Abstract
Mycothiol (MSH; AcCys-GlcN-Ins) is the glutathione analogue for mycobacteria. Mutations in MSH biosynthetic genes have been associated with resistance to isoniazid (INH) and ethionamide (ETH) in mycobacteria, but rigorous genetic studies are lacking, and those that have been conducted have yielded different results. In this study, we constructed independent null deletion mutants for all four genes involved in the MSH biosynthesis pathway (mshA, mshB, mshC, and mshD) in Mycobacterium smegmatis and made complementing constructs in integrating plasmids. The resulting set of strains was analyzed for levels of MSH, INH resistance, and ETH resistance. The mshA and mshC single deletion mutants were devoid of MSH production and resistant to INH, whereas the mshB deletion mutant produced decreased levels of MSH yet was sensitive to INH, suggesting that MSH biosynthesis is essential for INH susceptibility in M. smegmatis. Further evidence supporting this conclusion was generated by deleting the gene encoding the MSH S-conjugate amidase (mca) from the ΔmshB null mutant. This double mutant, ΔmshB Δmca, completely abolished MSH production and was resistant to INH. The mshA, mshC, and mshB single deletion mutants were also resistant to ETH, indicating that ETH resistance is modulated by the level of MSH in M. smegmatis. Surprisingly, the mshD deletion mutant lacked MSH production but was sensitive to both INH and ETH. The drug sensitivity was likely mediated by the compensated synthesis of N-formyl-Cys-GlcN-Ins, previously demonstrated to substitute for MSH in an mshD mutant of M. smegmatis. We conclude that MSH or N-formyl-Cys-GlcN-Ins is required for susceptibility to INH or ETH in M. smegmatis.
INTRODUCTION
The molecular mechanisms of resistance to the antituberculosis drugs isoniazid (INH) and ethionamide (ETH) are not fully understood. The increasing emergence of multidrug-resistant (MDR) tuberculosis (TB) and extensively drug-resistant (XDR) TB poses a major threat to the effective control of TB globally (39), which considerably increases the need to further explore mechanisms of resistance to INH or ETH.
INH is a front-line drug which has been most widely used for the treatment of active TB and latent infection since its discovery in 1952 (5, 9). The drug enters the mycobacterial cells by passive diffusion (3). INH is a prodrug activated by the mycobacterial katG-encoded catalase-peroxidase (41). Mutations in katG cause resistance to INH in mycobacteria and are the main mechanism of INH resistance in INH-resistant clinical isolates (10, 40). KatG-mediated INH activation produces a range of highly reactive species such as superoxide, hydroxyl radical (28), and isonicotinic-acyl radical (38). Isonicotinic-acyl radical, the putative active form of INH, covalently binds to the nicotinamide ring of NAD, forming the isonicotinoyl-NAD (INH-NAD) adduct, which subsequently inhibits the inhA-encoded enoyl-acyl carrier protein (ACP) reductase (24, 25). InhA is a NADH-dependent enzyme involved in the fatty acid biosynthesis type II (FASII) system, which catalyzes the reduction of the double bond at position 2 of a growing long-chain (16 or more carbon atoms) fatty acid during the fatty acid elongation process (20). Mycolic acids, whose synthesis derives from the FASII system, are important components of the outer part of the mycobacterial cell wall, where they form a hydrophobic barrier. Inhibition of InhA by the INH-NAD adduct in INH-treated mycobacteria leads to bacterial death through inhibition of mycolic acid biosynthesis (32, 35). Mutations within the promoter region of inhA confer resistance to INH by upregulating expression of target InhA via a drug titration mechanism (1). Resistance to INH can also be mediated by mutations in the structural region of the inhA gene, by lowering the InhA affinity to the INH-NAD adduct (25). In addition, mutations in inhA not only cause INH resistance, but they also confer cross-resistance to ETH, a structural analog of INH (1). ETH, a second-line antituberculosis drug most frequently used against MDR-TB (13), is also a prodrug and similar in action to INH. However, ETH requires activation by a different enzyme, the flavoprotein monooxygenase EthA (4, 37). Mutations in ethA confer resistance to ETH. ethA mutations have been found in both laboratory-derived and clinical ETH-resistant isolates (12).
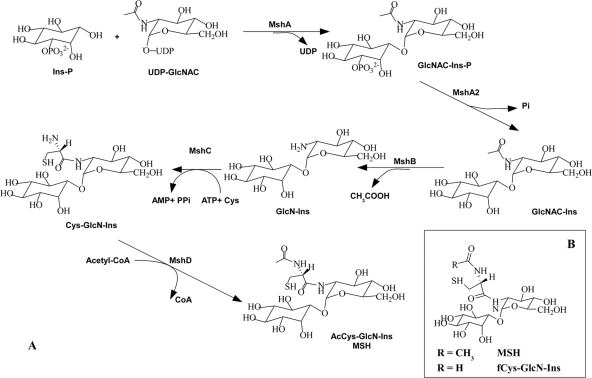
Recently, coresistance to both INH and ETH was also observed in spontaneous mycothiol glycosyltransferase (mshA) mutants from Mycobacterium tuberculosis which produced low levels of mycothiol (MSH; AcCys-GlcN-Ins) (34). MSH is the predominant low-molecular-weight thiol in mycobacteria, which produce no glutathione (GSH) (14). As a functional analog of GSH, MSH serves as an antioxidant thiol or detoxification agent (7, 16). Structurally different from GSH, the cysteine residue of MSH is acetylated and amide linked to disaccharide moieties composed of inositol and N-glucosamine (30). The MSH biosynthesis pathway in mycobacteria is illustrated in Fig. 1A (17). Except for MSH phosphatase mshA2, all other genes (mshA, MSH deacetylase mshB, MSH ligase mshC, and MSH acetyltransferase mshD) involved in MSH biosynthesis have been identified in mycobacteria (11, 15, 18, 27). Here, in order to clarify the underlying relationship between MSH biosynthesis and INH or ETH susceptibility in M. smegmatis, we precisely deleted each individual gene (mshA, mshB, mshC, and mshD) involved in the MSH biosynthesis pathway and made unmarked null deletion mutants. The deletion mutants as well as their complementary strains were assayed for MSH content and MICs of INH and ETH.
