Abstract
The in vitro interaction between triclosan and fluconazole against 24 azole-resistant clinical isolates of Candida albicans was evaluated by the microdilution checkerboard technique. The synergisms were verified by time-killing curves and agar diffusion tests in selected strains. Antagonistic activity was not detected.
TEXT
Candida albicans is the primary cause of opportunistic fungal disease in humans. It is predominantly found at low levels among the normal oral flora but can thrive in immunocompromised individuals (16, 25). Fluconazole has been used successfully as a prophylactic and a first-line therapeutic antifungal agent (5, 6, 19). However, the increase in azole use has precipitated a rise in drug resistance in clinical isolates. Triclosan, a chlorinated aromatic compound, has antimicrobial (4, 8, 20), antiparasitic (26), and anti-inflammatory (1, 24) activities. It has been used in personal care products (2). Combination therapy can improve the efficacy of antimicrobial therapy for infections recalcitrant to most treatments. Therefore, we aimed to assess the presence of combination effects with triclosan and fluconazole in C. albicans.
A total of 24 clinical isolates of fluconazole-resistant C. albicans were used in this study, and C. albicans ATCC 10231, C. parapsilosis ATCC 90018, and C. krusei ATCC 6258 were used as quality controls. The drugs were purchased from Sigma (Sigma-Aldrich).
The drug MICs were determined by broth microdilution according to CLSI method M27-A (3) with an inoculum of 2.5 × 103 CFU/ml. The plates were incubated at 35°C, and the optical density (OD) value was determined at 492 nm after 48 h, a modification to the CLSI reference method. All experiments were conducted in triplicate, and the median MIC-1 endpoint value, which represents an 80% reduction in turbidity, and MIC-2 endpoint value, which represents a 50% reduction in turbidity, were calculated (3). The drug interactions were analyzed using the fractional inhibitory concentration index (FICI) and ΔE models based on the Loewe additivity and Bliss independence theories, respectively (14, 21). The FICIs were defined as the sum of the MICs of each drug used in the combination divided by the MIC of the drug used alone. Synergy and antagonism were defined by FICIs of ≤0.5 and >4, respectively (15). The ΔE model was calculated as the sums of the percentages of all statistically significant (SS) synergistic (∑SYN) and antagonistic (∑ANT) interactions. Interactions that were <100% and >200% SS interactions were considered weak and strong, respectively. Interactions that were 100 to 200% SS interactions were considered moderate (14). The numbers of SS synergistic and antagonistic combinations were calculated for each strain.
A 100-μl sample of 106 CFU/ml C. albicans YL345, which exhibited the best synergistic effect, was spread onto a yeast extract-peptone-dextrose agar surface. Subsequently, 6-mm-diameter paper disks, impregnated with drugs or dimethyl sulfoxide (DMSO) alone, were placed onto the surface. The inhibition zones were measured using a dial caliper after a 48-h incubation at 35°C. The tests were performed in duplicate (9, 18).
The time-kill curves were conducted in an RPMI 1640 medium with 105 CFU/ml C. albicans YL345. At different time points after the drug incubation, 100 μl of the tube contents was subcultured in serial dilutions (10−1, 10−2, 10−3, and 10−4) on Sabouraud dextrose agar plates. Colony counts were determined after a 48-h incubation at 35°C. The results were reported as the mean ± standard deviation of all three replicates conducted for each compound, alone and in combination. The synergism and antagonism were defined as respective increases or decreases of ≥2 log10 CFU/ml in antifungal activity produced by the drug combination compared with the more active agent alone after 24 h (10, 12).
The checkerboard results are summarized in Table 1. The MIC-2 endpoint values for fluconazole and triclosan in C. albicans ranged from 4 to 32 μg/ml and from 32 to 64 μg/ml, respectively. The drug combination markedly reduced the MIC-2 endpoints of fluconazole and triclosan to 1 to 2 μg/ml and 4 to 8 μg/ml, respectively. A previous report has stated that the in vivo triclosan concentration in saliva was about 13 μg/ml at 10 min after brushing with toothpaste, and the duration of activity of triclosan at a concentration of 10 μg/ml in saliva was about 0.7 h (13). From our data, the MIC-2 values of triclosan against azole-resistant C. albicans strains were 4 to 8 μg/ml when it was combined with fluconazole. A concentration of triclosan in saliva between 10 and 13 μg/ml was adequate to inhibit an azole-resistant strain when the two drugs were combined.
Table 1.
Checkerboard analysis of in vitro interaction between TCL and FLC against 24 clinical isolates of C. albicansa
| Drug | Median MIC-2 endpoint (range) of drug (μg/ml) |
Median MIC-1 endpoint (range) of drug (μg/ml) |
||
|---|---|---|---|---|
| Alone | In combination | Alone | In combination | |
| FLC | 16 (4–32) | 1 (1–2) | 256 (64–>512) | 2 (1–4) |
| TCL | 32 (32–64) | 8 (4–8) | 64 (32–64) | 8 (8–16) |
TCL, triclosan; FLC, fluconazole.
