Abstract
In response to the need for new antiviral agents, dendrimer-based molecules have been recognized as having a large number of potential therapeutic applications. They include peptide-derivatized dendrimers, which are hyperbranched synthetic well-defined molecules which consist of a peptidyl branching core and covalently attached surface functional peptides. However, few studies have addressed their applications as direct-acting antiviral agents. Here, we report on the ability of the peptide dendrimer SB105 and its derivative, SB105_A10, to directly inhibit herpes simplex virus 1 (HSV-1) and HSV-2 in vitro replication, with favorable selective indexes discerned for both compounds. An analysis of their mode of action revealed that SB105 and SB105_A10 prevent HSV-1 and HSV-2 attachment to target cells, whereas SB104, a dendrimer with a different amino acid sequence within the functional group and minimal antiviral activity, was ineffective in blocking HSV attachment. Moreover, both SB105 and SB105_A10 retained their ability to inhibit HSV adsorption at pH 3.0 and 4.0 and in the presence of 10% human serum proteins, conditions mimicking the physiological properties of the vagina, a potential therapeutic location for such compounds. The inhibition of HSV adsorption is likely to stem from the ability of SB105_A10 to bind to the glycosaminoglycan moiety of cell surface heparan sulfate proteoglycans, thereby blocking virion attachment to target cells. Finally, when combined with acyclovir in checkerboard experiments SB105_A10 exhibited highly synergistic activity. Taken together, these findings suggest that SB105 and SB105_A10 are promising candidates for the development of novel topical microbicides for the prevention of HSV infections.
INTRODUCTION
Herpes simplex viruses (HSV) are responsible for a wide variety of clinical manifestations, ranging from asymptomatic infection or mild mucocutaneous lesions on the lips, cornea, genitals, or skin, up to more severe, and even life-threatening, infections, including encephalitis, neonatal infections, and progressive or visceral disease in the immunocompromised hosts (30). There are two serotypes of HSV, HSV-1 and HSV-2, which can infect either oral or genital sites. Following primary infection, which targets oral and genital mucosa, HSV establishes latent infections in the neurons of the sensory ganglia from where they may, or may not, reactivate, causing recurrent lesions at the site of primary infection. HSV-1 frequently reactivates from oral sites, whereas HSV-2 is more likely to reactivate from genital sites. Even if HSV infections are often subclinical, their incidence and severity have increased over the past decades due to the increasing number of immunocompromised patients (30). In particular, the impact of genital herpes as a public health threat is amplified because of its epidemiological synergy with HIV (30).
Synthetic nucleoside analogues targeting viral DNA polymerase, such as acyclovir (ACV), famciclovir (FAM), and valacyclovir (VCV), are routinely used as a standard treatment of symptomatic HSV infections (8, 12, 30, 38, 39). However, their clinical use in immunocompromised patients receiving long-term prophylactic treatment may lead to incidence of treatment failures due to the development of antiviral-resistant virus strains (12, 37, 39). Moreover, to date, none of the currently approved antiviral drugs has been able to eliminate an established latent infection. Thus, these limitations highlight the need to develop new anti-HSV agents with antiviral activity based on alternative mechanisms of action. Of the alternative potential viral targets being explored, prophylactic administration of agents that inhibit HSV attachment and/or entry represents a particularly attractive antiviral strategy since it prevents the establishment of infection. Molecules with this mode of action could provide the starting point for the development of topical microbicides that block transmission at the mucosal surface, thereby providing a realistic method of prophylactic intervention (16).
In response to the increasing need for improved antiviral drugs, dendrimer-based molecules have been recognized as having a large number of potential therapeutic applications (7, 22). The basic structure of these macromolecules is formed by a central core that give rise to a shell of branches presenting functional groups, represented by carbohydrates, anions, or peptides that are able to interact with biological surfaces or receptors. Thus, their hyperbranched structures enable a given molecular motif to be exposed in a highly multivalent fashion, thus offering an efficient means of presenting multiple ligands, or sites of contact, on a single molecule (7). This feature provides an array of options for interfering with the infectivity of a virus particle and/or with the multivalent binding interactions between a virus and its target cell. In fact, most of the antiviral dendrimers identified to date have been found to inhibit infection by blocking attachment of the virus to its target cell following their binding to either the virus or the cell surface (13, 31). Thus, the ability of dendrimers to prevent virus-cell binding has prompted the suggestion that they might also be used as topical microbicides for reducing the spread of sexually transmitted infections (31). Proof of this concept has been provided from the development of polyanionic dendrimers, such as SPL7013, a lysine-based dendrimer with naphthalene disulfonic acid surface groups, currently undergoing the expanded safety/phase IIa stage of clinical development as a vaginal microbicide (known as ViVagel) for the prevention of the transmission of sexually transmitted viral diseases (4, 11, 21, 32, 39).
In contrast, the development of peptide-derivatized antiviral dendrimers consisting of a peptidyl branching core and covalently attached surface peptide units does not appear to have received a level of attention comparable to that of carbohydrate- or polyanionic-derivatized dendrimers.
Thus, the aim of this study was to characterize new anti-HSV agents using a library of peptide-derivatized dendrimers. Dendrimers were derived from the M6 prototype, a tetrabranched dendrimer based on a lysine core which tethers four 10-mer peptide chains in lysine α and ε positions and which has been shown to be endowed with favorable characteristics for the development of new antibacterial and/or antiviral drugs (26, 27). We report on the ability of the dendrimeric derivatives SB105 and SB105_A10 to potently inhibit the in vitro replication of HSV-1 and HSV-2 by a mechanism that involves the inhibition of virion attachment to cell-surface heparan sulfate proteoglycans (HSPGs). These results indicate that SB105 and SB105_A10 are promising new candidates for the development of novel topical microbicides for the prevention of HSV-1 and HSV-2 infections.
MATERIALS AND METHODS
Dendrimer synthesis.
Dendrimers were synthesized as described previously (19). For a detailed description of their synthesis, see the supplemental material. All test compounds were dissolved in phosphate-buffered saline (PBS).
Cells, culture conditions and viruses.
African green monkey kidney cells (Vero) (ATCC CCL-81) were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Low-passage human embryonic lung fibroblasts (HELFs) were isolated at the Department of Public Health and Microbiology, University of Turin, Turin, Italy. The culture medium for Vero and HELF cells was Eagle's minimal essential medium (MEM; Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL), 1 mM sodium pyruvate, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate.
Clinical isolates of HSV-1 and HSV-2 sensitive to ACV were kindly provided by V. Ghisetti, Amedeo di Savoia Hospital, Turin, Italy. They were propagated and titrated by plaque assay on Vero cells as described previously (18). The recombinant laboratory strains HSV-1 KCZ and HSV-2 LacZ viruses contain the Escherichia coli LacZ reporter gene under the transcriptional control of the human cytomegalovirus immediate-early promoter (HCMV-LacZ), which replaces the glycoprotein gC coding sequence gene (17, 24). They were kindly provided by R. Manservigi (University of Ferrara) and propagated and titrated by plaque assay on Vero cells (17, 24).
