Abstract
The specifically targeted antimicrobial peptide (STAMP) C16G2 was developed to target the cariogenic oral pathogen Streptococcus mutans. Because the design of this peptide was novel, we sought to better understand the mechanism through which it functioned. Compared to antimicrobial peptides (AMPs) with wide spectra of activity, the STAMP C16G2 has demonstrated specificity for S. mutans in a mixed-culture environment, resulting in the complete killing of S. mutans while having minimal effect on the other streptococci. In the current study, we sought to further confirm the selectivity of C16G2 and also compare its membrane activity to that of melittin B, a classical toxic AMP, in order to determine the STAMP's mechanism of cell killing. Disruption of S. mutans cell membranes by C16G2 was demonstrated by increased SYTOX green uptake and ATP efflux from the cells similar to those of melittin B. Treatment with C16G2 also resulted in a loss of membrane potential as measured by DiSC(3)5 fluorescence. In comparison, the individual moieties of C16G2 demonstrated no specificity and limited antimicrobial activity compared to those of the STAMP C16G2. The data suggest that C16G2 has a mechanism of action similar to that of traditional AMPs and kills S. mutans through disruption of the cell membrane, allowing small molecules to leak out of the cell, which is followed by a loss of membrane potential and cell death. Interestingly, this membrane activity is rapid and potent against S. mutans, but not other noncariogenic oral streptococci.
INTRODUCTION
The treatment of dental caries has changed little in the past 50 years. Cariogenesis is innocuous in its pathogenesis, leading to tooth loss and health issues that affect every part of the human body (23, 26). Dental caries are not life threatening but result in a reduction in the quality of life and a significant financial burden on those affected, costing over $90 billion annually in the United States alone (2, 10). Currently, treatment consists of management through extraction or restoration of the affected tooth, while caries prevention centers on treating the symptoms of the disease (such as reducing demineralization through the use of fluoride) or treating the total bacterial load in the oral cavity by nonselective means such as aseptic mouth rinses or indiscriminant topical antibiotics (1, 16, 37). Though effective in the short term, current interventions only slow caries progression. Despite our best efforts, dental cariogenesis remains entrenched within all age and socio-economic groups, affecting uninsured and Native American populations most severely in the United States (10). Clearly, a novel approach to treating caries is required to truly prevent this disease. Evidence suggests that the majority of cavities are caused by Streptococcus mutans, a Gram-positive facultative anaerobic bacterium (5, 6, 22), which produces lactic acid that lowers the pH of the microenvironment, leading to dental caries through the erosion of enamel. As S. mutans represents a minority pathogenic constituent species in the polymicrobial oral cavity, an approach can be undertaken whereby S. mutans is removed selectively from the oral flora. In contrast to current aseptic interventions, this selective approach will result in protective colonization effects associated with noncariogenic oral flora that overtake S. mutans colonization sites or antagonize the growth of the bacterium directly. Additionally, noncariogenic flora have been shown to inhibit and even prevent exogenous S. mutans colonization in vitro and in vivo (18, 19). To achieve targeted killing of S. mutans, we have developed a narrow-spectrum therapeutic, known as a specifically targeted antimicrobial peptide (STAMP) that effectively kills S. mutans while leaving other bacteria in the environment unaffected. Consisting of three functionally independent, yet conjoined domains, the STAMP against S. mutans was designed by utilizing CSPC16, a truncated version of the S. mutans competence stimulating peptide (CSP) pheromone, as the STAMP targeting domain for effective accumulation on the S. mutans cell surface (8). The STAMP killing domain, G2, was designed as a truncated version of the broad-spectrum killing peptide novispirin G10. Within the final molecule, (dubbed C16G2), the two regions are joined together by a flexible tri-glycine linker region.
