose of oritavancin against Staphylococcus Aureus clinical isolates in an in vitro pharmacokinetic (PK)/pharmacodynamic (PD) model (IVPM), abstr. A1-1359. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September 2010 [Google Scholar]
- 14. Bhavnani S. M., et al. 2009. Use of pharmacokinetics-pharmacodynamics to support oritavancin dose selection for patients with complicated skin and skin-structure infection: clinical confirmation of proof of concept, abstr. A1-1288. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009 [Google Scholar]
- 15. Bhavnani S. M., Owen J. S., Loutit J. S., Porter S. B., Ambrose P. G. 2004. Pharmacokinetics, safety, and tolerability of ascending single intravenous doses of oritavancin administered to healthy human subjects. Diagn. Microbiol. Infect. Dis. 50:95–102 [DOI] [PubMed] [Google Scholar]
- 16. Bhavnani S. M., et al. 2008. Pharmacokinetic-pharmacodynamic (PK-PD) target attainment (TA) as decision support for oritavancin (ORI) susceptibility breakpoints for Staphylococcus aureus (SA), abstr. A-994. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet., Washington, DC, 25 to 28 October 2008 [Google Scholar]
- 17. Boylan C. J., et al. 2003. Pharmacodynamics of oritavancin (LY333328) in a neutropenic-mouse thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 47:1700–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clinical and Laboratory Standards Institute 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 19. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing. Approved standard M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 20. Cohen E., et al. 2002. Once-daily versus twice-daily intravenous administration of vancomycin for infections in hospitalized patients. J. Antimicrob. Chemother. 49:155–160 [DOI] [PubMed] [Google Scholar]
- 21. Craig W. A., Andes D. R. 2004. Activity of oritavancin (O) versus vancomycin (V) in the neutropenic murine thigh- and lung-infection models, abstr. A-1863. Abstr. 44th Annu. Intersci. Conf. Antimicrob. Agents Chemother., Washington, DC, 30 October to 2 November 2004 [Google Scholar]
- 22. Cubist Pharmaceuticals 2010. Daptomycin for injection. Package insert. Cubist Pharmaceuticals, Lexington, MA [Google Scholar]
- 23. Ellis-Grosse E. J., Babinchak T., Dartois N., Rose G., Loh E. 2005. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin. Infect. Dis. 41(Suppl. 5):S341–S353 [DOI] [PubMed] [Google Scholar]
- 24. Fetterly G. J., et al. 2005. Pharmacokinetics of oritavancin in plasma and skin blister fluid following administration of a 200-milligram dose for 3 days or a single 800-milligram dose. Antimicrob. Agents Chemother. 49:148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Food and Drug Administration 2010, posting date. Guidance for industry acute bacterial skin and skin structure infections: developing drugs for treatment (DRAFT GUIDANCE). U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Silver Spring, MD [Google Scholar]
- 26. Food and Drug Administration 1998. Guidance for industry: uncomplicated and complicated skin and skin structure infections—developing antimicrobial drugs for treatment. Food and Drug Administration, Silver Spring, MD [Google Scholar]
- 27. Girard D., Finegan S. M., Dunne M. W., Lame M. E. 2005. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J. Antimicrob. Chemother. 56:365–371 [DOI] [PubMed] [Google Scholar]
- 28. Hartman C. S., Wasilewski M. M., Bates B. M. 2008. Oritavancin in the treatment of complicated skin and skin structure infections (cSSSI): combined results of two phase 3 multinational trials, abstr. L-1514. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother (ICAAC)-Infect. Dis. Soc. Am. (ISDA) 46th Annu. Meet., Washington, DC, 25 to 28 October 2008 [Google Scholar]
