Abstract
The recommended treatment for herpes simplex encephalitis (HSE) remains intravenous acyclovir. In resource-poor countries, however, intravenous formulations are usually unavailable or unaffordable. We report the penetration of acyclovir into the cerebrospinal fluid (CSF) in patients with HSE, treated with the oral prodrug valacyclovir at 1,000 mg three times daily. The oral therapy achieved adequate acyclovir concentrations in the CSF and may be an acceptable early treatment for suspected HSE in resource-limited settings.
TEXT
Viral encephalitis is a common neurological condition in southern Vietnam, accounting for more than 200 admissions annually to the Hospital for Tropical Diseases (HTD) in Ho Chi Minh City. Herpes simplex virus type 1 (HSV-1) is the etiological agent in 10% of all viral encephalitis cases (3). HSV-1 is the most common cause of encephalitis in adults worldwide, with a high acute-case fatality rate and devastating neurological sequelae in a significant proportion of survivors (13). The recommended treatment is intravenous acyclovir, 10 mg/kg of body weight, three times daily for 21 days (2, 8, 13).
Acyclovir is a purine nucleoside analogue with activity against human herpesviruses, including HSV. Oral acyclovir has poor oral bioavailability (approximately 15 to 30%) and low penetration into the central nervous system (CNS). Hence, the treatment of HSV encephalitis, which requires higher drug levels to achieve antiviral efficacy, is dependent on the use of intravenous acyclovir. However, this regimen is unavailable and unaffordable for most patients in resource-limited settings, including in Vietnam ($3,700 for a full treatment course in Vietnam and rarely available).
Valacyclovir, the prodrug l-valyl ester of acyclovir, is converted in vivo to acyclovir by hepatic and plasma esterase. Oral bioavailability of acyclovir is 3- to 5-fold higher (about 54%) when given as the prodrug than when given as acyclovir (10). Orally administered valacyclovir is very well tolerated, with few reported adverse events, even at a dose of 2,000 mg four times daily (12). Valacyclovir is 20 times less expensive than intravenous acyclovir ($185 for a full treatment course in Vietnam) and is readily available in pharmacies in countries with limited resources. We investigated the pharmacokinetics of orally administered valacyclovir in the plasma and cerebrospinal fluid (CSF) of patients with herpes simplex encephalitis (HSE).
Adult patients aged ≥18 years with a presumptive clinical diagnosis of encephalitis were eligible to enter the study. All patients underwent routine diagnostic investigations that included CSF cell count, chemistry, microscopy with Gram, Ziehl-Neelsen, and India ink stains, and routine culture for bacterial and fungal pathogens. Patients were excluded when an alternative microbiological diagnosis was made or renal impairment was proven. All patients or patients' relatives gave informed consent prior to entering the study. Ethical approvals were obtained from the Scientific and Ethical Committees of the HTD and the Oxford Tropical Research Ethics Committee (OXTREC). Treatment was initiated immediately upon enrollment (Valtrex, 500 mg; GlaxoSmithKline) at 1,000 mg three times daily for a total of 21 days and was stopped if 2 consecutive HSV PCR tests were negative after 5 days.
Venous blood samples (1.5 ml) were obtained prior to and 1, 2, 3, 4, 6, and 8 h after dose administration on day 0. On days 2, 10, and 20, blood samples were collected at predose and at 2 h postdose. Each blood sample was immediately centrifuged upon venous collection. Plasma was collected and stored at −80°C. Two milliliters of CSF was obtained from all patients 2 h postdose on days 0, 2, 10, and 20 and stored at −80°C until use. Acyclovir concentrations in plasma and CSF were measured by high-performance liquid chromatography (HPLC) with UV detection after solid-phase extraction according to standard methods (6, 11). The limit of quantification was 50 ng/ml, and the interassay variation was less than 10% in both plasma and CSF.
Nine patients (5 males, 4 females) were enrolled in the study. Two patients had negative CSF HSV PCR tests, and valacyclovir was discontinued. One patient withdrew from the study. Two patients died on days 2 and 3 after study enrollment (both had positive HSV-1 PCR, and acyclovir CSF concentrations were not different from those of other patients). The other four patients had a positive PCR for HSV-1 and received the full 21-day course of valacyclovir. No serious adverse events were observed in the four patients. Routine hematology and biochemistry tests were within normal ranges, and no drug-related adverse events were observed throughout the study.
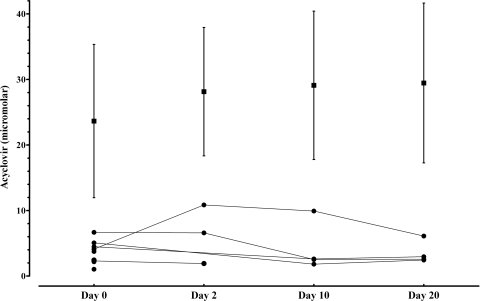
In all patients, the 2-h-postdose concentrations of acyclovir in plasma remained stable from day 2 to the end of the treatment, with the mean steady-state concentration around 28.1 ± 9.8 μM, whereas the 2-h-postdose concentrations in CSF reached maximum levels on day 2 (6.5 ± 4.5 μM) and then on days 10 (4.2 ± 3.8 μM) and 20 (3.5 ± 1.7 μM μM) decreased to concentrations similar to and lower than that of the first day of treatment (3.6 ± 1.7 μM) (Fig. 1).