Antimicrobial peptides (AMPs) and lipopeptides have been used for decades to prevent food spoilage and as topical antimicrobials, respectively (7, 39, 41). The exact mechanism through which AMPs kill targeted bacteria is not well understood and likely varies peptide by peptide, but various methods of membrane disruption and subsequent interference with intracellular targets are thought to be the main processes responsible (13, 27, 31, 32, 40). Classical examples are melittin and its analogs, such as melittin B, which are broad-spectrum cationic peptides derived from bee venom that effectively kill both Gram-positive and -negative bacteria (11, 33), as well as demonstrate potent cytotoxic activity. Melittin B initially interacts with cells through attraction to the anionic electrostatic charge of biological membranes (17). As melittin B accumulates on the cell surface, it disrupts lipid bilayers, interrupts membrane potential, and facilitates leakage of ions and small molecules into the environment (4, 25, 35). In contrast to melittin's nonspecific spectrum of activity, C16G2 has specific antimicrobial effects, targeting S. mutans preferentially, and displaying much less activity against other oral streptococci or Gram-negative bacteria. Though the STAMP's antimicrobial in vitro activity has been described partially (8, 21), it remains unclear if C16G2 targets bacterial membranes as part of the mechanism of action and if the STAMP is truly selectively permeabilizing S. mutans, as hypothesized previously (8). In particular, because C16G2 is novel, in that its antimicrobial killing region is conjoined to a non-AMP peptide region of equal size, it may well have a different mode of action compared with “classical” linear cationic AMPs. In this study, we investigated the mode of action of C16G2 and its ability to specifically target and disrupt the membrane of S. mutans in comparison to the wide-spectrum AMP melittin B (3, 17, 25, 38), which was utilized as a positive control for expected membrane disruption activity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains S. mutans UA140, S. mutans ATCC 104495, S. mutans JM11, S. sanguinis ATCC 10556, S. gordonii ATCC 10558, and S. salivarius K12 were grown in Todd-Hewitt (TH) broth at 37°C under anaerobic conditions (5% H2, 5%CO2, 90% N2).
Peptides utilized.
Peptides used in this study were synthesized, purified, and confirmed by CPC Scientific (Sunnyvale, CA) and GL Biochem (Shanghai, China) by standard 9-fluorenylmethyloxycarbonyl (Fmoc) solid-phase synthesis methods. Reported purities of >95% for C16G2 (CJ-06-01057), CSPC16 (CJ-12-01773), and G2 (CJ-12-01776) and >90% for melittin B (P101217-WY052272) were obtained by reverse-phase high-pressure liquid chromatography (RP-HPLC) by the vendor (data not shown).
MIC and MBC.
Antibacterial growth inhibition assays were performed using broth microdilution methods, as described previously (8, 28). Briefly, bacterial cells were grown overnight to an optical density at 600 nm (OD600) of 0.75 to 0.8 (corresponding to ∼1 × 108 CFU/ml, Cary 50 UV-Vis spectrophotometer; Agilent, Santa Clara, CA) and then diluted to ∼1 × 105 CFU/ml in TH broth. An appropriate volume of peptide stock solution (5 to 20 mg/ml, made in water or methanol, depending on solubility) was then added to the first column of the plate to give 64, 48, or 40 μM followed by serial 1:2 dilutions across the plate to give wells containing peptides ranging from 64 to 0.625 μM. The plates were then incubated at 37°C under anaerobic conditions for 16 to 20 h, and the MIC was determined as the concentration of peptide present in the last clear well after visual inspection. Up to 5% (vol/vol) methanol was found not to be antimicrobial (data not shown; 14). MICs were determined in triplicate, at minimum. Bactericidal assays were performed by plating the entire volume of each MIC reaction well on TH agar and then incubated at 37°C under anaerobic conditions for 24 h. Colonies were counted, and the minimal bactericidal concentration (MBC) was determined as the well with the lowest concentration of peptide that resulted in a 99.9% reduction in the number of CFU/ml.
Mixed species killing kinetics.
Bacterial cultures (S. mutans JM11, spectinomycin resistant; S gordonii; S. sanguinis; and S. salivarius) were grown to mid-exponential phase and diluted together to ∼1 × 106 CFU/ml in TH broth. Peptides were added to each well of a 96-well plate to a final concentration of 6.25 μM in a 300-μl total volume. An aliquot was removed at each time point, diluted 1:50 in TH broth, and plated on TH agar or TH agar plus 800 μg/ml spectinomycin for the number of surviving S. mutans JM11 CFU/ml, after appropriate dilution. Plates were incubated anaerobically at 37°C for 24 h before counting colonies. The detection level of the assay was 10 CFU/ml.
SYTOX green incorporation in biofilms.
Streptococci grown overnight were diluted to ∼1 × 105 CFU/ml in TH broth with 1% (wt/vol) sucrose in a 96-well plate and grown for 6 h to form biofilms. Medium was then removed, and biofilms were washed with a solution containing 10 mM HEPES and 150 mM NaCl. SYTOX green (Invitrogen, Carlsbad, CA) was then added to a final concentration of 2.5 μM and incubated for 10 min. Peptide at 3.125 μM was then added to each sample and incubated for an additional 10 min. After the supernatants with excess dye were removed from biofilms, they were visualized by fluorescence microscopy (Leica DMI6000B microscope and Leica DFC340FX camera). To quantify the level of SYTOX green, green pixels were selected from each image utilizing GIMP software (http://www.gimp.org), adjusted for any background green fluorescence, and expressed as fluorescence intensity units (15, 20).