Vesicular stomatitis virus (VSV), serotype Indiana, and a clinical isolate of adenovirus were propagated and titrated on HELF cells as described previously (19).
Antiviral assays.
To screen the mini-library of dendrimeric derivatives (Fig. 1) for anti-HSV activity, Vero cells were seeded in 24-well plates at a density of 70 × 103 cells. After 24 h, the cells were treated with 2 μM concentrations of different dendrimeric peptides 1 h prior to and during infection with a clinical isolate of HSV-1 at a multiplicity of infection (MOI) of 0.1 PFU/cell. Following virus adsorption (2 h at 37°C), cultures were maintained in medium containing the corresponding peptides and then incubated until control cultures displayed extensive cytopathology. At 48 h postinfection (p.i.), the cells and supernatants from the antiviral assay were harvested and disrupted by sonication. The extent of virus replication was then assessed by titrating the infectivity of supernatants of cell suspensions on Vero cells.
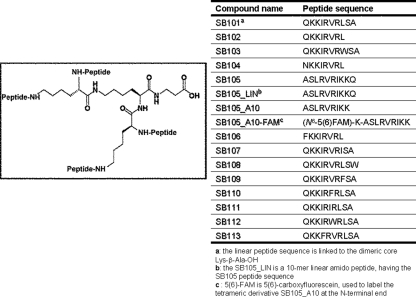
Fig. 1.
Structures of the peptide derivatized-dendrimers. The generic structure of the peptide dendrimers is shown. All the SB102-113 derivatives are dendrimeric molecules; each one bears four copies of a linear 10-mer peptide (sequences in table) linked to a lysine tetrameric core, with the exception of the SB101 (with a Lys-β-Ala-OH dimeric core) and SB105_LIN (with a 10-mer linear amido peptide core).
For the virus yield reduction assay, both untreated cells and those incubated with different concentrations of dendrimers for 1 h before infection were infected with clinical isolates of HSV-1 and HSV-2 or recombinant HSV-1 KCZ or HSV-2 LacZ at an MOI of 0.1. Following virus adsorption (2 h at 37°C), cultures were maintained in medium containing the corresponding peptides and then incubated for 48 h p.i. until control cultures displayed extensive cytopathology. Thereafter, the cells and supernatants from the antiviral assay were harvested and disrupted by sonication. The extent of virus replication was then assessed by titrating the infectivity of supernatants of cell suspensions on Vero cells. Plaques were microscopically counted, and the mean plaque counts for each drug concentration was expressed as a percentage of the mean plaque count for the control virus. The number of plaques was plotted as a function of drug concentration; concentrations producing 50% and 90% reductions in plaque formation (IC50 and IC90) were determined.
To determine cell viability, Vero cells were exposed to increasing concentrations of peptides. After 3 days of incubation, the number of viable cells was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method, as previously described (25).
To evaluate the stability of selected dendrimers at different pHs (11), SB105 and SB105_A10 were incubated in phosphate-buffered saline solutions of different pHs, ranging from pH 3 to pH 9, for 2 h at 37°C as previously described (11). Thereafter, different concentrations of pH-treated dendrimers were incubated with confluent Vero cell monolayers for 1 h at physiological pH. Cells were then infected at physiological pH with HSV-1 or HSV-2 at an MOI of 0.1 for 2 h at 37°C. At 48 h p.i., the cells and supernatants from the antiviral assay were harvested and disrupted by sonication. The extent of virus replication was then assessed by titrating the infectivity of supernatants of cell suspensions on Vero cells.
To investigate the activity of SB105 and SB105_A10 in the presence of human serum proteins, dendrimers were incubated in medium supplemented with 10% human serum (obtained from HSV-negative donors) for 1 h or 18 h at 37°C as described previously (11). Different concentrations of serum-exposed dendrimers were then used in virus yield reduction assays. After the pretreatment with the dendrimers in the presence of 10% human serum, the cells were infected at an MOI of 0.1 (2 h at 37°C) and maintained in medium containing 10% human serum and the corresponding peptides until control cultures displayed extensive cytopathology. At 48 h p.i., the extent of virus replication was then assessed by titrating the infectivity of supernatants of cell suspensions on Vero cells.
Immunoblotting.
Whole-cell protein extracts were prepared as previously described (6, 18). Proteins were separated by 8% SDS-PAGE and then transferred to Immobilon-P membranes (Millipore). Filters were blocked overnight in 5% nonfat dry milk in 10 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 0.1% Tween 20 and immunostained with mouse anti-HSV-1/2 ICP27 monoclonal antibody (MAb) (clone H1113; Virusys Corporation) (diluted 1:200), mouse anti-HSV-2 ICP8 MAb (clone 4E6; Virusys Corporation) (diluted 1:500), mouse anti-HSV-1 ICP8 MAb (clone 10A3; Abcam) (diluted 1:500), mouse anti-HSV-1/2 gD MAb (clone 2C10; Virusys Corporation) (diluted 1:2,000), or mouse anti-actin MAb (Chemicon International) (diluted 1:2,000). Immunocomplexes were detected with a sheep anti-mouse immunoglobulin Ab conjugated to horseradish peroxidase (Amersham) and visualized by enhanced chemiluminescence (SuperSignal; Pierce).
Attachment and entry assays.
The effect of dendrimeric peptides on viral attachment was assessed as previously described (35). Briefly, prechilled Vero cell monolayers were treated with various concentrations of dendrimers or heparin for 30 min at 4°C and infected with precooled HSV-1 or HSV-2 at a MOI of 0.004 in the presence of the compounds for 3 h at 4°. Cells were then washed three times with cold MEM to remove unattached virus and compounds, overlaid with 1.2% methylcellulose, and incubated for 2 days at 37°C. Plates were then fixed and colored with crystal violet, and plaques were microscopically counted. One lane per plate was used as the positive control: the same amount of virus was allowed to attach to the cells for 3 h at 4°C as described above, and after washing steps, the monolayer was overlaid with 1.2% methylcellulose. The number of plaques produced by this control well was set to 100%. Next, one lane per plate was used as a control to confirm that incubation at 4°C allowed viral attachment and not viral entry. In order to establish this, the cells to which virus had been preattached at 4°C were treated with cold acidic glycine (100 mM glycine, 150 mM NaCl [pH 3]) for 2 min to inactivate attached but not yet penetrated virus before being overlaid with 1.2% methylcellulose. This resulted in 100% inhibition of plaque formation in untreated cells, indicating that no virus had entered into cells during the attachment period.
