Abstract
The antimalarial drug chloroquine is eliminated to a significant extent by renal tubular secretion. The molecular mechanism of renal chloroquine secretion remains unknown. We hypothesized that organic cation transporter 2 (OCT2) and multidrug and toxin extrusion protein 1 (MATE1), localized in the basolateral and luminal membranes of proximal tubule cells, respectively, are involved in chloroquine transport. The interaction of chloroquine with both transporters was investigated using single-transfected human embryonic kidney 293 (HEK293)-MATE1 cells in uptake experiments and single-transfected Madin-Darby canine kidney II (MDCK)-OCT2 and MDCK-MATE1 cells as well as double-transfected MDCK-OCT2-MATE1 cells grown as polarized monolayers on transwell filters. In HEK293-MATE1 cells, chloroquine competitively inhibited MATE1-mediated metformin uptake (Ki = 2.8 μM). Cellular accumulation of chloroquine was significantly lower (P < 0.001) and transcellular chloroquine transport was significantly increased (P < 0.001) in MDCK-MATE1 and MDCK-OCT2-MATE1 cells compared to vector control cells after basal addition of chloroquine (0.1 to 10 μM). In contrast, no difference in cellular accumulation or transcellular transport of chloroquine was observed between MDCK-OCT2 and vector control cells. In line with an oppositely directed proton gradient acting as a driving force for MATE1, basal-to-apical transport of chloroquine by MDCK-OCT2-MATE1 cells increased with decreasing apical pH from 7.8 to 6.0. Transcellular transport of chloroquine by MDCK-OCT2-MATE1 cells was inhibited by cimetidine, trimethoprim, and amitriptyline. Our data demonstrate that chloroquine is a substrate and potent competitive inhibitor of MATE1, whereas OCT2 seems to play no role in chloroquine uptake. Concomitantly administered MATE1 inhibitors are likely to modify the renal secretion of chloroquine.
INTRODUCTION
Chloroquine is considered an important antimalarial drug (44). Failure of chloroquine-based antimalarial therapy is still associated with a significant mortality rate and has been recognized as a major threat to public health (4). In recent years, significant progress has been made in our understanding of treatment failure related to resistance mechanisms of the parasites, such as active efflux of the drug from the digestive vacuole (23). In contrast, investigations of possible mechanisms of treatment failure related to the host (i.e., molecular mechanisms of possible drug interactions that affect the pharmacokinetics and tolerability of chloroquine in humans) remain limited.
Chloroquine is widely used and relatively well tolerated; however, a narrow therapeutic index and a considerable interindividual variability of plasma concentrations remain important clinical issues (1, 2, 10, 13, 32). Chloroquine is extensively distributed to diverse tissues with a high apparent volume of distribution (13). During long-term use, tissue accumulation of chloroquine due to cumulative overdose may cause adverse reactions such as retinopathy or cardiomyopathy (6, 22, 32). Thus, decreased systemic chloroquine elimination may increase the risk of adverse reactions.
After oral intake, a fraction of 40% to 70% of chloroquine is eliminated unchanged by the kidney (5, 13). Impairment of renal function increases the elimination half-life of chloroquine, and a dose reduction in the case of renal insufficiency may be necessary (33). The main mechanism of renal chloroquine elimination is tubular secretion; i.e., chloroquine is secreted by the epithelial cells surrounding the tubular lumen of the nephron into the urine. Accordingly, renal chloroquine elimination exceeds the glomerular filtration rate by severalfold (13, 31, 43). Chloroquine is a weak base, and acidification of urine increases the renal elimination of chloroquine (15). However, the molecular mechanisms of renal tubular chloroquine secretion remain unknown.
The dependence of renal chloroquine elimination on urine pH suggests an involvement of multidrug and toxin extrusion protein 1 (MATE1) in its tubular secretion. MATE1 is a proton-substrate antiporter expressed in both the kidney and liver (30). MATE1 is localized in the luminal membranes of renal tubules and the canalicular membranes of hepatocytes, where it mediates the export of cations into urine and bile, respectively (30). The transport direction depends on the direction of the transmembrane proton gradient: MATE1 may function as uptake or export transporter. Accordingly, its export function is increased by acidification of the extracellular environment, whereas extracellular alkalinization or intracellular acidification increases MATE1-mediated uptake of substrates (20, 24, 30, 37).
Several drugs that are transported by MATE1, for example, metformin or cimetidine, are also substrates of organic cation transporter 2 (OCT2), a membrane transporter that is localized in the basolateral membranes of proximal renal tubular cells (19, 26, 28, 37). It is assumed that the coordinate activity of OCT2-mediated uptake from the blood and export into the urine by MATE1 is responsible for renal tubular secretion of these substances (20, 38).
Chloroquine has already been described as an inhibitor of OCT2, albeit with a low inhibitory potency (46). Whether chloroquine is also a substrate of OCT2 is not yet known. As reported previously, we and others have established OCT2-MATE1 Madin-Darby canine kidney II (MDCK) cell lines stably expressing OCT2 and MATE1 in order to characterize the role of these cation transporters in the renal secretion of their substrates (20, 34). Using these double-transfected cells together with their respective control cell lines, we investigated whether renal secretion of the antimalarial drug chloroquine is mediated by the OCT2-MATE1 system.
MATERIALS AND METHODS
Materials.