ATP leakage assay.
ATP leakage was detected using a luminescence assay. S. mutans was grown to mid-log phase and diluted to ∼1 × 106 cells/ml. Aliquots of 250 μl of cells were exposed to a final concentration of 6.25 μM peptide and incubated at ambient temperature for 5 min. After incubation, 250 μl of luciferase buffer (10% ethylene glycol, 1 mM HEPES, 1 mM MgCl2, 1 mM d-luciferin, and 5 mg/ml luciferase) was added to one sample at a time and shaken vigorously for 3 s before luminescence was measured on a GloMax luminometer (Promega, Sunnyvale, CA) with interpolation for 6 s.
Cytoplasmic-membrane depolarization with DiSC(3)5.
Depolarization of cytoplasmic membranes was determined using the membrane potential-sensitive cyanine dye DiSC3(5) (34) by a modification of the method of Wu et al. (40). Briefly, mid-log-phase bacteria were diluted in 25% TH broth, 100 mM KCl, and 0.4 mM DiSC3(5) to an OD600 of 0.05. Cells were incubated ∼40 min until a stable reduction in fluorescence was observed due to DiSC3(5) uptake and quenching in the cell. A 200-μl aliquot of cell suspension was placed in a glass-bottom 96-well plate with 3.125 μM peptide. Fluorescence was monitored on a Cary Eclipse fluorescence spectrophotometer (Agilent, Santa Clara, CA) at an excitation wavelength of 622 nm and emission wavelength of 670 nm.
Statistical analysis.
Student's t test was performed using Microsoft (Redmond, WA) Excel 2007, where noted.
RESULTS
Comparison of C16G2 and melittin B selectivity against oral bacteria.
Previously, we determined C16G2 MIC activity against S. mutans strains UA159, ATCC 25175, and T8, along with a small collection of other oral bacteria (8). In order to fully evaluate the selective potency of C16G2, we further examined the MIC activity of the STAMP and STAMP components against S. mutans strains and closely related noncariogenic oral streptococci. As shown in Table 1, we found that the MIC of C16G2 was 2- to 5-fold lower for S. mutans than for the other streptococcal species tested. The killing domain, G2, was not as effective and had MICs that were approximately 2-fold higher for the non-mutans streptococcal strains and up to 8-fold higher for the S. mutans strains. Treatment with melittin B resulted in similar MICs for all streptococcus species. The targeting region CSPC16 showed no killing at the maximum dilution for all the streptococcal strains tested. The minimum bactericidal concentration (MBC) of the peptides was also evaluated against UA140 and 104495. The MBC of C16G2 was 20 μM, which was approximately 4-fold higher than the MIC for both strains. This was significantly lower than those of the killing domain G2 and the targeting domain CSPC16, which had MBCs of 48 μM and >64 μM, respectively. Melittin B had an MBC of 12 μM against both S. mutans strains.
Table 1.
Antimicrobial peptide MIC
| Straina | Peptide (μM) |
|||
|---|---|---|---|---|
| C16G2 | CSPC16 | G2 | MelBb | |
| S. mutans UA140 | 5.2 ± 1.6 | >64 | 33.3 ± 12.9 | 10.4 ± 3.2 |
| S. mutans ATCC 104495 | 4.2 ± 1.6 | >64 | 33.3 ± 12.9 | 6.3 |
| S. salivarius K12 | 9.4 ± 3.4 | >64 | 16.7 ± 6.5 | 3.7 ± 1.3 |
| S. sanguinis ATCC 10556 | 25 | >64 | >50 | 6.3 |
| S. gordonii ATCC 10558 | 25 | >64 | >50 | 5.2 ± 1.6 |
n = 6.
MelB, melittin B.