To assess the effect of dendrimeric peptides on viral entry, prechilled Vero cell monolayers were infected with precooled HSV-1 or HSV-2 at a MOI of 0.004 for 3 h at 4°C to allow viral attachment. Cells were then washed with cold MEM three times to remove unattached virus and treated with various concentrations of dendrimers or heparin for 3 h at 37°C prior to inactivation of extracellular virus with acidic glycine for 2 min at room temperature (RT), as previously described (35). Subsequently, the cells were washed with warm medium three times to return the pH to neutral and overlaid with 1.2% methylcellulose and incubated for 2 days at 37°C. Plates were then fixed and colored with crystal violet, and plaques were microscopically counted. One lane per plate was used as the positive control: the same amount of virus was allowed to attach for 3 h at 4°C, and then cells were washed and the entry phase was allowed in the absence of drugs at 37°C for 3 h before inactivation of the virus that had not entered the cells. The monolayer was overlaid with 1.2% methylcellulose, and the number of plaques produced by these cells was set to 100%.
Virucidal assay.
To assess the effect of dendrimeric peptides on viral infectivity, dendrimers (1 μM) were added to aliquots of HSV-1 or HSV-2 (104 PFU), and the virus-dendrimers samples were then incubated at either 4 or 37°C for various lengths of time as previously described (35). After incubation, the samples were diluted with medium to reduce the concentration of peptide to one that was not active in an antiviral assay (0.02 μM). The MOI of HSV-1 and HSV-2 after dilution was of 0.01. The viruses were then titrated on Vero cells. Plaques were microscopically counted, and the mean plaque counts for each dendrimer concentration were expressed as PFU/ml on a log10 scale.
Heparinase assay.
Vero cell monolayers were pretreated with 2.5 U of heparinase I (heparin lyase I) or heparitinase I (heparin lyase III) (Sigma) in PBS containing 1 mM CaCl2, 1.5 mM MgCl2, 0.1% glucose, and 0.1% bovine serum albumin or with buffer alone for 1 h at 37°C as described previously (36). Cells were then washed three times with medium prior to the binding of fluorescein-conjugated SB105_A10 dendrimer (SB105_A10–6-carboxyfluorescein [FAM]). SB105_A10-FAM was then added alone or in the presence of a 10-fold molar excess of SB105_A10 or heparin for a further 1 h at 4°C. Detection of bound fluorescent SB105_A10-FAM was performed with fixed cells (4% formaldehyde for 10 min on ice) by fluorescence microscopy following counterstaining with Evans blue (Sigma). Images were recorded with an Olympus Fluoview-IX70 inverted confocal laser scanning microscope.
Drug combination analysis.
To evaluate the combined inhibitory effects of SB105_A10 and acyclovir (ACV) on HSV-1 and HSV-2 replication, checkerboard combination studies were performed using plaque reduction assays. Briefly, Vero cells seeded in 48-well plates were pretreated and then treated in quadruplicate with SB105_A10 at concentrations of 1, 0.5, 0.25, 0.125, 0.0625, 0.031, 0.015, and 0 μM 1 h prior to and during infection with a clinical isolate of HSV-1 or HSV-2 (60 PFU/well). Following virus adsorption (2 h at 37°C), the cells were overlaid with 1.2% methylcellulose containing the different concentrations of SB105_A10 in combination with ACV at 1, 0.5, 0.1, 0.05, 0.01, 0.005, 0.001, and 0 μM. At 48 h p.i., cells were fixed and stained with crystal violet and the plaques counted. The data were analyzed using an isobologram according to previously reported methods (15, 23). In this analysis, the 50% effective concentrations (EC50s) were used to calculate the fractional inhibitory concentration (FIC) as follows: FICx = (EC50 of compound “X” in combination/EC50 of compound alone); FICy = (EC50 of compound “Y” in combination/EC50 of compound alone). A FIC index (which corresponds to the sum of the FIC values of the combined compounds [FIC index = FICx +/FICy]) between 0.5 and 4 describes a nonsynergism, a FIC index of ≤0.5 indicates synergism, and a FIC index of >4 indicates antagonism. Data from drug combination studies were also evaluated using the MacSynergy II analysis program for multiple drug interactions (29).
Data analysis.
All data were generated from duplicate wells in at least three independent experiments. The effects of dendrimeric peptides at different concentrations were expressed as PFU/ml on a log10 scale or as the mean plaque count for each drug concentration expressed as a percentage of the mean plaque count of the control virus. Concentrations producing 50% and 90% reductions in plaque formation (IC50 and IC90) were calculated by nonlinear regression using the computer program PRISM, version 4.0 (GraphPad Software, Inc.).
RESULTS
Inhibition of HSV-1 and HSV-2 replication by SB105 and SB105_A10 dendrimers.
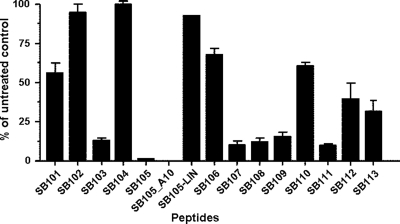
To identify novel peptide-derivatized dendrimers capable of inhibiting HSV replication in vitro, we generated a minilibrary of 15 linear, dimeric, and dendrimeric peptides derived from the M6 prototype (26). Figure 1 shows the generic structures of the multimeric lysine core and the sequences of the anchored surface linear peptides. The novel peptide-derivatized dendrimers composing the minilibrary were selected using a virus yield reduction assay in which test compounds were present in cell medium before, during, and after viral adsorption. Several of the tested peptide-derivatized dendrimers showed moderate activity against a clinical isolate of HSV-1 (Fig. 2). However, two of them, SB105 and SB105_A10, decreased HSV-1 replication by over 90% and were thus selected for further analysis. Furthermore, an unbranched peptide with the same sequence as SB105 (Fig. 1, SB105-LIN) only showed a minimal level of inhibitory activity (Fig. 2), thus suggesting that the dendrimer structure itself is required to confer the anti-HSV-1 activity to the specific amino acids comprising the SB105 surface groups.
Fig. 2.
Identification of peptide-derivatized dendrimers with anti-HSV activity. Vero cells were mock infected or infected with HSV-1 (MOI of 0.1) and, where indicated, the cells were pretreated and treated with 4 μM concentrations of the different dendrimeric peptides 1 h prior to and during infection until an extensive viral cytopathic effect was observed in the untreated controls. The extent of HSV-1 replication was then assessed by titrating the infectivity of supernatants of cell suspensions by a standard plaque assay on Vero cells. The data shown in each column are the means ± standard deviations (SD; error bars) from three independent experiments performed in duplicate.
