Abstract
The aim of the present study was to investigate the epidemiological link of multidrug-resistant Klebsiella oxytoca isolates causing community-onset infections among patients attending our outpatient department and to investigate the underlying resistance mechanisms. The isolates were tested by agar dilution MICs, phenotypic carbapenemase testing, enterobacterial repetitive intergenic consensus-PCR, and pulsed-field gel electrophoresis (PFGE). PCR assays and nucleotide sequencing were employed for the identification of bla gene types and the mapping of the integron-containing metallo-β-lactamase (MBL) gene. During the study period (January 2005 to April 2007), nine broad-spectrum cephalosporin-resistant K. oxytoca clinical isolates were prospectively collected from separate outpatients with urinary tract infections. In all cases, the patients had been hospitalized or exposed to health care facilities during the preceding year. Molecular typing revealed that all isolates belonged to the same K. oxytoca clonal type, which contained five PFGE subtypes. A novel chromosomal OXY-2 β-lactamase type variant (OXY-2-9) was detected in all isolates, but no mutations in the promoter region justifying blaOXY gene overproduction were detected. In addition, all isolates harbored the plasmidic CMY-31 (LAT-4) AmpC cephalosporinase, while three of them harbored VIM-1 MBL in a class 1 integron structure. This is the first study to present the dissemination in the community of multidrug-resistant K. oxytoca isolates causing extrahospital infections.
INTRODUCTION
Klebsiella oxytoca is an opportunistic pathogen that has been implicated in clusters of infections and hospital outbreaks, particularly among specific medical units (28, 31). Wild-type K. oxytoca strains produce a chromosomally encoded class A β-lactamase (OXY), which is evolutionary diversified into six major groups (OXY-1 to OXY-6) and several variants within the group (7). The enzyme is constitutively expressed at low levels, conferring resistance to amino- and carboxypenicillins but no significant resistance to other β-lactams (12). In 10 to 20% of K. oxytoca strains, the overproduction of blaOXY occurs due to distinct point mutations in the −35 and −10 promoter regions of the gene (8), conferring additional resistance to penicillins, the combinations of β-lactams with β-lactamase inhibitors, cephalosporins and aztreonam, plus a variable level of reduced susceptibility to cefotaxime, without affecting susceptibility to ceftazidime (9, 12). Discrepancies in terms of substrate hydrolysis have been reported (14). This antimicrobial profile, and especially the sparing of ceftazidime from hydrolysis, is a distinguishing factor separating OXY overproducers from those harboring extended-spectrum β-lactamases (ESBLs), plasmidic AmpC cephalosporinases, or carbapenemases.
The acquisition of an ESBL is the most common acquired mechanism of resistance to broad-spectrum cephalosporins in K. oxytoca (20, 21), while plasmidic AmpC cephalosporinases are less frequently detected in this species (29). Class A KPC and class B metallo-β-lactamase (MBL) carbapenemases also occasionally have been reported among K. oxytoca isolates in the hospital environment (2, 5).
In our clinical laboratory, preliminary susceptibility data have shown that a number of community-onset infections were due to multidrug-resistant K. oxytoca isolates. This incident prompted the design of the present survey, in which we investigated the epidemiological characteristics of infections caused in the community by broad-spectrum cephalosporin-resistant K. oxytoca and the underlying mechanisms of resistance to β-lactams.
MATERIALS AND METHODS
Patients and definitions.
During the survey period (January 2005 to April 2007), broad-spectrum cephalosporin-resistant K. oxytoca clinical isolates causing community-onset infections were selected for further investigation. The clinical isolates were prospectively collected from patients who had proceeded to the outpatient department of Serres General Hospital. Patients with community-onset infection due to broad-spectrum cephalosporin-resistant K. oxytoca isolates were defined as those who were referred from the community and had a clinically significant isolation of cefotaxime-, ceftriaxone-, and/or ceftazidime-nonsusceptible (MIC, >8 μg/ml) K. oxytoca strain (4). The community-onset infection was defined as health care associated if the patient was hospitalized or exposed to health care facilities the preceding year.
Patients' demographic characteristics and medical history, in terms of underlying diseases, surgery interventions, prior hospitalization or exposure to health care facilities, permanent urinary catheter usage, and previous antibiotic consumption, were recorded.
Identification and susceptibility testing of bacterial isolates.
