Abstract
There are currently few or no published data on the amount of cerebrospinal fluid (CSF) penetration of daptomycin in patients with suspected or documented neurosurgical infections. We conducted a prospective study, assessing the pharmacokinetics and CSF penetration of a single intravenous daptomycin dose administered at 10 mg/kg, based on total body weight (TBW), in six neurosurgical patients with indwelling external CSF shunts with suspected or documented meningitis or ventriculitis. Each patient had four blood and CSF samples drawn simultaneously at specific times after the end of infusion: 30 min, 6 h, 12 h, and 24 h. Pharmacokinetic parameters of daptomycin in serum were calculated using standard noncompartmental methods, and daptomycin was assayed using high-performance liquid chromatography (for serum) or liquid chromatography with mass spectrometry (for CSF). The mean (± standard deviation [SD]) maximum measured daptomycin concentrations were 93.7 ± 17.3 mg/liter in serum at 0.5 h postinfusion and 0.461 ± 0.51 mg/liter in CSF at 6 h postinfusion. The mean (± SD) daptomycin minimum concentrations were 13.8 ± 4.8 mg/liter in serum at 24 h postinfusion and 0.126 ± 0.12 mg/liter in CSF at 0.5 h postinfusion. The mean daptomycin penetration, determined by the area under the concentration-time curve in CSF (AUCCSF)/(AUCserum ratio), was 0.8%. Corrected for protein binding, the overall CSF penetration was 11.5%. Additional pharmacokinetic studies evaluating multiple and/or higher dosages of daptomycin are necessary in human subjects to better characterize the CSF penetration of daptomycin in neurosurgical patients.
INTRODUCTION
Infections of the central nervous system (CNS) in neurosurgical patients are a serious complication associated with high morbidity, mortality, and prolonged length of stay in the intensive care unit (ICU) (38, 40). Typically, neurosurgical devices such as external and internal ventricular cerebrospinal fluid (CSF) drains may lead to infections in these patients. Multidrug-resistant Gram-positive organisms, particularly methicillin-resistant Staphylococcus aureus (MRSA), are becoming increasingly problematic pathogens in patients with neurosurgical infections (17, 22, 25). Treatment of these infections poses challenges not only due to antimicrobial resistance but also due to the difficulty in achieving therapeutic concentrations at the site of infection primarily due to the presence of the blood-brain barrier (6, 27).
Daptomycin may be a potential option for the treatment of infections due to multidrug-resistant Gram-positive pathogens. It is currently approved by the U.S. Food and Drug Administration (FDA) at 4 mg/kg/day and 6 mg/kg/day for the treatment of skin and soft structure infections and S. aureus bacteremia, including right-sided endocarditis, respectively (3, 15). Based upon its concentration-dependent activity, higher dosages may increase the degree and rapidity of bacterial killing, especially for hard-to-treat infections such as those of the CNS (1, 37). Case reports and postmarketing surveillance data suggest that higher dosages of daptomycin (range, ≥6 to 10 mg/kg/day) may be safe and efficacious (14, 20, 23, 24, 29).
There are currently few or no published data on the pharmacokinetics of daptomycin at doses as high as 10 mg/kg. In addition, no human studies have explored the extent of CNS penetration in adults. The purpose of this study was to assess the pharmacokinetics and CNS penetration of a single intravenous (i.v.) daptomycin dose administered at 10 mg/kg in patients with indwelling external CSF shunts who had suspected or documented meningitis or ventriculitis.