Unlabeled metformin, chloroquine, cimetidine, levofloxacin, primaquine, amitriptyline, lamivudine, verapamil, and trimethoprim were obtained from Sigma-Aldrich (Taufkirchen, Germany). Unlabeled 1-methyl-4-phenylpyridinium (MPP+) was obtained from Biotrend (Cologne, Germany). [14C]metformin (0.03 Ci/mmol) and [3H]MPP+ (80 Ci/mmol) were purchased from American Radiolabeled Chemicals (St. Louis, MO), and [3H]chloroquine (5 Ci/mmol; 20 Ci/mmol) was obtained from Biotrend (Cologne, Germany). Poly-d-lysine hydrobromide (PDL) was from Sigma-Aldrich (Taufkirchen, Germany). Sodium butyrate was purchased from Merck KGaA (Darmstadt, Germany). G418 (Geneticin) disulfate and hygromycin were obtained from Invitrogen (Karlsruhe, Germany). All other chemicals, unless stated otherwise, were from Carl Roth GmbH & Co. KG (Karlsruhe, Germany) and were of the highest grade available.
Cell lines.
Human embryonic kidney 293 (HEK293) cells were transfected with the vector pMATE1.31 (20) using Lipofectamine (Invitrogen) according to the manufacturer's instructions. After Geneticin (800 μg/ml) selection, single colonies were characterized for SLC47A1 mRNA (encoding human MATE1) and MATE1 protein expression using quantitative reverse transcription-PCR (qRT-PCR) (LightCycler system; Roche) and immunoblot analysis. Vector-transfected HEK293 control cells were established by the same method using the respective expression plasmid without an insert for transfection. Single-transfected MDCK-OCT2 and MDCK-MATE1 cells as well as double-transfected MDCK-OCT2-MATE1 cells were previously established and characterized regarding protein expression, protein localization, and functionality in our laboratory (3, 20).
Cell culture.
Cells were cultured in minimum essential medium containing 10% heat-inactivated fetal bovine serum, 2 mM nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C and 5% CO2. For HEK293-MATE1 and MDCK-MATE1 cells 800 μg/ml Geneticin was added, for MDCK-OCT2 cells 250 μg/ml hygromycin was added, and for MDCK-OCT2-MATE1 cells 800 μg/ml Geneticin and 250 μg/ml hygromycin were added. Cells were routinely subcultured by trypsinization using a 0.05% trypsin–0.02% EDTA solution. All cell culture medium supplements were purchased from Invitrogen GmbH (Karlsruhe, Germany).
Transport studies.
The incubation medium for the transport experiments contained 142 mM NaCl, 5 mM KCl, 1 mM K2HPO4, 1.2 mM MgSO4, 1.5 mM CaCl2, 5 mM glucose, and 12.5 mM HEPES. The pH was adjusted by addition of NaOH or HCl. [14C]metformin, [3H]chloroquine, or [3H]MPP+ was dissolved in incubation medium, and unlabeled metformin (50 μM, 100 μM, 250 μM, or 1,000 μM), chloroquine (0.1 μM, 1 μM, 10 μM, or 100 μM), or MPP+ (50 μM) was added. Metformin, chloroquine, MPP+, primaquine, and amitriptyline were dissolved in water; cimetidine was dissolved in ethanol; verapamil was dissolved in methanol; and trimethoprim and levofloxacin were dissolved in dimethyl sulfoxide. Experiments with light-sensitive substances were performed under light protection.
Transport studies using HEK293-MATE1 cells.
Cells were seeded in PDL (0.1 mg/ml)-coated 12-well plates (Cellstar 12-well cell culture plate; Greiner Bio-One) at a density of 0.7 × 106 cells per well, grown to confluence for 2 days, and incubated with 10 mM sodium butyrate for 24 h to enhance protein expression (9). Before the uptake experiments were started, cells were washed with incubation medium (pH 7.8) at 37°C. Thereafter, cells were incubated for 10 min with incubation medium (pH 7.8, 500 μl per well) containing [14C]metformin. The incubation time of 10 min was chosen in analogy to previous important work (24). In order to characterize chloroquine and trimethoprim as inhibitors of MATE1-mediated [14C]metformin (100 μM) uptake, chloroquine or trimethoprim was added at increasing concentrations (chloroquine, 0.01 to 1,000 μM; trimethoprim, 0.01 to 250 μM). For determination of Ki value and type of inhibition, the effect of chloroquine (0.5 μM, 5 μM, and 50 μM) on uptake of [14C]metformin (50 μM, 250 μM, and 1,000 μM) was examined. The medium was immediately removed at the end of the incubation period, and cell monolayers were rapidly rinsed three times with ice-cold incubation medium (pH 7.3) before being lysed with 0.2% sodium dodecyl sulfate (SDS). The intracellular accumulation of radioactivity was detected using a liquid scintillation counter (Tricarb2800; Perkin-Elmer Life Sciences GmbH, Rodgau-Jügesheim, Germany), and protein concentrations in the cell lysates were measured by bicinchoninic acid assay (Pierce BCA protein assay kit; Thermo Scientific, Rockford, IL) for protein normalization of transport data.
Transport studies using MDCK-OCT2, MDCK-MATE1, and MDCK-OCT2-MATE1 cells.