Identification and initial susceptibility testing were performed with the Microscan system (Dade Behring Inc., West Sacramento, CA). Identification was confirmed with the API 20NE system (bioMérieux, Marcy l' Étoile, France). The preliminary antibiotic resistance profile was confirmed by the agar dilution method using CLSI interpretative criteria (4). MICs of β-lactams (aztreonam, cefoxitin, ceftriaxone, cefotaxime, ceftazidime, cefepime, imipenem, meropenem, and piperacillin-tazobactam), aminoglycosides (amikacin and gentamicin), tigecycline, trimethoprim, and ciprofloxacin were determined.
For screening the production of an MBL and/or KPC carbapenemase, the MBL Etest (AB Biodisk, Solna, Sweden) and the combined disk test using meropenem with and without EDTA and/or phenyl boronic acid (25) were used.
ERIC-PCR and PFGE.
The epidemiological relationship was analyzed initially by enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) with the primer ERIC2. Pulsed-field gel electrophoresis (PFGE) of XbaI-digested genomic DNA was performed with a contour-clamped homogeneous electric field DRII system (Bio-Rad, Hemel Hempstead, United Kingdom) as described elsewhere (26), and banding patterns were compared visually by following previous criteria (22).
Conjugation experiments.
The potential for the conjugational transfer of cephalosporin or carbapenem resistance was examined in biparental matings using Escherichia coli strain 26R793 (Lac− Rifr) as the recipient. Donor and recipient cells were mixed in a ratio of 1:5 in LB broth, and transconjugants were screened on MacConkey agar plates containing rifampin (150 μg/ml) and ceftazidime (1 μg/ml) or imipenem (1 μg/ml).
PCR assays and sequencing analysis.
The detection of the chromosomal blaOXY group genes and their promoters was performed by PCR, followed by DNA sequencing. PCR was performed using the primers 383 (5′-GGG GAT CCA GCC GGG GCC AA-3′) and S (5′-CGG GCC TGT TCC CGG GTT AA-3′), which amplify the whole blaOXY gene along with the promoter regions and produce an amplicon of 1,139 bp (14).
The possible carriage of other β-lactamase genes was tested by PCR using a panel of primers for the detection of ESBL (blaTEM, blaSHV, blaCTX-M, and blaGES/IBC) and carbapenemase (blaVIM, blaIMP, blaKPC, and blaOXA-48) genes (18, 26, 27). Plasmid-mediated AmpCs were screened in single PCRs that identify six family-specific AmpC genes within Gram-negative pathogens (17). Primers amplifying the whole blaVIM and blaCMY regions also were used for sequencing purposes (6, 30). Integron mapping was performed by using PCR assays combining primers specific for 5′-conserved segment (CS) and 3′-CS sequences (13) with primers specific for blaVIM, aacA, dhfrA, aadA, qacEΔ1, and sul genes.
PCR products were purified using ExoSAP-IT reagent (USB Corporation, Cleveland, OH) and used as templates for nucleotide sequencing on both strands with an ABI Prism 377 DNA sequencer (Perkin-Elmer, Applied Biosystems, Foster City, CA). The sequences obtained were compared to sequences available in GenBank.
RESULTS
Clinical isolates and antimicrobial susceptibility profiles.
Sixty-seven K. oxytoca isolates were collected from patients proceeding to the outpatient department during the study period. Nine of them exhibited reduced susceptibility to at least one of the expanded-spectrum cephalosporins and were selected for further testing. All of these isolates were recovered from urine specimens of separate patients who proceeded to the outpatient clinic of the hospital with urinary tract infection.
The antimicrobial susceptibility patterns of the K. oxytoca isolates are listed in Table 1. All nine isolates exhibited resistance to the expanded-spectrum cephalosporins tested. More specifically, the cefotaxime and ceftazidime MICs against these isolates ranged from 64 to >128 μg/ml, and ceftriaxone MICs ranged from 32 to >128 μg/ml. Two different resistance profiles were detected for the remaining β-lactams. Three isolates exhibited resistance to imipenem (MICs of 8 to 16 μg/ml), meropenem (MIC of 8 μg/ml), and cefepime (MICs of 16 to 64 μg/ml); additionally, they were resistant to aztreonam (MICs of 32 to 64 μg/ml), cefoxitin (MIC of >128 μg/ml), and piperacillin-tazobactam (MIC of >128 μg/ml). The remaining six isolates exhibited imipenem, meropenem, and cefepime MICs ranging from 0.25 to 1 μg/ml, 0.25 to 1 μg/ml, and 0.5 to 4 μg/ml, respectively. These isolates were resistant to cefoxitin (MIC of >128 μg/ml) and aztreonam (MICs of 32 to >128 μg/ml) and exhibited various levels of susceptibility to piperacillin-tazobactam (MICs of 16 to >128 μg/ml). All nine isolates remained susceptible to gentamicin and tigecycline, while various susceptibility profiles for amikacin, trimethoprim, and ciprofloxacin were detected.