Fig. 1.
Acyclovir concentrations 2 h postdose in plasma (mean [▪] + standard deviation) and in CSF (individual plots [•]). Two CSF samples (on day 2) were not taken at the correct time postdose and were excluded from the analysis.
The CSF/plasma concentration ratio was calculated to determine the blood/CSF levels of acyclovir over the treatment period (Table 1). The concentrations of acyclovir in plasma remained stable from the beginning of steady state (day 2) to the end of the treatment, whereas the concentrations in CSF decreased after day 2. The 2-h-postdose CSF/plasma acyclovir concentration ratio was 22.9% on day 2, 14.5% on day 10, and 12.0% on day 20. The acyclovir CSF/plasma ratio dropped to approximately 50% within 18 days of steady state.
Table 1.
Acyclovir concentrations at 2 h postdose in plasma and CSF
| Day | Acyclovir concn (μM; mean ± SD) in: |
CSF/plasma acyclovir concn (%) | |
|---|---|---|---|
| Plasma | CSF | ||
| 0 | 23.7 ± 11.7 | 3.6 ± 1.7 | 15.1 |
| 2 | 28.1 ± 9.8 | 6.5 ± 4.5 | 22.9 |
| 10 | 29.1 ± 11.3 | 4.2 ± 3.8 | 14.5 |
| 20 | 29.5 ± 12.2 | 3.5 ± 1.7 | 12 |
The albumin ratio between CSF and plasma is frequently used as a surrogate marker of brain inflammation resulting in an impaired blood-brain barrier (BBB). The influence of the BBB function on the penetration of acyclovir in the CSF was investigated. We found that an increase in albumin CSF/plasma ratios was correlated with higher acyclovir CSF levels (Pearson's r = 0.53; P = 0.02). This correlation is partially weak (95% confidence interval range, 0.11 to 0.79) but can be explained by the fact that albumin ratios were highest at day 0, when acyclovir concentrations in the CSF were not yet at steady state (ranges of albumin CSF/plasma ratio × 1,000 were 2.3 to 10.5 at day 0, 0.6 to 5.7 at day 2, 1.9 to 3.0 at day 10, and 1.0 to 2.4 at day 20).
Our study showed that orally administered valacyclovir at 1,000 mg three times daily in patients with HSV-1 encephalitis achieved a high mean CSF peak concentration of 6.5 ± 4.5 μM on day 2. This is higher than the reported 50% inhibitory concentration (IC50) for clinical isolates of HSV-1 and HSV-2 (range, 0.1 to 3.9 μM) after day 2 of therapy (1, 7). All patients remaining in the study at day 10 (n = 4) had negative PCR tests, supporting an effective exposure of acyclovir in the CSF. Over the period of treatment, the CSF acyclovir concentrations found were higher than concentrations previously reported, using the same dosage of valacyclovir but in patients with multiple sclerosis. In the study by Lycke et al. (5), the acyclovir CSF/plasma ratio 2 h postdose at steady state was 11%, and the mean CSF concentration was 2.5 ± 0.7 μM. In our study in CSF, acyclovir concentrations did not remain stable over the treatment period. A peak was found after 2 days of treatment, followed by a rapid decrease from days 2 to 10 and a slight decline from days 10 to 20. Interestingly, final CSF concentrations were similar to levels found in other studies where no evidence of BBB damage was shown (5). The mean CSF acyclovir concentration on day 20 of 3.5 ± 1.7 μM was still higher than the IC50 of most clinical isolates of HSV-1 and HSV-2.
The differences in acyclovir concentration kinetics in CSF compared to those of plasma during the treatment suggest a dynamic process of repair of the BBB during treatment for acute HSV encephalitis. The acute-phase injury leads to damage to the BBB function, increasing its permeability to acyclovir, resulting in the peak concentration seen early in the treatment course (day 2). After 10 days of treatment, the lower concentrations of acyclovir in CSF compared to baseline levels suggest that the disease-induced permeability of the BBB had been partially corrected. As the active inflammation in the CNS decreases, the BBB function returns to normal, and drug levels in the CSF are expected to be lower. The hypothesis that the BBB is impaired during acute HSV encephalitis and recovers its function at the end of the disease course is similarly supported by Lycke et al (5). In their study, patients had normal renal function and no evidence of BBB dysfunction, and the CSF concentration at 2 h (2.5 ± 0.9 μM) was similar to the levels on day 20 of valacyclovir at the same dose regimen as that used in our study.
