Abstract
Ceftaroline exhibits in vitro activity against extended-spectrum β-lactamase (ESBL)-, AmpC-, and KPC-producing Enterobacteriaceae when combined with the novel β-lactamase inhibitor NXL104. The purpose of this study was to evaluate the efficacy of a human-simulated regimen of ceftaroline plus NXL104 against Enterobacteriaceae in a murine thigh infection model.
Twelve Enterobacteriaceae isolates were tested with neutropenic ICR mice. Seven of these isolates were also tested with immunocompetent mice. Doses were given to simulate human free-drug exposures of ceftaroline (600 mg) plus NXL104 (600 mg) every 8 h over 24 h by targeting the percentage of time that free drug concentrations remain above the MIC, ƒT>MIC. The change in log10 CFU/ml compared with 0 h controls was observed after 24 h. Human-simulated exposures were achieved against all isolates (MICs of ≤0.015 to 1 μg/ml) in both the neutropenic and the immunocompetent host models, which was equivalent to a ƒT>MIC of 100%. A 0.5 to ≥2 log CFU reduction was observed in the neutropenic thigh infection model. Furthermore, significantly greater reductions in bacterial density were observed for five of seven isolates studied in an immunocompetent model than in the neutropenic-host model. Regardless of immune status, ceftaroline (600 mg) combined with NXL104 (600 mg) every 8 h provided predictable efficacy against ESBL-, non-ESBL-, and KPC-producing isolates with an MIC of ≤1 μg/ml and could be useful in combating the growing threat of resistant Enterobacteriaceae.
INTRODUCTION
Resistance among Gram-negative bacilli is a clinically important problem that is evolving worldwide (10). Much of the increase among Enterobacteriaceae can be attributed to the production of β-lactamases, particularly extended-spectrum β-lactamases (ESBLs), such as TEM, SHV, and CTX-M, or AmpC-type β-lactamases. These enzymes lead to reduced susceptibility to many antimicrobials, including the cephalosporins. Of even more recent concern is the production of KPC-type β-lactamases seen most commonly in Klebsiella spp. (18). These enzymes, classified as class A carbapenemases, markedly increase the MICs of carbapenems in addition to most other available antibiotics. The propensity for Enterobacteriaceae to be the causative pathogens in many common infections, such as pneumonia, bacteremia, and urinary tract and intra-abdominal infections, highlights the potential impact of these enzymes clinically (17).
Ceftaroline (CPT) is a novel cephalosporin with high affinity for the altered penicillin-binding protein PBP2′ or PBP2a present in MRSA and PBP2x and has a broad spectrum of activity against resistant Gram-positive organisms and many common Gram-negative organisms (9). Nonetheless, like all other cephalosporins, it exhibits reduced in vitro activity against ESBL-, AmpC-, and KPC-producing Enterobacteriaceae isolates (8, 14, 16, 19). However, when ceftaroline is combined with the novel non-β-lactam β-lactamase inhibitor NXL104, the in vitro activity of ceftaroline against Enterobacteriaceae is enhanced (15). NXL104 has a broad spectrum of inhibitory activity, neutralizing most class A ESBLs, carbapenemases, and class C (AmpC) β-lactamases (7, 12, 20). As this inhibitor moves further along in the developmental process, it is important to assess the efficacy of the combination when given at the proposed human dose against a broad sampling of these β-lactamase-producing Enterobacteriaceae. The purpose of the current study is to evaluate the efficacy of human-simulated exposures of ceftaroline (600 mg) every 8 h combined with NXL104 (600 mg) every 8 h in both the neutropenic- and the immunocompetent murine thigh infection models using Enterobacteriaceae isolates exhibiting a variety of resistance mechanisms.
MATERIALS AND METHODS
Antimicrobial test agents.
