Abstract
Increases in community-acquired infections caused by extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae have important implications for hospital infection control and empirical antibiotic therapy protocols. We developed and validated a tool for identifying patients harboring these organisms at hospital admission. We retrospectively analyzed chart data for 849 adult inpatients. The derivation cohort included 339 patients admitted to a large hospital in Rome during 2008, with (n = 113) or without (n = 226) culture positivity for ESBL-producing Escherichia coli, Klebsiella spp., or Proteus mirabilis within 48 h after admission. Logistic-regression-based prediction scores were calculated based on variables independently associated with the outcome. The model was validated in a second cohort (n = 510) selected with identical criteria in hospitals in Genoa and Turin during 2009. Prediction scores were based on the following six variables (reported with odds ratio for study outcome and the 95% confidence intervals in brackets): recent (≤12 months before admission) hospitalization (5.69 [2.94 to 10.99]), transfer from another health care facility (5.61 [1.65 to 19.08]), Charlson comorbidity score ≥ 4 (3.80 [1.90 to 7.59]), recent (≤3 months before admission) β-lactam and/or fluoroquinolone treatment (3.68 [1.96 to 6.91]), recent urinary catheterization (3.52 [1.96 to 6.91]), and age ≥ 70 years (3.20 [1.79 to 5.70]). The model displayed good calibration and good-to-excellent discrimination in the derivation and validation sets (Hosmer-Lemshow χ2 = 15.28 and 14.07; P = 0.17 and 0.23; areas under the receiver-operating characteristic curve, 0.83 and 0.92). It reliably identified patients likely to be harboring ESBL-producing Enterobacteriaceae at hospital admission who may need special infection control measures. Further study is needed to confirm this model's potential as a guide for prescribing empirical antibiotic therapy.
INTRODUCTION
In the last 2 decades, intensive use of broad-spectrum cephalosporins has led to the emergence of antibiotic-resistant strains of Enterobacteriaceae (predominantly Klebsiella pneumoniae and Escherichia coli) that produce extended-spectrum β-lactamases (ESBLs) (8, 12, 20, 21, 27). These strains are widespread throughout the world, but the prevalence and phenotypic characteristics of clinical isolates varies from area to area (7, 9).
Several studies suggest that infections caused by ESBL-producing bacteria have an important clinical impact, and the increasing prevalence of these organisms in hospitals has been well documented (9, 12, 34). In addition, they have recently been reported to cause urinary tract and bloodstream infections in nonhospitalized patients (1–3, 10, 14, 16, 18, 23, 24). An unrecognized influx of community-acquired ESBL-producing organisms into hospital settings could have important consequences. For one thing, patients admitted with these infections require special monitoring and infection control measures to prevent the spread of these organisms within the healthcare facility. Furthermore, there is obviously a substantial risk that the infecting pathogen will be resistant to empirically prescribed antimicrobial protocols normally used for community-acquired infections, which often include oxyimino cephalosporins. Many ESBL-producing organisms contain plasmids (sometimes the same ones encoding ESBL production) that confer resistance to other antimicrobial agents as well. In these cases, carbapenems are often the only drugs that are effective (9, 31–33). Failure to provide adequate treatment in the initial stages of bloodstream infections caused by ESBL-producing Enterobacteriaceae has already been linked to a markedly increased risk of mortality (26, 31). Our aim was to develop and validate a reliable, easy-to-use clinical prediction rule that could be used at hospital admission to identify patients likely to harbor these organisms.
MATERIALS AND METHODS
Setting and study design.
To identify risk factors for isolation of ESBL-producing Enterobacteriaceae (i.e., E. coli, Klebsiella spp., or Proteus mirabilis) (ESBL-EKP) from clinical samples shortly after hospital admission, we conducted a case-control study. Patient cohorts were identified via databases maintained by the microbiology laboratories in three large, full-service teaching hospitals in Italy, each with a yearly admission rate of about 50,000 patients: the Catholic University Hospital, a 1,600-bed hospital located in Rome; San Martino University Hospital, a 1,500-bed hospital in Genoa; and the San Giovanni Battista-Molinette Hospital, a 1,200-bed facility located in Turin.
The derivation cohort consisted of adult inpatients admitted to the Catholic University Hospital between 1 January and 31 December 2008. The case group comprised those whose records showed at least one isolation of an ESBL-EKP from samples collected within 48 h of hospital admission. Rectal swab screening was not routinely performed in any of the hospitals included in the study. Therefore, the study focused on the isolation of ESBL-EKP from clinical culture samples. If more than one isolation was reported for the same patient, only the first was included in the study (index culture). Patients admitted with a known history of ESBL-EKP infection were excluded. For each case identified, we included two controls (matched for hospital ward and month of admission) with no reports of culture positivity for Enterobacteriaceae during their hospitalization. These individuals were randomly selected from lists of patients admitted to the hospital during the study period.
The validation cohort (cases and controls) consisted of hospitalized individuals that were prospectively enrolled in San Martino University Hospital or San Giovanni Battista-Molinette Hospital between 1 June and 31 December 2009. The inclusion and exclusion criteria were identical to those used for the derivation cohort, with the exception that four control subjects were chosen for each case patient.
