Abstract
Efavirenz-based antiretroviral regimen is preferred during rifampin-containing tuberculosis therapy. However, current pharmacokinetic data are insufficient to guide optimized concurrent dosing. This study aimed to better characterize the effects of rifampin on efavirenz pharmacokinetics. Subjects were randomized to receive 600 mg efavirenz/day or 600 mg efavirenz with 600 mg rifampin/day for 8 days, with plasma samples collected for pharmacokinetic analysis over 24 h on day 8. Treatments were then crossed over after at least a 2-week washout period, and procedures were repeated. Efavirenz concentrations were determined by high-performance liquid chromatography (HPLC), and pharmacokinetic parameters were estimated by noncompartmental analysis. Efavirenz pharmacokinetic differences between treatment periods were evaluated by paired t test. The coefficients of variation in efavirenz plasma AUC0-24 (area under the concentration-time curve from 0 to 24 h) were 50% and 56% in the absence and presence of rifampin, respectively. Of the 11 evaluable subjects (6 white, 5 black; 6 women, 5 men), the geometric mean AUC0-24 ratio on/off rifampin (90% confidence interval) was 0.82 (0.72, 0.92), with individual AUC0-24 ratios varying from 0.55 to 1.18. Five subjects had a 24-hour efavirenz concentration (C24) of <1,000 ng/ml on rifampin. They were more likely to have received a lower dose in milligrams/kilogram of body weight and to have lower efavirenz AUC0-24 values in the basal state. Although rifampin resulted in a modest reduction in efavirenz plasma exposure in subjects as a whole, there was high variability in responses between subjects, suggesting that efavirenz dose adjustment with rifampin may need to be individualized. Body weight and genetic factors will be important covariates in dosing algorithms.
INTRODUCTION
Efavirenz is an essential component of preferred antiretroviral regimens in treatment of human immunodeficiency virus (HIV) infection in patients coinfected with tuberculosis (TB) (6, 32, 33). The standard adult dose of 600 mg daily is associated with considerable interindividual variability in plasma concentrations and clinical effects (10, 28, 38). There is even greater variability in efavirenz concentrations during coadministration with rifampin or rifampin-containing TB therapy (2, 13, 29). Efavirenz is primarily metabolized by hepatic CYP2B6, with some contributions from CYP3A4/5 (42), CYP2A6 (30), and UGT2B7 enzymes (1). Rifampin was shown to be a potent inducer of CYP2B6 activity when bupropion was used as a probe of enzyme activity (22). Among healthy volunteers, rifampin caused 26% and 20% reductions in mean efavirenz area under the curve (AUC) and maximum concentration (Cmax), respectively (2). Among HIV-TB-coinfected patients, mean reductions of 24% and 25% in efavirenz AUC and Cmax, respectively, were reported with rifampin coadministration. In the above-mentioned studies, some subjects had higher efavirenz plasma exposure with rifampin (compared with that when off rifampin), suggesting a lack of a significant induction effect (2, 24).
While the clinical significance of the interactions between efavirenz and rifampin is not entirely understood, to offset the mean reduction of efavirenz exposure with rifampin, some experts have recommended increasing the efavirenz dose to 800 mg/day, especially when body weight is greater than 50 kg (6, 33). An extensive review of published literature did not find adequate evidence to support a dose increase with rifampin coadministration because of current lack of understanding of the likelihood of under- or overdosing individuals (11). Prior drug-drug interaction studies failed to evaluate genetic contributions to the variability in the induction effect of rifampin (2, 24), were performed in the setting of clinical care, where patients were receiving other antituberculous drugs in addition to rifampin, or did not have an appropriate control period or comparator group (13, 20, 25, 35). To better characterize the effect of rifampin on efavirenz disposition as well as interindividual differences in response to rifampin, we conducted a randomized two-period crossover pharmacokinetic (PK) study in healthy African-American and Caucasian volunteers.
MATERIALS AND METHODS
Study subjects.
HIV-seronegative healthy volunteers, age 18 to 55 years, who identified themselves as either Caucasian or African-American, were enrolled. Subjects were excluded if a clinically significant medical condition or laboratory abnormality was detected. The Institutional Review Board of Miriam Hospital reviewed and approved the study, and all subjects signed written informed consent.
Study design.
An open-label, two-period crossover study was conducted. The subjects were randomized based on ethnicity to treatment with 600 mg efavirenz daily (period I) or 600 mg efavirenz plus 600 mg rifampin daily (period II) on days 1 to 8. After sampling for efavirenz plasma concentrations on day 8 and at least a 2-week washout period, subjects crossed over to the alternate treatment and the procedures were repeated. We enrolled equal numbers of Caucasians and African-Americans as well as males and females because of the reported ethnic and gender differences in CYP2B6 expression (21).