Analytical-grade ceftaroline (Forest Laboratories, Inc., New York, NY) and NXL104 (Forest Laboratories, Inc., New York, NY) were used for all in vivo analyses. Immediately prior to each in vivo experiment, ceftaroline and NXL104 were weighed and reconstituted with a solution of 1.9% l-arginine (lot L0003043; Forest Laboratories, Inc., New York, NY) in sterile water and then further diluted in normal saline to achieve the desired concentration. The combined ceftaroline and NXL104 solutions were stored under refrigeration until use and discarded 24 h after reconstitution.
Bacterial isolates.
Twelve clinical Enterobacteriaceae isolates with a variety of phenotypic and genotypic profiles were utilized in this study (Table 1). The resistance mechanisms (genotypic profile) and MICs (phenotypic profiles) determined by broth microdilution for ceftaroline, ceftaroline-NXL104 (1:1), and a number of other antibiotics were previously characterized for all isolates by R. Jones at JMI Laboratories, North Liberty, IA. Isolates were stored frozen at −80°C in double-strength skim milk (Remel, Lenexa, KS) and then subcultured twice onto Trypticase soy agar with 5% sheep blood (BD Biosciences, Sparks, MD) and grown for 18 to 24 h in ambient air at 35°C prior to use in the experiments.
Table 1.
Genotypic and phenotypic profiles of Enterobacteriaceae test isolates for ceftaroline and other compounds
| Isolate | Resistance mechanism | MIC (μg/ml)a |
|||
|---|---|---|---|---|---|
| CPT | CPT-NXL104 | PIP-TAZ | MEM | ||
| E. coli 356 | TEM-1 | 0.06 | ≤0.015 | 8 | ≤0.12 |
| E. coli 354 | WT | 0.06 | ≤0.03 | 2 | ≤0.12 |
| E. coli 357 | SHV-12 | 8 | 0.03 | 2 | ≤0.12 |
| K. pneumoniae 401 | CTX-M-2 | >128 | 0.06 | 2 | ≤0.06 |
| E. cloacae 47 | WT | 0.12 | 0.12 | 1 | ≤0.12 |
| E. coli 358 | FOX-5 | 16 | 0.12 | 16 | 0.03 |
| K. pneumoniae 399 | KPC-3 | >128 | 0.25 | >128 | 2 |
| E. coli 353 | CTX-M-14 | >256 | 0.25 | 4 | ≤0.12 |
| K. pneumoniae 402 | KPC-2 | >128 | 0.5 | >64 | >8 |
| E. coli 361 | CTX-M-2 | >256 | 0.5 | >64 | 0.12 |
| E. cloacae 49 | AmpC | 128 | 0.5 | >64 | 0.25 |
| K. pneumoniae 398 | KPC-2 | >128 | 1 | >64 | >8 |
Isolates with genotypic and phenotypic profiles provided by R. Jones, JMI Laboratories, North Liberty, IA.
CPT, ceftaroline; PIP-TAZ, piperacillin-tazobactam; MEM, meropenem.
Neutropenic-thigh-infection model.
Pathogen-free female ICR mice weighing 20 to 22 g were acquired from Harlan Laboratories (Indianapolis, IN). The study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee. Animals were maintained and used in accordance with National Research Council recommendations and were provided with food and water ad libitum. Mice were rendered transiently neutropenic by intraperitoneal injections of 150 and 100 mg cyclophosphamide/kg of body weight (Baxter, Deerfield, IL) given 4 days and 1 day, respectively, prior to inoculation (2). A single intraperitoneal injection of uranyl nitrate (5 mg/kg) was also administered 3 days prior to inoculation to induce a predictable degree of renal impairment and slow drug clearance (2). Each thigh was inoculated intramuscularly with 0.1 ml of a suspension of each isolate, freshly prepared from the second subculture of each organism and diluted in normal saline to achieve an inoculum of 107 CFU/ml 2 h prior to the initiation of antimicrobial therapy.
Immunocompetent-thigh-infection model.
Mice utilized in immunocompetent-host studies underwent the same procedure as neutropenic mice, but without the use of cyclophosphamide prior to inoculation. In addition, an inoculum of 108 CFU/ml was used to induce thigh infection.