To investigate chloroquine as a substrate of the OCT2-MATE1 system, we used MDCK-OCT2, MDCK-MATE1, and MDCK-OCT2-MATE1 cells grown as polarized epithelial monolayers on transwell filters. This experimental setup has been proposed as an appropriate in vitro model to evaluate the cooperative OCT2- and MATE1-mediated tubular transport of cationic compounds (20, 34, 40). The model allows quantification of intracellular chloroquine concentrations as well as of transcellular chloroquine transport (measured in the apical compartment) after addition of chloroquine to the basal compartment. Cellular accumulation and transcellular transport of [3H]chloroquine were measured as described previously (20). MDCK cells were seeded on porous membranes (ThinCert, 0.4-μm pore size; Greiner Bio-One, Frickenhausen, Germany) at a density of 0.5 × 106 cells per well. Cells were grown to confluence for 3 days, incubated with 10 mM sodium butyrate for 24 h, and subsequently used for transport experiments. Cell culture medium was removed from both sides of the monolayers, and cells were washed in incubation medium (pH 7.3) at 37°C. Subsequently, 1 ml incubation medium was added to the apical side of the monolayers, and experiments were started by addition of 1 ml incubation medium containing [3H]chloroquine (0.1 to 100 μM) to the basal side of the monolayers. As a positive control for the functionality of OCT2 in MDCK-OCT2 cells, [3H]MPP+ (50 μM) was added to the basal compartment, and cellular accumulation of MPP+ in MDCK-OCT2 and MDCK-vector control cells was measured as described previously (20). The pH of the medium on the basal side was 7.3; the pH in the apical compartment was 6.0 (unless indicated otherwise) to stimulate MATE1-mediated export as described previously (20). Various selected compounds which were previously characterized as inhibitors or substrates of OCT2 and/or MATE1 were added to the basal compartment in order to characterize them as inhibitors of transcellular transport of chloroquine (cimetidine, 0.1 to 300 μM; metformin, levofloxacin, lamivudine, primaquine, verapamil, amitriptyline, and trimethoprim, 10 μM or 100 μM). Primaquine was included as it is often coadministered with chloroquine in therapy. After incubation at 37°C in 5% CO2 for 30 min, 400-μl aliquots were taken from the apical compartment. The incubation medium was immediately removed at the end of the incubation period, and cell monolayers were rapidly rinsed three times with ice-cold incubation medium (pH 7.3). Filters were detached from the chambers, and cells were lysed with 0.2% SDS. The radioactivity of both the collected medium and solubilized cell monolayers was detected using a liquid scintillation counter (Tricarb2800; Perkin-Elmer Life Sciences GmbH, Rodgau-Jügesheim, Germany).
Data analysis.
Each concentration and each time point were investigated in two independent experiments with four to six separate wells in total. Data are presented as the mean ± standard deviation (SD) or with the respective 95% confidence interval (CI). Net transport was obtained by subtracting the transport in vector-transfected cells from that in OCT2- or MATE1-single-transfected or OCT2-MATE1-double-transfected cells. The percentage of uptake or transcellular transport inhibition was calculated from control experiments in the absence of inhibitor. The half-maximal inhibitory concentration (IC50) values for uptake or transcellular transport were calculated by fitting the data to a sigmoidal regression curve. For assessment of the type of inhibitory interaction of chloroquine with MATE1-mediated metformin uptake and for determination of the inhibition constant (Ki), the reciprocal velocity (1/V) of [14C]metformin uptake was plotted against the concentration of chloroquine according to the method of Dixon (9a). The uptake of [3H]MPP+ by MDCK-OCT2 and MDCK-vector control cells was analyzed with a two-tailed unpaired Student t test. Multiple comparisons were analyzed by one-way analysis of variance (ANOVA) followed by Tukey-Kramer's or Bonferroni's posttest. A value of P < 0.05 was required for statistical significance. The calculations were performed using GraphPad Prism (GraphPad Prism 5.01 for Windows, 2007; GraphPad Software, San Diego, CA).
RESULTS
Inhibition of MATE1-mediated metformin transport by chloroquine.
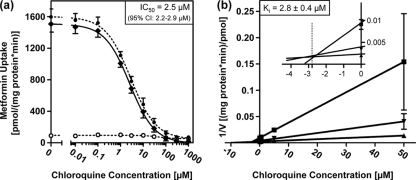
The effect of chloroquine on MATE1-mediated transport was investigated using the prototypic MATE1 substrate metformin in HEK293-MATE1 cells and HEK293-vector control cells (40). The extracellular pH was 7.8, and thus an outward gradient of H+ was established that stimulates the cellular uptake of typical cationic MATE1 substrates, such as metformin (24, 37). The addition of chloroquine caused a concentration-dependent inhibition of net metformin uptake (Fig. 1a). The IC50 was 2.5 μM (95% CI, 2.2 to 2.9 μM). To further investigate the type of inhibition, a Dixon plot analysis was performed (Fig. 1b). This analysis revealed a competitive inhibition of MATE1-mediated metformin uptake by chloroquine, with a Ki value of 2.8 ± 0.4 μM.
Fig. 1.
Inhibition of MATE1-mediated [14C]metformin transport (10 min) by chloroquine in HEK293 cells stably expressing MATE1 and in HEK293-vector control cells. Data are shown as the mean ± SD. (a) [14C]metformin (100 μM) uptake in the absence or presence of increasing concentrations of chloroquine. ○, uptake by HEK293-vector control cells; •, uptake by HEK293-MATE1 cells; ♦, net uptake, calculated by subtraction of uptake by HEK293-vector control cells from uptake by HEK293-MATE1 cells. (b) Dixon plot. [14C]metformin net transport at three different concentrations was determined in the absence or presence of increasing concentrations of chloroquine. Reciprocal velocity is plotted against the chloroquine concentration. The enlarged inset shows the intersection at −2.8 μM in the left upper quadrant. ▪, 50 μM [14C]metformin; ▾, 250 μM [14C]metformin; ▴, 1000 μM [14C]metformin.
Cellular accumulation and transcellular transport of chloroquine.
