Abstract
Relative to the cefotaxime-gentamicin combination, the moxifloxacin-cefotaxime combination significantly reduced microglial activation and immature oligodendrocyte cell death and delayed myelination in the developing white matter of neonatal rats with experimental Escherichia coli sepsis. These neuroprotective effects were not due to differences in in vivo bactericidal activities or in the systemic inflammatory responses and could be related to the intrinsic immunomodulatory properties of moxifloxacin. Molecular mechanisms underlying the neuroprotective effect of moxifloxacin remain to be elucidated.
TEXT
Preterm birth is frequently associated with cerebral white matter damage (WMD), leading to various neurodevelopmental disorders. WMD combines defective myelination and glial scarring and is detected in 20% of low-birth-weight neonates (5). The two main risk factors for WMD are hypoxia/ischemia and inflammation, often occurring during sepsis in preterm neonates (12). Escherichia coli is the leading cause of early sepsis in preterm infants (11). Despite significant advances in our understanding of the mechanisms underlying WMD, therapeutic options for preventing or repairing these lesions are extremely limited. Several fluoroquinolones have intrinsic anti-inflammatory properties, and we recently reported that single-agent therapy with ciprofloxacin, contrary to therapy with cefotaxime, was able to prevent delayed myelination in neonatal rats with E. coli sepsis (8). Here, we further explored the therapeutic potential of fluoroquinolones in this setting by comparing the neuroprotective effects of moxifloxacin (MOX) in combination with cefotaxime (CTX) to those of cefotaxime plus an aminoglycoside, the antibiotic combination most commonly used for neonatal sepsis. Despite the fact that MOX had a lower level of activity against E. coli than ciprofloxacin (MIC differing by one or two dilution steps), the choice of MOX was justified by its wider antibacterial spectrum (including group B Streptococcus, Listeria monocytogenes, and Ureaplasma spp., which are other pathogens in preterm infants) (1, 3, 4). Moreover, previously reported in vitro and in vivo studies suggested that MOX may exert a more marked anti-inflammatory activity than ciprofloxacin (2, 9).
We used a well-characterized neonatal rat E. coli sepsis model (8). Briefly, 5-day-old Sprague-Dawley rats (Charles River, Saint Germain sur l'Arbresle, France) were injected intraperitoneally with ∼6,000 CFU of E. coli strain C5, representative of the worldwide neonatal sepsis clone O18:K1:H7. The MICs of MOX, CTX, and gentamicin (GEN) for this strain, determined by the Etest (AB Biodisk, Solna, Sweden), were 0.12, 0.06, and 1 μg/ml, respectively. In this model, bacteremia is detectable in more than 90% of pups 7.5 h after challenge, and the mortality exceeds 90% at 24 h without treatment. The two antibiotic combinations were administrated subcutaneously at 7.5 h and 24 h postinoculation. The CTX (Sanofi, Paris, France) and GEN (Panpharma, Fougères, France) doses were 50 mg/kg and 5 mg/kg of body weight per injection, respectively, as described previously (6, 8). The optimal dose of MOX (Bayer, Puteaux, France) was determined in a pilot study based on a microbiological assay of serum levels (8). A dose of 15 mg/kg per injection was chosen, as it was associated with a mean peak serum concentration of 2.9 ± 0.1 mg/liter, similar to that observed during human therapy (10). Sham-infected pups were injected with normal saline. All experimental and animal housing procedures complied with Institut National de la Santé et de la Recherche Médicale guidelines and with the policies on the use of animals and humans in neuroscience research (revised and approved by the Society for Neuroscience in January 1995).
No difference in the levels of bacteremia were observed at 7.5 h postinoculation (immediately before treatment) between pups treated with CTX-GEN and those treated with CTX-MOX (6.1 × 104 ± 4.6 × 104 and 6.5 × 104 ± 3.2 × 104 CFU/ml, respectively). As early as 1 h after treatment initiation, bacteremia was no longer detected in either treatment group. Systemic inflammation was studied 3 and 17 h after treatment initiation by measuring pro- and anti-inflammatory cytokines (interleukin-1β [IL-1β], tumor necrosis factor alpha [TNF-α], IL-6, and IL-10) with the Luminex xMAP technique (multianalytic profiling) according to the manufacturer's guidelines (Bio-Rad, Marnes la Coquette, France) (8). No difference was found between the two treatments at either time point (see the figure in the supplemental material).
