Abstract
Rifampin coadministration dramatically reduces plasma lopinavir (LPV) concentrations. In healthy volunteers, doubling the dose of a lopinavir-ritonavir (LPV/r) capsule formulation overcame this interaction, but a subsequent study of double doses of the tablet formulation was stopped early owing to hepatotoxicity. However, healthy-volunteer study findings may not apply to HIV-infected adults. We evaluated the steady-state pharmacokinetics of LPV in HIV-infected adults virologically suppressed on an LPV/r regimen who were given rifampin, and the dose of the LPV/r tablet formulation was gradually increased. The steady-state pharmacokinetics of LPV/r were evaluated at baseline, a week after commencing rifampin, a week after the LPV/r dose was increased 1.5 times, and a week after the LPV/r dose was doubled. Twenty-one participants were enrolled. The median [interquartile range (IQR)] predose LPV concentrations (C0) were 8.1 (6.2 to 9.8) mg/liter at baseline, 1.7 (0.3 to 3.0) mg/liter after 7 days of rifampin, 5.9 (2.1 to 9.9) mg/liter with 1.5 times the dose of LPV/r, and 10.8 (7.0 to 13.1) mg/liter with double-dose LPV/r. There were no significant differences in the LPV area under the plasma concentration-time curve from 0 to 12 h (AUC0-12), C0, C12, maximum concentration of drug in serum (Cmax), or half-life (t1/2) between the baseline and double-dose LPV/r time points. Treatment was generally well tolerated, with two participants developing asymptomatic grade 3/4 transaminitis. Doubling the dose of the tablet formulation of LPV/r overcomes induction by rifampin. Less hepatotoxicity occurred in our cohort of HIV-infected participants than was reported in healthy-volunteer studies.
INTRODUCTION
Rifampin is a key component of tuberculosis treatment but also a potent inducer of many cytochrome P450 enzymes and the efflux pump p-glycoprotein (15). Protease inhibitors are substrates of both CYP 3A4 and p-glycoprotein, and the trough concentrations of all ritonavir-boosted protease inhibitors are reduced by more than 90% when standard doses are coadministered with rifampin (2). A healthy-volunteer study demonstrated that similar lopinavir (LPV) trough concentrations can be achieved either by adding ritonavir (RTV) to give a lopinavir/ritonavir ratio of 1:1 or by doubling the dose of the capsule formulation of lopinavir-ritonavir (LPV/r) (12).
Subsequent healthy-volunteer studies of the interaction between rifampin and adjusted doses of ritonavir-boosted saquinavir, atazanavir, and lopinavir (tablet formulation) were prematurely terminated because of high incidences of hepatotoxicity (6, 7, 16). These high rates of hepatotoxicity in healthy volunteers might not apply to patients with tuberculosis and HIV. First, in the healthy-volunteer studies, initiating rifampin prior to the protease inhibitor was associated with high rates of hepatotoxicity (6, 7, 16). In high-burden countries, protease inhibitors are used as part of the second-line antiretroviral treatment (ART) regimen; hence, most patients are established on the protease inhibitor before rifampin is initiated. Second, HIV infection may lower the risk of hepatotoxicity, as illustrated by the experience with rifampin and pyrazinamide for latent tuberculosis, which was well tolerated in HIV-infected individuals but was associated with high rates of hepatotoxicity in non-HIV-infected individuals (5, 9, 20).
We evaluated the steady-state pharmacokinetics of LPV and RTV in HIV-infected adults virologically suppressed on an LPV/r regimen who were given rifampin with the dose of LPV/r gradually increased to double the standard dose.
MATERIALS AND METHODS
Study design.