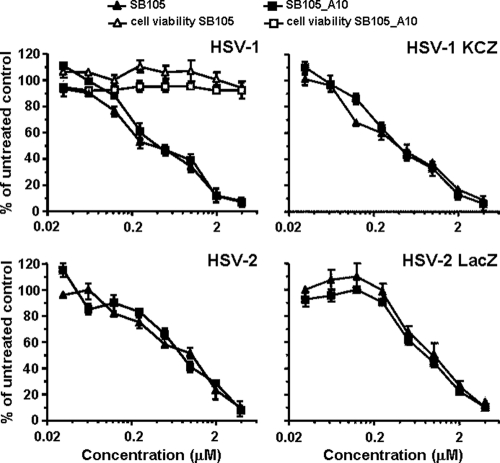
Pretreatment of Vero cells with SB105 and SB105_A10 peptides 1 h before infection produced a significant concentration-dependent inhibition of both clinical isolates of HSV-1 and HSV-2 and the recombinant HSV-1 KCZ and HSV-2 LacZ viruses at 2 days p.i. (Fig. 3). The IC50 and IC90 of both SB105 and SB105_A10 against HSV-1 replication were 0.4 and 3 μM, respectively. Against HSV-2 replication, they were 1 and 4 μM for SB105 and 0.8 and 4 μM for SB105_A10. To exclude the possibility that the antiviral activity of SB105 and SB105_A10 might be due to cytotoxicity, their effects on the viability of uninfected Vero cells were assessed using MTT assays. As shown in Fig. 3, their antiviral activity was not due to cytotoxicity of the target cells themselves, since the significant toxic effects were not observed at any concentration tested (maximal concentration tested, 100 μM). The selectively index (SI) of SB105 was greater than 250 and 100 for HSV-1 and HSV-2, respectively, whereas that of SB105_A10 was greater than 250 for HSV-1 and greater than 125 for HSV-2.
Fig. 3.
Antiviral activity of dendrimeric peptides on HSV-1 and HSV-2 replication. Vero cell monolayers were infected with clinical isolates of either HSV-1 or HSV-2 or the recombinant laboratory strain HSV-1 KCZ or HSV-2 LacZ at an MOI of 0.1 and, where indicated, the cells were treated with increasing concentrations of dendrimers 1 h before as well as during virus adsorption. These remained in the culture media throughout the experiment, until an extensive viral cytopathic effect was observed in the untreated controls. The extent of HSV replication was then assessed by titrating the infectivity of supernatants of Vero suspensions by standard plaque assay. Plaques were microscopically counted, and the mean plaque counts for each drug concentration were expressed as a percentage of the mean count of the control. The number of plaques was plotted as a function of drug concentration, and the concentrations producing 50 and 90% reductions in plaque formation (IC50 and IC90, respectively) were determined. The data shown represent means ± SD (error bars) from three independent experiments performed in duplicate. To determine cell viability, Vero cells were exposed to increasing concentrations of peptides. After 3 days of incubation, the number of viable cells was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method.
In contrast, the replication of a clinical isolate of adenovirus as well as of a VSV laboratory strain in HELF cells was not significantly affected by SB105 or SB105_A10, with IC50s of >50 μM for both viruses, thus supporting the specificity of the dendrimers' activities against herpesviruses.
SB105_A10 inhibits an immediate-early event in the HSV replication cycle.
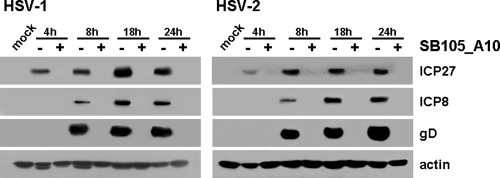
In order to obtain more insight into the nature of the antiviral activity of the selected peptide-derivatized dendrimers, we investigated the effects of SB105_A10 upon the gene expression program in both HSV-1 and HSV-2. To this end, total protein cell extracts were prepared from HSV-1- and HSV-2-infected Vero cells treated with SB105_A10 for various lengths of time postinfection. The expression levels of ICP27, ICP8, and gD were then examined by immunoblotting with specific antibodies and used as a reflection of the levels of immediate-early, early, and late HSV protein expression, respectively. As seen in Fig. 4, SB105_A10 blocked the expression of all HSV proteins assessed at all of the time points analyzed, thus indicating that SB105_A10 affects a very early stage in the HSV replication cycle, i.e., a stage that precedes the onset of IE gene expression. Consistent with this observation, the addition of either 2 μM SB105 or 2 μM SB105_A10 after 2 h of virus adsorption did not significantly reduce HSV-1 and HSV-2 replication, in contrast with the potent antiviral activity measured when the same dendrimers were applied up to 1 h prior to or at the time of infection (data not shown). These results support the view that the dendrimers target a very early phase of the HSV cycle, such as virus adsorption and/or entry.
Fig. 4.
SB105_A10 prevents HSV gene expression. Vero cells were infected with HSV-1 or HSV-2 at an MOI of 1, or mock infected (mock) and, where indicated, the cells were pretreated and treated with 2 μM SB105_A10 1 h prior to and during infection. Total cell extracts were prepared at increasing times p.i., fractionated by 8% SDS-PAGE (50 μg protein/lane), and analyzed by immunoblotting with anti-HSV-1/2 ICP27, anti-HSV-1/2 ICP8, and anti-HSV-1/2 gD. Actin immunodetection with a MAb served as an internal control.
SB105 and SB105_A10 inhibit HSV attachment.
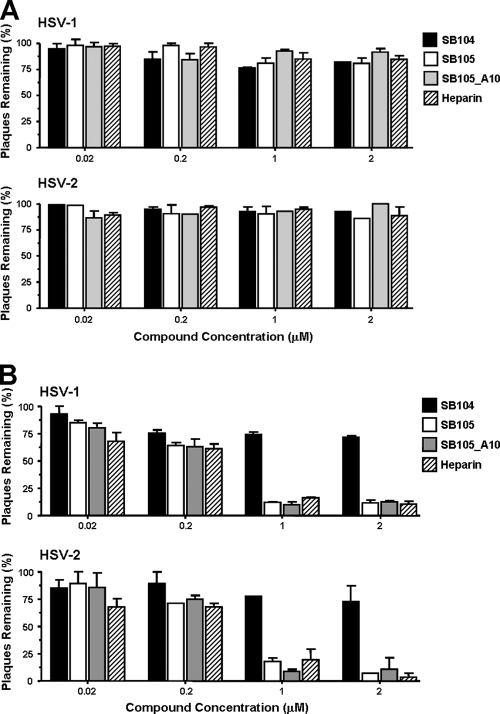
To investigate whether the inhibitory activities of SB105 and SB105_A10 were due to the inhibition of HSV entry into cells, prechilled Vero monolayers were infected with the clinical isolates of HSV-1 or of HSV-2 for 3 h at 4°C. SB105, SB105_A10, SB104 (as a negative control for inhibition of HSV-1 replication) (Fig. 2), or heparin (as a positive control for inhibition of viral attachment) was then added, and the cells were incubated at 37°C to allow viral entry. These experimental conditions permit synchronized virus penetration to occur following attachment at low temperatures (35). After 3 h at 37°C, any HSV virion that remained attached to the surface of the cells was inactivated by acidic glycine treatment. The cells were then overlaid with 1.2% methylcellulose to measure the infectivity of HSV-1 that had successfully entered into cells. As shown in Fig. 5 A, none of the dendrimers affected HSV-1 or HSV-2 entry at any of the concentrations examined.