Table 1.
Antimicrobial susceptibility patterns of the K. oxytoca clinical isolates causing community-onset infections
| Isolate | MICb (μg/ml) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPM | MER | ATM | CAZ | CRO | CTX | FEP | FOX | PTZ | GEN | AMK | CIP | TMP | TIG | |
| 1a | 16 | 8 | 64 | >128 | >128 | >128 | 16 | >128 | >128 | 4 | 16 | 4 | >128 | 2 |
| 2a | 16 | 8 | 32 | >128 | >128 | >128 | 64 | >128 | >128 | 4 | 16 | 8 | >128 | 1 |
| 3a | 8 | 8 | 32 | >128 | >128 | >128 | 32 | >128 | >128 | 4 | 8 | 1 | 128 | 0.25 |
| 4 | 0.5 | 0.25 | 128 | 64 | 32 | 64 | 2 | >128 | 16 | 0.5 | 8 | 4 | 0.25 | 1 |
| 5 | 1 | 0.5 | >128 | >128 | 128 | >128 | 4 | >128 | >128 | 1 | 128 | 64 | 0.25 | 2 |
| 6 | 1 | 0.25 | >128 | >128 | 128 | 128 | 2 | >128 | >128 | 0.5 | 64 | 4 | 0.12 | 0.5 |
| 7 | 1 | 1 | 64 | >128 | >128 | >128 | 2 | >128 | 32 | 0.25 | 64 | 32 | 0.12 | 1 |
| 8 | 0.25 | 0.25 | 32 | 128 | 64 | 128 | 1 | >128 | 16 | 0.25 | 32 | 8 | 0.25 | 0.25 |
| 9 | 0.5 | 0.25 | 128 | >128 | 128 | >128 | 0.5 | >128 | 16 | 4 | 16 | 4 | 128 | 0.5 |
VIM-1-producing isolate.
AMK, amikacin; ATM, aztreonam; CAZ, ceftazidime; CIP, ciprofloxacin; CRO, ceftriaxone; CTX, cefotaxime; FEP, cefepime; FOX, cefoxitin; GEN, gentamicin; IPM, imipenem; MER, meropenem; PTZ, piperacillin-tazobactam TIG, tigecycline; TMP, trimethoprim.
Phenotype testing for carbapenemase production showed that the carbapenem-resistant isolates were positive using the Etest MBL and the combined-disk test employing meropenem and EDTA; they were negative using the combined-disk test employing meropenem and phenyl boronic acid. The remaining six isolates were phenotypically negative for MBL and KPC production.
Patients' clinical and epidemiological characteristics.
The characteristics of the nine patients with community-onset infections due to broad-spectrum cephalosporin-resistant K. oxytoca are presented in Table 2. All outpatients had a history of previous hospitalization or exposure to health care facilities during the preceding year (mean time period after hospitalization, 60.5 days), and therefore their infections were defined as health care-associated community-onset infections. All but two of the patients had previous hospitalization in distinct periods (Table 2), and an issue of cross-infection was not identified. In the period between prior hospitalization and attendance to the outpatient department with community-onset infection, all patients remained at home and three of them were catheterized. During their previous hospitalization or after their hospital discharge, the patients had received antimicrobial regimens that included ciprofloxacin, cefuroxime, ticarcillin-clavulanate, piperacillin-tazobactam, or amikacin (Table 2). Based on the medical records, none of the patients had developed infection due to broad-spectrum cephalosporin-resistant K. oxytoca during previous hospitalization. K. oxytoca urinary tract infections were treated successfully with gentamicin (three times daily, intramuscularly [i.m.] or intravenously [i.v.]) as monotherapy or in combination with ciprofloxacin (twice daily, i.m. or per os [p.o.]) or cotrimoxazole (twice daily, p.o.). During the survey, none of the nine patients proceeded to the outpatient department with recurrent broad-spectrum cephalosporin-resistant K. oxytoca community-onset infection.