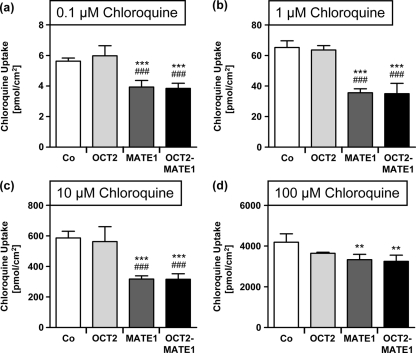
Next, the cellular accumulation and transcellular transport of chloroquine were analyzed in MDCK cells expressing the uptake transporter OCT2 or the export protein MATE1 or in double-transfected MDCK cells expressing OCT2 and MATE1 simultaneously. Chloroquine was added to the basal compartment. At all tested concentrations, cellular chloroquine accumulation was significantly decreased in MDCK-MATE1 and MDCK-OCT2-MATE1 cells compared to MDCK-vector control cells (Fig. 2) (P < 0.01). For concentrations of 0.1 to 10 μM, the cellular accumulation of chloroquine was also significantly decreased in MDCK-MATE1 and MDCK-OCT2-MATE1 cells compared to MDCK-OCT2 cells (Fig. 2) (P < 0.001). There was no significant difference between chloroquine concentrations in MDCK-OCT2 and MDCK-vector control cells. In addition, no significant difference in intracellular chloroquine concentrations was observed between MDCK-MATE1 and MDCK-OCT2-MATE1 cells.
Fig. 2.
[3H]chloroquine uptake by monolayers of MDCK-vector control (Co), MDCK-OCT2 (OCT2), MDCK-MATE1 (MATE1), and MDCK-OCT2-MATE1 (OCT2-MATE1) cells after 30 min. [3H]chloroquine was administered to the basal compartment at 0.1 μM (a), 1 μM (b), 10 μM (c), or 100 μM (d). Data are shown as the mean ± SD. Data were analyzed by one-way ANOVA followed by Tukey-Kramer's posttest. **, P < 0.01 versus MDCK-vector control; ***, P < 0.001 versus MDCK-vector control; ###, P < 0.001 versus MDCK-OCT2.
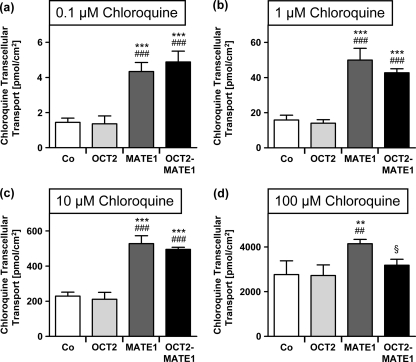
As shown in Fig. 3, MDCK-MATE1 and MDCK-OCT2-MATE1 cells showed significantly higher (P < 0.001) transcellular chloroquine transport than MDCK-OCT2 and MDCK-vector control cells when 0.1 to 10 μM chloroquine was added to the basal compartment. For 100 μM chloroquine, MDCK-MATE1 showed significantly higher (P < 0.05) transcellular transport than MDCK-OCT2-MATE1, MDCK-OCT2, and MDCK-vector control cells. No difference was observed between transcellular transport in MDCK-OCT2 cells and MDCK-vector control cells.
Fig. 3.
[3H]chloroquine transcellular transport from the basal to the apical compartment by monolayers of MDCK-vector control (Co), MDCK-OCT2 (OCT2), MDCK-MATE1 (MATE1), and MDCK-OCT2-MATE1 (OCT2-MATE1) cells after 30 min. [3H]chloroquine was administered to the basal compartment at 0.1 μM (a), 1 μM (b), 10 μM (c), or 100 μM (d). Data are shown as the mean ± SD. Data were analyzed by one-way ANOVA followed by Tukey-Kramer's posttest. **, P < 0.01 versus MDCK-vector control; ***, P < 0.001 versus MDCK-vector control; ##, P < 0.01 versus MDCK-OCT2; ###, P < 0.001 versus MDCK-OCT2; §, P < 0.05 versus MDCK-MATE1.
When MPP+ (50 μM) was added to the basal compartment as a positive control for the functionality of OCT2 in MDCK-OCT2 cells, cellular MPP+ accumulation in MDCK-OCT2 cells was significantly higher than that in MDCK-vector control cells (179 ± 23 pmol/cm2 versus 59 ± 12 pmol/cm2; P < 0.001).
pH dependence of transcellular transport of chloroquine.
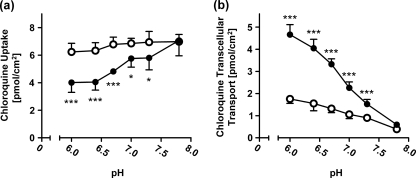
In order to mimic the situation in the kidney, we varied the pH in the apical compartment (corresponding to the renal tubular lumen) and applied a chloroquine concentration of 0.1 μM (which can be achieved in plasma during treatment with chloroquine [5]) on the basal side. As shown in Fig. 4, intracellular chloroquine concentrations increased and transcellular chloroquine transport decreased in MDCK-OCT2-MATE1 cells with increasing pH. In a range of pH 6.0 to 7.3, intracellular chloroquine concentrations were significantly lower (P < 0.05) and transcellular transport significantly higher (P < 0.001) in MDCK-OCT2-MATE1 cells than in MDCK-vector control cells. These differences between double-transfected cells and vector control cells were maximal at pH 6.0, continuously declined with increasing pH, and were abolished at pH 7.8.
Fig. 4.
pH dependence of intracellular [3H]chloroquine accumulation (a) and transcellular chloroquine transport from the basal to the apical compartment (b) by monolayers of MDCK-vector control (○) and MDCK-OCT2-MATE1 (•) cells. [3H]chloroquine (0.1 μM) was added to the basal compartment. The pH in the apical compartment was adjusted to 6.0, 6.4, 6.7, 7.0, 7.3, or 7.8. The pH in the basal compartment was 7.3 in all experiments. Data are shown as the mean ± SD. Data were analyzed by one-way ANOVA followed by Bonferroni's posttest (comparison of uptake or transcellular transport in OCT2-MATE1-double-transfected MDCK cells at a given apical pH with the respective control). *, P < 0.05; ***, P < 0.001.