The corresponding median FICI and ΔE values are shown in Table 2. The FICIs ranged from 0.125 to 0.375 and from 0.125 to 0.25 when analyzed using the MIC-2 and the MIC-1 endpoints, respectively. The ΔE values ranged from 116.8% to 589.2% when calculated using the MIC-2 endpoint. Antagonisms were not observed.
Table 2.
FICI and ΔE analyses of in vitro interaction between TCL and FLC against 24 clinical isolates of C. albicansa
| Endpoint type | Result according to nonparametric methodb |
||||
|---|---|---|---|---|---|
| FICI model |
ΔE model |
||||
| FICI, median (range) | INT | ∑SYN % (n) | ∑ANT % (n) | INT | |
| MIC-2 | 0.313 (0.125 to 0.375) | SYN (all isolates) | 116.8 (15) to 589.2 (22) | −28.3 (5) to −95.2 (12) | SYN (21 isolates), IND (3 isolates) |
| MIC-1 | 0.25 (0.125 to 0.25) | SYN (all isolates) | 50.1 (8) to 686.8 (24) | −8.3 (2) to −91.9 (7) | SYN (22 isolates), IND (2 isolates) |
TCL, triclosan; FLC, fluconazole.
INT, interpretation; SYN, synergism; ANT, antagonism; IND, indifference; n, number of interactions..
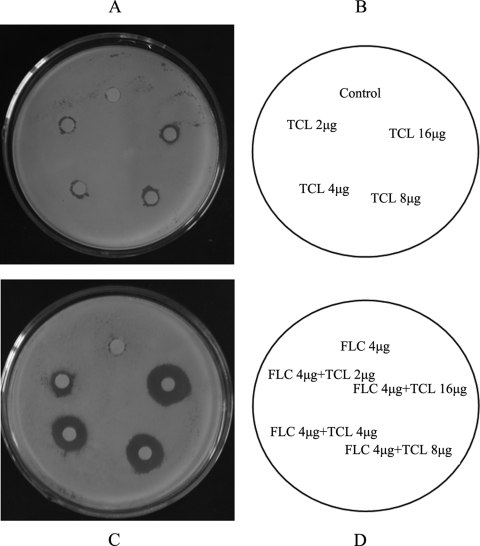
The synergism between fluconazole and triclosan was confirmed by agar diffusion tests (Fig. 1). The halo diameters produced by the combination were predominantly larger than ones produced by single-drug treatments. The sizes of the inhibition zones increased to 19.2, 18, 15.6, and 10.8 mm when 16 μg/ml fluconazole was combined with 16, 8, 4, and 2 μg/ml of triclosan, respectively.
Fig. 1.
Agar disk diffusion assay for FLC combined with TCL in C. albicans YL345. Panel B describes the image for panel A, and panel D describes the image for panel C.
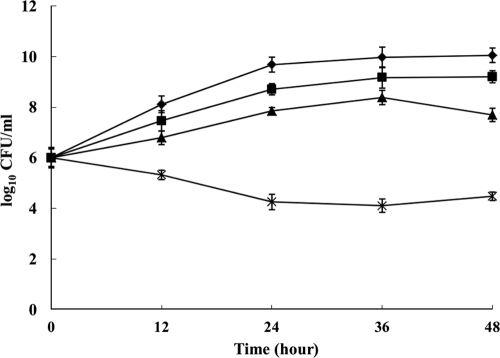
The time-kill curves verified the synergic combinations (Fig. 2). Triclosan and fluconazole did not significantly affect isolate growth when the drugs were used alone at 16 μg/ml and 4 μg/ml, respectively. The combination therapy yielded a 3.0-log10-CFU/ml decrease compared with triclosan alone after 24 h, wherein there was a significant difference (P < 0.01).
Fig. 2.
Time-kill curves for fluconazole (FLC) and triclosan (TCL) alone and in combination in clinical azole-resistant C. albicans YL345. ⧫, growth control; ▪, FLC; ▴, TCL; ×, FLC plus TCL.
Taken together, our findings indicate that triclosan exhibits an antifungal effect in vitro against azole-resistant C. albicans when combined with fluconazole. In the checkerboard assay, the FICI model has been frequently used to determine the interaction between antifungal drugs (7, 9, 12, 17, 21, 23). The ΔE model is a useful method for characterizing drug interactions. We verified the positive interactions using the agar diffusion test and time-kill curves, which were able to detect differences in the rate and degree of antifungal activity over time (11). An agar diffusion test can provide more visually convincing results. A combination treatment with triclosan has been previously demonstrated to significantly enhance the efficacy of triclosan against microbes (20, 22). In contrast to various previous reports (20, 22), triclosan is a better synergist to fluconazole against C. albicans.
In conclusion, the combination treatment of fluconazole and triclosan effectively synergizes against C. albicans. Our findings may provide an alternative approach to overcoming antifungal drug resistance. However, the mechanisms underlying the synergy must be further elucidated.