Pharmacokinetic sampling.
Pharmacokinetic blood sampling for efavirenz concentrations was performed on day 8 of drug(s) administration. The administration of efavirenz was switched to mornings 2 days prior to the pharmacokinetic sampling. The date and time of ingestion of study drugs were recorded in a diary and verified on the day of sampling. Blood samples were obtained at times 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 h relative to day 8 efavirenz dosing. Blood was centrifuged at 3,000 × g for 10 min, and the plasma was separated and stored in labeled tubes at −70°C until time of drug assay.
Pharmacokinetic analysis.
Efavirenz concentrations in plasma were measured using high-performance liquid chromatography (HPLC) with the UV light method (36). This method is validated over a range of 15 to 10,000 ng/ml and is accurate (90.4 to 110.5%) with intraday and interday precision of 2.3 to 8.3%. The assay is validated according to FDA guidelines and the laboratory is CLIA certified and participates in quarterly national and international external proficiency testing. Maximum efavirenz plasma concentration (Cmax), time to Cmax (Tmax), and concentration at 24 h (C24h) were obtained by visual inspection of the plasma efavirenz concentration-time profile for each patient. The 24-h-postdose sample for one subject during the rifampin-dosing phase was lost during centrifugation. Since PK sampling was performed at steady state, efavirenz concentration obtained at time zero (907.3 ng/ml) was considered C24h for this subject (subject identifier [ID] W6). Noncompartmental analysis was conducted using Win Nonlin Professional version 5.2.1 (Pharsight Corporation, Cary, NC). Area under the concentration-time curve from time zero to 24 h (AUC0-24) was calculated using the log-trapezoidal method. Apparent oral clearance of efavirenz (CL/F) was calculated by dividing the administered efavirenz daily dose (600 mg) by the AUC0-24. Clearance was normalized to body weight (CL/F/W) measured at study entry in kilograms.
Enzymes and nuclear receptor genotyping.
Subjects were genotyped for CYP2B6*6 (reference SNP accession no. rs3745274), CYP2B6*16 (rs28399499), CYP2A6*9B (rs8192726), CYP2A6*17 (rs28399454), pregnane X receptor (PXR; NR1I2) 63396C→T single nucleotide polymorphism (SNP) (rs2472677), and constitutive androsane receptor (CAR; NR1I3) G→A SNP (rs2307424) by using Applied Biosystems kits as we previously described. Genotypes for the UGT2B7*2 (rs7439366), UGT2B7*1c (rs28365062), and PXR variants rs3732360C→T were determined by genomic PCR amplification and sequencing as previously described with minor modifications (9, 16, 31). The SNPs were selected because they have been previously shown to be associated with efavirenz plasma concentrations or effect (18, 19, 43), as well as CYP3A4 and CYP2B6 activity (12, 31). The composite CYP2B6 516/983 genotype was defined by the number of minor allele polymorphisms of CYP2B6 516G→T and 983C→T SNPs (0 = extensive metabolizer, 1 = intermediate metabolizer, and 2 = slow metabolizer) (37).
Statistical analysis.
Univariate analyses of association between patient factors and efavirenz AUC0-24 values were assessed by the t test or Mann-Whitney rank sum test (sex, race, and history of alcohol use), analysis of variance (ANOVA) (three genotype groups), or Spearman rank order correlation test (age, body weight, and body mass index). Arithmetic means of efavirenz pharmacokinetic parameters, including Tmax, Cmax, C24h, AUC0-24, apparent oral clearance (CL/F), and weight-adjusted apparent oral clearance (CL/F/W), were compared by the use of the paired t test (Wilcoxon signed rank test; if data failed normality test). Geometric mean ratios (efavirenz plus rifampin/efavirenz alone) and 90% confidence intervals (90% CI) of log-transformed data were calculated using Win Nonlin Professional version 5.2.1 (Pharsight Corporation, Cary, NC). Finally, differences in demographic and genetic factors between patients with efavirenz C24h concentrations of <1,000 ng/ml (considered subtherapeutic) and >1,000 ng/ml were compared by t test or Mann-Whitney rank sum test (continuous data) or chi-square or Fisher's exact test (categorical data). A P value of <0.05 was considered significant.
RESULTS
Efavirenz pharmacokinetics in the study population.
Of the 13 volunteers who were initially enrolled, one subject developed a vasovagal episode during blood sampling and did not complete the study. Another subject (ID B3) had detectable efavirenz concentrations when efavirenz was administered alone but undetectable concentrations with rifampin coadministration. Although medication dosing was observed on the day of PK sampling, we could not rule out medication nonadherence, and the individual was excluded from further analysis.