The study was an open-label, sequential, four-period, multiple-dose trial in HIV-infected adults who were virologically suppressed (viral loads, <400 copies/ml) on LPV/r together with dual nucleoside reverse transcriptase inhibitors. We compared the steady-state pharmacokinetics of LPV and RTV using noncompartmental analysis under 4 sequential treatment conditions over a 12-h dosing interval in HIV-infected participants: a standard dose of LPV/r (400 mg/100 mg) every 12 hours (study day 1), after which rifampin at 600 mg daily was commenced; LPV/r in standard doses every 12 hours with rifampin (study day 8); 1.5 times the standard dose of LPV/r (600 mg/150 mg) every 12 hours with rifampin (study day 15); and twice the standard dose of LPV/r (800 mg/200 mg) every 12 hours with rifampin (study day 22). Dual nucleoside reverse transcriptase inhibitors were continued throughout with no dose adjustments. Treatment adherence was assessed by using a treatment diary and pill counts.
Study participants.
We recruited HIV-infected participants established on an LPV/r regimen (tablet formulation) from a South African antitretroviral clinic, the Hannan Crusaid Treatment Centre in Gugulethu, Cape Town, South Africa. We included medically stable HIV-infected adults older than 18 years with viral loads of <400 copies/ml. Exclusion criteria were abnormal creatinine, severe diarrhea, hepatic disease (defined as either alanine aminotransferase [ALT] at more than 1.5 times the upper limit of normal or a positive hepatitis B surface antigen or hepatitis C antibody test result), grade 3 or higher raised fasting cholesterol (>7.77 mmol/liter) or triglycerides (>8.49 mmol/liter), random glucose measurements of >11.1 mmol/liter, excessive alcohol consumption (in excess of 2 units per day or 14 units per week), symptoms or signs of tuberculosis, taking drugs other than the study drugs known to alter the pharmacokinetics of LPV, and pregnancy.
Pharmacokinetic assessment.
Participants were admitted overnight and fasted from 22h00. We observed the dose of LPV/r taken the evening before pharmacokinetic sampling and ensured it was 12 h before the predose sample the next morning. Intensive pharmacokinetic sampling was done predose and at 1.5 h, 2 h, 2.5 h, 3 h, 4 h, 5 h, 6 h, 8 h, and 12 h after observed dosing. Standardized meals were given between the 2-h and 2.5-h, 5-h and 6-h, and 8-h and 12-h sampling times.
We collected 4-ml blood samples in lithium heparin tubes that were kept on melting ice prior to separation. Within 1 h of sampling, the blood samples were centrifuged at 3,000 rpm for 10 min. Each plasma sample was aliquoted and stored at −80°C until the drug concentration was determined.
Safety monitoring.
We monitored ALT and total serum bilirubin 3 times a week from the day before rifampin was started until the end of the study period (study days 1, 4, 6, 8, 11, 13, 15, 18, 20, 22, 25, and 28). Electrocardiograms were done at baseline and repeated at days 15 and 22 after the LPV/r dose increases. All adverse events were recorded and graded according to the grading system of the Division of AIDS (4). Subjects were withdrawn from the study when they developed grade 3 or greater adverse events thought to be related to the study drugs.
Drug assays.
We used validated liquid chromatography tandem mass spectrometry (LC/MS-MS) to determine the LPV and RTV concentrations in the plasma samples. Lopinavir and ritonavir were assayed as previously described (17). The assay range for lopinavir was 0.05 to 20 μg/ml, and for ritonavir it was 0.025 to 5 μg/ml. Inter- and intraday coefficients of variation were below 10% for both drugs. The laboratory participates in the International Interlaboratory Control Program of Stichting Kwaliteitsbewaking Klinische Geneesmiddelanalyse en Toxicologie (KKGT) (Hague, Netherlands). LPV and RTV concentrations reported as below the limit of quantification (BLQ) were analyzed as the BLQ concentration divided by 2.
Ethical approval.
The study was approved by the University of Cape Town Human Research Ethics Committee. Each volunteer was informed of the objectives, nature, and potential risks of the study. Written informed consent was obtained from every participant.
Statistical analyses.
Stata version 11.0 (Stata Corporation, College Station, TX) was used to characterize the pharmacokinetic parameters of LPV and RTV using noncompartmental analyses. The area under the plasma concentration-time curve from 0 to 12 h (AUC0-12) was calculated from a 12-h dosing interval using the linear trapezoidal rule. The predose (C0) and 12-h (C12) LPV concentrations were determined directly from the concentration-time data.