Pharmacokinetic studies and determination of dosing regimen.
All mice were prepared and infected as described for the neutropenic-thigh-infection model. Single doses of combined ceftaroline and NXL104 (1:1 ratio) at 12.5, 25, and 50 mg/kg were administered in 0.2-ml volumes subcutaneously 2 h after inoculation. Groups of six mice were euthanized, and blood samples were collected via cardiac puncture at eight time points over an 8-h period. Plasma samples were prepared using serum samples separated by centrifugation and stored at −80°C until analysis. Ceftaroline and NXL104 concentrations were subsequently analyzed by Eurofins Medinet, Inc. (Chantilly, VA), using a validated liquid chromatography-tandem mass spectrometry (LC-MS-MS) assay. The upper and lower limits of quantitation for the ceftaroline assay were 20 μg/ml and 0.0075 μg/ml, respectively (R >0.999). The intraday percents coefficient of variation (%CV) for ceftaroline quality control samples of 16 μg/ml and 0.015 μg/ml were both <15%, and the interday %CVs were <5%. The upper and lower limits of quantitation for the NXL104 assay were 20 μg/ml and 0.05 μg/ml, respectively (R >0.999). The intraday %CVs for NXL104 quality control samples of 16 μg/ml and 0.15 μg/ml were <10%, and the interday %CVs were <10%.
Pharmacokinetic parameter values for single doses of ceftaroline and NXL104 in mice were calculated using first-order elimination and nonlinear least-squares techniques (WinNonlin version 5.2; Pharsight, Mountain View, CA). Compartment model selection was based on visual inspection of the fit and correlation between the observed and calculated concentrations based upon the Akaike's Information Criterion and correlation coefficient. A dosing regimen in mice that simulated the percentage of time that free drug concentrations remain above the MIC (%ƒT>MIC) for ceftaroline, as well as peak concentration (Cmax) and area under the curve (AUC), was developed using mean pharmacokinetic parameter values calculated from the individual parameter value estimates observed in humans following treatment with ceftaroline (600 mg) combined with NXL104 (600 mg) administered every 8 h as a 1-h infusion. Exposures for both drugs in humans were derived from pharmacokinetic data in healthy volunteers (data on file at Cerexa, Inc., Oakland, CA). The respective protein-binding values for ceftaroline and NXL104 were 20% and 5% in humans and 35% and 20% in mice (data on file at Cerexa, Inc., Oakland, CA). Confirmatory pharmacokinetic studies were conducted with both neutropenic and immunocompetent infected mice prior to the use of these regimens in pharmacodynamic analyses to ensure that target exposures were achieved with the simulated dosing regimen in both models. Infected, neutropenic mice were administered the human-simulated dosing regimen, and blood samples were collected from groups of six mice at eight time points over the 8-h dosing interval. Additionally, groups of six immunocompetent mice were included at select time points in order to confirm that the pharmacokinetics were similar in both models of infection.
In vivo efficacy.
Twelve isolates with a range of MICs from ≤0.015 to 1 μg/ml CPT-NXL104 were studied using the neutropenic thigh infection model. Seven of these isolates were also evaluated in an immunocompetent model in order to assess the efficacy of a human-simulated dosing regimen both with and without an intact immune system. Beginning 2 h after inoculation, groups of three mice were administered the human-simulated regimen of ceftaroline and NXL104 over a 24-h period. All doses were administered as 0.2-ml subcutaneous injections. Control animals were administered normal saline at the same volume, route, and frequency as the treatment regimen. Groups of three untreated control mice were euthanized by CO2 exposure, followed by cervical dislocation just prior to the initiation of therapy (0 h). All other treatment and control mice were sacrificed 24 h after the initiation of therapy. Mice that did not survive to 24 h were harvested at the time of expiration. Following sacrifice, thighs were removed and homogenized individually in 5 ml of normal saline. Serial dilutions of thigh homogenate were plated onto Trypticase soy agar with 5% sheep blood for determination of bacterial density. Efficacy, defined as the change in bacterial density, was calculated as the change in log10 CFU/ml obtained for ceftaroline- and NXL104-treated mice after 24 h from the 0-h control animals. A comparison of efficacy observed in neutropenic and immunocompetent thigh infection models for each isolate was made using a Student t test or a Mann-Whitney U test if data were not normally distributed. A P value of <0.05 was defined a priori as statistically significant.