MATERIALS AND METHODS
This was a prospective, nonrandomized study conducted in the neurointensive care unit (NICU) at Detroit Medical Center (Detroit, MI), a level I trauma center, from 2008 to 2010. The study protocol was approved by the Human Investigation Committee, and written informed consent was obtained for each patient. Inclusion criteria for adult patients included the presence of an indwelling external CSF access device (i.e., ventriculostomy or lumbar drain), creatinine clearance ≥30 ml/min, and the presence of inflamed meninges as a result of documented or suspected meningitis or ventriculitis. Patients with both clinical symptoms (fever, headache, meningismus, and altered mentation) and laboratory parameters (CSF leukocytosis, defined as a CSF white blood cell count [WBC] of >100/mm3, elevated CSF protein, defined as CSF protein of >100 mg/dl, reduced CSF glucose, defined as CSF glucose of <40 mg/dl, or a positive CSF Gram stain or culture) indicative of CNS infection were deemed to have definitive bacterial meningitis/ventriculitis. Inflamed meninges were defined as the presence of >5 leukocytes/mm3 of CSF. Patients experiencing either clinical symptoms alone or at least one of the aforementioned laboratory signs of CNS infection were classified as having suspected meningitis/ventriculitis. Exclusion criteria included patients with conditions known or suspected to alter pharmacokinetics (i.e., burn or cystic fibrosis patients), patients already receiving daptomycin for empirical or documented infection, patients with impaired renal function (defined as serum creatinine of ±20% baseline), pregnancy, hypersensitivity to daptomycin, or significantly elevated creatine phosphokinase (CPK) levels at baseline (defined as >250 U/liter). Daptomycin was administered as a single dose at 10 mg/kg, based on total body weight (TBW), over a 30-min i.v. infusion.
Each patient had four blood (5 to 10 ml) and CSF (1 ml) samples drawn simultaneously at specific times after the end of infusion: 30 min, 6 h, 12 h, and 24 h. After each blood draw, patients were monitored for any signs or symptoms associated with bleeding or discomfort. Side effects, including dose-related toxicities (i.e., elevated CPK and myopathy) were assessed at baseline and post-drug infusion. Initial patient demographic data were collected upon enrollment into the study.
The pharmacokinetic parameters of daptomycin in the serum were calculated using standard noncompartmental methods (4). The elimination rate constant (kel) and half-life (t1/2) were determined by log-linear regression analysis of the terminal portion of the serum concentration-time curve. All four measured concentrations were used in the estimation of kel, with a correlation coefficient ranging from 0.95 to 0.99 for the ln(concentration)-time relationship in individual subjects. Both AUC24 h and AUC0-∞ were calculated for plasma data, with the AUC0-∞ used in the calculation of serum clearance. However, AUC0-∞ could not be calculated for CSF concentrations, since an accurate assessment of the elimination rate constant in CSF could not be made. Therefore, AUC24 h was used to calculate the CSF/serum ratio. Clearance in the serum (CLS) and CSF (CLCSF) was calculated as dose/AUC0-∞, while the apparent volume of distribution was calculated as Cl/kel. Peak concentrations of drug in serum and CSF and the time from the end of infusion to peak concentrations in the serum and CSF were derived from the concentration-versus-time curve. The percent meningeal penetration was determined by the AUCCSF/AUCserum ratio. Due to the minimal protein present in the CSF and the high level of daptomycin protein binding (92%) (13), we determined the CSF penetration after correcting for protein binding as follows: % penetration = [(AUCCSF)/(fAUCserum)] × 100% (21).
Daptomycin was assayed in serum using a high-performance liquid chromatographic (HPLC) method based on that described by Dvorchik et al. (10). Briefly, samples were processed by the addition of 0.1% formic acid in methanol to precipitate plasma proteins. Following centrifugation, the supernatant was transferred to autosampler vials and injected. An isocratic mobile phase of 35% acetonitrile with 65% ammonium phosphate buffer (0.5%) was used at a flow rate of 1.0 ml/min. A Nova-Pak C8 column (3.9 by 150 mm; Waters) was used for separation. Daptomycin was detected at a wavelength of 224 nm (Waters 2996 photodiode array detector). The standard curve had an r2 of 0.995 and a between-day coefficient of variation of 6.7%. CSF samples were extracted using solid-phase extraction (HLB 1-ml/30-mg cartridge; Waters) and were assayed by Cubist Pharmaceuticals, Inc., using liquid chromatography with tandem mass spectrometry (LC/MS-MS), since concentrations were below the limit of detection for the conventional HPLC method. The LC/MS-MS instrumentation included an Applied Biosystems API-4000 coupled with an Agilent 1100 quaternary pump and a Leap CTC autosampler. The daptomycin analogue CB-183253 was used as the internal standard. The calibration curve ranged from 0.025 to 5.0 μg/ml. For the quality control samples at low (0.075 μg/ml), middle (0.5 μg/ml), and high (4.0 μg/ml) concentrations, the coefficient of variation ranged from 2.2 to 5.1%, while the accuracy was −9.3 to 15.4.