Normally distributed numerical data were described using means and standard deviations, and the t test for paired samples was used for hypothesis testing. The Fisher exact test was used for categorical data hypothesis testing. Numerical data that followed a nonnormal distribution were described using the median and interquartile range (IQR), and the Wilcoxon signed-rank test was used for hypothesis testing. Geometric mean ratios (90% confidence intervals [CIs]) were calculated to compare the AUC0-12 and the maximum concentration of drug in serum (Cmax) on study days 8, 15, and 22 to those on study day 1.
RESULTS
We enrolled 21 black African participants in the study, 18 of whom were female. The mean age ± standard deviation (SD) was 36.1 ± 7.1 years, the mean body mass index ±SD was 26.2 ± 5.8 kg/m2, and the median (IQR) CD4+ cell count was 564 (408 to 669) cells/mm3.
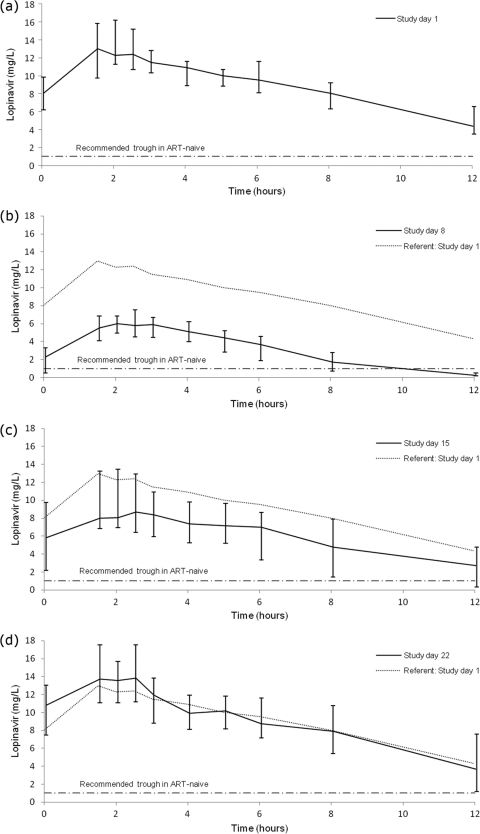
Figure 1 shows the median steady-state LPV concentrations over time measured on study days 1, 8, 15, and 22, and the pharmacokinetic parameters are summarized in Table 1.
Fig. 1.
Median steady-state lopinavir concentrations over time on study days 1 (a), 8 (b), 15 (c), and 22 (d). Shown are median and interquartile lopinavir concentrations over time under the following conditions: study day 1, standard dose of LPV/r (400 mg/100 mg every 12 hours) without rifampin (a); study day 8, standard dose of LPV/r and rifampin (600 mg daily) (b); study day 15, 1.5 times the standard dose of LPV/r and rifampin (c); and study day 22, twice the standard dose of LPV/r and rifampin (d).
Table 1.
Steady-state lopinavir pharmacokinetic parameters
| Study day | AUC0-12 (mg·h/liter) [median (IQR)] | Geometric mean ratio (90% CI)a | Cmax (mg/liter) [median (IQR)] | Geometric mean ratio (90% CI) | t1/2 (h) [median (IQR)] | C0 (mg/liter) [median (IQR)] | C12 (mg/liter) [median (IQR)] |
|---|---|---|---|---|---|---|---|
| 1 | 118.3 (91.3–126.1) | Referent | 15.1 (11.3–18.2) | Referent | 5.7 (4.6–6.7) | 8.1 (6.2–9.8) | 4.33 (3.5–6.5) |
| 8 | 38.2 (25.9–47.0) P < 0.001a,b | 0.32 (0.28–0.36) | 6.3 (5.5–7.9) P < 0.00a,b | 0.46 (0.42–0.50) | 1.6 (1.3–1.8) P < 0.001a,b | 1.7 (0.3–3.0) | 0.2 (0.1–0.4) |
| 15 | 69.2 (37.1–107.8) P < 0.001a,b | 0.63 (0.54–0.73) | 9.4 (7.0–14.4) P < 0.00a,b | 0.72 (0.63–0.83) | 2.9 (1.3–6.8) P < 0.01a,b | 5.9 (2.1–9.9) | 1.6 (0.1–4.4) |
| 22 | 101.6 (78.9–141.7) P = 0.45a,b | 1.02 (0.90–1.15) | 15.3 (12.2–20.2) P = 0.09a,b | 1.13 (0.97–1.31) | 5.0 (2.1–8.7) P = 0.5a,b | 10.8 (7.0–13.1) | 3.7 (1.2–7.7) |
Compared with study day 1.