Supplemental in vivo studies: determination of immune effects.
Because exposures achieved with a human-simulated regimen were expected to result in maximal efficacy (i.e., 100% ƒT>MIC for all isolates), additional in vivo pharmacodynamic studies were conducted to better characterize the role of immune status in producing efficacy with ceftaroline and NXL104 against three Enterobacteriaceae isolates. Combined ceftaroline and NXL104 (1:1 ratio) was administered subcutaneously at doses ranging from 0.5 to 30 mg/kg every 12 h for 24 h to both neutropenic and immunocompetent infected mice. The change in log10 CFU/ml compared with the level for 0-h controls was observed after 24 h. The ƒT>MIC for each dose administered was determined using the pharmacokinetic parameter values derived from the single-dose pharmacokinetic studies as described above. A comparison of efficacy observed at equivalent doses in neutropenic- and immunocompetent-thigh-infection models for each isolate was made using a Student t test or a Mann-Whitney U test if data were not normally distributed, and a P value of <0.05 was defined a priori as statistically significant.
RESULTS
Bacterial isolates.
The genotypic and phenotypic profiles, covering a range of CPT-NXL104 MICs, of the 12 Enterobacteriaceae isolates evaluated in this study are listed in Table 1. Although ceftaroline MICs (range: 0.06 to >256) for these isolates are elevated as expected because of their defined genotypic profiles, the addition of NXL104 to ceftaroline restored its activity (range, ≤0.015 to 1 μg/ml). In addition, ceftaroline and NXL104 MICs for the 12 isolates studied were low (≤1 μg/ml), but consistent with those collected and studied in surveys (15).
Pharmacokinetic determination.
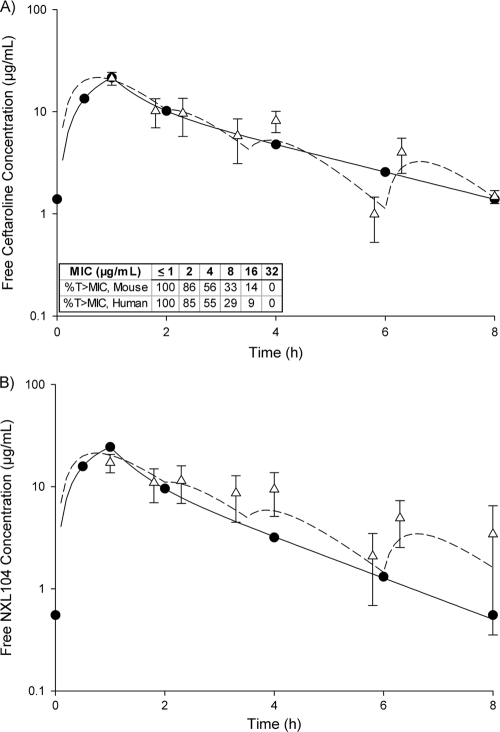
A one-compartment model was used to characterize the free concentration-time profiles of both ceftaroline and NXL104 in murine plasma. The pharmacokinetic parameter values for ceftaroline for single doses of combined ceftaroline and NXL104 tested in the murine model are listed in Table 2. Due to variations in pharmacokinetics observed when humans were compared to mice for ceftaroline and NXL104, it was determined that a ceftaroline/NXL104 dose ratio of 1:0.4 was needed in mice to achieve similar concentrations and exposures of both agents that are observed when a 1:1 ratio is administered in humans. The resulting regimen involved four doses of ceftaroline: 25 mg/kg administered at 0 h, followed by 3.125 mg/kg at 2, 3.5, and 6 h. NXL104 was combined with each ceftaroline dose at a ratio of 1:0.4 (ceftaroline/NXL104). The free concentration-time profiles of a 1-h infusion of ceftaroline (600 mg) combined with NXL104 (600 mg) every 8 h in humans, as well as the human-simulated murine regimen, are displayed in Fig. 1. This dosing