Variables analyzed.
Data were collected from patients' medical records and computerized hospital databases. For consistency's sake, the variables recorded for each cohort were defined in accordance with recent publications in this field (31–34). These variables included (i) patient demographics, (ii) source of admission (in particular, whether or not the patient had been transferred from another healthcare facility [acute care, long-term care, or nursing home]), (iii) underlying diseases and comorbidities present on admission (including solid tumors, hematological malignancies, liver disease, chronic renal failure, diabetes mellitus, chronic obstructive pulmonary disease, heart failure, cerebrovascular disease, solid organ transplantation, and AIDS), and (iv) recent medical history, including hospitalization for >2 days during the 12 months preceding admission, surgery or invasive procedures within the 30 days preceding admission (including the insertion of central venous catheters [CVCs], a nasogastric tube, or Foley catheters or endoscopy), immunosuppressive and/or corticosteroid therapy within the 3 months before admission, and antimicrobial therapy lasting >48 h during the 3 months preceding admission. For risk-factor analysis, the latter variable was dichotomized (any therapy versus no therapy). The impact of comorbidities was determined based on the Charlson comorbidity index (5).
Microbiological analyses.
Isolates were identified at the species level with the Vitek 2 (bioMérieux, Inc., Hazelwood, MO) and/or the Phoenix (Becton Dickinson Microbiology Systems) systems. ESBL production was detected by broth microdilution method according to the Clinical and Laboratory Standards Institute guidelines (6). K. pneumoniae ATCC 700603 and E. coli ATCC 25922 were used as positive and negative controls, respectively.
Statistical analysis.
Continuous variables were compared by using the Student t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. Categorical variables were evaluated with the χ2 or two-tailed Fisher exact test. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of any association that emerged. Values are expressed as means ± the standard deviations (SD) (continuous variables) or as percentages of the group from which they were derived (categorical variables). Two-tailed tests were used to determine statistical significance; a P value of <0.05 was considered significant.
Variables associated with ESBL-EKP isolation in the univariate analysis (P ≤ 0.10) were included in a logistic regression model, and a backward stepwise approach was used to identify independent predictors of ESBL-EKP isolation. Variables were retained in the final model if the P value was ≤0.05. The final regression model was transformed into a point-based rule. The weighted scores assigned to each variable were obtained by dividing each regression coefficient by half of the smallest coefficient and rounding to the nearest integer (28). The discriminatory power of the prediction rule in the derivation group was expressed as the area under the receiver-operator characteristic curve (ROC AUC). An AUC of 0.5 indicates no discriminative ability, and perfect discrimination (i.e., a test with 100% sensitivity and 100% specificity) is reflected by an AUC of 1. An AUC exceeding 0.8 is usually indicative of good to excellent prediction; those in the 0.7 to 0.8 and 0.6 to 0.7 ranges reflect moderate and low predictive power, respectively. The sensitivity and specificity of the prediction rule—each with 95% CIs—were calculated at different cutoff values. Positive and negative predictive values (PPV and NPV, respectively) were obtained with standard methods. Calibration was assessed by using the Hosmer-Lemeshow test for goodness of fit, which evaluates expected and observed probabilities in population deciles. The same methods were used to assess model discrimination and calibration in the validation cohort.
All statistical analyses were performed using the Intercooled Stata program, version 10, for Windows (Stata Corp., College Station, TX).
RESULTS
Derivation cohort.
A total of 115 patients with culture positivity for ESBL-EKP met the inclusion criteria for the derivation set study. Two were excluded because of missing data, leaving a total of 113 cases for analysis. Two control subjects were enrolled for each case, bringing the total number of patients in the derivation cohort to 339. The ESBL-EKP isolates (E. coli [n = 77, 68.1%], K. pneumoniae [n = 19, 16.8%], and P. mirabilis [n = 17, 15.1%]) were mainly recovered from urine specimens (n = 72, 63.7%). Blood (n = 19, 16.8%), skin and soft tissue (n = 14, 12.4%), respiratory tract (n = 8, 7.1%), biliary tract (n = 4, 3.5%), and surgical wound (n = 2, 1.8%) specimens accounted for the remaining isolates. In six cases the same microorganism was isolated simultaneously from two different sites.
The mean (± the SD) age of the patients in the case group was 65.9 ± 20.3 years, and more than half were older than 70 years (59/113, 52.2%). Sixty (53.1%) were women. The proportion of cases hospitalized on medical wards (80/113, 70.8%) was significantly higher than that for surgical wards (28/113, 24.7%) or intensive care units (5/113, 4.4%). Table 1 summarizes the main clinical and demographic characteristics of case patients included in the derivation cohort.
Table 1.