RESULTS
Six adult neurosurgical patients (four females and two males) were enrolled in the study. Daptomycin administration and the serum and CSF collection procedures were well tolerated, with no adverse events noted postinfusion of daptomycin. No CPK elevations (postinfusion range, 150 to 243 IU/liter) were noted in those patients with baseline CPK levels drawn (n = 4), with no patients experiencing myopathy or rhabdomyolysis. The median dose of daptomycin administered was 725 mg (range, 650 to 1,500 mg). Individual patients' baseline characteristics are displayed in Table 1. All six patients had underlying CNS diseases, including subarachnoid hemorrhage (n = 4), intracerebral hemorrhage (n = 1), and traumatic brain injury (n = 1). Five out of the six patients were administered antibiotics prior to and with daptomycin. All six patients required an indwelling CSF device during hospitalization in the ICU.
Table 1.
Individual patients' demographics and characteristics at baseline
| Patient | Age (yr) | Gender | Wt (kg) | Daptomycin dose (mg) | Creatinine clearance (ml/min) | APACHE-II severity score |
|---|---|---|---|---|---|---|
| 1 | 26 | Female | 70 | 700 | 127.2 | 12 |
| 2 | 45 | Male | 65 | 650 | 142.9 | 6 |
| 3 | 62 | Female | 63.2 | 650 | 78 | 8 |
| 4 | 50 | Female | 78 | 800 | 30 | 22 |
| 5 | 44 | Female | 157 | 1,500 | 92.3 | 14 |
| 6 | 67 | Male | 75 | 750 | 92.5 | 16 |
The mean (± SD) daptomycin serum concentrations at 0.5, 6, 12, and 24 h postinfusion were 93.7 ± 17.3 mg/liter, 43.3 ± 13.5 mg/liter, 27.0 ± 10.0 mg/liter, and 13.8 ± 4.8 mg/liter, respectively. The mean (± SD) daptomycin CSF concentrations at 0.5, 6, 12, and 24 h postinfusion were 0.126 ± 0.120 mg/liter, 0.461 ± 0.51 mg/liter, 0.442 ± 0.45 mg/liter, and 0.221 ± 0.150 mg/liter, respectively.
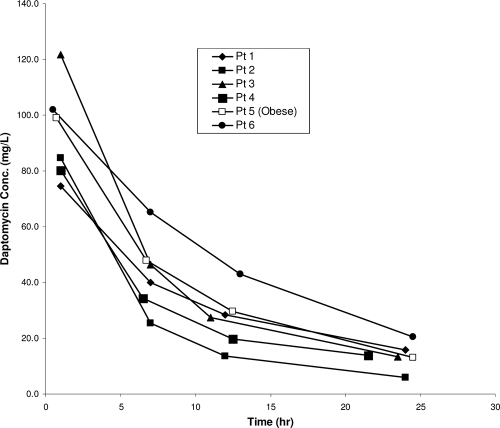
The mean (± SD) daptomycin exposures (AUC24 h values) were 906.0 ± 219.6 mg h/liter for serum and 8.3 mg h/liter for CSF, with a mean penetration ratio of 0.008, i.e., 0.8%. After correcting for daptomycin protein binding, the penetration of daptomycin into the CSF was determined to be 11.5% (21). The pharmacokinetic parameters for daptomycin in serum and CSF are reported in Table 2. Since there are limited data on the pharmacokinetics of high-dose daptomycin in obese patients, a subanalysis was performed on a 43-year-old morbidly obese African-American female (height, 65 in.; TBW, 157 kg; BMI, 57.6 kg/m2) who was administered a single i.v. dose of 1,500 mg of daptomycin. Nonobesity was defined as a BMI of 18 to 24.9 kg/m2, and morbid obesity was defined as a BMI of ≥40 kg/m2. Comparisons of pharmacokinetics in the five nonobese patients versus the obese patient revealed similar results. The AUC24 in serum and CSF in the nonobese patients averaged 893.1 mg h/liter and 8.5 mg h/liter, respectively, versus 970 mg h/liter and 7.40 mg h/liter, respectively, in the obese individual. The clearance, Vd, and t1/2 were also similar among the five nonobese patients versus those of the obese individual, with parameters as follows: clearance, 9.8 ml/h/kg versus 8.9 ml/h/kg; Vd, 0.12 liters/kg versus 0.11 liters/kg; and t1/2, 8.6 h versus 8.4 h. Figure 1 shows individual serum concentrations over time, illustrating that by providing a dose based on TBW, serum concentrations of daptomycin in the obese patient were quite similar to those found in nonobese patients.