There are very few data describing the CSF/plasma ratio obtained after an intravenous administration of acyclovir. When these have been described, they vary from 25% to 70% (14). In most cases, these levels were determined after a short treatment course, in small case series or case reports, and were rarely done on simultaneous sampling of blood and CSF (CSF levels were measured and the plasma values were predicted). A consistent mean value would be approximately 30% (4). Delivering the drug by the oral or intravenous route should not change the CSF/plasma ratio, unless there is a saturable mechanism (which would decrease the ratio) or a specific kinetic leading to quick accumulation in the CSF after the acute plasma exposure following intravenous (IV) administration of the drug. An important observation, previously reported (5, 9), is that concentrations in CSF vary less over dose intervals than those in plasma. These results suggest that acyclovir slowly crosses the blood-brain barrier and is then actively transported out of the CSF compartment and supports the idea that the plasma profile (IV versus oral) is unlikely to significantly affect the CSF/plasma ratio.
In conclusion, valacyclovir orally administered at 1,000 mg three times daily for 21 days in patients with herpes encephalitis achieved therapeutic levels in the CSF throughout the course of treatment. The higher levels of acyclovir in CSF at the early stage of treatment potentially reflected an impaired and more permeable BBB. In resource-poor settings, valacyclovir should be considered an alternative to intravenous acyclovir for treatment of herpes simplex encephalitis when the latter is not available or affordable. The maximum benefit of these drugs is when they are used early in the course of disease. Consideration should be given to making oral valacyclovir available and encouraging its use immediately after a patient presents with suspected encephalitis, particularly in provincial and rural settings where molecular diagnostic tests and intravenous acyclovir are not available.
Acknowledgments
This study was supported by the Wellcome Trust, United Kingdom.
We are grateful to the director, nurses, and technicians of the Hospital for Tropical Diseases, Ho Chi Minh City, Viet Nam, for their support and to all the patients who participated in the study for their contribution.
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Collins P. 1983. The spectrum of antiviral activities of acyclovir in vitro and in vivo. J. Antimicrob. Chemother. 12(Suppl. B):19–27 [DOI] [PubMed] [Google Scholar]
- 2. Crumpacker C. S., Gonzalez R. G., Makar R. S. 2003. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 26-2003. A 50-year-old Colombian man with fever and seizures. N. Engl. J. Med. 349:789–796 [DOI] [PubMed] [Google Scholar]
- 3. Le V. T., et al. 2010. Viral etiology of encephalitis in children in southern Vietnam: results of a one-year prospective descriptive study. PLoS Negl. Trop. Dis. 4:e854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lycke J., Andersen O., Svennerholm B., Appelgren L., Dahlof C. 1989. Acyclovir concentrations in serum and cerebrospinal fluid at steady state. J. Antimicrob. Chemother. 24:947–954 [DOI] [PubMed] [Google Scholar]
- 5. Lycke J., Malmestrom C., Stahle L. 2003. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob. Agents Chemother. 47:2438–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poirier J. M., Radembino N., Jaillon P. 1999. Determination of acyclovir in plasma by solid-phase extraction and column liquid chromatography. Ther. Drug Monit. 21:129–133 [DOI] [PubMed] [Google Scholar]
- 7. Sangdara A., Bhattarakosol P. 2008. Acyclovir susceptibility of herpes simplex virus isolates at King Chulalongkorn Memorial Hospital, Bangkok. J. Med. Assoc. Thai. 91:908–912 [PubMed] [Google Scholar]
- 8. Skoldenberg B., et al. 1984. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised multicentre study in consecutive Swedish patients. Lancet 2:707–711 [DOI] [PubMed] [Google Scholar]
- 9. Smith J. P., et al. 2010. Pharmacokinetics of acyclovir and its metabolites in cerebrospinal fluid and systemic circulation after administration of high-dose valacyclovir in subjects with normal and impaired renal function. Antimicrob. Agents Chemother. 54:1146–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soul-Lawton J., et al. 1995. Absolute bioavailability and metabolic disposition of valacyclovir, the l-valyl ester of acyclovir, following oral administration to humans. Antimicrob. Agents Chemother. 39:2759–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Svensson J. O., Barkholt L., Sawe J. 1997. Determination of acyclovir and its metabolite 9-carboxymethoxymethylguanine in serum and urine using solid-phase extraction and high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 690:363–366 [DOI] [PubMed] [Google Scholar]
- 12. Weller S., et al. 1993. Pharmacokinetics of the acyclovir pro-drug valacyclovir after escalating single- and multiple-dose administration to normal volunteers. Clin. Pharmacol. Ther. 54:595–605 [DOI] [PubMed] [Google Scholar]
- 13. Whitley R. J., et al. 1986. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N. Engl. J. Med. 314:144–149 [DOI] [PubMed] [Google Scholar]
- 14. Whitley R. J., Blum M. R., Barton N., de Miranda P. 1982. Pharmacokinetics of acyclovir in humans following intravenous administration. A model for the development of parenteral antivirals. Am. J. Med. 73:165–171 [DOI] [PubMed] [Google Scholar]