Comparison of characteristics of case patients in the derivation and validation groups
| Characteristicsa | No. (%) of patients |
P | |
|---|---|---|---|
| Derivation set (n = 113) | Validation set (n = 102) | ||
| Microorganism isolated | |||
| Escherichia coli | 77 (68.1) | 89 (87.2) | <0.001 |
| Klebsiella spp. | 19 (16.8) | 10 (9.8) | 0.13 |
| Proteus mirabilis | 17 (15.1) | 5 (4.9) | 0.01 |
| Isolate source | |||
| Blood | 19 (16.8) | 27 (26.5) | 0.08 |
| Urinary tract | 72 (63.7) | 54 (52.9) | 0.11 |
| Lower respiratory tract | 8 (7.1) | 11 (10.8) | 0.33 |
| Surgical wound | 2 (1.8) | 5 (4.9) | 0.19 |
| Skin and soft tissues | 14 (12.4) | 13 (12.8) | 0.94 |
| Biliary tract | 4 (3.5) | 6 (5.9) | 0.42 |
| Patient characteristics | |||
| Male patients | 53 (46.9) | 55 (53.9) | 0.30 |
| Patients >70 years old | 59 (52.2) | 66 (64.7) | 0.06 |
| Ward | |||
| Medicine | 80 (70.8) | 79 (77.5) | 0.27 |
| Surgery | 28 (24.8) | 21 (20.6) | 0.46 |
| Intensive care units | 5 (4.4) | 2 (1.9) | 0.31 |
| Comorbidities | |||
| Solid tumor | 29 (25.7) | 34 (33.3) | 0.22 |
| Hematological malignancy | 8 (7.1) | 8 (7.8) | 0.83 |
| Liver disease | 17 (15.0) | 13 (12.8) | 0.63 |
| Chronic renal failure | 23 (20.4) | 23 (22.6) | 0.69 |
| Diabetes mellitus | 29 (25.7) | 23 (22.6) | 0.59 |
| Chronic obstructive pulmonary disease | 20 (17.7) | 16 (15.7) | 0.69 |
| Heart failure | 56 (49.6) | 52 (50.9) | 0.83 |
| Cerebrovascular disease | 33 (29.2) | 30 (29.4) | 0.97 |
| Solid organ transplantation | 7 (6.2) | 2 (1.9) | 0.12 |
| AIDS | 4 (3.5) | 1 (0.9) | 0.21 |
| Charlson comorbidity index ≥ 4 | 37 (32.7) | 60 (58.8) | <0.001 |
| History | |||
| Recent hospitalization* | 92 (81.4) | 78 (76.5) | 0.37 |
| Admission from another healthcare facility | 14 (12.4) | 30 (29.4) | 0.002 |
| Recent bacterial infections† | 62 (54.9) | 36 (35.3) | 0.004 |
| Dialysis | 2 (1.7) | 3 (2.9) | 0.56 |
| Surgical procedures‡ | 30 (26.6) | 29 (28.4) | 0.76 |
| Central venous catheter‡ | 12 (10.6) | 22 (21.6) | 0.03 |
| Urinary catheterization‡ | 44 (38.9) | 53 (51.9) | 0.06 |
| Surgical drainage tube(s)‡ | 12 (10.6) | 6 (5.9) | 0.21 |
| Nasogastric tube‡ | 5 (4.4) | 6 (5.9) | 0.63 |
| Total parenteral nutrition‡ | 10 (8.9) | 18 (17.7) | 0.06 |
| Endoscopyb | 15 (13.3) | 9 (8.8) | 0.30 |
| Immunosuppressive therapy† | 8 (7.1) | 5 (4.9) | 0.50 |
| Corticosteroid therapy† | 24 (21.2) | 24 (23.5) | 0.69 |
| Radiotherapy† | 5 (4.4) | 3 (2.9) | 0.57 |
| Chemotherapy† | 13 (11.5) | 14 (13.7) | 0.62 |
| Recent antibiotic therapy† | |||
| In general (any drug) | 69 (61.1) | 56 (54.9) | 0.36 |
| By drug class | |||
| Aminoglycosides | 6 (5.3) | 6 (5.9) | 0.92 |
| β-Lactam-β-lactamase inhibitor | 8 (7.1) | 17 (16.7) | 0.04 |
| Fluoroquinolones | 24 (21.2) | 31 (30.4) | 0.18 |
| Oxyimino cephalosporins | 27 (23.9) | 13 (12.8) | 0.02 |
| Carbapenems | 9 (7.9) | 6 (5.9) | 0.49 |
| Others | 9 (7.9) | 14 (13.7) | 0.21 |
*, During the 12 months preceding index hospitalization; †, during the 3 months preceding index blood culture; ‡, during the 30 days preceding index blood culture.
This category includes esophagogastroduodenoscopy, colonoscopy, and endoscopic retrograde cholangiopancreatography, during the 30 days preceding index blood culture.
In univariate analysis, ESBL-EKP culture positivity within 48 h of hospital admission was significantly associated with age >70 years (P < 0.001), previous hospitalization (P < 0.001), and transfer from another healthcare facility (P < 0.001). Compared to controls, the case group had higher rates of diabetes (P = 0.01), chronic pulmonary obstructive diseases (P < 0.001), cerebrovascular disorders (P < 0.001), renal failure (P = 0.001), and Charlson comorbidity scores ≥ 4 (P < 0.001). Patients in this group were also more likely to have a recent history of urinary catheterization (P < 0.001) and of the following: steroid therapy (P < 0.001), antibiotic therapy (any drug) (P < 0.001), antibiotic therapy with β-lactams and/or fluoroquinolones (P < 0.001), immunosuppressive therapy (P = 0.02), or radiotherapy (P = 0.07).