Table 2.
Characteristics of outpatients infected with broad-spectrum cephalosporin-resistant K. oxytoca clinical isolatesa
| Isolate no. | Date of isolation (mo/yr) | Age (yr)/sex | Time after hospitalization (days) | Reason for previous hospitalization | Predisposing factor(s)/underlying disease | Previous consumption of antibioticsb | β-Lactamase gene | PFGE type |
|---|---|---|---|---|---|---|---|---|
| 1 | 1/2005 | 72/M | 15 | Renal lithiasis | Prostate cancer, diabetes mellitus | AMK, CXM | CMY-31, VIM-1 | Ia |
| 2 | 1/2005 | 77/F | 124 | Renal lithiasis | PTZ, CIP, CXM | CMY-31, VIM-1 | Ib | |
| 3 | 2/2005 | 79/M | 21 | Prostatectomy | Diabetes mellitus | PTZ, AMK, CXM | CMY-31, VIM-1 | Ia |
| 4 | 4/2005 | 71/M | 43 | Papillary transitional carcinoma | PIICA | TCA, CXM | CMY-31 | Ia |
| 5 | 5/2005 | 67/M | 12 | Prostate hypertrophy | PTZ, CIP | CMY-31 | Ia | |
| 6 | 1/2006 | 63/M | 197 | Renal lithiasis | PTZ, AMK, CIP | CMY-31 | Ia | |
| 7 | 9/2006 | 71/M | 18 | Papillary transitional carcinoma | PIICA | TCA, AMK | CMY-31 | Ic |
| 8 | 2/2007 | 82/M | 98 | Papillary transitional carcinoma | PIICA | TCA, CIP | CMY-31 | Id |
| 9 | 4/2007 | 68/F | 17 | Renal lithiasis | TCA, CXM | CMY-31 | Ie |
M, male; F, female; PIICA, previous intravesical instillation of chemotherapeutic agents; AMK, amikacin; CIP, ciprofloxacin; CXM, cefuroxime; PTZ, piperacillin-tazobactam; TCA, ticarcillin-clavulanate.
During previous hospitalization and/or after discharge.
Molecular typing.
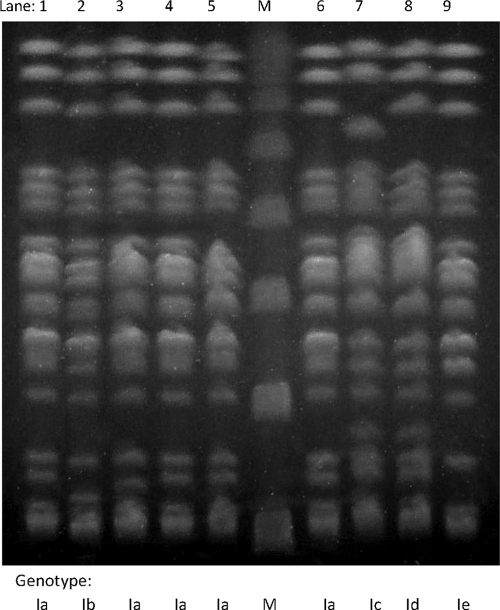
ERIC-PCR showed identical banding patterns for all nine K. oxytoca isolates, implying a single underlying genotype. Molecular typing using PFGE also clustered K. oxytoca isolates into a single PFGE clonal type (clone I). Nevertheless, the PFGE clone contained five subtypes differing by one to three bands from the predominant subtype Ia (Fig. 1). The subtype Ia was detected in five isolates (two MBL-positive and three MBL-negative isolates), while all of the remaining subtypes (Ib to Ie) were detected in single K. oxytoca isolates.
Fig. 1.
PFGE profiles of the nine K. oxytoca isolates of the study. Lanes 1 to 3, CMY-31- and VIM-1-producing K. oxytoca isolates; lanes 4 to 9, CMY-31-producing K. oxytoca isolates; lane M, multimers of a phage lambda DNA (48.5 kb) molecular mass marker.
Molecular testing for bla genes.