Fig. 5.
SB105 and SB105_A10 inhibit the first phases of the HSV replication cycle. (A) Dendrimers do not affect viral entry after virus adsorption. Prechilled Vero cells were infected with precooled HSV-1 or HSV-2 at a MOI of 0.004 for 3 h at 4°C to allow virion attachment to cells. Unattached virus was removed by washing, and cells were treated with various concentrations of SB105, SB105_A10, SB104, or heparin for 3 h at 37°C prior to inactivation of extracellular virus with acidic glycine for 2 min at RT. After further washing steps, cells were covered with 1.2% methylcellulose containing medium. At 48 h p.i., viral plaques were stained and counted. The results shown are means ± SD (error bars) from three independent experiments performed in duplicate. (B) SB105 and SB105_A10 prevent HSV attachment. Prechilled Vero cells were treated with various concentrations of SB105, SB105 A_10, SB104, or heparin at 4°C for 30 min and then infected with precooled HSV-1 or HSV-2 at a MOI of 0.004 for 3 h at 4°C in the presence of compounds as indicated. After virus adsorption, cells were overlaid with 1.2% methylcellulose and incubated at 37°C. Two days postinfection, viral plaques were stained and counted. The results shown are means ± SD (error bars) from three independent experiments performed in duplicate.
Next, to test the effects of the dendrimers on HSV attachment, prechilled Vero cell monolayers were infected with HSV-1 or HSV-2 in the presence of the dendrimers or heparin for 2 h at 4°C (a condition that is known to allow virus adsorption only). Cells were washed to remove the dendrimers and any unattached virus and covered with a solution of 1.2% methylcellulose in order to measure the infectivity of HSV that had successfully attached to the cells. As shown in Fig. 5B, SB105 and SB105_A10 clearly impaired the attachment of HSV in a concentration-dependent manner and to a similar degree as that observed with the virus yield reduction assay (Fig. 3). In contrast, SB104 showed only a minimal inhibitory activity against HSV entry, consistent with its lack of any significant antiviral activity when tested in the virus yield reduction assay (Fig. 2). As expected, heparin blocked the ability of HSV-1 to attach to Vero cells by preventing the virus from interacting with cell surface heparan sulfate (HS) (34).
Altogether, these findings indicate that the initial step of HSV attachment to target cells is prevented in the presence of SB105 or SB105_A10.
SB105 and SB105_A10 do not exert virucidal activity against HSV.
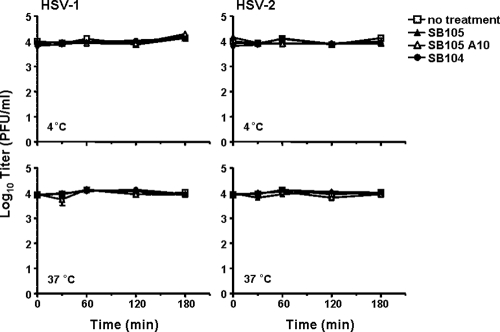
Since inhibition of HSV attachment might result from an irreversible dendrimer-induced inactivation of the virions, we explored the possibility that SB105 and SB105_A10 were interacting with HSV particles and inactivating viral infectivity. In order to do this, HSV-1 and HSV-2 aliquots were incubated with 1 μM SB105, SB105_A10, or SB104 at 4 or 37°C for various lengths of time. After incubation, the samples were diluted to reduce the dendrimer concentrations well below those which inhibit HSV replication (0.02 μM), and the infectivity of preincubated virions was titrated on Vero cells. As shown in Fig. 6, the preincubation of virions with dendrimers at either 4 or 37°C did not produce a detectable loss of HSV-1 infectivity. Thus, these results suggest that SB105 and SB105_A10 inhibit HSV infection only if present at the time of virus attachment to target cells.
Fig. 6.
Preincubation of SB105 and SB105_A10 with viruses does not affect HSV infectivity. HSV-1 and HSV-2 aliquots (104 PFU) were incubated at either 4 or 37°C for various lengths of time with no dendrimers (open squares) or 25 μM SB105 (closed triangles), SB105_A10 (opened triangles), or SB104 (closed circles). After incubation, the samples were diluted to reduce the dendrimer concentration below that which inhibits HSV replication (0.02 μM), and the virus was titrated on Vero cells. Plaques were microscopically counted and the mean plaque counts for each peptide concentration expressed as PFU/ml on a log10 scale. The data shown are means ± SD (error bars) from three independent experiments performed in duplicate.
SB105_A10 dendrimer binds to cell surface HSPGs.
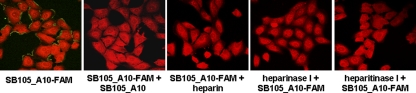
The specific amino acid sequence that functionalizes SB105 and SB105_A10 contains a cluster of basic amino acids and thus confers positive charges to the surface of these dendrimers. Since the initial contact between HSV and a cell is characterized by the binding of the virion glycoproteins gB and gC to negatively charged heparan sulfate (HS) moieties of cell surface proteoglycans (HSPGs) (5, 34, 37), we next investigated whether the antiviral activity of the selected dendrimers could be a consequence of their binding to HSPGs, thereby resulting in the inhibition of the first step of HSV infection. Vero cells were incubated with the fluorescent derivative SB105_A10-FAM for 2 h at 4°C and, as shown in Fig. 7, a fine punctate cell surface pattern of green fluorescence was observed, indicating its successful binding to cells. The staining was not due to nonspecific background, since fluorescence was prevented by the unlabeled SB105_A10 (Fig. 7). Moreover, SB105_A10-FAM binding to Vero cells was blocked by soluble heparin, as well as by the pretreatment of target cells with heparinase I or heparitinase I, which digest the glycosaminoglycans moieties of HSPGs (Fig. 7). These results indicate the ability of SB105_A10 to bind cellular HS and suggest the competitive inhibition of HSV-1 binding to cell surface HSPGs as the mechanism of action of SB105_A10 against HSV-1 infection.
Fig. 7.
SB105_A10 binds to cell surface heparan sulfate. Vero cell monolayers were left untreated or treated with heparinase I or heparitinase I (2.5 U/ml) for 1 h at 37°C, washed with medium, and chilled at 4°C. Next, SB105_A10-FAM (5 μM) was added alone or in the presence of a 10-fold molar excess of SB105_A10 or heparin (10 μg/ml) for a further 1 h at 4°C. Cells were then fixed, counterstained with Evans blue, and examined by fluorescence microscopy. The experiment was repeated three times, and representative images are presented.
Activity of SB105 and SB105_A10 at different pHs or in the presence of human serum proteins.