In logistic regression analysis, six variables were independently associated with isolation of ESBL-EKP within 48 h of hospital admission: previous hospitalization (P < 0.001), admission from another healthcare facility (P = 0.006), Charlson comorbidity score ≥ 4 (P < 0.001), previous therapy with β-lactams and/or fluoroquinolones (P < 0.001), recent history of urinary catheterization (P < 0.001), and age ≥70 years (P < 0.001) (Table 2).
Table 2.
Multivariate logistic regression analysis of risk factors for ESBL-producing Enterobacteriaceae isolation within 48 h of hospital admission in the derivation set, with corresponding point values
| Parameter | Regression coefficient | P | OR (95% CI) | Score |
|---|---|---|---|---|
| Recent hospitalizationa | 1.73 | <0.001 | 5.69 (2.94–10.99) | 3 |
| Admission from another healthcare facility | 1.72 | 0.006 | 5.61 (1.65–19.08) | 3 |
| Charlson comorbidity index ≥ 4 | 1.33 | <0.001 | 3.80 (1.90–7.59) | 2 |
| Previous therapy with β-lactams and/or fluoroquinolonesb | 1.30 | <0.001 | 3.68 (1.96–6.91) | 2 |
| History of urinary catheterizationc | 1.25 | <0.001 | 3.52 (1.96–6.91) | 2 |
| Age ≥70 years | 1.16 | <0.001 | 3.20 (1.79–5.70) | 2 |
During the 12 months preceding index hospitalization.
Includes treatment with β-lactam/β-lactamase inhibitor combinations, oxyiminocephlosporins, and/or fluoroquinolones during the 3 months preceding index admission.
During the 30 days preceding index blood culture.
Validation cohort.
From June through December 2009 in the two hospitals involved in the validation study, ESBL-EKPs were isolated within 48 h of hospitalization in 102 patients. For each case, four control patients were included, bringing the number of patients in the validation cohort to 510. Their baseline characteristics are summarized in Table 1. Compared to the derivation cohort, the validation cohort contained a higher percentage of E. coli (87.2% versus 68.1% in the derivation cohort, P < 0.001). The two cohorts were similar in terms of isolate sources, but significant differences were noted in mean Charlson comorbidity scores (P < 0.001), admission rates from other health care institutions (P = 0.002), and recent histories of the bacterial infections (P = 0.004), CVCs (P = 0.03), or treatment with β-lactam-β-lactamase inhibitors (P = 0.04) and/or oxyimino-cephalosporins (P = 0.02) (Table 1).
Construction and validation of the predictive scoring system. (i) Derivation set.
A weighted score was assigned to each risk factor found to be independently associated with isolation of ESBL-EKP within 48 h of hospital admission in the derivation set, as follows: previous hospitalization, 3 points; admission from another healthcare facility, 3 points; previous antibiotic therapy with β-lactams and/or fluoroquinolones, 2 points; Charlson comorbidity score ≥ 4, 2 points; age ≥70 years, 2 points; and recent history of urinary catheterization, 2 points (Table 2). The individual scores were added together to produce an overall score ranging from 0 to 14 points.
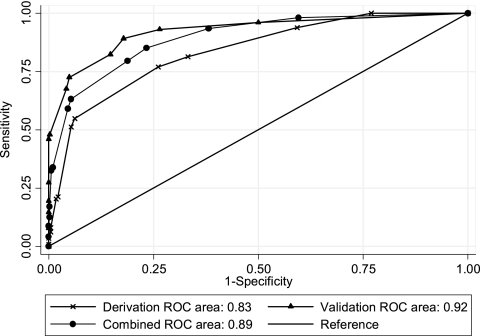
The distribution of overall scores among the cases and controls of the derivation cohort is summarized in Table 3. Scores of 0 were found exclusively among controls, as were the vast majority (85.1%) of scores of 2. The ROC AUC for these data was 0.83 (95% CI = 0.79 to 0.88), indicating that the model is an excellent predictor of ESBL-EKP isolation within the first 48 h of hospitalization (Fig. 1). The results of Hosmer-Lemshow chi-squared testing (χ2 = 15.28; P = 0.17) were indicative of good calibration.
Table 3.