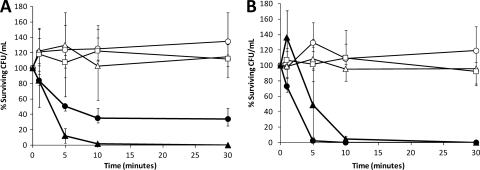
To further demonstrate the specificity of C16G2 for S. mutans or lack thereof for melittin B, we mixed planktonic-phase oral streptococcus species and determined the S. mutans killing kinetics of C16G2 compared to those of the targeting domain (CSPC16), killing domain (G2), and melittin B. As shown in Fig. 1, treatment with 6.25 μM melittin B resulted in a rapid and sustained reduction in the number of surviving CFU/ml for all streptococci. In contrast, treatment with C16G2 resulted in a striking decrease in the number of S. mutans CFU/ml (>2 log10) after 10 min (P = 0.01), while non-S. mutans streptococci decreased by 47% during this time frame (Fig. 1). No regrowth of S. mutans was observed during the 120-min duration of the experiment (data not shown), while the non-S. mutans streptococci rebounded to 80% of the number of input CFU/ml by 120 min (data not shown). Treatment with CSPC16 or G2 had an effect similar to that of the no-peptide control for all streptococci (Fig. 1). The targeting domain, CSPC16, also had no significant effect on S. mutans, while G2 treatment was associated with modest antimicrobial activity (Fig. 1B). These data suggest that C16G2 has a potency similar to that of melittin B, but, interestingly, only against S. mutans, while melittin B displayed robust activity against all streptococci examined, as expected.
Fig. 1.
Mixed species killing kinetics. Oral streptococci (S. mutans JM11, S. sanguinis ATCC 10556, S. gordonii ATCC 10558, and S. salivarius K12) were added at a concentration of ∼1 × 106 CFU/ml each in TH broth and incubated with 6.25 μM C16G2 (●), melittin B (▴), CSPC16 (△), G2 (□), or no peptide (○) followed by plating on TH agar (A) (total bacteria) and TH plus 800 μg/ml spectinomycin (B) (S. mutans). Numbers indicate the percentage of reduction in bacterial count relative to the number of input CFU/ml. At least 4 independent experiments were performed.
Analysis of helical characteristics.
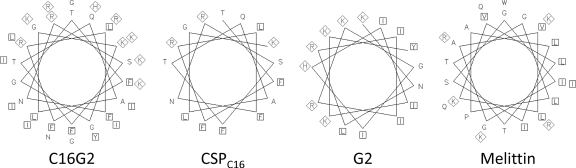
We investigated the predicted structural characteristics of C16G2, in comparison to its component peptides and melittin B (Table 1). A recent analysis of CSPs from streptococcus strains has demonstrated that CSP from S. mutans UA159 has a well-defined amphipathic α-helical structure spanning residues Leu4 to Gly20 and is necessary for receptor binding (36). This region spans the length of C16G2's CSPC16 domain (Tyr5 to Lys21 of CSP) (Table 2) and retains the features that are necessary for receptor binding (36) while retaining a cationic charge. The other C16G2 component, G2 (Table 1), contains deletions of Arg4 and Gly18 from the parent peptide novispirin G10 (8, 9, 30). Structural analysis demonstrated that G10 has an amphipathic, bent α-helical structure (30) which is likely retained in G2 since neither residue is part of the structural motifs. As shown in Fig. 2, after coupling the targeting and killing domains together with a flexible triple-glycine linker region, the C16G2 STAMP likely has an extended amphipathic α-helical/bent α-helical structure, based on helical-wheel projections. This projection compared favorably to melittin B, which has a known amphipathic and α-helical character that is required for membrane interaction. These results suggest that C16G2 may have a similar AMP-like arrangement of residues across the entire sequence (targeting and killing regions) that may be critical for antimicrobial activity.
Table 2.
Peptide characteristics
| Peptide | Sequence | Manufacturer | Purity (%) | Length (aa)a | MW | Charge (pH 7) | pI | Hydrophobicity moment | Angle |
|---|---|---|---|---|---|---|---|---|---|
| C16G2 | TFFRLFNRSFTQALGKGGGKNLRIIRKGIHIIKKY-NH2 | CPC Scientific | 95 | 35 | 4,080 | 10.1 | 12.9 | 993 | 188.4 |
| CSPC16 | TFFRLFNRSFTQALGK | CPC Scientific | 95 | 16 | 1,933 | 3.0 | 12.4 | 5.6 | 163.3 |
| CSP | SGSLSTFFRLFNRSFTQALGK | NA | NA | 21 | 2,365 | 3.0 | 12.4 | 6.14 | 301.9 |
| G2 | KNLRIIRKGIHIIKKY-NH2 | CPC Scientific | 95 | 16 | 1,993 | 7.1 | 12.4 | 5.42 | 114.5 |
| Novispirin G10 | KNLRRIIRKGIHIIKKYG | NA | NA | 18 | 2,206 | 7.1 | 12.2 | 7.35 | 192.9 |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ-NH2 | GL Biochem | 95 | 26 | 2,847 | 6.0 | 14.0 | 5.18 | 68.7 |
aa, amino acids.