Analysis of the mechanism of action of SB105 and SB105_A10 demonstrated their ability to prevent HSV infection. However, to evaluate their potential as candidate compounds for the development of topical microbicides for preventing sexual transmission of HSV-1 and HSV-2, the influence of specific physiological properties of the vagina, such as the pH and the presence of proteins, on their antiviral efficacy should be considered. Thus, to examine the stability of SB105 and SB105_A10 at different pHs (ranging between 3.0 and 9.0), the dendrimers were incubated in buffers of different pHs for 2 h at 37°C, and their EC50s were then measured using virus yield reduction assays at physiological pH. As reported in Table 1, the acidic treatment (pHs 3.0 and 4.0) for 2 h did not affect the stability of either SB105 or SB105_A10, since the effectiveness against HSV-1 or HSV-2 was similar to that observed for dendrimers incubated at neutral pH.
Table 1.
Stability of SB105 and SB105_A10 at different pHs
| Virus and pH | Antiviral activity (EC50 [μM])a |
|
|---|---|---|
| SB105 | SB105_A10 | |
| HSV-1 | ||
| 3.0 | 0.21 ± 0.04 | 0.27 ± 0.02 |
| 4.0 | 0.32 ± 0.05 | 0.25 ± 0.01 |
| 5.0 | 0.38 ± 0.02 | 0.44 ± 0.03 |
| 6.0 | 0.42 ± 0.02 | 0.46 ± 0.01 |
| 7.0 | 0.41 ± 0.02 | 0.46 ± 0.02 |
| 8.0 | 0.44 ± 0.01 | 0.36 ± 0.04 |
| 9.0 | 0.43 ± 0.01 | 0.40 ± 0.02 |
| HSV-2 | ||
| 3.0 | 0.78 ± 0.02 | 0.77 ± 0.05 |
| 4.0 | 0.89 ± 0.02 | 0.75 ± 0.02 |
| 5.0 | 1.05 ± 0.03 | 0.89 ± 0.01 |
| 6.0 | 1.09 ± 0.01 | 0.92 ± 0.04 |
| 7.0 | 1.13 ± 0.02 | 0.83 ± 0.02 |
| 8.0 | 1.19 ± 0.01 | 0.81 ± 0.01 |
| 9.0 | 1.00 ± 0.04 | 0.85 ± 0.05 |
Compound concentration that inhibits 50% of HSV replication as determined by a virus yield reduction assay in Vero cells as described in Materials and Methods. The values are means ± SD of data derived from three independent experiments performed in duplicate.
To evaluate the influence of the presence of human proteins on their antiviral activity, SB105 and SB105_A10 were incubated at 37°C with 10% human serum from HSV-negative donors for 1 or 18 h prior to using them to pretreat and treat cells in the virus yield reduction assay in the presence of 10% human serum protein. The dendrimers retained their activity against HSV-1 and HSV-2 in the presence of 10% human serum even after an overnight incubation at 37°C (Table 2). Altogether, these findings indicate that the stability of SB105 and SB105_A10 was not affected by specific parameters which characterize the vaginal environment.
Table 2.
Effect of human serum on SB105 and SB105_A10 antiviral activity
| Incubation timea and virus | Antiviral activity (EC50 [μM])b |
|
|---|---|---|
| SB105 | SB105_A10 | |
| 1 h | ||
| HSV-1 | 0.40 ± 0.01 | 0.39 ± 0.03 |
| HSV-2 | 1.03 ± 0.04 | 0.89 ± 0.02 |
| 18 h | ||
| HSV-1 | 0.36 ± 0.03 | 0.45 ± 0.02 |
| HSV-2 | 0.95 ± 0.02 | 0.83 ± 0.04 |
Treatment with 10% human serum for indicated incubation time.
Compound concentration that inhibits 50% of HSV replication as determined by virus yield reduction assay in Vero cells as described in Materials and Methods. The values are the means ± SD of data derived from three independent experiments performed in duplicate.
Synergistic effect of combined SB105_A10 and acyclovir treatment on HSV-1 replication.
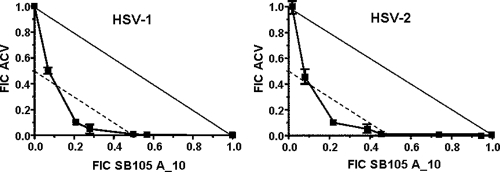
Since the above results indicate that the mode of action of SB105_A10 differs from that of the reference drug acyclovir, we wanted to investigate whether a combination of the two compounds would result in synergistic activity against HSV replication. To this end, the combined effects of SB105_A10 and ACV on the replication of HSV-1 and HSV-2 were evaluated using plaque reduction assays and then analyzed using the isobologram method (23, 15). Figure 8 shows isobologram representations of the SB105_A10-ACV interaction; in this figure, the solid lines represent the theoretical plot for an additive interaction between the two compounds (which is the expression of an independent antiviral effect), while the broken lines represent the theoretical plot for a significant synergistic interaction. The FIC values obtained for several combinations of SB105_A10 and ACV are located for both viruses under the plot representing synergism (FIC index, <0.5) and indicate that the two compounds interact in a synergistic manner, each reinforcing the other's antiviral activity against HSV-1 or HSV-2. Data from the SB105_A10-ACV combination were evaluated further using MacSynergy II software for multiple drug interactions (29). Synergy volumes of >100 μM2% were measured for the drug combination against both HSV-1 and HSV-2, thus confirming a significant synergy (data not shown).
Fig. 8.
Effects of combination of SB105_A10 and ACV on HSV replication. Vero cells were pretreated and treated with different concentrations of SB105_A10 prior to and during infection with clinical isolates of HSV-1 or HSV-2. Following virus adsorption, the cells were overlaid with 1.2% methylcellulose in the presence of SB105_A10 combined with different concentrations of ACV. Data for each compound are reported as the fractional inhibitory concentration (FIC), calculated by dividing the EC50 of compound “X” in combination with compound “Y” by the EC50 of compound “X” alone. The solid and broken lines represent the unit lines for FIC equal to 1 and 0.5, respectively. The FIC index, corresponding to the sum of the FIC values for compounds “X” and “Y,” is indicated for every combination of concentrations. A FIC index between 0.5 and 4 describes a nonsynergism, a FIC index of ≤0.5 indicates synergism, and a FIC index of >4 indicates antagonism. The results are from a single experiment performed in quadruplicate, representative of three independent experiments.
Cytotoxicity assays confirmed that none of these drug combinations exhibited toxic effects against growing Vero cells (data not shown), thus suggesting that their synergistic antiviral activity was not the result of an increased cytotoxicity but that it likely arose from the combined interference of two different phases of the HSV replication cycle.
DISCUSSION
This study shows that the peptide derivatized-dendrimers SB105 and SB105_A10 have a potent dose-dependent antiviral activity against both laboratory strains and clinical isolates of HSV-1 and HSV-2, the mechanism for which involves the inhibition of the initial virion attachment to HSPGs on the surface of target cells.