Distribution of scores in the derivation and validation sets
| Points | No. (%) of patients | |||||
|---|---|---|---|---|---|---|
| Derivation set | Validation set | |||||
| Cases | Controls | Total | Cases | Controls | Total | |
| 0 | 0 | 52 (100) | 52 | 4 (1.9) | 204 (98.1) | 208 |
| 2 | 7 (14.9) | 40 (85.1) | 47 | 3 (3.1) | 96 (96.9) | 99 |
| 3 | 14 (19.2) | 59 (80.8) | 73 | 4 (10.3) | 35 (89.7) | 39 |
| 4 | 5 (23.8) | 16 (76.2) | 21 | 7 (35) | 13 (65) | 20 |
| 5 | 25 (35.7) | 45 (64.3) | 70 | 10 (20) | 40 (80) | 50 |
| 6 | 4 (66.7) | 2 (33.3) | 6 | 5 (62.5) | 3 (37.5) | 8 |
| 7 | 34 (82.9) | 7 (17.1) | 41 | 20 (55.6) | 16 (44.4) | 36 |
| 8 | 1 (50) | 1 (50) | 2 | 2 (66.7) | 1 (33.3) | 3 |
| 9 | 14 (82.4) | 3 (17.7) | 17 | 19 (100) | 0 | 19 |
| 10 | 2 (100) | 0 | 2 | 8 (100) | 0 | 8 |
| 11 | 3 (75) | 1 (25) | 4 | 5 (100) | 0 | 5 |
| 12 | 3 (100) | 0 | 3 | 7 (100) | 0 | 7 |
| 14 | 1 (100) | 0 | 1 | 8 (100) | 0 | 8 |
| Total | 113 (33.3) | 226 (66.7) | 339 | 102 (20) | 408 (80) | 510 |
Fig. 1.
Receiver-operator characteristic curves (ROC AUC) for the scoring system in the derivation set, validation set, and combined populations.
Table 4 shows the prediction rules derived from this scoring system. Diagnostic performance parameters are reported for different cutoffs.
Table 4.
Model and risk score performance: derivation set (n = 339)
| Score | Model and risk score performancea |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | Se | Sp | PPV | NPV | Acc | |
| ≥2 | 113 | 174 | 52 | 0 | 100 | 23 | 39 | 100 | 49 |
| ≥3 | 106 | 134 | 92 | 7 | 94 | 41 | 44 | 93 | 58 |
| ≥4 | 92 | 75 | 151 | 21 | 81 | 67 | 55 | 88 | 72 |
| ≥5 | 87 | 59 | 167 | 26 | 77 | 74 | 60 | 87 | 75 |
| ≥6 | 62 | 14 | 212 | 51 | 55 | 94 | 82 | 81 | 81 |
| ≥7 | 58 | 12 | 214 | 55 | 51 | 95 | 83 | 80 | 80 |
| ≥8 | 24 | 5 | 221 | 89 | 21 | 98 | 83 | 71 | 72 |
| ≥9 | 22 | 4 | 222 | 91 | 19 | 98 | 85 | 71 | 72 |
| ≥10 | 9 | 1 | 225 | 104 | 8 | 100 | 90 | 68 | 69 |
| ≥11 | 7 | 1 | 225 | 106 | 6 | 100 | 88 | 68 | 68 |
| ≥12 | 4 | 0 | 226 | 109 | 4 | 100 | 100 | 67 | 68 |
TP, number of true positives; FP, number of false positives; FN, number of false negatives; TN, number of true negatives; Se, % sensitivity; Sp, % specificity; PPV, % positive predictive value; NPV, % negative predictive value; Acc, rate of accuracy (%) of the risk score model.
When high risk was defined as an overall score of ≥ 3, the scoring system had excellent sensitivity (94%), low specificity (41%), and an PPV and NPV of 44 and 93%, respectively, and an overall accuracy of 58%. Scores of ≥3 points were associated with an OR for early ESBL-EKP isolation of 10.39 (95% CI = 4.55 to 27.54, P < 0.001). When the cutoff was raised to 6, the sensitivity dropped (55%) and the specificity increased appreciably (94%). This cutoff level was associated with an PPV and NPV of 82 and 81%, an overall accuracy of 81%, and an OR for ESBL-EKP isolation of 18.40 (95% CI = 9.21 to 38.10, P < 0.001).
(ii) Validation set.
As shown in Table 3 and Fig. 1, when the prediction rule was applied in the validation cohort, the model once again exhibited excellent predictive power (ROC AUC = 0.92; 95% CI = 0.89 to 0.95), as well as good calibration (Hosmer-Lemeshow χ2 = 14.07; P = 0.23).
The prediction rules derived from the scoring system in the validation set are listed in Table 5 with diagnostic performance parameters for the main cutoffs. The ORs for ESBL-EKP isolation within 48 h of hospital admission were even higher than those observed in the derivation cohort: 37.69 (95% CI = 16.76 to 98.55, P < 0.001) for scores ≥ 3 and 51.27 (95% CI = 26.30 to 100.90, P < 0.001) for scores ≥ 6. The lower cutoff displayed excellent sensitivity but lost specificity; use of the higher cutoff markedly increased specificity and diminished sensitivity to some extent, but the overall accuracy was better than that associated with a cutoff of 3 (91% versus 77%).
Table 5.