We then examined WMD 5 days after treatment initiation. Pups were anesthetized with isoflurane and then infused with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). The brains were harvested and processed for immunohistochemistry with the primary antibodies listed in the table in the supplemental material, as previously described (8). The optical density of myelin basic protein (MBP)-positive fibers was measured within coronal sections of the lateral corpus callosum by using image analysis software (ImageJ; NIH), as previously reported (8). No difference was observed between sham-infected pups untreated or treated with either CTX-GEN or CTX-MOX in terms of the morphology or density of labeled cells, regardless of the antibody used. As expected, E. coli sepsis was associated with a marked increase in the density of activated microglial cells (OX 42 labeled) in the white matter of rats in both treatment groups (Fig. 1A). However, the density of activated microglial cells was significantly lower with CTX-MOX than with CTX-GEN (P < 0.001). Similarly, cell death assessment based on the cleaved-caspase-3-positive cell density was higher in CTX-GEN-treated pups than in sham-infected pups (P < 0.001), while no significant difference was observed between sham-infected pups and CTX-MOX-treated pups (Fig. 1B). Next, we investigated the impact of these findings on the oligodendroglial lineage and subsequent myelination of the developing white matter.
FIG. 1.
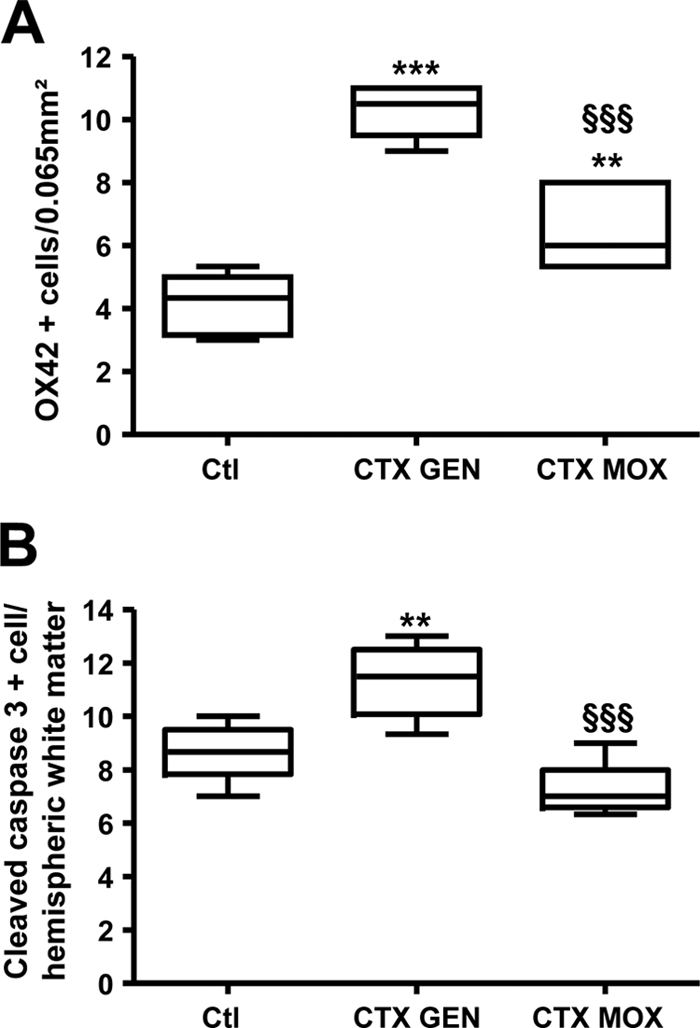
Density of OX42-positive microglial cells (A) and cleaved-caspase-3-positive apoptotic cells (B) in the hemispheric and cingulate white matter, respectively, in sham-infected control pups (Ctl) (n = 8) and CTX-GEN-treated (n = 8) and CTX-MOX-treated (n = 8) infected animals 5 days after treatment initiation. **, P < 0.01; ***, P < 0.001 (CTX-GEN versus control, determined by one-way analysis of variance [ANOVA] with Newman-Keuls correction). §§§, P < 0.001 (CTX-MOX versus CTX-GEN, determined by one-way ANOVA with Newman-Keuls correction).
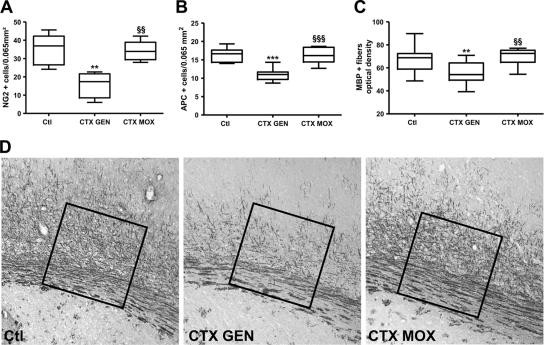
No difference in the densities of NG2-positive cells (immature oligodendrocytes) was observed between sham-infected and CTX-MOX-treated infected pups. In contrast, the density of NG2-positive cells was markedly lower in CTX-GEN-treated infected animals than in the sham-infected controls (P < 0.01) (Fig. 2A). Similar results were obtained with the adenomatous polyposis coli (APC) marker (mature oligodendrocytes) (P < 0.001) (Fig. 2B). Finally, compared to CTX-GEN, CTX-MOX significantly reduced the myelination delay (Fig. 2C and D).