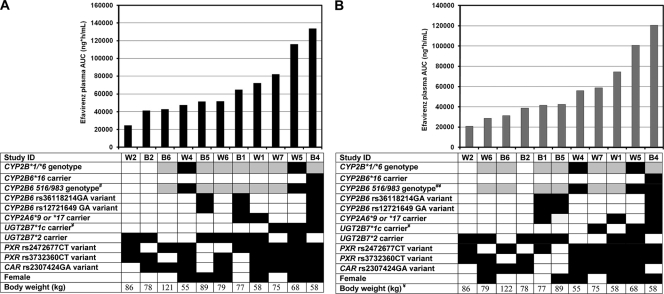
The final study population of 11 subjects included 6 Caucasians and 5 African-Americans and 6 females and 5 males. The mean (standard deviation [SD]) age was 42.6 (7.5) years, body weight was 76.9 (18.9) kilograms, and body mass index (BMI) was 26.9 (5.7) kg/m2. The coefficients of variation (CV) in efavirenz plasma AUC0-24 were 50% and 56% in the absence and presence of rifampin, respectively. The distribution of efavirenz plasma AUC0-24 in the absence and presence of rifampin and the relationship with subject factors are shown in Fig. 1 A and B, respectively. UGT2B7*1c carriers, compared to noncarriers, had higher efavirenz AUC0-24 values in the basal state (mean, 110,489 versus 49,496 ng·h/ml; P < 0.001) and in the induced state (mean, 93,415 versus 41,773 ng·h/ml; P = 0.006). CYP2B6 516/983 genotype status was significantly associated with efavirenz AUC0-24 in the induced state (mean, 92,507, 46,200, and 29,853 ng·h/ml for subjects with slow, intermediate, and fast metabolizer genotypes, respectively; P = 0.024). A similar relationship was observed in the basal state, but the differences between genotype groups did not reach statistical significance (P = 0.057). There was an inverse relationship between body weight and efavirenz AUC0-24 in the induced state (correlation coefficient = −0.764; P = 0.005). Age, body mass index, sex, ethnicity, and other genetic factors evaluated were not significantly associated with efavirenz AUC0-24 in either treatment period (P > 0.05) (Fig. 1).
Fig. 1.
Distribution of efavirenz AUC0-24 values in increasing order and relationship with individual factors in the absence (A) and presence (B) of rifampin. #, Composite CYP2B6 516/983 genotype was defined by the number of minor allele polymorphisms of CYP2B6 516G→T and 983C→T SNPs; ¥, relationship between subject factor and efavirenz AUC0-24 values is significant (P < 0.05). Differences between groups were examined by the Mann-Whitney rank sum test (2 genotype groups) or by ANOVA (3 genotype groups), and relationship with body weight was examined by Spearman rank order correlation test. Shading code for genetic factors: white, homozygous reference; gray, heterozygous; black, homozygous variant or carrier.
Effect of rifampin on efavirenz pharmacokinetics.
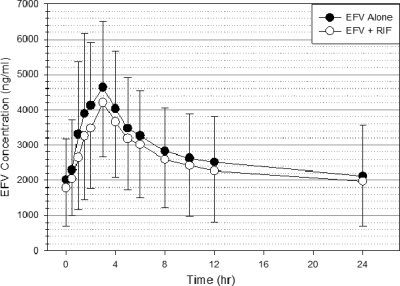
The plasma concentration-time profile for efavirenz in the absence and presence of rifampin is shown in Fig. 2. Mean efavirenz plasma concentrations at all sampling time points were lower when efavirenz was administered with rifampin, but there was wide intersubject variability. Efavirenz mean C24h and AUC0-24 values were lower with rifampin coadministration than when efavirenz was administered alone (1,722 versus 2,116 ng/ml, P = 0.014, and 55,810 versus 66,251 ng·h/ml, P = 0.010, respectively). The differences in mean values between the two periods for Tmax, Cmax, and CL/F were not significant (data not shown in tables). Similar trends were observed in the pharmacokinetics parameters in the absence and presence of rifampin when we excluded the subject W6, in whom the C24h sample was lost during period II (data not shown). The geometric means and geometric mean ratios (on/off rifampin) with 90% CI for the efavirenz plasma pharmacokinetic parameters are summarized in Table 1. The geometric mean ratios (90% CI) for Cmax and AUC0-24 were 0.84 (0.71, 1.00) and 0.82 (0.72, 0.92), respectively. The result did not change significantly after a repeat analysis with subject W6 excluded (data not shown).
Fig. 2.