Fig. 2.
Helical wheel projections of the STAMP C16G2 and its moieties. Hydrophobic and charged residues are indicated by squares and diamonds, respectively.
Examination of peptide-mediated membrane permeability.
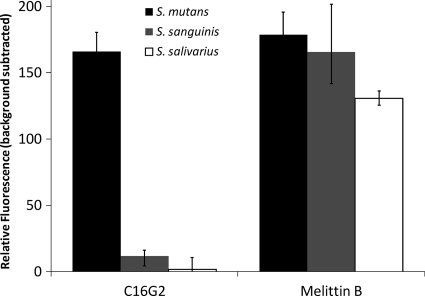
We used the fluorescent dye SYTOX green to study the membrane permeabilizing effect of C16G2 and melittin B on S. mutans biofilms. When the lipid bilayer is disrupted, the normally cell-impermeant SYTOX green enters the cell and binds to the genomic DNA and can be detected by fluorescence imaging techniques. As shown in Fig. 3, when cells were treated with 3.1 μM C16G2, it effectively disrupted the membranes of S. mutans biofilms, resulting in visible levels of dye uptake (Fig. 3) similar to those of melittin B-treated biofilms (P = 0.05). However, S. sanguinis and S. salivarius biofilms treated with melittin B had 14 to 75 times more SYTOX green incorporation, respectively, compared to those treated with C16G2 (Fig. 3), for which levels did not differ significantly from the background (P = 0.01). These results indicate that C16G2 specifically permeabilizes S. mutans biofilms as efficiently as melittin B but has very little activity against the other streptococci tested.
Fig. 3.
SYTOX green dye incorporation in streptococcus biofilms. Biofilms were grown for 6 h, washed with 10 mM HEPES and 150 mM NaCl, and then incubated with 2.5 μM SYTOX green and 3.125 μM of either C16G2 or melittin B. Relative fluorescence values presented represent the average of three experiments, with standard deviation.
Investigation of metabolite leakage.
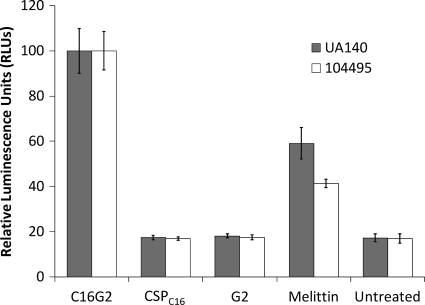
One of the mechanisms through which AMPs kill cells is by forming pores in biological membranes, resulting in the leakage of small molecules (12). In bacterial cells, ATP is maintained intracellularly at a concentration of 1 aM per cell. Release of ATP into the environment can be detected by chemiluminescence, which indicates the disruption of the cell membrane. As shown in Fig. 4, the release levels of ATP from S. mutans UA140 and 104495 after membrane disruption with the melittin B, C16G2, and STAMP targeting and killing moieties were compared. Cultures of both S. mutans strains treated with 6.25 μM C16G2 or melittin B had levels of luminescence that were significantly higher than those of cells treated with either the targeting domain CSPC16 or the killing domain G2 (Fig. 4) (P = 0.01). Both CSPC16- and G2-treated cultures were similar to no-peptide-treated cultures for both strains, indicating a lack of membrane disruption (Fig. 4) (P = 0.01). Treatment with the STAMP C16G2 resulted in significantly higher luminescence than that of melittin B, indicating that more ATP was released by C16G2 against S. mutans (Fig. 4) (P = 0.01). These results suggest that C16G2 likely targets the bacterial membrane as its primary mode of action, and interestingly, the STAMP appears more potent than melittin B in promoting ATP leakage from S. mutans, which may account for the observed C16G2 MIC that was lower than that of melittin B (Table 1).
Fig. 4.
ATP efflux from S. mutans detected by relative luminescence. Planktonic cells were grown to mid-log phase and adjusted to ∼1 × 107 CFU/ml and treated with 6.25 μM of the peptides indicated. ATP leakage was detected by mixing the treated cells with a luciferase reagent and measuring photon output. Data presented represent an average of three separate reactions, with standard deviation.