PCR testing showed that all nine isolates harbored a chromosomal blaOXY gene. Sequencing analysis revealed that in all cases the blaOXY gene belonged to the blaOXY-2 group but differed from the reference blaOXY-2-1 gene (GenBank accession no. Z49084) in nucleotides that resulted in three amino acid substitutions at Ambler positions 153, 158, and 255. The new OXY-2 variant was characterized as OXY-2-9 (GenBank accession no. FJ754667) and also differed from the remaining OXY-2 variants (OXY-2-2 to OXY-2-8) by two to four amino acid substitutions. In the region of the promoters known to be responsible for the hyperproduction of OXY enzymes, no mutations were determined in any of our sequences.
All isolates also were found to harbor a plasmidic AmpC that was characterized by sequence analysis as CMY-31 (LAT-4) cephalosporinase. In addition, PCR and sequencing analysis identified a blaVIM-1 allele in the three phenotypically MBL-positive K. oxytoca isolates but not in the remaining K. oxytoca isolates. PCR testing for other bla genes, including various carbapenemases, ESBLs, and plasmidic AmpC genes, was negative in all cases. Conjugation experiments failed to demonstrate the transfer of CMY-31 or VIM-1 in any of our isolates.
Integron structure.
PCR mapping revealed a class 1 integron structure only among the blaVIM-1-positive K. oxytoca isolates. The integron had, in all three isolates, a variable region of approximately 1.5 kb in size that included (from 5′ to 3′) blaVIM-1 and aac(6′)-IIc gene cassettes, which is similar to the structure of In87 described previously in a VIM-1-producing Enterobacter cloacae isolate from Greece (10). The blaVIM-1 cassette (including the 81 nucleotides of the 59-base element) was identical to the one described originally in E. coli (15). The aac(6′)-IIc gene cassette was located downstream of the blaVIM-1 gene cassette, and it was identical to that previously sequenced from P. aeruginosa (GenBank accession no. AF162771). At the 5′CS, a strong P1 promoter followed directly by an inactive P2 promoter (without a GGG insertion prior to the −10 hexamer) was identified.
DISCUSSION
K. oxytoca is a nosocomial pathogen that occasionally is implicated in community-onset clinical infections (3). The present study reports, for the first time, the emergence of extrahospital infections due to multidrug-resistant K. oxytoca isolates. Given the fact that all patients had been hospitalized during the preceding 7 months, these community-onset infections were characterized as being health care associated. Moreover, molecular typing indicated that all K. oxytoca isolates were clonally related, providing stronger evidence that the transmission occurred during patients' hospitalization. It should be noted that during the study period, three broad-spectrum cephalosporin-resistant K. oxytoca isolates were recovered sporadically from clinical specimens of hospitalized patients. They belonged to the same PFGE type as those recovered from community-onset infections and carried OXY-2-9, CMY-31, and, in one case, VIM-1 (data not shown).
The common phylogenetic origin of the multidrug-resistant K. oxytoca isolates was further supported by the fact that all isolates harbored the same variant of the intrinsic blaOXY chromosomal gene. In the present study, a novel variant of the blaOXY-2 β-lactamase group, blaOXY-2-9, was documented that has three nucleotide substitutions in the original blaOXY-2 gene. Until recently, eight variants had been described within the OXY-2 group (7). The blaOXY-2-9 coding regions and promoter sequences were indistinguishable in all of our K. oxytoca isolates, supporting the hypothesis of a common ancestor. Moreover, in all isolates, substitutions in the promoter sequence of the blaOXY-2-9 gene that could lead to OXY enzyme overproduction (8, 9) were not detected.
Therefore, the observed resistance to broad-spectrum cephalosporins was attributed to the presence of the plasmidic CMY-31 (LAT-4) AmpC, which was detected in all cases. This cephalosporinase originally was detected among cefoxitin-resistant E. coli isolates in Greek hospitals (11) and has been described rarely in other hospital settings (1). It is phylogenetically and functionally similar to CMY-2, one of the most widespread plasmid-mediated cephalosporinases in Enterobacteriaceae, causing high-level resistance to expanded-spectrum cephalosporins and aztreonam (32).
Interestingly, the three isolates that exhibited carbapenem resistance harbored the blaVIM-1 gene. A blaVIM-1-producing K. oxytoca clinical isolate also has been isolated recently in a Greek hospital, but the respective gene was integrated in a different integron structure (16). The present study describes the first transmission in the community of carbapenemase-producing K. oxytoca isolates that causes extrahospital infections. The VIM-1-encoding integron structure most likely has been acquired by an AmpC-producing K. oxytoca strain that became established in the hospital setting and then colonized patients before leaving the hospital.