Unintended interactions between HSPGs and their protein partners may evolve in pathophysiological conditions, such as chronic inflammation, tumor growth, angiogenesis, or microbial infections (3). Thus, molecules that interfere with the interactions between negatively charged HS chains and their binding partners could represent interesting candidates for the development of pharmacological agents for the treatment of diseases that involve HSPG-protein interactions (33). With regard to HSV infections, in which virus adsorption is mediated by the binding of virion glycoproteins gC and gB to HSPGs (5, 37), natural antimicrobial proteins, such as lactoferrin and its N-terminal pepsin-derived fragment lactoferricin, as well as small chemical antagonists, such as surfen, have been reported to exert antiviral activities via mechanisms of action that involve their binding to HSPGs, thereby blocking HSV entry (14, 33). SB105 and SB105_A10 appear to belong to this category of antiviral agents since they are specific for viruses with replication cycles that initiate with the binding of virion proteins to cell surface HPSGs. In fact, infection of herpesviruses, such as HSV-1, HSV-2 (this study), and HCMV (19), as well as that of pseudovirions of several human papillomavirus (HPV) types (10), is inhibited by SB105 and SB105_A10. In contrast, virus infection for which virion attachment to target cells does not depend on HSPGs, such as that with adenovirus or VSV (1, 20), is not significantly affected.
In this study, we identified the anti-HSV activity for two peptide-derivatized dendrimers developed from the M6 prototype (26). The M6 peptide, synthesized in dendrimeric tetrabranched form (multiple antigen peptide [MAP]), has a tetrameric lysine core that tethers four 10-mer peptide chains with the sequence QKKIRVRLSA in lysine α and ε positions. It demonstrates antibacterial activity, not only against members of the Enterobacteriaceae but also against recent clinical isolates of multidrug-resistant Pseudomonas aeruginosa, by binding lipopolysaccharide and causing perforation of cell membranes without destroying external cell morphology. The same dendrimeric peptide has also been shown to be highly resistant to blood proteases and to have low hemolytic activity and little cytotoxic effect against eukaryotic cells, making it a promising candidate for the development of new antibacterial/antiviral drugs (27). Here, we observed that the anti-HSV activity of SB105 and SB105_A10 depends on the specific amino acid sequence of the peptide surface groups attached to the dendrimer scaffold, as variations of this sequence were found to reduce or abolish their antiviral action. In fact, the screening of 15 distinct dendrimer compounds with differences in the amino acid sequences of their surface groups led to the selection of SB105 and its derivative SB105_A10 in which the C-terminal glutamine residue of the functional ASLRVRIKKQ group was removed. The basic amino acid residues of the ASLRVRIKKQ peptide (which functionalizes the SB105 and SB105_A10 dendrimers) endow the dendrimers' surfaces with positive charges. This predicted polycationic structure of SB105 and SB105_A10 led us to hypothesize that they may interact with the negatively charged carboxyl/sulfate groups of the HS chains and thus block the binding of HSV to its cellular receptors. We therefore examined their ability to inhibit the early stages of HSV replication, i.e., attachment and penetration. Indeed, the results from attachment and entry assays and from immunofluorescence and HS chain enzymatic depletion experiments suggested that dendrimers prevent HSV-1 and HSV-2 binding to cell surface HSPGs.
Although the exact mechanism by which these dendrimers interact with HPSGs remain to be established, it is likely that ionic interactions between their cluster of positively charged amino acids and the negatively charged sulfate and carboxyl groups in HS constitute the major binding interactions. It is therefore unlikely that they are able to form complexes with HSV envelope glycoproteins, as has been suggested for some sulfonated dendrimers (32). Consistent with this hypothesis, we observed that SB105_A10 binds to cells in a heparan sulfate-dependent manner, since this interaction was prevented both by the homologous polysaccharide heparin and by heparinase/heparitinase treatment of target cells (Fig. 7) (19).
As the global incidence, morbidity, and mortality of viral sexually transmitted infections (STIs) are very significant, the development of new, safe, topically applied microbicides for the prevention of STIs is a high priority. Therefore, attachment/entry inhibitors that block virus shedding and transmission by close personal contact may provide a realistic method of microbicide intervention. Furthermore, given the ability of HSV to establish latency and reactivate frequently, the ideal microbicide to prevent its transmission should prevent the establishment of infection. From this perspective, agents like SB105 and SB105_A10, which prevent attachment to target cells, may be most advantageous. The suitability of this strategy is demonstrated by numerous negatively charged polyanions that in recent years have been selected for development as candidate microbicides, as they bind HSV envelope components and block virion attachment and entry into target cells (28). Of these, dendrimers and dendrimer-like molecules have recently been generated with surfaces formed of negative charges and screened for potential antiherpesvirus activities (12, 31). Indeed, in vitro analysis has revealed that sulfonated and carboxylated polylysine dendrimers effectively block the HSV-1 and -2 infection of cells, and nonprimate animal studies have shown that they were able to protect animals against an intravaginal HSV-2 challenge (4, 2, 11). However, in addition to inhibiting HSV attachment, the sulfonated dendrimers were also shown to act at a postentry level by apparently inhibiting HSV DNA synthesis, which suggests that they could be used not only for prevention of HSV infection but also for inhibiting viral replication (11). On the basis of these results, SPL7013, a lysine-based dendrimer with naphthalene disulfonic acid surface groups, is currently being developed as a candidate topical microbicide, known as ViVagel, for the prevention of both HIV-1 and HSV-2 transmission (32).
Topical microbicides are applied directly to the genital tract to protect against the acquisition of STIs. However, their activity may be affected by the unique physiological properties of the vagina. We therefore tested the stability of SB105 and SB105_A10 at various pHs and in the presence of proteins. The results showed that these treatments did not significantly reduce their stability, thus suggesting that these dendrimers may be suitable for vaginal application without loss of their antiviral activity. Finally, besides their anti-HSV activity, SB105 and SB105_A10 also have recently been shown to be active against pseudovirions of HPV-6, -16, and -18, as well as against HIV-1 infections (10; A. Giuliani, S. Landolfo, D. Lembo, D. Gibellini, G. Pirri, L, Pizzuto, and G. Gribaudo, Italian patent application MI2009A001425). Therefore, these dendrimers have the potential for further development as the active ingredient of broad-spectrum microbicides with the goal of preventing transmission of the major viral STIs.
In conclusion, the results of this study indicate that SB105 and SB105_A10 could form attractive candidates for a new class of antiviral drugs that exert their effects via a novel pathway targeting virus attachment and that they might possess advantageous features, such as activity against a broad range of viruses and the low risk of emergence of drug-resistant strains. Moreover, they could also be considered for combinatorial drug treatment with nucleoside analogues, such as ACV, for the therapeutic management of HSV infection. The potent in vitro anti-HSV activities of SB105 and SB105_A10 calls for further studies to be performed to evaluate their efficacy and safety in murine models of acute infection, in order to validate their development as novel microbicides for the prevention of HSV infection and other viral STIs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from Italian Ministry of University and Scientific Research to G.G. (PRIN 2007) and S.L. (PRIN 2008) and the Piedmont Region to G.G and S.L. (Ricerca Sanitaria Finalizzata 2008 and 2009).