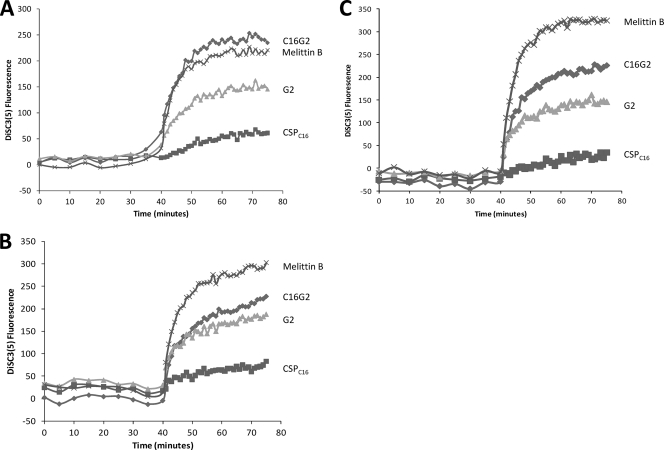
Determination of membrane depolarization after peptide treatment.
Disruption of bacterial cell membranes with AMPs can result in a loss of membrane potential (ΔΨ) leading to cell death (12). To observe the depolarizing effects, we used the fluorescent dye DiSC3(5), which is sensitive to membrane potential and is quenched in intact membranes. Melittin B has been shown to efficiently depolarize membranes by these methods (29), and was used as a positive control in this experiment. As shown in Fig. 5A, C16G2 treatment was associated with a large increase in fluorescence (equal to that of melittin B) for S. mutans cultures but, interestingly, did not induce such an effect against S. gordonii or S. salivarius (Fig. 5B and C). In comparison, melittin B treatment resulted in the largest increase (compared with those with other peptides) in fluorescence for both S. gordonii and S. salivarius (Fig. 5B and C). The killing peptide G2 had approximately the same effect on all three bacteria, but at a lower level of membrane depolarization than that for C16G2 or melittin B. The targeting peptide CSPC16 did not result in a significant fluorescence increase compared to those for mock-treated controls for all bacteria tested. The difference in the rate of ΔΨ interruption between streptococci after C16G2 treatment suggests that the STAMP had depolarizing effects selective for S. mutans (compared to activity against S. salivarius and S. gordonii) that were at a level similar to those of a known depolarizing AMP. These results again indicate that C16G2 actively disrupts the membrane of S. mutans as its primary mode of action.
Fig. 5.
Membrane depolarization of Streptococcus spp. Oral streptococci, S. mutans (A), S. gordonii (B), and S. salivarius (C), were incubated with DiSC3(5), and the fluorescence increase as a result of loss of ΔΨ was monitored. After stabilization, cultures were treated with peptide as indicated. Experiments were performed in duplicate.
DISCUSSION
In this study we sought to characterize the mode of action of the STAMP C16G2. In contrast to conventional antimicrobial peptides with wide spectra of activity, C16G2 was rationally designed to target S. mutans by utilizing CSPC16, a peptide derived from an S. mutans-specific CSP, as a means for selective delivery of the AMP G2 (8, 9). By systematically comparing C16G2 with the known AMP melittin B (12, 13, 24), we were able to determine that C16G2 has membrane-disrupting activity that results in loss of ΔΨ and cell death, at a level of potency similar to that of melittin B. Interestingly, and unlike that of melittin B, C16G2's robust membrane activity was found to be S. mutans specific and dependent on the CSPC16 targeting region, as G2 alone had only modest and wide-spectrum antimicrobial effects. These mechanistic data are in strong alignment with the killing kinetics and MIC and MBC data presented here and described previously (8, 21), confirming that C16G2 selectively affects S. mutans and S. mutans biofilms even in mixed microbial communities.
Sequence analysis of C16G2 suggests that it is an amphipathic and cationic α-helical peptide that is similar to other AMPs (13). The hydrophobic moment of C16G2 is considerably greater than that of its individual moieties due to the stacking of hydrophobic residues in the STAMP (Table 1; Fig. 1). It seems likely that the amphipathic characteristic shared between C16G2 and AMPs results in the STAMP functioning as a membrane disrupting peptide, but with greater specificity for its target (Fig. 2 and 3).