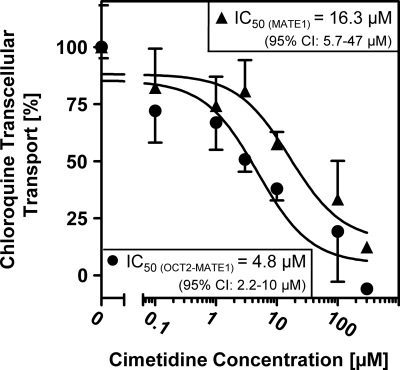
Inhibition of transcellular transport of chloroquine.
Cimetidine has been described as both a substrate and inhibitor of OCT2 and MATE1 (35, 37, 40, 46). The addition of cimetidine to the basal compartment caused a concentration-dependent inhibition of net transcellular chloroquine transport by MDCK-MATE1 and MDCK-OCT2-MATE1 cells (Fig. 5). IC50s were calculated to be 16.3 μM for MDCK-MATE1 cells (95% CI, 5.7 to 47 μM) and 4.8 μM for MDCK-OCT2-MATE1 cells (95% CI, 2.2 to 10 μM). Additionally, several substances were screened as inhibitors of net transcellular chloroquine transport in MDCK-OCT2-MATE1 cells (Table 1). No significant effect of metformin, levofloxacin, lamivudine, primaquine, or verapamil was observed. However, amitriptyline (100 μM, inhibition of 31.8% ± 7.1% [P < 0.01]) and trimethoprim (10 μM, inhibition of 44.2% ± 23%; 100 μM, inhibition of 87.5% ± 6.0% [P < 0.001]) caused significant inhibition of net transcellular chloroquine transport in MDCK-OCT2-MATE1 cells.
Fig. 5.
[3H]chloroquine transcellular net transport from the basal to the apical compartment by monolayers of MDCK-MATE1 (▴) and MDCK-OCT2-MATE1 (•) cells in the absence or presence of increasing concentrations of cimetidine. Net transport was calculated by subtraction of transport by MDCK-vector control cells from uptake by MDCK-MATE1 or MDCK-OCT2-MATE1 cells. [3H]chloroquine (0.1 μM) and cimetidine were administered to the basal compartment. Data are shown as the mean ± SD.
Table 1.
Inhibition of chloroquine (0.1 μM) net transcellular transport in double-transfected MDCK-OCT2-MATE1 cells by various compounds
| Compound | CAS registry no. | Concn (μM) | Net chloroquine transport (%)a |
|
|---|---|---|---|---|
| Mean | SD | |||
| None | 100 | 6.6 | ||
| Metformin | 1115-70-4 | 10 | 99.5 | 11 |
| 100 | 92.9 | 17 | ||
| Levofloxacin | 100986-85-4 | 10 | 82.3 | 20 |
| 100 | 83.5 | 18 | ||
| Lamivudine | 134678-17-4 | 10 | 90.0 | 9.0 |
| 100 | 94.5 | 9.2 | ||
| Primaquine | 63-45-6 | 10 | 88.5 | 9.7 |
| 100 | 74.6 | 21 | ||
| Verapamil | 23313-68-0 | 10 | 96.8 | 15 |
| 100 | 89.4 | 17 | ||
| Amitriptyline | 549-18-8 | 10 | 87.8 | 5.1 |
| 100 | 68.2** | 7.1 | ||
| Trimethoprim | 738-70-5 | 10 | 55.8*** | 23 |
| 100 | 12.5*** | 6.0 | ||
**, P < 0.01; ***, P < 0.001.
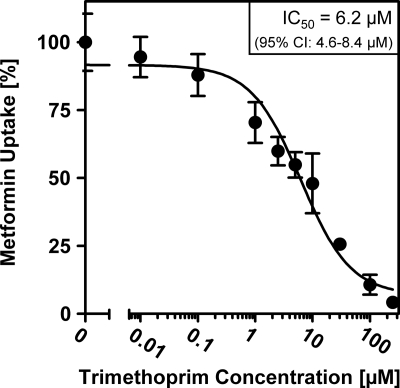
Inhibition of MATE1-mediated metformin transport by trimethoprim.
A screen test of drugs for their inhibitory interaction with MATE1, recently performed by Meyer zu Schwabedissen et al. (24), suggested that trimethoprim might be a strong inhibitor of MATE1. In our experiments, trimethoprim was identified as a potent inhibitor of the transcellular transport of chloroquine. In order to further characterize the inhibitory potency of trimethoprim for MATE1-mediated transport, we investigated trimethoprim as an inhibitor of the MATE1-mediated uptake of the model substrate metformin. We found that trimethoprim caused a concentration-dependent inhibition of net metformin uptake in HEK293-MATE1 cells, with an IC50 of 6.2 μM (95% CI, 4.6 to 8.4 μM) (Fig. 6).
Fig. 6.
Inhibition of MATE1-mediated [14C]metformin (100 μM) net uptake (10 min) by trimethoprim in HEK293 cells stably expressing MATE1. Net uptake was calculated by subtraction of uptake by HEK293-vector control cells from uptake by HEK293-MATE1 cells. Data are shown as the mean ± SD.
DISCUSSION
Our major finding is that MATE1, but not OCT2, is an important determinant of renal secretion of the antimalarial drug chloroquine. We also could demonstrate that MATE1 enhances transcellular transport of chloroquine in a pH-dependent manner. Finally, certain inhibitors which affect polarized chloroquine transport were identified.
An important issue in malaria therapy is that chloroquine plasma concentrations show considerable interindividual variability (1, 10). The underlying reasons are not entirely understood. Therapeutically recommended chloroquine plasma concentrations for the treatment of nonresistant malaria are in the range of approximately 300 to 600 nM (5). While lower exposure is associated with treatment failure, supratherapeutic concentrations increase the risk of adverse events (2, 29).