Acknowledgments
We are grateful to T. C. White and Y. Y. Jiang for providing the isolates.
This work was supported by the National Nature Science Foundation of China (grant no. 30871889).
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Barkvoll P., Rølla G. 1994. Triclosan protects the skin against dermatitis caused by sodium lauryl sulphate exposure. J. Clin. Periodontol. 21:717–719 [DOI] [PubMed] [Google Scholar]
- 2. Bhargava H. N., Leonard P. A. 1996. Triclosan: applications and safety. Am. J. Infect. Control 24:209–218 [DOI] [PubMed] [Google Scholar]
- 3. Clinical Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 3rd ed., M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4. Darouiche R. O., Mansouri M. D., Gawande P. V., Madhyastha S. 2009. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination. J. Antimicrob. Chemother. 64:88–93 [DOI] [PubMed] [Google Scholar]
- 5. Graybill J. R. 1997. Editorial response: can we agree on the treatment of candidiasis? Clin. Infect. Dis. 25:60–62 [DOI] [PubMed] [Google Scholar]
- 6. Greenspan D. 1994. Treatment of oropharyngeal candidiasis in HIV-positive patients. J. Am. Acad. Dermatol. 31:S51–S55 [DOI] [PubMed] [Google Scholar]
- 7. Guo N., et al. 2009. Antifungal activity of thymol against clinical isolates of fluconazole-sensitive and -resistant Candida albicans. J. Med. Microbiol. 58:1074–1079 [DOI] [PubMed] [Google Scholar]
- 8. Jones R. D., Jampani H. B., Newman J. L., Lee A. S. 2000. Triclosan: a review of effectiveness and safety in health care settings. Am. J. Infect. Control. 28:184–196 [PubMed] [Google Scholar]
- 9. Kiraz N., et al. 2009. Antifungal activity of caspofungin in combination with amphotericin B against Candida glabrata: comparison of disk diffusion, Etest, and time-kill methods. Antimicrob. Agents Chemother. 53:788–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klepser M. E., Ernst E. J., Lewis R. E., Ernst M. E., Pfaller M. A. 1998. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob. Agents Chemother. 42:1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis R. E., Diekema D. J., Messer S. A., Pfaller M. A., Klepser M. E. 2002. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 49:345–351 [DOI] [PubMed] [Google Scholar]
- 12. Li Y., et al. 2008. In vitro interaction between azoles and cyclosporin A against clinical isolates of Candida albicans determined by the chequerboard method and time-kill curves. J. Antimicrob. Chemother. 61:577–585 [DOI] [PubMed] [Google Scholar]
- 13. Loftsson T., Leeves N., Bjornsdottir B., Duffy L., Masson M. 1999. Effect of cyclodextrins and polymers on triclosan availability and substantivity in toothpastes in vivo. J. Pharm. Sci. 88:1254–1258 [DOI] [PubMed] [Google Scholar]
- 14. Meletiadis J., Mouton J. W., Meis J. F., Verweij P. E. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Odds F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 16. Patel M., Shackleton J. A., Coogan M. M., Galpin J. 2008. Antifungal effect of mouth rinses on oral Candida counts and salivary flow in treatment-naïve HIV-infected patients. AIDS Patient Care STDs 22:613–618 [DOI] [PubMed] [Google Scholar]
- 17. Philip A., et al. 2005. In vitro synergy testing of anidulafungin with itraconazole, voriconazole, and amphotericin B against Aspergillus spp. and Fusarium spp. Antimicrob. Agents Chemother. 49:3572–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quan H., et al. 2006. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob. Agents Chemother. 50:1096–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rex J. H., et al. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325–1330 [DOI] [PubMed] [Google Scholar]
- 20. Sharma S., Ramya T. N., Surolia A., Surolia N. 2003. Triclosan as a systemic antibacterial agent in a mouse model of acute bacterial challenge. Antimicrob. Agents Chemother. 47:3859–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun S., et al. 2008. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob. Agents Chemother. 52:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tambe S. M., Sampath L., Modak S. M. 2001. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J. Antimicrob. Chemother. 47:589–598 [DOI] [PubMed] [Google Scholar]
- 23. Te Dorsthorst D. T., et al. 2002. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 46:2982–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waaler S. M., Rölla G., Skjörland K. K., Ogaard B. 1993. Effects of oral rinsing with triclosan and sodium lauryl sulfate on dental plaque formation: a pilot study. Scand. J. Dent. Res. 101:192–195 [DOI] [PubMed] [Google Scholar]
- 25. Willocks L., et al. 1991. Fluconazole resistance in AIDS patients. J. Antimicrob. Chemother. 28:937–939 [DOI] [PubMed] [Google Scholar]
- 26. Zimhony O., Vilchèze C., Jacobs W. R., Jr. 2004. Characterization of Mycobacterium smegmatis expressing the Mycobacterium tuberculosis fatty acid synthase I (fas1) gene. J. Bacteriol. 186:4051–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]