Mean efavirenz concentration-time profile in 11 healthy volunteers measured on day 8 of the treatment period either in the absence (black circles) or presence (white circles) of rifampin. Error bars indicate standard deviations of the means.
Table 1.
Geometric mean and geometric mean ratio (90% CI) of efavirenz steady-state pharmacokinetic parameter estimates in the absence and presence of efavirenz in healthy volunteersa
| Pharmacokinetic parameter | Geometric mean (90% CI) |
Geometric mean ratio (EFV-RIF/EFV) (90% CI) | |
|---|---|---|---|
| EFV alone (n = 11) | EFV-RIF (n = 11) | ||
| Tmax (h) | 2.46 (2.10, 2.89) | 2.30 (1.56, 3.39) | 0.99 (0.76, 1.29) |
| Cmax (ng/ml) | 4,571 (652, 5,721) | 3,882 (3,034, 4,966) | 0.84 (0.71, 1.00) |
| C24h (ng/ml) | 1,766 (1,262, 2,472) | 1,435 (1,026, 2,009) | 0.81 (0.73, 0.89) |
| AUC0-24 (ng·h/ml) | 59,429 (45,604, 77,466) | 48,865 (36,475, 65,464) | 0.82 (0.72, 0.92) |
| CL/F (ml/h) | 2,958 (1,380, 6,339) | 4,656 (3,112, 6,966) | 1.55 (1.15, 2.11) |
CI, confidence interval; EFV, efavirenz; RIF, rifampin; n, number of subjects; Cmax, peak concentration; Tmax, time to Cmax; C24h, 24-h-postdose concentration; AUC0-24, total area under the curve from time zero to 24 h; CL/F, apparent oral clearance.
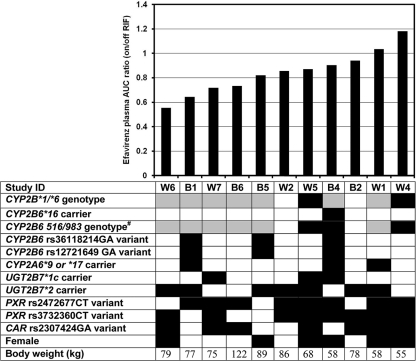
Individual efavirenz AUC0-24 ratios (on/off rifampin) ranged from 0.55 to 1.18 (Fig. 3), with an arithmetic mean (SD) of 0.84 (0.18). There was a trend toward an inverse relationship between the AUC0-24 ratio and body weight (correlation coefficient = −0.591; P = 0.051). We found no significant relationship between the AUC0-24 ratio and age, sex, ethnicity, and the genetic factors evaluated.
Fig. 3.
Distribution of efavirenz AUC0-24 (with rifampin/no rifampin) ratios in increasing order and relationship with individual factors. #, composite CYP2B6 516/983 genotype was defined by the number of minor allele polymorphisms of CYP2B6 516G→T and 983C→T SNPs. Shading code for genetic factors: white, homozygous reference; gray, heterozygous; black, homozygous variant or carrier.
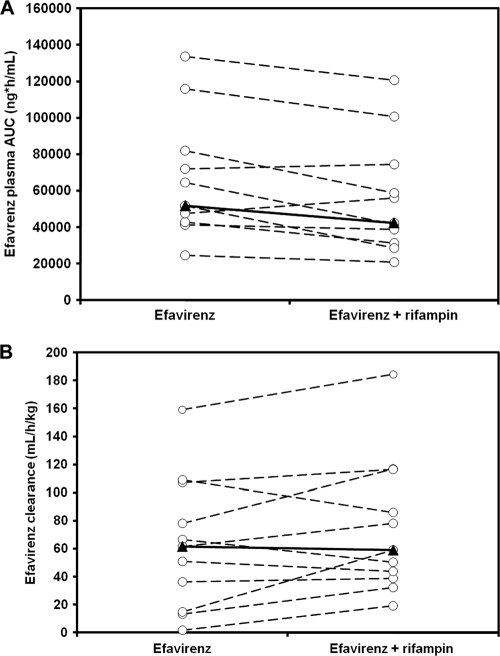
The changes in efavirenz AUC0-24 or weight-normalized CL/F between the two periods appeared to be minimal in a majority of the subjects (Fig. 4). Ten subjects showed a 6 to 44% decrease in efavirenz AUC0-24 values with rifampin cotreatment. Two subjects (W1 and W4) had slight increases in efavirenz AUC0-24 values of 3 and 18%, respectively (Fig. 4A). The percent change in CL/F from baseline varied from +1,041% to −24% (mean [SD] of 139.1% [313.8%]). Three subjects (W1, W4, and B6) had paradoxical decreases in efavirenz CL/F of 24.1, 21.1, and 14.0% with rifampin cotreatment compared to when efavirenz was administered alone (Fig. 4B).