Table 2.
Daptomycin pharmacokinetic parameters
| Patient | AUC24 h, serum (mg h/liter) | AUC, CSF (mg h/liter) | AUC ratio (CSF/serum) | Clearance (ml/h/kg) | V (liters/kg) | t1/2 (h) | k (1/h) |
|---|---|---|---|---|---|---|---|
| 1 | 838.8 | 1.01 | 0.001 | 9.66 | 0.15 | 10.6 | 0.065 |
| 2 | 676.3 | 3.61 | 0.005 | 13.68 | 0.13 | 6.5 | 0.107 |
| 3 | 1,001.3 | 17.12 | 0.017 | 9.00 | 0.10 | 7.4 | 0.094 |
| 4 | 690.9 | 1.47 | 0.002 | 10.49 | 0.13 | 8.3 | 0.083 |
| 5 | 970.0 | 7.40 | 0.008 | 8.86 | 0.11 | 8.4 | 0.083 |
| 6 | 1,258.5 | 19.35 | 0.015 | 6.38 | 0.10 | 10.4 | 0.067 |
| Mean | 906.0 | 8.3 | 0.008 | 9.7 | 0.12 | 8.6 | 0.083 |
| SD | 219.6 | 8.0 | 0.007 | 2.4 | 0.02 | 1.6 | 0.016 |
Fig. 1.
Serum concentrations of daptomycin in an obese patient and nonobese patients (n = 5) following administration of a single 10-mg/kg dose based on TBW.
DISCUSSION
This is the first study evaluating daptomycin CSF pharmacokinetics in humans. Limited studies have assessed daptomycin CSF penetration in animal models. All animal models have evaluated daptomycin penetration after a single 15-mg/kg dose, which is equivalent to 6 mg/kg in humans (8). Cottagnoud et al. demonstrated in a rabbit pneumococcal meningitis model that the penetration into inflamed meninges ranged from 4.37 to 7.53% after a single dose of 15 mg/kg of daptomycin. The CSF daptomycin level peaked at 5.2 mg/liter at hour 4 and then decreased to 3.2 mg/liter at hour 8 (8). Furthermore, Gerber et al. revealed that in a methicillin-susceptible S. aureus (MSSA) rabbit meningitis model the penetration of a single dose of 15 mg/kg of daptomycin was 5% into inflamed meninges versus 2% into noninflamed meninges (16). The addition of dexamethasone significantly reduced the penetration from 6% to 2% after a single 15-mg/kg dose of daptomycin into a rabbit pneumococcal meningitis model (12). Most recently, Riser et al. reported on the CSF penetration of daptomycin dosed at 9 mg/kg/day in a 54-year-old patient with suspected MSSA meningitis. The investigators drew a single serum and CSF trough concentration prior to the third dose, revealing concentrations of 11.21 mg/liter and 0.52 mg/liter, respectively, and concluded that the CSF penetration of daptomycin was approximately 5% (35).
Our results revealed that the mean daptomycin peak CSF concentration of 0.461 mg/liter achieved 6 h postinfusion was significantly lower in humans (mean penetration of 0.8% as determined by the AUCCSF/AUCserum ratio) than that previously reported in animal studies. The differences in penetration in our study versus that in the animal studies could be due to the variations in the degree of inflammation of the meninges in our six human subjects, which may have influenced the penetration of daptomycin into the CSF. Furthermore, five out of the six patients were on effective meningitis antibiotics prior to and with daptomycin, which would affect meningeal inflammation. However, if we account for the potential differences in protein binding between CSF and serum, the CSF penetration of daptomycin was 11.5%.