Model and risk score performance: validation set (n = 510)
| Score | Model and risk score performancea | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | Se | Sp | PPV | NPV | Acc | |
| ≥2 | 98 | 204 | 204 | 4 | 96 | 50 | 32 | 98 | 59 |
| ≥3 | 95 | 108 | 300 | 7 | 93 | 74 | 47 | 98 | 77 |
| ≥4 | 91 | 73 | 335 | 11 | 89 | 82 | 55 | 97 | 84 |
| ≥5 | 84 | 60 | 348 | 18 | 82 | 85 | 58 | 95 | 85 |
| ≥6 | 74 | 20 | 388 | 28 | 73 | 95 | 79 | 93 | 91 |
| ≥7 | 69 | 17 | 391 | 33 | 68 | 96 | 80 | 92 | 90 |
| ≥8 | 49 | 1 | 407 | 53 | 48 | 100 | 98 | 88 | 89 |
| ≥9 | 47 | 0 | 408 | 55 | 46 | 100 | 100 | 88 | 89 |
| ≥10 | 28 | 0 | 408 | 74 | 27 | 100 | 100 | 85 | 85 |
| ≥11 | 20 | 0 | 408 | 82 | 20 | 100 | 100 | 83 | 84 |
| ≥12 | 15 | 0 | 408 | 87 | 15 | 100 | 100 | 82 | 83 |
TP, number of true positives; FP, number of false positives; FN, number of false negatives; TN, number of true negatives; % Se, sensitivity; % Sp, specificity; PPV, % positive predictive value; NPV, % negative predictive value; Acc, rate of accuracy (%) of the risk score model.
Application of the model in the combined cohort.
When we combined the two cohorts (n = 849), the predictive effects of the model were similar to those observed in the derivation set. The ORs for early isolation of ESBL-producing Enterobacteriaceae associated with scores of ≥3 and ≥6 were 23.25 (95% CI = 13.11 to 44.18, P < 0.001) and 30.37 (95% CI = 19.10 to 48.66, P < 0.001), respectively. The lower cutoff displayed a sensitivity, specificity, PPV, NPV, and overall accuracy of 93%, 62, 45, 97, and 70, respectively. The corresponding figures for the 6-point cutoff were 63, 95, 80, 88, and 87%, respectively. In the combined cohort, the prediction rule had an ROC AUC of 0.89 (95% CI = 0.87 to 0.92) (Fig. 1) and a Hosmer-Lemeshow χ2 of 10.19 (P = 0.51).
DISCUSSION
In recent years, ESBL-EKPs have been increasingly implicated as causes of both hospital and community-acquired infections (1–3, 9, 7, 10, 14, 16, 18, 22–24, 34,). Their role in the latter type of infections implies that reservoirs of these pathogens exist outside hospitals and not only among individuals with frequent healthcare contacts. Indeed, Mesa et al. reported relatively high isolation rates in livestock, food, and human sewage, with a general prevalence of 6.6% (19). The prevalence of fecal carriers varies, with reports up to 13.1 and 15.4% among healthy individuals and outpatients, respectively (15, 35). A recent study found ESBL-producing E. coli in the feces of 67.9% of patients with community-acquired urinary tract infections (UTIs) caused by these organisms, and fecal carriage was also increased in these patients' relatives (27.4% for those living in the same household, 15.6% for those living in other households, and 7.4% in nonrelatives) (25).
This widespread occurrence of ESBL-EKP in the community has important implications for the management of infections in hospital settings. For one thing, current policies on the empirical treatment of serious community-acquired infections that might be caused by Enterobacteriaceae (e.g., complicated UTIs and intra-abdominal infections) might need to be revised. Second, early identification of patients likely to be colonized and/or infected with these bacteria is also an important step in the prevention or containment of their spread among hospitalized patients. There is a pressing need for an easy-to-use risk stratification tool that can be used at hospital admission. However, while the clinical impact of serious infections caused by ESBL-producing Enterobacteriaceae in inpatient populations has been well documented (1, 16, 23), few studies have analyzed risk factors for the entry of ESBL-producing organisms into hospitals (2–4, 10, 14, 18, 24).
Our study was conducted in three medical centers where serious infections caused by strains of ESBL-EKP are increasing in frequency. The results demonstrated that patients harboring these organisms can be reliably identified on admission by the application of a simple clinical prediction rule. This type of risk stratification has proved to be an important strategy for improving clinical decisions and infection control (11, 13, 17, 29, 30). Our score is, to the best of our knowledge, the first one that specifically identifies probable carriers of ESBL-EKP among new admissions.
The multivariate model identified six factors associated with the isolation of ESBL-EKP within the first 48 h of hospitalization. They include previous therapy with β-lactams and/or fluoroquinolones, previous hospitalization, transfer from another healthcare facility, a Charlson comorbidity score of ≥4, recent history of urinary catheterization, and an age of ≥70 years.
Predictors of admission with ESBL-EKP infection/colonization that were identified by our multivariate models include factors associated with healthcare-related environmental exposure to ESBL-producing organisms and others reflecting increased susceptibility to bacterial colonization of the gastrointestinal tract, i.e., the recent use of antibiotic therapy. However, the emergence of ESBL-producing bacteria (particularly those producing CTX-M-type β-lactamases) in patients who have not had recent contact with the healthcare system can confuse the strategies based solely on such risk factors. Our score also includes important variables such as patient age, recent history of urinary catheterization, and the presence of comorbidities.