Fig. 4.
Efavirenz AUC0-24 (A) and weight-adjusted apparent oral clearance (B) in 12 healthy volunteers in the absence and presence of rifampin. Efavirenz and rifampin were administered at standard dosages, and patients were in steady state. Rifampin coadministration caused a mean reduction in AUC0-24 values by 16% and a mean increase in apparent oral clearance by 139%. Open circles with dotted lines represent individuals, and triangles with solid lines are the medians.
Factors associated with efavirenz C24h of <1,000 ng/ml in the presence of rifampin.
Five of the 11 subjects had efavirenz C24h of <1,000 ng/ml during rifampin cotreatment, while only one subject had efavirenz C24h of <1,000 ng/ml in the absence of rifampin. Given that the suggested minimum effective plasma concentration of efavirenz is 1,000 ng/ml (28, 32), we sought to identify individual factors associated with efavirenz C24h of <1,000 ng/ml during rifampin coadministration. Body weight-adjusted efavirenz dose in milligram/kilogram and lower baseline efavirenz plasma AUC or C24h values were significantly associated with efavirenz C24h of <1,000 ng/ml (Table 2). Both subjects with CYP2B6 516/983 fast metabolizer genotypes in the study population had C24h of <1,000 ng/ml, and two of the three individuals with slow metabolizer genotypes had C24h of >4,000 ng/ml, which is considered the upper limit of the therapeutic range (Table 2).
Table 2.
Comparison of characteristic of patients who had 24-h-postdose efavirenz concentrations <1,000 ng/ml and >1,000 ng/ml during concurrent administration with rifampina
| Characteristic | C24 < 1,000 ng/ml (n = 5) | C24 > 1,000 ng/ml (n = 6) | P value |
|---|---|---|---|
| Mean (SD) age (years) | 42.2 (5.5) | 43.0 (9.4) | 0.871 |
| Mean (SD) body wt (kg) | 88.6 (19.0) | 67.2 (13.3) | 0.052 |
| Mean (SD) body mass index (kg/m2) | 29.0 (5.6) | 25.2 (5.8) | 0.294 |
| Dose/body wt (mg/kg) | 7.0 (1.2) | 9.2 (1.6) | 0.034 |
| Sex | 0.080 | ||
| No. of males (%) | 4 (80.0) | 1 (20.0) | |
| No. of females (%) | 1 (16.7) | 5 (83.3) | |
| Race | 0.567 | ||
| No. of African-Americans (%) | 3 (60.0) | 2 (40.0) | |
| No. of Caucasians (%) | 2 (33.3) | 4 (66.7) | |
| CYP2B6 516/983 genotype | 0.084 | ||
| Extensive (%) | 2 (100.0) | 0 (0.0) | |
| Intermediate (%) | 3 (50.0) | 3 (50.0) | |
| Slow (%) | 0 (0.0) | 3 (100) | |
| History of alcohol use | 1.00 | ||
| No (%) | 2 (50.0) | 2 (50.0) | |
| Yes (%) | 3 (42.9) | 4 (57.1) | |
| Mean (SD) baseline efavirenz AUC (ng·h/ml) | 44,965 (14,720) | 83,768 (34,655) | 0.046 |
| Mean (SD) baseline efavirenz C24h (ng/ml) | 1,163 (336) | 2,910 (1,555) | 0.037 |
| Mean (SD) AUC0-24 ratio (on/off rifampin) | 0.75 (0.16) | 0.92 (0.164) | 0.104 |
C24h, 24-hour post-dose concentration; AUC0-24, total area under the curve from time zero to 24 h.
Safety and tolerability.
The administration of efavirenz alone or with rifampin for 8 days was well tolerated in this study. Central nervous system (CNS) side effects of efavirenz were evaluated using the CNS symptom experience questionnaire designed for the AIDS Clinical Trials Group study A5097s (7). The self-administered questionnaire included 34 questions (scaled from 0 to 4), with a range of scores from 0 to 136. The change in CNS symptom test scores when efavirenz was administered alone varied from −2 to +16, with four subjects showing no changes in scores. During the period of efavirenz and rifampin coadministration, the changes in test scores varied from −16 to +38, with two subjects reporting no changes. One subject (ID B4) with the highest efavirenz exposure had the highest increase in symptom test scores during both periods. Symptoms that were considered by subject B4 to be extreme (scale 4) included restless sleep, waking up a lot, intense dreams, and trouble going to sleep. There was no discontinuation of study medications due to adverse events by any of the study subjects.