The exact mechanism by which C16G2 is selective for S. mutans remains unclear, but it may involve early membrane-binding or partition steps that are governed by CSPC16. As support for this hypothesis, it is known that when dissolved in liquid media, melittin B and other AMPs have extended linear conformations, taking on amphipathic α-helical structures only when dissolved in hydrophobic solvents or in lipid bilayers (12). This behavior is also present in CSP, the parent peptide of the targeting domain CSPC16 (36); as CSP binds to the cell membrane, it folds into an amphipathic α-helical peptide that can properly interact with the ComD receptor extracellular domain of S. mutans and relay competence signals. We have demonstrated that ComD has no role in the targeting of the STAMP C16G2 to S. mutans (8), but the proper folding of CSPC16 on the surface of S. mutans may retain a role in sequestering and retaining STAMP on the S. mutans surface independent of the receptor. It is likely that a myriad of membrane structures exist in an S. mutans-specific composition that can be recognized by the pheromone fragment, as well as C16G2. Additionally, should CSPC16 behave like CSP, it will have a linear conformation in solution and will not take on its α-helical conformation until interaction with the cell membrane of S. mutans. This has a direct impact on the antimicrobial mechanism, as the α-helical conformation and the amphipathic characteristics of the C16G2 are then fully aligned. In this hypothesis, against untargeted oral bacteria, CSPC16 would lack avidity or hydrophobic interactions with the membrane, resulting in poor binding and/or retention, as well as a lack of α-helical adoption, resulting in decreased hydrophobic moment and membrane activity. Further experiments will be necessary to determine if CSPC16 adopts an S. mutans-specific α-helical structure and if this plays a role in the STAMP activity.
In conclusion, our data demonstrate that treatment of S. mutans with the STAMP C16G2 results in disruption of the cell membrane, allowing efflux of its intracellular contents and uptake of a cell impermeable dye. This disruption is more potent against S. mutans than that caused by melittin B but interestingly does not occur at a high level when other noncariogenic oral streptococci are treated with the STAMP. The overall amphipathic character of C16G2, which may be selectively inducible on the S. mutans surface, may facilitate this specific membrane activity.
ACKNOWLEDGMENTS
This work was supported by funds from C3 Jian, Inc., and NIH grants (DE019970 and DE020102).
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Anderson M. H., Shi W. 2006. A probiotic approach to caries management. Pediatr. Dent. 28:151–153,192–198. [PubMed] [Google Scholar]
- 2. Anonymous. 2008. National health expenditure projections 2008–2018. Centers for Medicare and Medicaid Services, Office of the Actuary, Baltimore, MD [Google Scholar]
- 3. Bechinger B. 1997. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 156:197–211 [DOI] [PubMed] [Google Scholar]
- 4. Bechinger B. 1999. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim. Biophys. Acta 1462:157–183 [DOI] [PubMed] [Google Scholar]
- 5. Beighton D. 2005. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent. Oral Epidemiol. 33:248–255 [DOI] [PubMed] [Google Scholar]
- 6. Corby P. M., et al. 2005. Microbial risk indicators of early childhood caries. J. Clin. Microbiol. 43:5753–5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowell N. D., Allen A. R., Jarvis B. 1971. The in vivo effect of nisin on the microflora of the oral cavity. J. Appl. Bacteriol. 34:787–791 [DOI] [PubMed] [Google Scholar]
- 8. Eckert R., et al. 2006. Targeted killing of Streptococcus mutans by a pheromone-guided “smart” antimicrobial peptide. Antimicrob. Agents Chemother. 50:3651–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eckert R., et al. 2006. Adding selectivity to antimicrobial peptides: rational design of a multidomain peptide against Pseudomonas spp. Antimicrob. Agents Chemother. 50:1480–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evans C. A., Kleinman D. V. 2000. The Surgeon General's report on America's oral health: opportunities for the dental profession. J. Am. Dent. Assoc. 131:1721–1728 [DOI] [PubMed] [Google Scholar]
- 11. Fennell J. F., Shipman W. H., Cole L. J. 1967. Antibacterial action of a bee venom fraction (melittin) against a penicillin-resistant staphylococcus and other microorganisms, p. 1–25 U.S. Naval Radiological Defense Laboratory, Biological and Medical Sciences Division, San Francisco, CA: [DOI] [PubMed] [Google Scholar]
- 12. Hancock R. E. W., Chapple D. S. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hancock R. E. W., Lehrer R. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82–88 [DOI] [PubMed] [Google Scholar]