It is noteworthy that no recurrent community-onset infections were detected among outpatients, similarly to the community-onset infections caused by MBL-producing Proteus mirabilis in our region (23). However, the mean time period between hospital discharge and the onset of the symptoms was notably longer for K. oxytoca infections than P. mirabilis infections (60.5 versus 43.7 days, respectively), implying a relatively milder nature of K. oxytoca infections. Supporting evidence for this hypothesis is that despite the presence of factors adverse to antimicrobial effectiveness, such as renal lithiasis and papillary transitional carcinoma of urinary bladder, all outpatients were treated successfully with antimicrobial regimens. In contrast, previous studies have shown that MBL-producing Pseudomonas aeruginosa and Klebsiella pneumoniae strains are frequently associated with recurrent community-onset infections (19, 24).
In summary, a unique clone of multidrug-resistant K. oxytoca carrying a novel chromosomal OXY-2 variant was responsible for causing community-onset health care-associated infections in our community. These clonal isolates displayed enhanced resistance to all expanded-spectrum cephalosporins and aztreonam, which was attributed to the acquisition of a plasmidic AmpC. The additional resistance to carbapenems and cefepime that was observed in some isolates was attributed to the VIM-1 MBL. The epidemiological surveillance for colonization with multidrug-resistant isolates before hospital discharge seems of importance to the prevention of clusters of infection by multidrug-resistant pathogens in the extrahospital setting.
Footnotes
Published ahead of print on 9 May 2011.
REFERENCES
- 1. Adler H., et al. 2008. Plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking inducible chromosomal AmpC genes: prevalence at a Swiss university hospital and occurrence of the different molecular types in Switzerland. J. Antimicrob. Chemother. 61:457–458 [DOI] [PubMed] [Google Scholar]
- 2. Carattoli A., et al. 2010. Complete nucleotide sequence of the IncN plasmid pKOX105 encoding VIM-1, QnrS1 and SHV-12 proteins in Enterobacteriaceae from Bolzano, Italy compared with IncN plasmids encoding KPC enzymes in the U. S. A. J. Antimicrob. Chemother. 65:2070–2075 [DOI] [PubMed] [Google Scholar]
- 3. Chen J. Y., Chen P. S., Chen Y. P., Lee W. T., Lin L. J. 2006. Community-acquired Klebsiella oxytoca endocarditis: a case report. J. Infect. 52:129–131 [DOI] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. CLSI M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Conejo M. C., et al. 2010. Isolation of multidrug-resistant Klebsiella oxytoca carrying blaIMP-8, associated with OXY hyperproduction, in the intensive care unit of a community hospital in Spain. J. Antimicrob. Chemother. 65:1071–1073 [DOI] [PubMed] [Google Scholar]
- 6. Doi Y., et al. 2009. Reduced susceptibility to cefepime among Escherichia coli clinical isolates producing novel variants of CMY-2 β-lactamase. Antimicrob. Agents Chemother. 53:3159–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fevre C., et al. 2005. Six groups of the OXY β-lactamase evolved over millions of years in Klebsiella oxytoca. Antimicrob. Agents Chemother. 49:3453–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fournier B., Gravel A., Hooper D. C., Roy P. 1999. Strength and regulation of the different promoters for chromosomal β-lactamase of Klebsiella oxytoca. Antimicrob. Agents Chemother. 43:850–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fournier B., Lagrange P. H., Philippon A. 1996. β-Lactamase gene promoters of 71 clinical strains of Klebsiella oxytoca. Antimicrob. Agents Chemother. 40:460–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galani I., Souli M., Chryssouli Z., Orlandou K., Giamarellou H. 2005. Characterization of a new integron containing bla(VIM-1) and aac(6′)-IIc in an Enterobacter cloacae clinical isolate from Greece. J. Antimicrob. Chemother. 55:634–638 [DOI] [PubMed] [Google Scholar]
- 11. Gazouli M., Tzouvelekis L. S., Vatopoulos A. C., Tzelepi E. 1998. Transferable class C β-lactamases in Escherichia coli strains isolated in Greek hospitals and characterization of two enzyme variants (LAT-3 and LAT-4) closely related to Citrobacter freundii AmpC β-lactamase. J. Antimicrob. Chemother. 42:419–425 [DOI] [PubMed] [Google Scholar]