DISCUSSION
The findings of this study indicate a modest decrease in mean efavirenz plasma exposure with rifampin coadministration in the subjects as a whole, as well as a wide variability in response. The mean decrease in efavirenz AUC0-24 values of 16% in this crossover study is numerically lower than the 26% reduction reported in a healthy volunteer study (2) and the 24% decrease in Spanish HIV-TB-coinfected patients (24). However, the mean decrease in efavirenz AUC0-24 values was significant (P < 0.05) in our and the previous healthy-volunteer studies (2) but was not in the study among HIV-TB-coinfected patients (24). A common finding in the previous pharmacokinetic studies (2, 24) and ours is wide interindividual variability in the rifampin effect, with some subjects showing no decrease in efavirenz plasma exposure with rifampin therapy. In our study, the reduction in efavirenz AUC0-24 values with rifampin ranged from a decrease of 44% to an increase of 18%. The large deviation (both positive and negative) from the average in the current and previous studies might explain the modest effect of rifampin in the study populations as a whole. The apparent lack of induction of efavirenz metabolism by rifampin-containing anti-TB therapy in some subjects is contrary to the expected effect of rifampin, but it is consistent with the bimodal effects of rifampin-containing anti-TB therapy on efavirenz plasma concentrations observed in HIV-TB-coinfected patients (13, 17, 35).
The clinical implications of the induction or lack of induction effect of rifampin on efavirenz pharmacokinetics are not entirely clear. Many patients achieve adequate efficacy and tolerability with 600 mg or 800 mg efavirenz daily during coadministration with rifampin (3, 23, 27). However, 800 mg efavirenz daily was associated with a high frequency of central nervous system (CNS) and hepatic toxicities associated with supratherapeutic plasma concentrations in black native Africans (3). Furthermore, a reduced efavirenz dose to 200 mg daily or discontinuation of efavirenz has been necessary in some HIV-TB-coinfected patients during concurrent rifampin-containing TB therapy because of intolerable CNS toxicities (14, 15, 41). In contrast, other HIV-TB-coinfected patients have required increased doses up to 1,600 mg daily to achieve desired plasma concentrations and virologic suppression during concurrent therapy (4). The above-mentioned clinical studies or case reports indicate that a better understanding of the individual differences in the efavirenz pharmacokinetic response to rifampin coadministration is necessary for rational decisions about efavirenz dose adjustment with rifampin therapy, as one dose adjustment will not fit all patients.
Nearly one-half of the subjects in this study had efavirenz trough concentrations considered to be subtherapeutic during the period of rifampin coadministration, but only one of the 11 patients had a subtherapeutic trough concentration in the absence of rifampin. Although the minimum effective efavirenz plasma concentration is still controversial, our findings suggest that some but not all individuals are likely underdosed using the current standard fixed dose. Both CYP2B6 516/983 “fast metabolizers” as well as higher body weight appeared to predict a lower efavirenz concentration with rifampin therapy. The data from this controlled pharmacokinetic study support the findings of several studies demonstrating a relationship between efavirenz concentrations and body weight (24, 26, 39), as well as with CYP2B6 516G→T polymorphisms (8, 19, 20, 34, 40).
The main limitation of our study was the small number of subjects with known functional genetic variants. This limited our ability to draw any firm conclusions about lack of association with some of the genetic factors, especially for PXR and CAR. We also did not assay for efavirenz metabolites and could not use the metabolite-to-parent AUC ratio as an index of the activity of enzymes mediating the main or accessory pathways in each subject to assess the magnitude of induction. Notwithstanding, this controlled crossover study provides insights into the interindividual variability in response to the induction effects of rifampin on efavirenz disposition. Individuals with larger body weights, UGT2B7*1c noncarriers, and those with the CYP2B6 extensive metabolizing genotype had lower efavirenz AUC0-24 values with rifampin coadministration. Our findings suggest that genetic factors and body weight may be important factors and should be taken into consideration for developing dosing algorithms or when designing larger studies to determine the appropriate dose of efavirenz with rifampin coadministration.
ACKNOWLEDGMENTS
This research was supported in part by a K23 developmental award (NIH K23 AI071760) to A.K. and by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases (U01 AI069472). M.H.C. was supported by NIH grant R01-GM-61834 from the National Institute of General Medical Sciences (Bethesda, MD).
A.K. and A.D.M.K. previously received research grants from Bristol-Myers Squibb not related to this research. K.T.T. receives research funding from Merck and Co., Inc., and GlaxoSmithKline. J.B.D., P.P., J.K., M.H.C., and D.J.G. report no conflicts of interest.