Chloroquine pharmacokinetics are determined by extensive tissue distribution and elimination via liver and kidney (5, 13). To an extent of 40% to 70%, chloroquine is eliminated by renal elimination of the unchanged substance, and a dose reduction in renal insufficiency may be necessary (5, 13, 33). Renal chloroquine clearance exceeds the glomerular filtration rate by severalfold, which is in agreement with tubular secretion being the principal mechanism of renal elimination (13, 31, 43). In the liver, chloroquine is N dealkylated into its main metabolite N-monodesethylchloroquine, which also shows antimalarial activity (12, 18).
Differences in the activity of drug transporters have been suspected to underlie the interindividual variability in chloroquine pharmacokinetics and therapeutic efficacy (17). However, the relevance of drug transporters of the host for chloroquine therapy is not clear (17), as recent research was instead focused on transporter-mediated mechanisms of resistance in the malaria parasite (23).
In our present work, we investigated chloroquine as a substrate of the uptake transporter OCT2 and the efflux transporter MATE1, which are localized in the basolateral and luminal membranes of proximal renal tubule cells, respectively (26, 30). In transcellular transport experiments, decreased intracellular and increased apical concentrations of chloroquine in MDCK cells overexpressing MATE1 reflect MATE1-mediated chloroquine export from the cells into the apical compartment. In contrast, overexpression of OCT2 did not enhance cellular uptake or transcellular transport of chloroquine, whereas MPP+ as a positive control showed increased accumulation in MDCK-OCT2 cells compared to MDCK-vector control cells. The lack of a significant contribution of OCT2 to cellular chloroquine uptake is in accordance with results recently published by Zheng et al., who found no effect of OCT2 inhibitors on the uptake of chloroquine by MDCK cells (45). Therefore, while OCT2 seems to play no role in the uptake of chloroquine, MATE1 mediates its cellular efflux.
Consequently, reduced function of MATE1 might impair renal clearance of chloroquine. A decrease in renal chloroquine clearance has been shown to increase the chloroquine elimination half-life (33), which may increase the risk of adverse events. Single-nucleotide polymorphisms causing a reduction of MATE1-mediated transport of MATE1 model substrates such as metformin or tetraethylammonium have been identified in vitro (8, 24). It is possible that functionally relevant genetic variants of the SLC47A1 gene (encoding MATE1) may impair systemic chloroquine clearance. However, the impact of such variants on chloroquine pharmacokinetics remains to be investigated.
We also studied the effect of pH modification on MATE1-mediated chloroquine efflux. In agreement with MATE1 function depending on the transmembrane proton gradient, we observed a clear dependence of transcellular chloroquine transport on the pH in the apical compartment. These in vitro results provide an explanation for in vivo data showing that acidification of urine increases renal elimination of chloroquine (15).
MATE1-mediated renal chloroquine secretion might be influenced not only by luminal pH or genetic variants but also by concomitantly administered substances that inhibit MATE1. Cimetidine has been identified as a substrate and an inhibitor of both OCT2 and MATE1 (35, 37, 40, 46). In our experiments, the basal addition of cimetidine inhibited transcellular chloroquine transport in both MDCK-MATE1-single-transfected and MDCK-OCT2-MATE1-double-transfected cells. The lower IC50 in double-transfected cells can be explained by higher intracellular cimetidine concentrations due to OCT2-mediated cimetidine uptake (40). In vivo, chloroquine elimination is inhibited by concomitantly administered cimetidine (11). So far, this has been attributed to the inhibition of hepatic metabolism of chloroquine by cimetidine, but our results indicate that inhibition of renal secretion might also contribute to this interaction.
To allow for the possibility that other inhibitors of MATE1 are also substrates of OCT2, we used cells overexpressing OCT2 in addition to MATE1 in order to achieve higher intracellular concentrations of the respective inhibitors. No significant inhibition of transcellular chloroquine transport was observed for metformin, levofloxacin, lamivudine, primaquine, and verapamil. However, amitriptyline (100 μM) caused significant inhibition of transcellular chloroquine transport. Amitriptyline is an inhibitor of OCT2, but an interaction with MATE1 has not been described so far (46). Our results suggest that amitriptyline inhibits MATE1-mediated transport.
Trimethoprim caused a strong inhibition of transcellular chloroquine transport. Trimethoprim has been reported to increase the plasma concentrations of several cations, such as lamivudine, zidovudine, or procainamide, in vivo by inhibiting renal tubular secretion (7, 21, 25, 36, 42). However, the mechanism of this interaction is not entirely clear. Inhibition of OCT2 by trimethoprim has been suggested, but the in vitro-estimated IC50s for this interaction vary in a range from 21 to 1,318 μM for different substrates (16, 41, 46). Here we demonstrate that trimethoprim inhibits MATE1-mediated metformin uptake with an IC50 of 6.2 μM. This is in line with previous results reported by Meyer zu Schwabedissen et al. (24), who reported a marked inhibition of MATE1-mediated uptake by 25 μM trimethoprim. Considering the low IC50 for inhibition of MATE1-mediated metformin transport, it is likely that an interaction with MATE1 is a major mechanism underlying the clinically observed inhibition of renal cation secretion by trimethoprim.
Using the model substrate metformin, we also characterized chloroquine as a potent inhibitor of MATE1-mediated transport. The Dixon plot analysis of the interaction of chloroquine with metformin demonstrated a competitive inhibition mechanism.