Antibiotic penetration into the CSF is highly variable and dependent upon the lipophilic properties of the drug, its molecular size, its protein binding, and the degree of meningeal inflammation. Previous studies with various other antibiotics report a wide range of achievable penetration. For instance, the penetration of penicillin in the CSF, even with inflamed meninges, has ranged from 2 to 15% (9, 19, 30, 36, 39), whereas vancomycin penetration has ranged from 14 to 30% depending on meningeal inflammation (2, 7, 32, 34), and broad-spectrum cephalosporins, such as cefotaxime and ceftriaxone, have had limited CSF penetration, with a range of 4 to 17% (18, 33). Accordingly, the preferred antimicrobial to treat CNS infections is small and moderately lipophilic and has a low level of protein binding (30).
Another important variable for antimicrobials used for the treatment of meningitis is the relationship of the achievable CSF concentration to the infecting pathogen's MIC. Daptomycin MICs for Gram-positive organisms (e.g., S. aureus, S. pneumoniae, etc.) generally range between 0.1 and 1 mg/liter. CSF concentrations below those achieved in the serum may be adequate for microbiological eradication when the MICs are low. Lipman et al. (26) reported clinical improvement in a patient treated with high dosing (400 mg i.v. every 8 h) of ciprofloxacin for Pseudomonas aeruginosa meningitis, with CSF concentrations peaking at 0.9 mg/liter and persisting 1 week later. The authors suggested that higher dosing of the ciprofloxacin might yield severalfold-higher peak concentrations over the MIC and ultimately improve the killing activity of the drug. Given the concentration-dependent characteristics of daptomycin, a study evaluating doses of >10 mg/kg/day would be helpful to determine whether higher peak CSF concentrations are achievable.
Although we did not observe CPK elevations or signs and symptoms of muscle toxicity after the single 10-mg/kg dose in our patient group, these patients were monitored for only a short period of time (pre- and post-daptomycin infusion), and thus it is possible that a delayed reaction may have occurred which was unnoticed. However, there are not only safety data available for dosages up to 12 mg/kg/day in healthy volunteers (5) but also several studies that have evaluated the safety of daptomycin in patients at doses exceeding 6 mg/kg/day (range, >6 to 10 mg/kg/day). In a majority of these studies, patients have tolerated high-dose daptomycin for relatively long treatment periods without significant changes in CPK or signs and symptoms of muscle toxicity (14, 21, 24, 28, 29).
There are limited pharmacokinetic data for daptomycin in obese patients, especially at higher than currently recommended doses. Our results with a 10-mg/kg dose are similar to the findings of the few analyses performed on obese patients at lower doses. Dvorchik and Damphousse evaluated the pharmacokinetics of daptomycin dosed at 4 mg/kg according to TBW in six moderately obese, morbidly obese, and matched nonobese patients (11). The authors found that the TBW-normalized drug clearance was 20 to 25% lower in the morbidly obese subjects than in the nonobese subjects (7.82 ml/h/kg versus 10.19 ml/h/kg; P < 0.05). Likewise, the TBW-normalized Vd was significantly lower in the morbidly obese group than in the nonobese group (0.09 liters/kg versus 0.11 liters/kg; P < 0.05). Dvorchik and Damphousse concluded that daptomycin should be dosed based on TBW, with no adjustments necessary for obese patients. We found similar drug clearances in the obese patient and the nonobese patients when adjusted for TBW (8.9 ml/h/kg versus 9.8 ml/h/kg). Additionally, the AUC and Cmax were higher in the obese patient than in the nonobese patients, since the obese patient received a higher dose of daptomycin; however, the half-lives and clearance were similar among the two populations. This similarity in half-life suggests that the same dosing interval may be appropriate for obese and nonobese patients.
Pai and colleagues performed a similar study; however, they used the four-variable modification of diet in renal disease (MDRD) equation to determine glomerular filtration rate (GFR) (31). The authors found that the mean absolute volume was 22% higher and the total serum daptomycin clearance was 12% higher in the morbidly obese group than in the normal-weight group. The authors concluded that administering daptomycin based on TBW in morbidly obese patients correlated best with the volume of distribution.
In conclusion, we found that a single dose of i.v. daptomycin dosed at 10 mg/kg had minimal penetration into the CSF in patients with suspected or documented meningitis. Furthermore, we determined that daptomycin based on TBW, even at higher doses, may be appropriate in obese patients. Additional pharmacokinetic studies in obese patients and evaluating multiple and/or higher dosages of daptomycin are necessary in human subjects in order to better characterize the CSF penetration of daptomycin in neurosurgical patients.