regimen was repeated in mice every 8 h for 24 h. The human-simulated regimen of ceftaroline utilized in mice matched the pharmacodynamic parameter of interest, ƒT>MIC, in humans up to MICs of 32 μg/ml (Fig. 1A). In addition, the ƒCmax (μg/ml) and ƒAUC0-8 (μg·h/ml) values for ceftaroline attained with the human-simulated regimen in mice were 21.2 and 55.0, respectively, and were similar to those predicted for humans (21.6 and 52.0, respectively). The ƒCmax (μg/ml) and ƒAUC0-8 (μg·h/ml) values of NXL104 attained with the human-simulated regimen in mice were 17.2 and 60.6, respectively, and were relatively similar to those predicted for humans (24.4 and 47.0, respectively). The ƒT>MIC achieved for NXL104 with the human-simulated regimen in mice was also similar to that observed in humans over the range of combined ceftaroline/NXL104 MICs studied (≥81% at an MIC of 1 μg/ml and 100% at an MIC of ≤0.5 μg/ml). In addition, concentrations observed with the human-simulated regimen were similar between the neutropenic- and immunocompetent-host models at select time points.
Table 2.
Total drug pharmacokinetic parameter values for ceftaroline in mouse sera following single doses of combined ceftaroline and NXL104 (1:1)a
| Dose (mg/kg) | Cmax (μg/ml) | Tmax (h) | AUC0-24 (μg·h/ml) | V (liters/kg) | kel (h−1) | t1/2 (h) | CL (liters/h/kg) |
|---|---|---|---|---|---|---|---|
| 12.5 | 16.64 | 0.73 | 33.10 | 0.28 | 1.37 | 0.51 | 0.38 |
| 25 | 31.31 | 0.51 | 47.27 | 0.42 | 1.25 | 0.55 | 0.53 |
| 50 | 71.11 | 0.29 | 113.48 | 0.56 | 0.79 | 0.88 | 0.44 |
AUC0-24, area under the concentration-time curve for 0 to 24 h;
CL, clearance; Cmax, peak concentration; kel, elimination rate constant; t1/2, half-life; Tmax, time of peak concentration; V, volume of distribution.
Fig. 1.
Free concentration-time profiles for combined ceftaroline (600 mg) (A) and NXL104 (600 mg) (B) in humans (solid lines and black circles) and mice (dotted line and white triangles). Murine data are presented as means ± standard deviations. The ƒT>MICs achieved for ceftaroline with the human-simulated regimen in mice and corresponding targets in humans are listed in the table under graph A.
In vivo efficacy.
The mean (± standard deviation) bacterial densities for 0-h control mice at the start of dosing were 5.98 ± 0.28 log10 CFU/ml per thigh in neutropenic mice and 7.08 ± 0.31 log10 CFU/ml in immunocompetent mice, which increased by averages of 3.1 ± 0.9 log10 CFU/ml and 1.7 ± 0.7 log10 CFU/ml, respectively, after 24 h. Although all mice that received treatment survived to the 24-h time point, a number of control mice failed to survive to 24 h, due to the aggressive virulence of the isolates studied. Bacterial densities obtained from these animals were similar to those in animals that survived to 24 h and were included in the data analysis.