- 12. Gheorghiu R., Yuan M., Hall L. M., Livermore D. M. 1997. Bases of variation in resistance to β-lactams in Klebsiella oxytoca isolates hyperproducing K1 β-lactamase. J. Antimicrob. Chemother. 40:533–541 [DOI] [PubMed] [Google Scholar]
- 13. Lévesque C., Piché L., Larose C., Roy P. H. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mammeri H., Poirel L., Nordmann P. 2003. In vivo selection of a chromosomally encoded β-lactamase variant conferring ceftazidime resistance in Klebsiella oxytoca. Antimicrob. Agents Chemother. 47:3739–3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miriagou V., Tzelepi E., Gianneli D., Tzouvelekis L. S. 2003. Escherichia coli with a self-transferable, multiresistant plasmid coding for metallo-β-lactamase VIM-1. Antimicrob. Agents Chemother. 47:395–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miriagou V., et al. 2008. Emergence of Serratia liquefaciens and Klebsiella oxytoca with metallo-β-lactamase-encoding IncW plasmids: further spread of the blaVIM-1-carrying integron In-e541. Int. J. Antimicrob. Agents 32:540–541 [DOI] [PubMed] [Google Scholar]
- 17. Pérez-Pérez F. J., Hanson N. D. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poirel L., Héritier C., Tolün V., Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poulou A., et al. 2010. Recurrent healthcare-associated community-onset infections due to Klebsiella pneumoniae producing VIM-1 metallo-β-lactamase. J. Antimicrob. Chemother. 65:2538–2542 [DOI] [PubMed] [Google Scholar]
- 20. Romero E. D., et al. 2007. Prevalence of clinical isolates of Escherichia coli and Klebsiella spp. producing multiple extended-spectrum β-lactamases. Diagn. Microbiol. Infect. Dis. 59:433–437 [DOI] [PubMed] [Google Scholar]
- 21. Sturm P. D., et al. 2010. Prevalence, molecular characterization, and phenotypic confirmation of extended-spectrum β-lactamases in Escherichia coli, Klebsiella pneumoniae, and Klebsiella oxytoca at the Radboud University Nijmegen Medical Centre in The Netherlands. Microb. Drug Resist. 16:55–60 [DOI] [PubMed] [Google Scholar]
- 22. Tenover F. C., et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsakris A., et al. 2007. Transmission in the community of clonal Proteus mirabilis carrying VIM-1 metallo-β-lactamase. J. Antimicrob. Chemother. 60:136–139 [DOI] [PubMed] [Google Scholar]
- 24. Tsakris A., et al. 2009. Large dissemination of VIM-2-metallo-β-lactamase-producing Pseudomonas aeruginosa strains causing health care-associated community-onset infections. J. Clin. Microbiol. 47:3524–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsakris A., et al. 2010. A simple phenotypic method for the differentiation of metallo-β-lactamases and class-A KPC carbapenemases in Enterobacteriaceae clinical isolates. J. Antimicrob. Chemother. 65:1664–1671 [DOI] [PubMed] [Google Scholar]
- 26. Tsakris A., et al. 2000. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J. Clin. Microbiol. 38:1290–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tzelepi E., et al. 2003. Extended-spectrum β-lactamase types in Klebsiella pneumoniae and Escherichia coli in two Greek hospitals. Int. J. Antimicrob. Agents 21:285–288 [DOI] [PubMed] [Google Scholar]
- 28. Watson J. T., et al. 2005. Outbreak of catheter-associated Klebsiella oxytoca and Enterobacter cloacae bloodstream infections in an oncology chemotherapy center. Arch. Intern. Med. 165:2639–2643 [DOI] [PubMed] [Google Scholar]
- 29. Yamasaki K., et al. 2010. Laboratory surveillance for prospective plasmid-mediated AmpC β-lactamases in the Kinki region of Japan. J. Clin. Microbiol. 48:3267–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan J. J., et al. 2001. Metallo-β-lactamases in clinical Pseudomonas isolates in Taiwan and identification of VIM-3, a novel variant of the VIM-2 enzyme. Antimicrob. Agents Chemother. 45:2224–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zárate M. S., et al. 2008. Outbreak of OXY-2-producing Klebsiella oxytoca in a renal transplant unit. J. Clin. Microbiol. 46:2099–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zioga A., et al. 2009. CMY-31 and CMY-36 cephalosporinases encoded by ColE1-like plasmids. Antimicrob. Agents Chemother. 53:1256–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]