The score is simple to calculate and constructed from variables that are readily available at the time of admission, such as demographic characteristics, elements of the patient history, and routine clinical findings. This enhances its practical value in clinical settings, and its consistent use might conceivably reduce the subsequent need for surveillance cultures. It provided good discrimination of risk in both the derivation and validation sets, with similar ROC AUCs, and the fact that the two cohorts came from different hospitals and were hospitalized during different time periods increases the likelihood that our findings can be generalized to a broad range of patients and acute-care facilities.
When a threshold of ≥6 was used, the specificity of prediction was more than 94% in both the derivation and validation sets, and the PPV and NPV were, respectively, 82 and 81% in the derivation set and 79 and 93% in the validation set. Although sensitivity was low (55 and 73% in the derivation and validation sets, respectively), the high specificity of the prediction could improve targeting of high-risk patients. Conventional measures used to identify inpatients colonized by antibiotic resistant strains of bacteria (e.g., rectal swabs) could be limited to this subset of individuals, thereby reducing workloads as well as costs. In addition, high-risk patients could be empirically subjected to appropriate infection control measures while the screening cultures are being processed.
Inappropriate antimicrobial drug therapy during the empirical phase of treatment is the main risk factor for mortality in patients with severe infections caused by ESBL-EKP (8, 12, 21, 31–33), including those that are community acquired (1, 23). Use of our scoring system with the lower threshold (≥3) could provide useful information for prescribing empirical therapy. In the derivation set, the sensitivity (94%) and NPV (93%) of our model were very high, while the specificity (41%) and PPV (44%) were low. Similar figures emerged in the validation set. If patients with scores ≥ 3 initially receive broad-spectrum antibiotic treatment that includes an agent active against ESBL-EKP (e.g., carbapenems), the probability of inappropriate therapy should be very low (<10%). However, because the PPV associated with this cutoff is low, this type of treatment would also be administered to about half the patients with infections caused by bacteria other than ESBL-producing Enterobacteriaceae. Therefore, a more appropriate strategy might be to use drugs likely to be effective against ESBL producers when the patient's score is ≥3 and (i) the infection is suspected to be serious and/or (ii) the patients are already severely ill, situations in which initially inappropriate antibiotic therapy carries a high risk of mortality. In either case, however, as soon as microbiological data become available, antibiotic treatment should be de-escalated whenever appropriate to prevent the subsequent emergence of multidrug-resistant bacteria. It is important to note that application of the scoring system in empirical treatment decision-making processes needs further validation against a different type of control population, i.e., hospitalized patients suspected of infection (including more severe infections such as bacteremia) whose cultures did not grow ESBL-EKPs.
Our study has a number of other limitations. First, the data set we used included relatively few patients harboring ESBL-EKP isolates. This may have led us to underestimate the role of certain factors and, although our findings are statistically significant, our conclusions do need to be confirmed in a larger clinical trial. Second, because rectal swab screening for ESBL-EKP was not performed on admission in any of the hospitals involved in the present study, the control group might conceivably have included some colonized patients without clinical manifestations. This might also have facilitated the underestimation of some risk factors. Third, considerable variability in community-onset ESBL-EKP infections has been observed between different countries and within different subregions of the same area. Consequently, our results might not be applicable in all parts of the world (18, 22). For example, international travel has been reported as a major risk factor for developing an ESBL-producing E. coli infection in certain parts of the world (e.g., Canada and New Zealand) (18), but this variable was not even analyzed in our scoring system.
In conclusion, we have developed and validated a novel scoring system that can reliably identify patients likely to be harboring ESBL-producing Enterobacteriaceae on hospital admission. The score is based on six easy-to-define variables that are readily available at the time of hospital admission. Proper use of this tool should minimize the time required to identify patients harboring these organisms and allow more rapid application of measures designed to prevent the spread of these resistant strains within the inpatient population. Future efforts should focus on quantifying its value as a risk assessment tool compared to the clinical judgment of hospitalists, which is likely to be highly variable from one setting to another.
ACKNOWLEDGMENT
This study was partially supported by grants from the Italian Ministry for University and Scientific Research (Fondi Ateneo Linea D-1 2009-2010).
Footnotes
Published ahead of print on 2 May 2011.