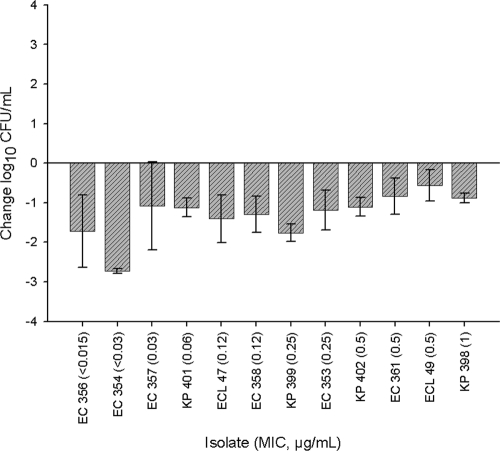
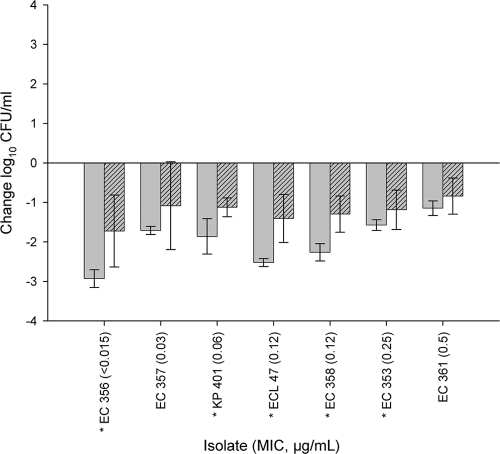
The human-simulated regimen of ceftaroline-NXL104 produced a 0.5- to ≥2-log10-CFU/ml reduction after 24 h against all isolates in the neutropenic thigh infection model (Fig. 2). In addition, a statistically greater reduction in log10 CFU/ml was observed at 24 h in the immunocompetent model than in the neutropenic model for five of the seven isolates that were studied in both models (Fig. 3).
Fig. 2.
Change in log10 CFU/ml for human-simulated ceftaroline and NXL104 at 24 h for 12 Enterobacteriaceae isolates with an MIC range of <0.015 to 1 μg/ml in a neutropenic-thigh-infection model. Each value is the mean ± standard deviation for 6 to 12 infected thighs for an individual isolate.
Fig. 3.
Comparative efficacies of human-simulated ceftaroline and NXL104 at 24 h compared with the level for 0-h controls for seven Enterobacteriaceae isolates in immunocompetent (solid bars)- and neutropenic (patterned bars)-thigh-infection models. Each value is the mean ± standard deviation for 6 to 12 infected thighs for an individual isolate. Asterisks indicate statistically significant differences (P < 0.05).
Supplemental in vivo studies.
The results of the dose-ranging studies conducted in both models of infection are presented in Fig. 4. Reductions in bacterial density were significantly greater in the immunocompetent model than in the neutropenic model at equivalent doses of 2.5 and 15 mg/kg. In addition, the ƒT>MIC required for a reduction in bacterial density in the immunocompetent-host model ranged from 23% to 52%, compared with 56% to 61% in the neutropenic-host model.
Fig. 4.
Comparative efficacies of various doses of ceftaroline and NXL104 at 24 h compared with 0-h controls for E. coli 356 (CPT-NXL104 MIC < 0.015 μg/ml) (A), K. pneumoniae 401 (0.06 μg/ml) (B), and E. coli 361 (0.5 μg/ml) (C) in immunocompetent (solid bars)- and neutropenic (patterned bars)-thigh-infection models. Each value is the mean ± standard deviation for five or six infected thighs. Asterisks indicate statistically significant differences at a given dose (P < 0.05).
DISCUSSION
Resistance among Enterobacteriaceae resulting from the production of ESBLs and KPCs is a growing problem, both in the United States and abroad (10, 18). In this study, we sought to determine if a human-simulated regimen of a novel cephalosporin, ceftaroline, was effective against these organisms when combined with the novel non-β-lactam β-lactamase inhibitor NXL104 in a murine thigh infection model. This combination was efficacious against all ESBL-, non-ESBL-, and KPC-producing isolates tested in both neutropenic- and immunocompetent-host models of infection.