Wilcoxon signed-rank test.
LPV trough (C0) concentrations below the recommended lower limit for ART-naïve patients (1 mg/liter) (1, 11) occurred in 0/21 study subjects on day 1, 10/21 (P < 0.01) on day 8, 2/20 (P = 0.23) on day 15, and 0/18 on day 22. The proportion of participants with subtherapeutic LPV C12 concentrations was higher on all study days than the number of those with subtherapeutic LPV C0 concentrations: 2/21 on day 1, 18/21 (P < 0.01) on day 8, 10/20 (P < 0.01) on day 15, and 4/18 (P = 0.39) on day 22.
Table 2 summarizes the steady-state ritonavir pharmacokinetic parameters.
Table 2.
Steady-state ritonavir pharmacokinetic parameters
| Study day | AUC0-12 (mg·h/liter) [median (IQR)] | Geometric mean ratio (90% CI) | Cmax (mg/liter) [median (IQR)] | Geometric mean ratio (90% CI) | t1/2 (h) (mean ± SD) | C0 (mg/liter) [median (IQR)] | C12 (mg/liter) [median (IQR)] |
|---|---|---|---|---|---|---|---|
| 1 | 5.8 (3.9–8.5) | Referent | 1.2 (0.8–1.9) | Referent | 3.5 (3.2–4.6) | 0.33 (0.18–0.55) | 0.13 (0.10–0.18) |
| 8 | 2.2 (1.5–3.7) P < 0.00a,b | 0.39 (0.34–0.42) | 0.5 (0.4–0.9) P < 0.001a,b | 0.44 (0.38–0.51) | 2.6 (2.2–2.8) P < 0.001a,b | 0.09 (0.03–0.16) | 0.03 (0.01–0.05) |
| 15 | 4.8 (2.5–9.6) P = 0.63a,b | 0.85 (0.71–1.01) | 1.1 (0.7–2.2) P = 0.60a,b | 0.90 (0.73–1.11) | 2.7 (1.9–3.2) P < 0.001a,b | 0.31 (0.10–0.70) | 0.06 (0.03–0.15) |
| 22 | 7.6 (4.2–13.2) P = 0.004a,b | 1.42 (1.20–1.67) | 2.0 (1.4–3.1) P = 0.002a,b | 1.54 (1.27–1.88) | 2.8 (2.4–3.3) P < 0.003a,b | 0.63 (0.37–1.11) | 0.11 (0.05–0.25) |
Compared with study day 1.
Wilcoxon signed-rank test.
Nineteen of the 21 participants completed the study. Two participants were withdrawn from the study owing to grade 3/4 asymptomatic transaminitis; one developed grade 3 transaminitis on the standard dose of LPV/r and rifampin, the other on 1.5 times the standard dose of LPV/r and rifampin. In both participants, the transaminitis resolved after LPV/r and rifampin were withdrawn. Another participant withdrew consent after developing grade 2 nausea on 1.5 times the standard dose of LPV/r and rifampin. Other adverse events were mild but frequent: 6 participants developed grade 1/2 transaminitis, 2 grade 1 hyperbilirubinaemia, 8 grade 1/2 nausea, 2 grade 1/2 diarrhea, and 1 PR interval prolongation (0.198 to 2.14 ms) on double the standard dose of LPV/r. All adverse events resolved. On routine viral-load measurement, all but 2 participants remained virologically suppressed. Adherence was measured at least 3 times a week during the study period by using participant questioning and correlating pill counts with recorded doses in the treatment diary. All participants had 100% adherence during the study period using these measures. Subsequent to the pharmacokinetic study, 2 participants had detectable viral loads measured at their routine clinic visits, which were ascribed to poor adherence.