Renal tubular efflux mediated by MATE1 is assumed to be the final step in renal tubular secretion of drugs such as metformin or cimetidine (38). Inhibition or deletion of MATE1 decreases renal elimination of these drugs and increases intracellular drug concentrations in renal tubular cells (14, 39, 40). This may potentiate nephrotoxic effects of drugs such as cisplatin (27). Whether chloroquine affects the pharmacokinetics of concomitantly administered drugs by inhibition of MATE1 needs to be clarified. Therapeutically recommended plasma concentrations for the treatment of malaria would not be expected to affect MATE1-mediated metformin transport in vivo. However, it should be considered that MATE1 is localized in the renal luminal and hepatocellular canalicular membranes. With regard to inhibition of MATE1-mediated secretion of other drugs into the urine or into the bile, the concentration of unbound chloroquine in the cytosol may therefore be more relevant than the plasma concentration. To our knowledge, the concentration of unbound chloroquine achieved in the cytosol during therapy is not known. However, our experiments showing a significant transcellular transport in MATE1-overexpressing cells indicate that relevant free concentrations can be achieved.
In conclusion, we have identified chloroquine as a substrate and competitive inhibitor of MATE1. Our findings also provide an explanation for the known effects of urinary pH on renal chloroquine elimination. A decrease in MATE1 function due to genetic variants or concomitantly administered drugs that inhibit MATE1 is likely to affect renal chloroquine elimination and possibly influence adverse drug reactions or therapeutic effects of the antimalarial drug chloroquine.
ACKNOWLEDGMENTS
The authors declare no conflict of interest.
We thank Claudia Hoffmann for excellent technical assistance.
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Aderounmu A. F., Salako L. A., Lindström B., Walker O., Ekman L. 1986. Comparison of the pharmacokinetics of chloroquine after single intravenous and intramuscular administration in healthy Africans. Br. J. Clin. Pharmacol. 22:559–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. AlKadi H. O. 2007. Antimalarial drug toxicity: a review. Chemotherapy 53:385–391 [DOI] [PubMed] [Google Scholar]
- 3. Bachmakov I., et al. 2009. Interaction of beta-blockers with the renal uptake transporter OCT2. Diabetes Obes. Metab. 11:1080–1083 [DOI] [PubMed] [Google Scholar]
- 4. Baird J. K. 2005. Effectiveness of antimalarial drugs. N. Engl. J. Med. 352:1565–1577 [DOI] [PubMed] [Google Scholar]
- 5. Bayer Vital GmbH 2008. Resochin Tabletten 250 mg/ Resochin junior Tabletten 81 mg. Prescribing information. Bayer Vital GmbH, Leverkusen, Germany [Google Scholar]
- 6. Cervera A., Espinosa G., Font J., Ingelmo M. 2001. Cardiac toxicity secondary to long term treatment with chloroquine. Ann. Rheum. Dis. 60:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatton J. Y., et al. 1992. Trimethoprim, alone or in combination with sulphamethoxazole, decreases the renal excretion of zidovudine and its glucuronide. Br. J. Clin. Pharmacol. 34:551–554 [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y., et al. 2009. Genetic variants in multidrug and toxic compound extrusion-1, hMATE1, alter transport function. Pharmacogenomics J. 9:127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui Y., et al. 1999. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 55:929–937 [PubMed] [Google Scholar]
- 9a. Dixon M. 1953. The determination of enzyme inhibitor constants. Biochem. J. 55:170–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edwards G., et al. 1988. Pharmacokinetics of chloroquine in Thais: plasma and red-cell concentrations following an intravenous infusion to healthy subjects and patients with Plasmodium vivax malaria. Br. J. Clin. Pharmacol. 25:477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ette E. I., Brown-Awala E. A., Essien E. E. 1987. Chloroquine elimination in humans: effect of low-dose cimetidine. J. Clin. Pharmacol. 27:813–816 [DOI] [PubMed] [Google Scholar]
- 12. Fu S., et al. 1986. In vitro activity of chloroquine, the two enantiomers of chloroquine, desethylchloroquine and pyronaridine against Plasmodium falciparum. Br. J. Clin. Pharmacol. 22:93–96 [PMC free article] [PubMed] [Google Scholar]
- 13. Gustafsson L. L., et al. 1983. Disposition of chloroquine in man after single intravenous and oral doses. Br. J. Clin. Pharmacol. 15:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito S., et al. 2010. Potent and specific inhibition of mMate1-mediated efflux of type I organic cations in the liver and kidney by pyrimethamine. J. Pharmacol. Exp. Ther. 333:341–350 [DOI] [PubMed] [Google Scholar]
- 15. Jailer J. W., Rosenfeld M., Shannon J. A. 1947. The influence of orally administered alkali and acid on the renal excretion of quinacrine, chloroquine and santoquine. J. Clin. Invest. 26:1168–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jung N., et al. 2008. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab. Dispos. 36:1616–1623 [DOI] [PubMed] [Google Scholar]
- 17. Kerb R., et al. 2009. Pharmacogenetics of antimalarial drugs: effect on metabolism and transport. Lancet Infect. Dis. 9:760–774 [DOI] [PubMed] [Google Scholar]
- 18. Kim K. A., Park J. Y., Lee J. S., Lim S. 2003. Cytochrome P450 2C8 and CYP3A4/5 are involved in chloroquine metabolism in human liver microsomes. Arch. Pharm. Res. 26:631–637 [DOI] [PubMed] [Google Scholar]
- 19. Kimura N., et al. 2005. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab. Pharmacokinet. 20:379–386 [DOI] [PubMed] [Google Scholar]