A ƒT>MIC of 100% was achieved with the human-simulated regimen, and efficacy against all 12 isolates was observed in the neutropenic model, regardless of the resistance mechanism. It is not surprising that efficacy against all isolates was observed, as ƒT>MIC was well above 60% to 70%, the predicted target for attaining maximal bactericidal effects with a cephalosporin (4, 5, 13, 21). In a study of ceftaroline alone against Escherichia coli and Klebsiella pneumoniae isolates (MIC, 1 to 2 μg/ml) in a neutropenic thigh infection model, the mean ƒT>MIC needed for a 2 log10 CFU/ml reduction was 54% ± 3% (3). For the two isolates with MICs of 0.12 μg/ml, similar efficacies were noted between the ESBL (E. coli 358; FOX-5) and non-ESBL (Enterobacter cloacae 47; wild type [WT]) producers (∼1 log10 CFU/ml reduction in neutropenic mice for both isolates at 24 h), demonstrating that NXL104 has the ability to restore activity to ceftaroline against ESBL-producing organisms. This finding confirms the in vitro observations of NXL104 restoring susceptibility of ESBL (including CTX-M)- and carbapenemase-producing organisms to certain β-lactam agents (7, 12, 20).
Although in vivo pharmacodynamic studies are typically conducted using neutropenic hosts, it is also important to understand how a host's immune system might enhance the efficacy observed with a particular agent, as these agents will undoubtedly be used clinically in both immunocompetent and immunocompromised patients. As a result of the efficacy observed in the neutropenic model where the human-simulated exposure already paralleled 100% ƒT>MIC for the isolates tested, only moderate increases in bacterial reduction were noted in the immunocompetent animals. Regardless of immunocompetency, the observed efficacy with the regimen using 600 mg every 8 h exceeds both the static and the 1-log-CFU/ml reduction values that have been utilized for predicting efficacy in humans (1, 6).
Since 100% ƒT>MIC against all isolates was achieved with the human-simulated regimen, additional dose-ranging studies were conducted to better characterize the role of immune status on efficacy observed with ceftaroline-NXL104. When the combination was studied over a range of doses against three of these isolates, significant differences in bacterial density were observed between the two models when equivalent doses were administered. Furthermore, the ƒT>MICs required for stasis and a 1 log10 CFU/ml reduction in bacterial density were lower in the immunocompetent-host model (23% to 38% and 37% to 52%, respectively) than in the neutropenic-host model (42% to 48% and 56%, respectively). Although this was by no means a full dose-ranging study, the pharmacodynamic targets of stasis and a 1 log10 CFU/ml reduction observed were consistent with those previously reported for ceftaroline monotherapy in a neutropenic model (28% ± 9% and 41% ± 11%, respectively) (3). These findings suggest that the immune status of the host plays a vital role in the efficacy observed in vivo.
Although a pharmacodynamic parameter predictive of efficacy has yet to be determined for NXL104 or for β-lactamase inhibitors in general, best efforts were made to simulate the human pharmacokinetics of NXL104 within the murine model by matching ƒAUC, ƒCmax, and ƒT>MIC. Efficacy was observed for all isolates in the current study, with a simulated 1:1 ratio (2.5:1 in mice) of ceftaroline to NXL104. In a study of combined ceftaroline and NXL104 in a murine septicemia model, improved efficacy over ceftaroline alone was observed when mice were administered doses at three different ratios, 1:1, 2:1, and 4:1 (11).
The combination of these novel antimicrobial agents is particularly promising given the inadequacies in coverage against ESBL- and KPC-producing Gram-negative organisms for β-lactams alone. Regardless of immune status, ceftaroline (600 mg) combined with NXL104 (600 mg) every 8 h was efficacious against not only a non-ESBL-producing isolate but ESBL- and KPC-producing isolates as well. The combination of ceftaroline and NXL104 could be a much needed addition to the dwindling armamentarium of antimicrobials with efficacy against resistant Enterobacteriaceae.
ACKNOWLEDGMENTS
We thank Debora Santini, Lindsay Tuttle, Jennifer Hull, Henry Christensen, Pam Tessier, Catharine Bulik, and Rebecca Keel for their assistance with the animal experimentation.
This work was supported by Cerexa, Inc., Oakland, CA (a wholly owned subsidiary of Forest Laboratories, Inc., New York, NY).