DISCUSSION
This is the first study of the steady-state pharmacokinetics of adjusted doses of LPV/r with rifampin in HIV-infected adults. Therapeutic LPV C0 trough concentrations were achieved in all participants by doubling the dose of the tablet formulation of LPV/r, although 18/20 participants achieved therapeutic LPV C0 trough concentrations with 1.5 times the dose of LPV/r (600 mg/150 mg every 12 hours). In our cohort, we consistently found a higher proportion of participants with subtherapeutic C12 trough concentrations than with subtherapeutic C0 trough concentrations. Subtherapeutic LPV C12 trough concentrations were noted on all study days.The combination of LPV/r and rifampin was relatively well tolerated in our cohort of HIV-infected individuals compared with previous healthy-volunteer studies, but this cannot be extrapolated to treating patients with tuberculosis, as we have safety data for only 22 days.
LPV is a substrate of both CYP 3A4 and p-glycoprotein, which are inhibited by RTV (22). The increased dose of RTV partially offsets the induction effect of rifampin and, together with the increased dose of LPV, sufficiently overcomes induction by rifampin. However, we report pharmacokinetic measurements only; the effect of the increased dose of LPV/r with rifampin on the virological response is unknown.
Most patients achieved therapeutic LPV C0 trough concentrations with 1.5 times the dose of LPV/r (600 mg/150 mg every 12 hours), making a dose-down strategy with therapeutic drug monitoring of LPV an option in patients who do not tolerate double-dose LPV/r (800 mg/150 mg every 12 hours).
For patients on protease inhibitors who have no other antiretroviral options, two strategies can be followed when treating tuberculosis: adjusting the doses of the protease inhibitor or replacing rifampin with rifabutin. In high-burden countries, rifabutin is seldom an option, owing to its high current cost and complex dosing schedule and the widespread use of fixed-dose combinations containing rifampin for treating tuberculosis. The safety and pharmacokinetics of adjusted-dose LPV/r in combination with rifampin has been studied in two healthy-volunteer studies. La Porte et al. studied the LPV/r capsule formulation and demonstrated that the induction of rifampin can be overcome by either doubling the dose of LPV/r or increasing the RTV component to the same dose as LPV (12). Two of 10 volunteers in the LVP/r 800-mg/200-mg arm and 5 of 9 volunteers in the LPV/r 400-mg/400-mg arm were prematurely discontinued owing to hepatotoxicity. A subsequent study by Nijland et al. evaluating the pharmacokinetics of an adjusted-dose LPV/r tablet formulation with rifampin was prematurely terminated owing to very high rates of hepatotoxicity (16).
There are several possible reasons why lower rates of hepatotoxicity were seen in our study. First, HIV infection may be associated with a lower risk of hepatotoxicity. High rates of hepatotoxicity occurred in non-HIV-infected individuals compared with HIV-infected individuals in studies using rifampin and pyrazinamide to treat latent tuberculosis (5, 9, 20). HIV-tuberculosis-coinfected patients tolerated the combination of rifampin and saquinavir-ritonavir relatively well (14, 18), but high rates of hepatotoxicity were seen in healthy volunteers treated with this combination (6). The lower risk of hepatotoxicity in HIV-infected patients might be explained by an attenuated immune response, which is thought to play an important role in idiosyncratic drug-induced hepatocellular reactions (13). Secondly, we slowly escalated the dose of LPV/r over 2 weeks. High rates of hepatotoxicity occurred in healthy volunteers when double doses of lopinavir-ritonavir were given without dose escalations in combination with rifampin (16). Lastly, we initiated rifampin in HIV-infected participants established on LPV/r. High rates of hepatotoxicity occurred in the healthy-volunteer studies where rifampin was introduced prior to the protease inhibitors (6, 7, 16). Rifampin preinduction may rapidly generate protease inhibitor metabolites that are hepatotoxic (7). In high-burden countries, rifampin-based antitubercular therapy will usually be commenced in patients already on protease inhibitors, given that protease inhibitors are used in second-line ART regimens.