ACKNOWLEDGMENTS
This study was funded by Cubist Pharmaceuticals.
R.K., J.N.C., D.J.E., D.P., and W.M.C. have no conflicts. M.J.R. has received research grants from Astellas, Cubist, Forest, and Pfizer; has served as a consultant to Astellas, Cubist, Forest, and Ortho-McNeil; and has served on speaker's bureaus for Cubist, Novartis, and Pfizer.
Footnotes
Published ahead of print on 18 April 2011.
REFERENCES
- 1. Akins R. L., Rybak M. J. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albanese J., et al. 2000. Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob. Agents Chemother. 44:1356–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arbeit R. D., Maki D., Tally F. P., Campanaro E., Eisenstein B. I. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673–1681 [DOI] [PubMed] [Google Scholar]
- 4. Benet L. Z., Galeazzi R. L. 1979. Noncompartmental determination of the steady-state volume of distribution. J. Pharm. Sci. 68:1071–1074 [DOI] [PubMed] [Google Scholar]
- 5. Benvenuto M., Benziger D. P., Yankelev S., Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradbury M. W. 1984. The structure and function of the blood-brain barrier. Fed. Proc. 43:186–190 [PubMed] [Google Scholar]
- 7. Caricato A., et al. 2006. Levels of vancomycin in the cerebral interstitial fluid after severe head injury. Intensive Care Med. 32:325–328 [DOI] [PubMed] [Google Scholar]
- 8. Cottagnoud P., et al. 2004. Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 48:3928–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Louvois J., Gortvai P., Hurley R. 1977. Antibiotic treatment of abscesses of the central nervous system. Br. Med. J. ii:985–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dvorchik B. H., Brazier D., DeBruin M. F., Arbeit R. D. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dvorchik B. H., Damphousse D. 2005. The pharmacokinetics of daptomycin in moderately obese, morbidly obese, and matched nonobese subjects. J. Clin. Pharmacol. 45:48–56 [DOI] [PubMed] [Google Scholar]
- 12. Egermann U., et al. 2009. Combination of daptomycin plus ceftriaxone is more active than vancomycin plus ceftriaxone in experimental meningitis after addition of dexamethasone. Antimicrob. Agents Chemother. 53:3030–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenstein B. I. 2004. Lipopeptides, focusing on daptomycin, for the treatment of Gram-positive infections. Expert Opin. Invest. Drugs 13:1159–1169 [DOI] [PubMed] [Google Scholar]
- 14. Figueroa D. A., et al. 2009. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin. Infect. Dis. 49:177–180 [DOI] [PubMed] [Google Scholar]
- 15. Fowler V. G., Jr., et al. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653–665 [DOI] [PubMed] [Google Scholar]
- 16. Gerber P., Stucki A., Acosta F., Cottagnoud M., Cottagnoud P. 2006. Daptomycin is more efficacious than vancomycin against a methicillin-susceptible Staphylococcus aureus in experimental meningitis. J. Antimicrob. Chemother. 57:720–723 [DOI] [PubMed] [Google Scholar]
- 17. Gnanalingham K. K., Elsaghier A., Kibbler C., Shieff C. 2003. The impact of methicillin-resistant Staphylococcus aureus in a neurosurgical unit: a growing problem. J. Neurosurg. 98:8–13 [DOI] [PubMed] [Google Scholar]
- 18. Humbert G., Leroy A., Nair S. R., Cherubin C. E. 1984. Concentrations of cefotaxime and the desacetyl metabolite in serum and CSF of patients with meningitis. J. Antimicrob. Chemother. 13:487–494 [DOI] [PubMed] [Google Scholar]
- 19. Karlsson M., Hammers-Berggren S., Lindquist L., Stiernstedt G., Svenungsson B. 1994. Comparison of intravenous penicillin G and oral doxycycline for treatment of Lyme neuroborreliosis. Neurology 44:1203–1207 [DOI] [PubMed] [Google Scholar]
- 20. Katz D. E., et al. 2008. A pilot study of high-dose short duration daptomycin for the treatment of patients with complicated skin and skin structure infections caused by gram-positive bacteria. Int. J. Clin. Pract. 62:1455–1464 [DOI] [PubMed] [Google Scholar]