We thank the study participants and the nurses and staff of the ACTG unit and the Immunology Center Laboratory for all their valuable assistance in recruitment, evaluation of subjects, and obtaining, handling, and processing the pharmacokinetic samples. We also thank Heyward Hull at UNC for guidance on the statistical analysis. The University of North Carolina at Chapel Hill, Center for AIDS Research 9P30 AI50410, Clinical Pharmacology and Analytical Chemistry Core, analyzed the efavirenz concentrations.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Belanger A. S., et al. 2009. Glucuronidation of the antiretroviral drug efavirenz (EFV) by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine (AZT). Drug Metab. Dispos. 37:1793–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benedek I. H., et al. 1998. Pharmacokinetic interaction between efavirenz (EFV) and rifampin (RIF) after multiple oral doses in healthy volunteers, abstr. 42280. 12th World AIDS Conference, Geneva, Switzerland, 28 June to 3 July 1998. [Google Scholar]
- 3. Brennan-Benson P., Lyus R., Harrison T., Pakianathan M., Macallan D. 2005. Pharmacokinetic interactions between efavirenz and rifampicin in the treatment of HIV and tuberculosis: one size does not fit all. AIDS 19:1541–1543 [DOI] [PubMed] [Google Scholar]
- 4. Cabrera S. E., et al. 2008. Efavirenz-rifampicin interaction: therapeutic drug monitoring to efavirenz dosage optimization in HIV/TBC patients. AIDS 22:2549–2551 [DOI] [PubMed] [Google Scholar]
- 5. CDER 2003. Guidance for industry bioavailability and bioequivalence studies for orally administered drug products—general considerations. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), Rockville, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation /Guidances/ucm070124.pdf. [Google Scholar]
- 6.Centers for Disease Control Prevention. Managing drug interactions in the treatment of HIV-related tuberculosis. CDC, Atlanta, GA.: 2007. http://www.cdc.gov/tb/publications/guidelines/TB_HIV_Drugs/PDF/tbhiv.pdf. [Google Scholar]
- 7. Clifford D. B., et al. 2005. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann. Intern. Med. 143:714–721 [DOI] [PubMed] [Google Scholar]
- 8. Cohen K., et al. 2009. Effect of rifampicin-based antitubercular therapy and the cytochrome P450 2B6 516G>T polymorphism on efavirenz concentrations in adults in South Africa. Antivir. Ther. 14:687–695 [PMC free article] [PubMed] [Google Scholar]
- 9. Court M. H., et al. 2003. Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab. Dispos. 31:1125–1133 [DOI] [PubMed] [Google Scholar]
- 10. Csajka C., et al. 2003. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin. Pharmacol. Ther. 73:20–30 [DOI] [PubMed] [Google Scholar]
- 11. DiGiacinto J. L., Chan-Tack K. M., Robertson S. M., Reynolds K. S., Struble K. A. 2008. Are literature references sufficient for dose recommendations? An FDA case study of efavirenz and rifampin. J. Clin. Pharmacol. 48:518–523 [DOI] [PubMed] [Google Scholar]
- 12. Faucette S. R., et al. 2006. Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J. Pharmacol. Exp. Ther. 317:1200–1209 [DOI] [PubMed] [Google Scholar]
- 13. Friedland G., Khoo S., Jack C., Lalloo U. 2006. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J. Antimicrob. Chemother. 58:1299–1302 [DOI] [PubMed] [Google Scholar]
- 14. Gatanaga H., et al. 2007. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin. Infect. Dis. 45:1230–1237 [DOI] [PubMed] [Google Scholar]
- 15. Gatanaga H., Oka S. 2009. Successful genotype-tailored treatment with small-dose efavirenz. AIDS 23:433–434 [DOI] [PubMed] [Google Scholar]
- 16. Kwara A., et al. 2009. Interindividual variability in pharmacokinetics of generic nucleoside reverse transcriptase inhibitors in TB/HIV-coinfected Ghanaian patients: UGT2B7*1c is associated with faster zidovudine clearance and glucuronidation. J. Clin. Pharmacol. 49:1079–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwara A., Lartey M., Sagoe K. W., Court M. H. 2011. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS 25:388–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kwara A., Lartey M., Sagoe K. W., Kenu E., Court M. H. 2009. CYP2B6, CYP2A6 and UGT2B7 genetic polymorphisms are predictors of efavirenz mid-dose concentration in HIV-infected patients. AIDS 23:2101–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwara A., Lartey M., Sagoe K. W., Rzek N. L., Court M. H. 2009. CYP2B6 (c.516G→T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br. J. Clin. Pharmacol. 67:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwara A., et al. 2008. Pharmacokinetics of efavirenz when coadministered with rifampin in TB/HIV coinfected patients: pharmacogenetic effect of CYP2B6 variation. J. Clin. Pharmacol. 48:1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lamba V., et al. 2003. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J. Pharmacol. Exp. Ther. 307:906–922 [DOI] [PubMed] [Google Scholar]