Diurnal variation of protease inhibitors has been reported previously (8, 10). Absorption differences due to the effect of food may account for the differences in our C0 and C12 trough concentrations. Our cohort received a meal before the observed C0 dose was taken, while the observed C12 dose was taken after a 10-h fast.
Our study findings have several limitations. First, we assessed the effect of rifampin on LPV/r concentrations only. Tuberculosis is treated with combination antituberculosis drugs, including isoniazid, which is an inhibitor of CYP 3A4 (3). Both LPV/r pharmacokinetics and hepatotoxicity may be different when administered with rifampin and isoniazid. Second, there may also be a disease effect of tuberculosis on both LPV/r concentrations and hepatotoxicity. Third, we evaluated hepatotoxicity for only 3 weeks. It is possible that high rates of hepatotoxicity may occur later during treatment. There may also be a carryover effect on toxicity owing to the sequential study design, with the last treatment period most affected. Fourth, RTV exposure is known to be higher in females, and our cohort was predominantly female. A greater pharmacokinetic success rate may therefore be seen in female patients (19, 21). Finally, our cohort consisted of HIV-infected participants who were virologically suppressed with high CD4+ counts. The risk of hepatotoxicity in this cohort may differ from that in HIV-infected individuals with tuberculosis and various degrees of immunosuppression.
In conclusion, we have described the first evaluation of steady-state pharmacokinetics of adjusted-dose LPV/r and rifampin in HIV-infected adults. We showed that it is possible to overcome the induction effect of rifampin by doubling the dose of LPV/r to 800 mg/200 mg. Compared with previous healthy-volunteer studies, our cohort of HIV-infected adults tolerated the combination of LPV/r and rifampin relatively well. Future research should study the tolerability and effectiveness of double-dose LPV/r in HIV-infected patients with tuberculosis.
ACKNOWLEDGMENTS
Our study was funded by the European and Developing Countries Clinical Trials Partnership (EDCTP). H.M. and G.M. received partial support from SATBAT through the Fogarty International Center (U2RTW007370/3 and 5U2RTW007373). E.H.D. received partial support from the Fogarty International Centre/USNIH (U2RTW007373 ICOHRTA).
We acknowledge the staff and patients of the Hannan Crusaid Treatment Centre who enthusiastically participated in the study.
Footnotes
Published ahead of print on 2 May 2011.
REFERENCES
- 1. Ananworanich J., et al. 2005. Pharmacokinetics and 24-week efficacy/safety of dual boosted saquinavir/lopinavir/ritonavir in nucleoside-pretreated children. Pediatr. Infect. Dis. J. 24:874–879 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control Prevention 2007. Managing drug interactions in the treatment of HIV-related tuberculosis. http://www.cdc.gov/tb/publications/guidelines/HIV_AIDS.htm Accessed 13 August 2010
- 3. Desta Z., Soukhova N. V., Flockhart D. A. 2001. Inhibition of cytochrome P450 (CYP450) isoforms by isoniazid: potent inhibition of CYP2C19 and CYP3A. Antimicrob. Agents Chemother. 45:382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Division of AIDS, National Institute of Allergy Infectious Diseases, National Institutes of Health 2004. Division of AIDS table for grading the severity of adult and pediatric adverse events. http://www.ucdmc.ucdavis.edu/clinicaltrials/documents/DAIDS_AE_GradingTable_FinalDec2004.pdf Accessed 13 August 2010
- 5. Gordin F. M., et al. 2004. Hepatotoxicity of rifampin and pyrazinamide in the treatment of latent tuberculosis infection in HIV-infected persons: is it different than in HIV-uninfected persons? Clin. Infect. Dis. 39:561–565 [DOI] [PubMed] [Google Scholar]