- 14. He J., et al. 2007. Novel synthetic antimicrobial peptides against Streptococcus mutans. Antimicrob. Agents Chemother. 51:1351–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He J., et al. 2010. Systematic approach to optimizing specifically targeted antimicrobial peptides against Streptococcus mutans. Antimicrob. Agents Chemother. 54:2143–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He X., Lux R., Kuramitsu H. K., Anderson M. H., Shi W. 2009. Achieving probiotic effects via modulating oral microbial ecology. Adv. Dent. Res. 21:53–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klocek G., Schulthess T., Shai Y., Seelig J. 2009. Thermodynamics of melittin binding to lipid bilayers. Aggregation and pore formation. Biochemistry 48:2586–2596 [DOI] [PubMed] [Google Scholar]
- 18. Kreth J., Zhang Y., Herzberg M. C. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J. Bacteriol. 190:4632–4640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuramitsu H. K., He X., Lux R., Anderson M. H., Shi W. 2007. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 71:653–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li L., et al. 2010. Design and characterization of an acid-activated antimicrobial peptide. Chem. Biol. Drug Des. 75:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li L. N., et al. 2010. Targeted antimicrobial therapy against Streptococcus mutans establishes protective noncariogenic oral biofilms and reduces subsequent infection. Int. J. Oral Sci. 2:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loesche W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakano K., et al. 2009. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol. Immunol. 24:64–68 [DOI] [PubMed] [Google Scholar]
- 24. Oren Z., Shai Y. 1998. Mode of action of linear amphipathic α-helical antimicrobial peptides. Biopolymers 47:451–463 [DOI] [PubMed] [Google Scholar]
- 25. Oren Z., Shai Y. 1997. Selective lysis of bacteria but not mammalian cells by diastereomers of melittin: structure-function study. Biochemistry 36:1826–1835 [DOI] [PubMed] [Google Scholar]
- 26. Parker M. T., Ball L. C. 1976. Streptococci and aerococci associated with systemic infection in man. J. Med. Microbiol. 9:275–302 [DOI] [PubMed] [Google Scholar]
- 27. Peschel A., Sahl H.-G. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4:529–536 [DOI] [PubMed] [Google Scholar]
- 28. Qi F., et al. 2005. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol. Lett. 251:321–326 [DOI] [PubMed] [Google Scholar]
- 29. Rathinakumar R., Walkenhorst W. F., Wimley W. C. 2009. Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: the importance of interfacial activity. J. Am. Chem. Soc. 131:7609–7617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sawai M. V., et al. 2002. Impact of single-residue mutations on the structure and function of ovispirin/novispirin antimicrobial peptides. Protein Eng. 15:225–232 [DOI] [PubMed] [Google Scholar]
- 31. Schüller F., Benz R., Sahl H.-G. 1989. The peptide antibiotic subtilin acts by formation of voltage-dependent multi-state pores in bacterial and artificial membranes. Eur. J. Biochem. 182:181–186 [DOI] [PubMed] [Google Scholar]
- 32. Shai Y. 1995. Molecular recognition between membrane-spanning polypeptides. Trends Biochem. Sci. 20:460–464 [DOI] [PubMed] [Google Scholar]
- 33. Shin S. Y., Hahm K.-S. 2004. A short α-helical antimicrobial peptide with antibacterial selectivity. Biotechnol. Lett. 26:735–739 [DOI] [PubMed] [Google Scholar]
- 34. Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. 1974. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry 13:3315–3330 [DOI] [PubMed] [Google Scholar]
- 35. Smith R., et al. 1994. Structure and orientation of the pore-forming peptide melittin, in lipid bilayers. J. Mol. Biol. 241:456–466 [DOI] [PubMed] [Google Scholar]
- 36. Tian X., et al. 2009. A method for structure-activity analysis of quorum-sensing signaling peptides from naturally transformable streptococci. Biol. Proced. Online 11:207–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsang P. W., et al. 2006. A medical approach to the diagnosis and treatment of dental caries. AHIP Cover. 47:38–42 [PubMed] [Google Scholar]
- 38. van den Bogaart G., Guzmán J. V., Mika J. T., Poolman B. 2008. On the mechanism of pore formation by melittin. J. Biol. Chem. 283:33854–33857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woodworth J. R., Nyhart E. H., Jr., Brier G. L., Wolny J. D., Black H. R. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu M., Maier E., Benz R., Hancock R. E. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235–7242 [DOI] [PubMed] [Google Scholar]
- 41. Zendo T., Yoneyama F., Sonomoto K. 2010. Lactococcal membrane-permeabilizing antimicrobial peptides. Appl. Microbiol. Biotechnol. 88:1–9 [DOI] [PubMed] [Google Scholar]