We thank Roberto Manservigi and Valeria Ghisetti for the provision of HSV-1 and HSV-2 strains and Mark N. Prichard for help with the synergy calculation using MacSynergy II software.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Berk A. J. 2007. Adenoviridae: the viruses and their replication, p. 2355–2394 In Knipe D. M., et al. (ed.), Fields virology, 5th ed., vol. 2. Lippincott, Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2. Bernstein D. I., et al. 2003. Evaluation of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob. Agents Chemother. 47:3784–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bishop J. R., Schuksz M., Esko J. D. 2007. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446:1030–1037 [DOI] [PubMed] [Google Scholar]
- 4. Bourne N., et al. 2000. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 44:2471–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campadelli-Fiume G., et al. 2007. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev. Med. Virol. 17:313–326 [DOI] [PubMed] [Google Scholar]
- 6. Caposio P., Luganini A., Hahn G., Landolfo S., Gribaudo G. 2007. Activation of the virus-induced IKK/NF-κB signaling axis is critical for the replication of human cytomegalovirus in quiescent cells. Cell. Microbiol. 9:2040–2054 [DOI] [PubMed] [Google Scholar]
- 7. Cloninger M. J. 2002. Biological applications of dendrimers. Curr. Opin. Chem. Biol. 6:742–748 [DOI] [PubMed] [Google Scholar]
- 8. Coen D. M., Schaffer P. A. 2003. Antiherpesvirus drugs: a promising spectrum of new drugs and drug targets. Nat. Rev. Drug Discov. 2:278–288 [DOI] [PubMed] [Google Scholar]
- 9. Reference deleted.
- 10. Donalisio M., et al. 2010. Identification of a dendrimeric heparan sulfate-binding peptide that inhibits infectivity of genital types of human papillomaviruses. Antimicrob. Agents Chemother. 54:4290–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gong E., et al. 2005. Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex virus. Antiviral Res. 68:139–146 [DOI] [PubMed] [Google Scholar]
- 12. Greco A., Diaz J.-J., Thouvenot D., Morfin F. 2007. Novel targets for the development of anti-herpes compounds. Infect. Disord. Drug Targets 7:11–18 [DOI] [PubMed] [Google Scholar]
- 13. Heegaard P. M., Boas U. 2006. Dendrimer based anti-infective and anti-inflammatory drugs. Recent Pat. Antiinfect. Drug Discov. 1:331–351 [DOI] [PubMed] [Google Scholar]
- 14. Jenssen H. 2005. Anti herpes simplex virus activity of lactoferrin/lactoferricin—an example of antiviral activity of antimicrobial protein/peptide. Cell. Mol. Life. Sci. 62:3002–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson M. D., MacDougall C., Ostrosky-Zeichner L., Perfect J. R., Rex J. H. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keller M. J., Tujama A., Carlucci M. J., Herold B. C. 2005. Topical microbicides for prevention of genital herpes infection. J. Antimicrob. Chemother. 55:420–423 [DOI] [PubMed] [Google Scholar]
- 17. Laquerre S., et al. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J. Virol. 72:6119–6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luganini A., Caposio P., Landolfo S., Gribaudo G. 2008. Phosphorothioate-modified oligodeoxynucleotides inhibit human cytomegalovirus replication by blocking virus entry. Antimicrob. Agents Chemother. 52:1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luganini A., et al. 2010. Peptide-derivatized dendrimers inhibit human cytomegalovirus infection by blocking virus binding to cell surface heparan sulfate. Antiviral Res. 85:532–540 [DOI] [PubMed] [Google Scholar]
- 20. Lyles D. S., Rupprecht C. E. 2007. Rhabdoviridae, p. 1363–1408 In Knipe D. M., et al. (ed.), Fields virology, 5th ed., vol. 1. Lippincott, Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 21. McCarthy T. D., et al. 2005. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2:312–318 [DOI] [PubMed] [Google Scholar]
- 22. Niederhafner P., et al. 2005. Peptide dendrimers. J. Pept. Sci. 11:757–788 [DOI] [PubMed] [Google Scholar]
- 23. Odds F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 24. Oyan A. M., et al. 1993. Resistance of herpes simplex virus type 2 to neomycin maps to the N-terminal portion of glycoprotein C. J. Virol. 67:2434–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pauwels R., et al. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309–321 [DOI] [PubMed] [Google Scholar]
- 26. Pini A., et al. 2005. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob. Agents Chemother. 49:2665–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pini A., et al. 2007. Characterization of the branched antimicrobial peptide M6 by analyzing its mechanism of action and in vivo toxicity. J. Pept. Sci. 13:393–399 [DOI] [PubMed] [Google Scholar]
- 28. Pinna D., et al. 2008. Inhibition of herpes simplex virus types 1 and 2 in vitro infection by sulfated derivatives of Escherichia coli K5 polysaccharide. Antimicrob. Agents Chemother. 52:3078–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prichard M. N., Shipman C., Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 14:181–205 [DOI] [PubMed] [Google Scholar]
- 30. Roizman B., Knipe D. M., Whitley R. J. 2007. Herpes simplex viruses, p. 2501–2601 In Knipe D. M., et al. (ed.), Fields virology, 5th ed., vol. 2. Lippincott, Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 31. Rosa Borges A., Schengrund C.-L. 2005. Dendrimers and antivirals: a review. Curr. Drug Targets Infect. Disord. 5:247–254 [DOI] [PubMed] [Google Scholar]
- 32. Rupp R., Rosenthal S. L., Stanberry L. R. 2007. VivaGel (SPL7013 gel): a candidate dendrimer-microbicide for the prevention of HIV and HSV infection. Int. J. Nanomed. 2:561–566 [PMC free article] [PubMed] [Google Scholar]
- 33. Schuksz M., et al. 2008. Surfen, a small molecule antagonist of heparan sulfate. Proc. Natl. Acad. Sci. U. S. A. 105:13075–13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shieh M., WuDunn D., Montgomery R., Esko J., Spear P. 1992. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116:1273–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shogan B., et al. 2006. Virucidal activity of a GT-rich oligonucleotide against herpes simplex virus mediated by glycoprotein B. J. Virol. 80:4740–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Silvestri M. E., Sundqvist V. A. 2001. An investigation into the heparin-binding properties of a synthetic peptide deduced from antigenic domain 2 of human cytomegalovirus glycoprotein B. Scand. J. Immunol. 53:282–289 [DOI] [PubMed] [Google Scholar]
- 37. Spear P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. 6:401–410 [DOI] [PubMed] [Google Scholar]
- 38. Superti F., Ammendolia M. G., Marchetti M. 2008. New advances in anti-HSV chemotherapy. Curr. Med. Chem. 15:900–911 [DOI] [PubMed] [Google Scholar]
- 39. Whitley R. 2006. New approaches to the therapy of HSV infections. Herpes 13:53–55 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.