- 6. Grange S., Schutz M., Schmitt C., Riek M., Gaudeul-Ehrhart E. 2005. Unexpected hepatotoxicity observed in a healthy volunteer study on the effects of multiple dose rifampicin on the steady-state pharmacokinetics of ritonavir-boosted sequinavir and vice versa, abstr. 35. Sixth Int. Workshop Clin. Pharmacol. HIV Ther., Montreal, Quebec, Canada, 28 to 30 April 2005 [Google Scholar]
- 7. Haas D. W., et al. 2009. Hepatotoxicity and gastrointestinal intolerance when healthy volunteers taking rifampin add twice-daily atazanavir and ritonavir. J. Acquir. Immune Defic. Syndr. 50:290–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsu A., et al. 1997. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 41:898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jasmer R. M., et al. 2002. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann. Intern. Med. 137:640–647 [DOI] [PubMed] [Google Scholar]
- 10. Justesen U. S., Pedersen C. 2002. Diurnal variation of plasma protease inhibitor concentrations. AIDS 16:2487–2489 [DOI] [PubMed] [Google Scholar]
- 11. La Porte C. J. L., et al. 2006. Updated guideline to perform therapeutic drug monitoring for antiviral agents. Rev. Antivir. Ther. 3:4–14 [Google Scholar]
- 12. La Porte C. J., et al. 2004. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob. Agents Chemother. 48:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee W. M. 2003. Drug-induced hepatotoxicity. N. Engl. J. Med. 349:474–485 [DOI] [PubMed] [Google Scholar]
- 14. Losso M. H., Lourtau L. D., Toibaro J. J., Saenz C., Gonzalez C. 2004. The use of saquinavir/ritonavir 1000/100 mg twice daily in patients with tuberculosis receiving rifampin. Antivir. Ther. 9:1031–1033 [PubMed] [Google Scholar]
- 15. Niemi M., Backman J. T., Fromm M. F., Neuvonen P. J., Kivisto K. T. 2003. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin. Pharmacokinet. 42:819–850 [DOI] [PubMed] [Google Scholar]
- 16. Nijland H. M., et al. 2008. High incidence of adverse events in healthy volunteers receiving rifampicin and adjusted doses of lopinavir/ritonavir tablets. AIDS 22:931–935 [DOI] [PubMed] [Google Scholar]
- 17. Ren Y., et al. 2008. Effect of rifampicin on lopinavir pharmacokinetics in HIV-infected children with tuberculosis. J. Acquir. Immune Defic. Syndr. 47:566–569 [DOI] [PubMed] [Google Scholar]
- 18. Ribera E., et al. 2007. Pharmacokinetic interaction between rifampicin and the once-daily combination of saquinavir and low-dose ritonavir in HIV-infected patients with tuberculosis. J. Antimicrob. Chemother. 59:690–697 [DOI] [PubMed] [Google Scholar]
- 19. Sekar V., Ryan R., Schaible D., Mazikewich A., Mrus J. 2008. Pharmacokinetic profile of darunavir co-administered with low-dose ritonavir in treatment-experienced women and men with HIV infection: 4-week analysis in a substudy of the GRACE (gender, race and clinical experience) study, abstr. O16, p. 46 Abstr. Ninth Int. Workshop Clin. Pharmacol. HIV Ther., New Orleans, LA, 7 to 9 April 2008 [Google Scholar]
- 20. Tortajada C., et al. 2005. Is the combination of pyrazinamide plus rifampicin safe for treating latent tuberculosis infection in persons not infected by the human immunodeficiency virus? Int. J. Tuberc. Lung Dis. 9:276–281 [PubMed] [Google Scholar]
- 21. Umeh O. C., et al. 13 January 2011. Sex differences in lopinavir and ritonavir pharmacokinetics among HIV-infected women and men. J. Clin. Pharmacol. [Epub ahead of print.] doi:10.1177/0091270010388650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou S., et al. 2004. Therapeutic drugs that behave as mechanism-based inhibitors of cytochrome P450 3A4. Curr. Drug Metab. 5:415–442 [DOI] [PubMed] [Google Scholar]