- 21. Kim A., et al. 2008. In vivo microdialysis study of the penetration of daptomycin into soft tissues in diabetic versus healthy volunteers. Antimicrob. Agents Chemother. 52:3941–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korinek A. M. 1997. Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord. Service Epidemiologie Hygiene et Prevention. Neurosurgery 41:1073–1081 [DOI] [PubMed] [Google Scholar]
- 23. Kullar R., et al. High-dose daptomycin for complicated Gram-positive infections, poster P1984. Progr. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis., Helsinki, Finland, 16 to 19 May 2009 [Google Scholar]
- 24. Kullar R., et al. 2011. High-dose daptomycin for treatment of complicated gram-positive infections: a large, multicenter, retrospective study. Pharmacotherapy 31:527–536 [DOI] [PubMed] [Google Scholar]
- 25. Lietard C., Thebaud V., Besson G., Lejeune B. 2008. Risk factors for neurosurgical site infections: an 18-month prospective survey. J. Neurosurg. 109:729–734 [DOI] [PubMed] [Google Scholar]
- 26. Lipman J., Allworth A., Wallis S. C. 2000. Cerebrospinal fluid penetration of high doses of intravenous ciprofloxacin in meningitis. Clin. Infect. Dis. 31:1131–1133 [DOI] [PubMed] [Google Scholar]
- 27. Lutsar I., McCracken G. H., Jr., Friedland I. R. 1998. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin. Infect. Dis. 27:1117–1127,1128–1129 [DOI] [PubMed] [Google Scholar]
- 28. Mohr J. F., Friedrich L. V., Yankelev S., Lamp K. C. 2009. Daptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE). Int. J. Antimicrob. Agents 33:543–548 [DOI] [PubMed] [Google Scholar]
- 29. Moise P. A., Hershberger E., Amodio-Groton M. I., Lamp K. C. 2009. Safety and clinical outcomes when utilizing high-dose (> or =8 mg/kg) daptomycin therapy. Ann. Pharmacother. 43:1211–1219 [DOI] [PubMed] [Google Scholar]
- 30. Nau R., Sorgel F., Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 23:858–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pai M. P., et al. 2007. Influence of morbid obesity on the single-dose pharmacokinetics of daptomycin. Antimicrob. Agents Chemother. 51:2741–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfausler B., et al. 2003. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J. Neurosurg. 98:1040–1044 [DOI] [PubMed] [Google Scholar]
- 33. Pfister H. W., Preac-Mursic V., Wilske B., Einhaupl K. M. 1989. Cefotaxime vs penicillin G for acute neurologic manifestations in Lyme borreliosis. A prospective randomized study. Arch. Neurol. 46:1190–1194 [DOI] [PubMed] [Google Scholar]
- 34. Ricard J. D., et al. 2007. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin. Infect. Dis. 44:250–255 [DOI] [PubMed] [Google Scholar]
- 35. Riser M. S., Bland C. M., Rudisill C. N., Bookstaver P. B. Cerebrospinal fluid penetration of high-dose daptomycin in suspected Staphylococcus aureus meningitis. Ann. Pharmacother 44:1832–1835 [DOI] [PubMed] [Google Scholar]
- 36. Rockowitz J., Tunkel A. R. 1995. Bacterial meningitis. Practical guidelines for management. Drugs 50:838–853 [DOI] [PubMed] [Google Scholar]
- 37. Rose W. E., et al. 2008. Daptomycin activity against Staphylococcus aureus following vancomycin exposure in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:831–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sellner J., Tauber M. G., Leib S. L. Pathogenesis and pathophysiology of bacterial CNS infections. Handb. Clin. Neurol. 96C:1–16 [DOI] [PubMed] [Google Scholar]
- 39. van der Valk P. G., Kraai E. J., van Voorst Vader P. C., Haaxma-Reiche H., Snijder J. A. 1988. Penicillin concentrations in cerebrospinal fluid (CSF) during repository treatment regimen for syphilis. Genitourin. Med. 64:223–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zunt J. R. Infections of the central nervous system in the neurosurgical patient. Handb. Clin. Neurol. 96C:125–141 [DOI] [PubMed] [Google Scholar]