- 29. Heine H. S., et al. 2008. Efficacy of oritavancin in a murine model of Bacillus anthracis spore inhalation anthrax. Antimicrob. Agents Chemother. 52:3350–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim S. J., et al. 2008. Oritavancin exhibits dual mode of action to inhibit cell-wall biosynthesis in Staphylococcus aureus. J. Mol. Biol. 377:281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klevens R. M., et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 32. Lehoux D., et al. 2009. Comparative efficacy of oritavancin against methicillin-sensitive and -resistant Staphylococcus aureus strains in a neutropenic mouse thigh-infection model, abstr. B-1324. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009 [Google Scholar]
- 33. Lemaire S., et al. 2008. Activities of antistaphylococcal antibiotics towards the extracellular and intraphagocytic forms of Staphylococcus aureus isolates from a patient with persistent bacteraemia and endocarditis. Clin. Microbiol. Infect. 14:766–777 [DOI] [PubMed] [Google Scholar]
- 34. McKay G. A., et al. 2009. Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. J. Antimicrob. Chemother. 63:1191–1199 [DOI] [PubMed] [Google Scholar]
- 35. Moeck G., Milata A. F. F. J., McClure T., Walker K. J., Parr J. T. 2010. Comparison of oritavancin activity against Staphylococcus aureus isolates from a 2007–2008 phase 2 study and from 1999–2002 phase 3 studies of complicated skin and skin structure infection, abstr. P-936. Twentieth Eur. Congr. Clin. Microbiol. Infect. Dis., Vienna, Austria, 10 to 13 April 2010 [Google Scholar]
- 36. Moriarty S., Wasilewski M. M., Rosen A., Perry M. 2008. Incidence of histamine-like infusion reactions (HLIRs) in 2 phase 3 studies comparing oritavancin (ORI) with vancomycin (VAN) in the treatment of complicated skin and skin structure infections (cSSSI), poster L-1515. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother (ICAAC)-Infect. Dis. Soc. Am. (ISDA) 46th Annu. Meet., Washington, DC, 25 to 28 October 2008 [Google Scholar]
- 37. Nguyen H. A., et al. 2009. Intracellular activity of antibiotics in a model of human THP-1 macrophages infected by a Staphylococcus aureus small-colony variant strain isolated from a cystic fibrosis patient: study of antibiotic combinations. Antimicrob. Agents Chemother. 53:1443–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okusanya O. O., et al. 2009. Pharmacokinetics (PK) and pharmacokinetics-pharmacodynamics of oritavancin against Staphylococcus aureus using data from a neutropenic murine thigh-infection model, abstr. A1-1287. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 39. Patti G. J., et al. 2009. Vancomycin and oritavancin have different modes of action in Enterococcus faecium. J. Mol. Biol. 392:1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pfizer, Inc 2010. Linezolid for injection. Prescribing information. Pfizer, Inc., New York, NY [Google Scholar]
- 41. Rubino C. M., et al. 2008. Use of pharmacokinetic-pharmacodynamic principles for decision support for short-course oritavancin dosing regimens for complicated skin and skin structure infections, abstr. O152. Eighteenth Eur. Congr. Clin. Microbiol. Infect. Dis., Barcelona, Spain [Google Scholar]
- 42. Rubino C. M., et al. 2009. Oritavancin population pharmacokinetics in healthy subjects and patients with complicated skin and skin structure infections or bacteremia. Antimicrob. Agents Chemother. 53:4422–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stevens D. L., et al. 2005. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 41:1373–1406 [DOI] [PubMed] [Google Scholar]
- 44. Stevens D. L., et al. 2002. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin. Infect. Dis. 34:1481–1490 [DOI] [PubMed] [Google Scholar]
- 45. Stryjewski M. E., et al. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683–1693 [DOI] [PubMed] [Google Scholar]
- 46. Theravance, Inc 2009. Telavancin for injection. Prescribing information. Theravance, Inc., San Francisco, CA [Google Scholar]
- 47. Weigelt J., et al. 2005. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob. Agents Chemother. 49:2260–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. World Health Organization 2001. Global strategy for the containment of antimicrobial resistance. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/drugresist/en/EGlobal_Strat.pdf [Google Scholar]