- 20. König J., Zolk O., Singer K., Hoffmann C., Fromm M. F. 30 September 2010. Double-transfected MDCK cells expressing human OCT1/MATE1 or OCT2/MATE1: determinants of uptake and transcellular translocation of organic cations. Br. J. Pharmacol. [Epub ahead of print.] doi: 10.1111/j.1476-5381.2010.01052.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kosoglou T., Rocci M. L., Jr., Vlasses P. H. 1988. Trimethoprim alters the disposition of procainamide and N-acetylprocainamide. Clin. Pharmacol. Ther. 44:467–477 [DOI] [PubMed] [Google Scholar]
- 22. Leecharoen S., Wangkaew S., Louthrenoo W. 2007. Ocular side effects of chloroquine in patients with rheumatoid arthritis, systemic lupus erythematosus and scleroderma. J. Med. Assoc. Thai. 90:52–58 [PubMed] [Google Scholar]
- 23. Martin R. E., et al. 2009. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science 325:1680–1682 [DOI] [PubMed] [Google Scholar]
- 24. Meyer zu Schwabedissen H. E., Verstuyft C., Kroemer H. K., Becquemont L., Kim R. B. 2010. Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. Am. J. Physiol. Renal Physiol. 298:F997–F1005 [DOI] [PubMed] [Google Scholar]
- 25. Moore K. H., et al. 1996. Pharmacokinetics of lamivudine administered alone and with trimethoprim-sulfamethoxazole. Clin. Pharmacol. Ther. 59:550–558 [DOI] [PubMed] [Google Scholar]
- 26. Motohashi H., et al. 2002. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 13:866–874 [DOI] [PubMed] [Google Scholar]
- 27. Nakamura T., Yonezawa A., Hashimoto S., Katsura T., Inui K. 2010. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem. Pharmacol. 80:1762–1767 [DOI] [PubMed] [Google Scholar]
- 28. Nies A. T., Herrmann E., Brom M., Keppler D. 2008. Vectorial transport of the plant alkaloid berberine by double-transfected cells expressing the human organic cation transporter 1 (OCT1, SLC22A1) and the efflux pump MDR1 P-glycoprotein (ABCB1). Naunyn Schmiedebergs Arch. Pharmacol. 376:449–461 [DOI] [PubMed] [Google Scholar]
- 29. Obua C., et al. 2008. Population pharmacokinetics of chloroquine and sulfadoxine and treatment response in children with malaria: suggestions for an improved dose regimen. Br. J. Clin. Pharmacol. 65:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Otsuka M., et al. 2005. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. U. S. A. 102:17923–17928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rengelshausen J., et al. 2004. Pharmacokinetic interaction of chloroquine and methylene blue combination against malaria. Eur. J. Clin. Pharmacol. 60:709–715 [DOI] [PubMed] [Google Scholar]
- 32. Ruiz-Irastorza G., Ramos-Casals M., Brito-Zeron P., Khamashta M. A. 2010. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann. Rheum. Dis. 69:20–28 [DOI] [PubMed] [Google Scholar]
- 33. Salako L. A., Walker O., Iyun A. O. 1984. Pharmacokinetics of chloroquine in renal insufficiency. Afr. J. Med. Med. Sci. 13:177–182 [PubMed] [Google Scholar]
- 34. Sato T., et al. 2008. Transcellular transport of organic cations in double-transfected MDCK cells expressing human organic cation transporters hOCT1/hMATE1 and hOCT2/hMATE1. Biochem. Pharmacol. 76:894–903 [DOI] [PubMed] [Google Scholar]
- 35. Tahara H., et al. 2005. A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J. Pharmacol. Exp. Ther. 315:337–345 [DOI] [PubMed] [Google Scholar]
- 36. Takubo T., Kato T., Kinami J., Hanada K., Ogata H. 2000. Effect of trimethoprim on the renal clearance of lamivudine in rats. J. Pharm. Pharmacol. 52:315–320 [DOI] [PubMed] [Google Scholar]
- 37. Tanihara Y., et al. 2007. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters. Biochem. Pharmacol. 74:359–371 [DOI] [PubMed] [Google Scholar]
- 38. Terada T., Inui K. 2008. Physiological and pharmacokinetic roles of H+/organic cation antiporters (MATE/SLC47A). Biochem. Pharmacol. 75:1689–1696 [DOI] [PubMed] [Google Scholar]
- 39. Tsuda M., et al. 2009. Targeted disruption of the multidrug and toxin extrusion 1 (mate1) gene in mice reduces renal secretion of metformin. Mol. Pharmacol. 75:1280–1286 [DOI] [PubMed] [Google Scholar]
- 40. Tsuda M., et al. 2009. Involvement of human multidrug and toxin extrusion 1 in the drug interaction between cimetidine and metformin in renal epithelial cells. J. Pharmacol. Exp. Ther. 329:185–191 [DOI] [PubMed] [Google Scholar]
- 41. Urakami Y., Kimura N., Okuda M., Inui K. 2004. Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm. Res. 21:976–981 [DOI] [PubMed] [Google Scholar]
- 42. Vlasses P. H., et al. 1989. Trimethoprim inhibition of the renal clearance of procainamide and N-acetylprocainamide. Arch. Intern. Med. 149:1350–1353 [PubMed] [Google Scholar]
- 43. Walker O., Salako L. A., Alván G., Ericsson Ö, Sjöqvist F. 1987. The disposition of chloroquine in healthy Nigerians after single intravenous and oral doses. Br. J. Clin. Pharmacol. 23:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. WHO 2010. Guidelines for the treatment of malaria, 2nd edition WHO Press, World Health Organization, Geneva, Switzerland [Google Scholar]
- 45. Zheng N., Zhang X., Rosania G. R. 14 December 2010. The effect of phospholipidosis on the cellular pharmacokinetics of chloroquine. J. Pharmacol. Exp. Ther. [Epub ahead of print.] doi: 10.1124/jpet.110.175679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zolk O., Solbach T. F., König J., Fromm M. F. 2009. Structural determinants of inhibitor interaction with the human organic cation transporter OCT2 (SLC22A2). Naunyn Schmiedebergs Arch. Pharmacol. 379:337–348 [DOI] [PubMed] [Google Scholar]