- 22. Loboz K. K., et al. 2006. Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin. Pharmacol. Ther. 80:75–84 [DOI] [PubMed] [Google Scholar]
- 23. Lopez-Cortes L. F., et al. 2006. Efavirenz trough levels are not associated with virological failure throughout therapy with 800 mg daily and a rifampicin-containing antituberculosis regimen. J. Antimicrob. Chemother. 58:1017–1023 [DOI] [PubMed] [Google Scholar]
- 24. Lopez-Cortes L. F., et al. 2002. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin. Pharmacokinet. 41:681–690 [DOI] [PubMed] [Google Scholar]
- 25. Manosuthi W., et al. 2006. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. AIDS 20:131–132 [DOI] [PubMed] [Google Scholar]
- 26. Manosuthi W., et al. 2009. Body weight cutoff for daily dosage of efavirenz and 60-week efficacy of efavirenz-based regimen in human immunodeficiency virus and tuberculosis coinfected patients receiving rifampin. Antimicrob. Agents Chemother. 53:4545–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manosuthi W., et al. 2005. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. AIDS 19:1481–1486 [DOI] [PubMed] [Google Scholar]
- 28. Marzolini C., et al. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71–75 [DOI] [PubMed] [Google Scholar]
- 29. Matteelli A., et al. 2007. Multiple-dose pharmacokinetics of efavirenz with and without the use of rifampicin in HIV-positive patients. Curr. HIV Res. 5:349–353 [DOI] [PubMed] [Google Scholar]
- 30. Ogburn E. T., Jones D. R., Masters A. R., Xu C., Guo Y., Desta Z. 2010. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 (CYP) 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab. Dispos. 38:1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oleson L., von Moltke L. L., Greenblatt D. J., Court M. H. 2010. Identification of polymorphisms in the 3′-untranslated region of the human pregnane X receptor (PXR) gene associated with variability in cytochrome P450 3A (CYP3A) metabolism. Xenobiotica 40:146–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Panel on Antiretroviral Guidelines for Adults Adolescents 2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 33. Pozniak A. L., et al. 2005. BHIVA treatment guidelines for tuberculosis (TB)/HIV infection 2005. HIV Med. 6(Suppl. 2):62–83 [DOI] [PubMed] [Google Scholar]
- 34. Ramachandran G., et al. 2009. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob. Agents Chemother. 53:863–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ren Y., et al. 2009. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J. Acquir. Immune Defic. Syndr. 50:439–443 [DOI] [PubMed] [Google Scholar]
- 36. Rezk N. L., Crutchley R. D., Yeh R. F., Kashuba A. D. 2006. Full validation of an analytical method for the HIV-protease inhibitor atazanavir in combination with 8 other antiretroviral agents and its applicability to therapeutic drug monitoring. Ther. Drug Monit. 28:517–525 [DOI] [PubMed] [Google Scholar]
- 37. Ribaudo H. J., et al. 2010. Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group study. J. Infect. Dis. 202:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stahle L., Moberg L., Svensson J. O., Sonnerborg A. 2004. Efavirenz plasma concentrations in HIV-infected patients: inter-and intraindividual variability and clinical effects. Ther. Drug Monit. 26:267–270 [DOI] [PubMed] [Google Scholar]
- 39. Stohr W., et al. 2008. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir. Ther. 13:675–685 [PubMed] [Google Scholar]
- 40. Uttayamakul S., et al. 2010. Effects of CYP2B6 G516T polymorphisms on plasma efavirenz and nevirapine levels when co-administered with rifampicin in HIV/TB co-infected Thai adults. AIDS Res. Ther. 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Luin M., et al. 2009. Efavirenz dose reduction to 200 mg once daily in a patient treated with rifampicin. AIDS 23:742–744 [DOI] [PubMed] [Google Scholar]
- 42. Ward B. A., et al. 2003. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J. Pharmacol. Exp. Ther. 306:287–300 [DOI] [PubMed] [Google Scholar]
- 43. Wyen C., et al. , on behalf of the German Competence Network for HIV/AIDS 2009. Association of a constitutive androstane receptor polymorphism (rs2307424) with treatment discontinuation in HIV+ patients receiving efavirenz, abstr. PS12/6. 12th European AIDS Conference, 11 to 14 November 2009, Cologne, Germany http://onlinelibrary.wiley.com/doi/10.1111/j.1468-1293.2009.00789.x/pdf. [Google Scholar]