FIG. 2.
Densities of NG2-positive cells (A), APC-positive cells (B), and MBP-positive fibers (optical density) (C and D) in the lateral corpus callosum of sham-infected control pups (Ctl) (n = 6 to 10) and CTX-GM-treated (n = 6 to 15) or CTX-MOX-treated (n = 6 to 9) infected animals 5 days after treatment initiation. The area in which the MBP optical density was measured is indicated by the squares in D. **, P < 0.01; ***, P < 0.001 (CTX-GEN versus the control group, determined by one-way ANOVA with Newman-Keuls correction). §§, P < 0.01; §§§, P < 0.001 (CTX-MOX versus CTX-GEN, determined by one-way ANOVA with Newman-Keuls correction).
Together, these results strongly suggest that during E. coli sepsis, CTX-MOX treatment reduces microglial activation and apoptotic cell death significantly more effectively than does CTX-GEN in the developing white matter of E. coli-infected rat pups. These short-term effects may prevent the deleterious impact on the oligodendroglial lineage and subsequent changes in myelination related to E. coli sepsis. The benefits of CTX-MOX compared to CTX-GEN were not related to differences in bacterial killing or in the systemic inflammatory response and might therefore be due to an intrinsic immunomodulatory effect of MOX on the developing brain. Whether this effect is related to the modulation of neurotrophic factor production, inducible nitric oxide synthase expression (as observed previously with ciprofloxacin), or semaphorin expression balance (8) remains to be determined.
Fluoroquinolones, although not approved for use in neonates, have been successfully used to treat multidrug-resistant and severe Gram-negative infections without major adverse effects in infants (7). MOX has a synergistic bactericidal action against group B Streptococcus when combined with cefotaxime (4), was previously proposed as an alternative treatment for listeriosis (3), and has a low MIC for Ureaplasma spp. (1). The CTX-MOX combination may therefore be an option for the probabilistic treatment of early-onset bacterial infection in preterm infants. Moxifloxacin should be further investigated as a potential candidate for the prevention of white matter damage associated with E. coli sepsis and to delineate molecular mechanisms underlying its neuroprotective effect.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 18 April 2011.
REFERENCES
- 1. Biran V., et al. 2010. Ureaplasma parvum meningitis in a full-term newborn. Pediatr. Infect. Dis. J. 29:60–64 [DOI] [PubMed] [Google Scholar]
- 2. Dalhoff A., Shalit I. 2003. Immunomodulatory effects of quinolones. Lancet Infect. Dis. 3:359–371 [DOI] [PubMed] [Google Scholar]
- 3. Grayo S., et al. 2008. Rapid eradication of Listeria monocytogenes by moxifloxacin in a murine model of central nervous system listeriosis. Antimicrob. Agents Chemother. 52:3210–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jacquier H., et al. 2007. In vitro activity of moxifloxacin (8-methoxyquinolone) alone or in combination with cefotaxime against group B streptococci. Pathol. Biol. 55:412–417 [DOI] [PubMed] [Google Scholar]
- 5. Khwaja O., Volpe J. J. 2008. Pathogenesis of cerebral white matter injury of prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 93:F153–F161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim K. S. 1985. Comparison of cefotaxime, imipenem-cilastatin, ampicillin-gentamicin, and ampicillin-chloramphenicol in the treatment of experimental Escherichia coli bacteremia and meningitis. Antimicrob. Agents Chemother. 28:433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leibovitz E. 2006. The use of fluoroquinolones in children. Curr. Opin. Pediatr. 18:64–70 [DOI] [PubMed] [Google Scholar]
- 8. Loron G., et al. 27 October 2010, posting date Ciprofloxacin prevents myelination delay in neonatal rats subjected to E. coli sepsis. Ann. Neurol. doi:10.1002/ana.22190 [DOI] [PubMed] [Google Scholar]
- 9. Shalit I., et al. 2002. Immunomodulatory and protective effects of moxifloxacin against Candida albicans-induced bronchopneumonia in mice injected with cyclophosphamide. Antimicrob. Agents Chemother. 46:2442–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stass H., Kubitza D. 1999. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J. Antimicrob. Chemother. 43(Suppl. B):83–90 [DOI] [PubMed] [Google Scholar]
- 11. Stoll B. J., et al. 2005. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of Gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr. Infect. Dis. J. 24:635–639 [DOI] [PubMed] [Google Scholar]
- 12. Volpe J. J. 2008. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J. Pediatr. 153:160–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.