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Ambrose P. G., et al. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 [DOI] [PubMed] [Google Scholar]
- 2. Andes D., Craig W. A. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andes D., Craig W. A. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeRyke C. A., Lee S. Y., Kuti J. L., Nicolau D. P. 2006. Optimizing dosing strategies of antibacterials utilizing pharmacodynamic principles. Drugs 66:1–14 [DOI] [PubMed] [Google Scholar]
- 5. Drusano G. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug'. Nat. Rev. Microbiol. 2:289–300 [DOI] [PubMed] [Google Scholar]
- 6. Drusano G. L. 2010. Pharmacodynamics of ceftaroline fosamil for complicated skin and skin structure infection: rationale for improved anti-methicillin-resistant Staphylococcus aureus activity. J. Antimicrob. Chemother. 65(Suppl. 4):iv33–iv39 [DOI] [PubMed] [Google Scholar]
- 7. Endimiani A., Choudhary Y., Bonomo R. A. 2009. In vitro activity of NXL104 in combination with β-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob. Agents Chemother. 53:3599–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ge Y., Biek D., Talbot G. H., Sahm D. F. 2008. In vitro profiling of ceftaroline against a collection of recent bacterial clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:3398–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishikawa T., et al. 2003. TAK-599, a novel N-phosphono type prodrug of anti-MRSA cephalosporin T-91825: synthesis, physiochemical and pharmacological properties. Bioorg. Med. Chem. 11:2427–2437 [DOI] [PubMed] [Google Scholar]
- 10. Lautenbach E., Polk R. 2007. Resistant gram-negative bacilli: a neglected healthcare crisis? Am. J. Health Syst. Pharm. 64:S3–S21 [DOI] [PubMed] [Google Scholar]
- 11. Levasseur P., et al. In vivo antibacterial efficacy of ceftaroline combined with the β-lactamase inhibitor NXL 104 in a murine septicemia model, abstract B-1339a. 2009 Abstr. 49th Annu. Meet. Intersci. Conf. Antimicrob. Agents Chemother. [Google Scholar]
- 12. Livermore D. M., Mushtaq S., Warner M., Miossec C., Woodford N. 2008. NXL104 combinations versus Enterobacteriaceae with CTX-M extended-spectrum β-lactamases and carbapenemases. J. Antimicrob. Chemother. 62:1053–1056 [DOI] [PubMed] [Google Scholar]
- 13. Lodise P., Lomaestro B. M., Drusano G. L. 2006. Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on β-lactam antibiotics. Pharmacotherapy 26:1320–1332 [DOI] [PubMed] [Google Scholar]
- 14. Mushtaq S., Livermore D. M. 2010. AmpC induction by ceftaroline. J. Antimicrob. Chemother. 65:586–588 [DOI] [PubMed] [Google Scholar]
- 15. Mushtaq S., Warner M., Williams G., Critchley I., Livermore D. M. 2010. Activity of chequerboard combinations of ceftaroline and NXL 104 versus β-lactamase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 65:1428–1432 [DOI] [PubMed] [Google Scholar]
- 16. Mushtaq S., Warner M., Yigong G., Kaniga K., Livermore D. M. 2007. In vitro activity of ceftaroline (PPI-0903M, T-91825) against bacteria with defined resistance mechanisms and phenotypes. J. Antimicrob. Chemother. 60:300–311 [DOI] [PubMed] [Google Scholar]
- 17. National Nosocomial Infections Surveillance System 2004. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470–485 [DOI] [PubMed] [Google Scholar]
- 18. Nordmann P., Cuzon G., Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236 [DOI] [PubMed] [Google Scholar]
- 19. Sader H. S., Fritsche T. R., Kaniga K., Ge Y., Jones R. N. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stachyra T., et al. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J. Antimicrob. Chemother. 64:326–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turnidge J. D. 1998. The pharmacodynamics of β-lactams. Clin. Infect. Dis. 27:10–22 [DOI] [PubMed] [Google Scholar]