REFERENCES
- 1. Apisarnthanarak A., Kiratisin P., Mundy L. M. 2008. Predictors of mortality from community-onset bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Control Hosp. Epidemiol. 29:671–674 [DOI] [PubMed] [Google Scholar]
- 2. Azap O. K., et al. 2010. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin. Microbiol. Infect. 16:147–151 [DOI] [PubMed] [Google Scholar]
- 3. Ben-Ami R., et al. 2009. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin. Infect. Dis. 49:682–690 [DOI] [PubMed] [Google Scholar]
- 4. Ben-Ami R., et al. 2006. Influx of extended-spectrum beta-lactamase-producing Enterobacteriaceae into the hospital. Clin. Infect. Dis. 42:925–934 [DOI] [PubMed] [Google Scholar]
- 5. Charlson M. E., Pompei P., Ales K. L., MacKenzie C. R. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383 [DOI] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. M100–S19. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Coque T. M., Baquero F., Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Eur. Surveill. 13 [PubMed] [Google Scholar]
- 8. Cosgrove S. E. 2006. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 42(Suppl. 2):S82–S89 [DOI] [PubMed] [Google Scholar]
- 9. Falagas M. E., Karageorgopoulos D. E. 2009. Extended-spectrum beta-lactamase-producing organisms. J. Hosp. Infect. 73:345–354 [DOI] [PubMed] [Google Scholar]
- 10. Friedmann R., et al. 2009. Prospective evaluation of colonization with extended-spectrum beta-lactamase (ESBL)-producing enterobacteriaceae among patients at hospital admission and of subsequent colonization with ESBL-producing enterobacteriaceae among patients during hospitalization. Infect. Control Hosp. Epidemiol. 30:534–542 [DOI] [PubMed] [Google Scholar]
- 11. Furuno J. P., et al. 2004. Prediction rules to identify patients with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci upon hospital admission. Am. J. Infect. Control. 32:436–440 [DOI] [PubMed] [Google Scholar]
- 12. Giske C. G., et al. 2008. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob. Agents Chemother. 52:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harbarth S., et al. 2006. Evaluating the probability of previously unknown carriage of MRSA at hospital admission. Am. J. Med. 119:275.e15–275.e23 [DOI] [PubMed] [Google Scholar]
- 14. Hawser S. P., et al. 2010. Incidence and antimicrobial susceptibility of Escherichia coli and Klebsiella pneumoniae with extended-spectrum beta-lactamases in community- and hospital-associated intra-abdominal infections in Europe: results of the 2008 Study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob. Agents Chemother. 54:3043–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kader A. A., Kumar A., Kamath K. A. 2007. Fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in patients and asymptomatic healthy individuals. Infect. Control Hosp. Epidemiol. 28:1114–1116 [DOI] [PubMed] [Google Scholar]
- 16. Kang C. I., et al. 2008. Clinical features and outcome of community-onset bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 27:85–88 [DOI] [PubMed] [Google Scholar]
- 17. Laupacis A., Sekar N., Stiell I. G. 1997. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA 277:488–494 [PubMed] [Google Scholar]
- 18. Laupland K. B., Church D. L., Vidakovich J., Mucenski M., Pitout J. D. 2008. Community-onset extended-spectrum beta-lactamase (ESBL) producing Escherichia coli: importance of international travel. J. Infect. 57:441–448 [DOI] [PubMed] [Google Scholar]
- 19. Mesa R. J., et al. 2006. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 58:211–215 [DOI] [PubMed] [Google Scholar]
- 20. Ortega M., et al. 2009. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J. Antimicrob. Chemother. 63:568–574 [DOI] [PubMed] [Google Scholar]
- 21. Peralta G., et al. 2007. Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia. J. Antimicrob. Chemother. 60:855–863 [DOI] [PubMed] [Google Scholar]
- 22. Pitout J. D., Laupland K. B. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8:159–166 [DOI] [PubMed] [Google Scholar]
- 23. Rodríguez-Baño J., et al. 2010. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin. Infect. Dis. 50:40–48 [DOI] [PubMed] [Google Scholar]
- 24. Rodríguez-Baño J., et al. 2008. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch. Intern. Med. 168:1897–1902 [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez-Baño J., López-Cerero L., Navarro M. D., Díaz de Alba P., Pascual A. 2008. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J. Antimicrob. Chemother. 62:1142–1149 [DOI] [PubMed] [Google Scholar]
- 26. Schwaber M. J., Carmeli Y. 2007. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J. Antimicrob. Chemother. 60:913–920 [DOI] [PubMed] [Google Scholar]
- 27. Song K. H., et al. 2009. Clinical outcomes of spontaneous bacterial peritonitis due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species: a retrospective matched case-control study. BMC Infect. Dis. 9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sullivan L. M., Massaro J. M., D'Agostino Sr R. B. 2004. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat. Med. 23:1631–1660 [DOI] [PubMed] [Google Scholar]
- 29. Tacconelli E., Karchmer A. W., Yokoe D., D'Agata E. M. 2004. Preventing the influx of vancomycin-resistant enterococci into health care institutions, by use of a simple validated prediction rule. Clin. Infect. Dis. 39:964–970 [DOI] [PubMed] [Google Scholar]
- 30. Tacconelli E., Cataldo M. A., De Angelis G., Cauda R. 2007. Risk scoring and bloodstream infections. Int. J. Antimicrob. Agents 30(Suppl. 1):S88–S92 [DOI] [PubMed] [Google Scholar]
- 31. Tumbarello M., et al. 2008. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli: risk factors for inadequate initial antimicrobial therapy. Antimicrob. Agents Chemother. 52:3244–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tumbarello M., et al. 2007. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob. Agents Chemother. 51:1987–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tumbarello M., et al. 2006. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob. Agents Chemother. 50:498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tumbarello M., et al. 2010. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-beta-lactamase production and inadequate initial antibiotic therapy. Antimicrob. Agents Chemother. 54:4085–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Valverde A., et al. 2004. Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during non-outbreak situations in Spain. J. Clin. Microbiol. 42:4769–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]