Fig. 1.
(A) MSH biosynthesis pathway. The MSH glycosyltransferase MshA links inositol-1-phosphate (Ins-P) to N-acetylglucosamine (GlcNAc). The product is dephosphorylated and deacetylated by the phosphatase MshA2 and the deacetylase MshB, respectively. The ligase MshC condenses cysteine and GlcN-Ins in an ATP-dependent manner, and the acetyltransferase MshD synthesizes MSH (AcCys-GlcN-Ins). CoA, coenzyme A. (B) Structure of fCys-GlcN-Ins.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. smegmatis mc2155 (29) and its derived mutants were grown in 7H9 medium (Difco) supplemented with 0.05% Tween 80, 0.2% glycerol, and 10% ADS (0.081% NaCl, 0.5% bovine albumin fraction V, 0.2% dextrose). Escherichia coli strains DH5α and HB101 were cultured with LB medium (Difco). Kanamycin (20 μg/ml for M. smegmatis, 40 μg/ml for E. coli) and hygromycin (100 μg/ml for M. smegmatis, 150 μg/ml for E. coli; Roche) were added to culture medium as needed. All chemicals used in this study were purchased from Sigma unless specified otherwise.
Generation of gene deletion mutants.
mc2155-derived mutants were generated by allelic exchange using specialized transduction (2). DNA fragments containing ∼50 bp of target gene, with ∼1,000 bp of upstream and downstream flanking DNA sequence, were PCR amplified (NEB F530L, Phusion high-fidelity DNA polymerase) using the oligonucleotides listed in Table 1. The subsequent upstream and downstream arms were digested with the indicated restriction enzymes (Table 1) and cloned into Van91I-digested p0004s vector arms. P0004s is a pJSC347 (26)-derived cosmid containing lambda phage cos sites, a hyg resistance marker, and a sacB cassette (T. Hsu and W. R. Jacobs, Jr., unpublished data). The resulting allelic exchange substrates were digested with PacI and ligated with PacI-digested temperature-sensitive mycobacteriophage phAE159 (Hsu and Jacobs, unpublished) derived from TM4. Phage was packaged using a MaxPlax packaging extract (Epicenter Biotechnologies) for construction of shuttle phasmids in the E. coli HB101 strain. The obtained phasmids were electroporated into mc2155 for phage propagation. Phage was delivered into mc2155 as previously described (2). Subsequently, target genes were deleted and replaced with a γδres-sacB-hyg-γδres cassette, using hygromycin for selection. After Hygr selection and confirmation of the correct deletion/replacement, the γδres-sacB-hyg-γδres cassette of the deletion/replacement allele was removed. Removal was accomplished by infection of TM4-derived phage phAE201, which expresses the γδ-resolvase enzyme (Hsu and Jacobs, unpublished), and plating on sucrose (5%, wt/vol) for counterselection. Both marked and unmarked mutants were verified by PCR and sequencing of predicted junctions. For construction of ΔmshB Δmca double null deletion mutants, the mca gene was exchanged from the mc2155 ΔmshB unmarked mutant using specialized transduction, and the γδres-sacB-hyg-γδres cassette was subsequently removed.
Table 1.
Oligonucleotides used for generation of allelic exchange substrates
| Primer | Sequence | Restriction enzyme used |
|---|---|---|
| mshA | ||
| LL | 5′-TTTTTTTTCCATAAATTGGATCTGCTCGGCCGCACGCTC-3′ | Van91I |
| LR | 5′-TTTTTTTTCCATTTCTTGGTGCGCCAGCGGAGAGGTGTG-3′ | Van91I |
| RL | 5′-TTTTTTTTGCATAGATTGCCGTTCCGGACGCCGGTTCTC-3′ | BstAPI |
| RR | 5′-TTTTTTTTGCATCTTTTGCCGGCGTCCTGGCTGTACTTG-3′ | BstAPI |
| mshB | ||
| LL | 5′-TTTTTTTTGCATAAATTGCGGCGACGACAACACCGTGAC-3′ | BstAPI |
| LR | 5′-TTTTTTTTGCATTTCTTGCCCGGTGGTCAGGGTCTCGTC-3′ | BstAPI |
| RL | 5′-TTTTTTTTGCATAGATTGCGGGCCACGGGATTCCCGAGG-3′ | BstAPI |
| RR | 5′-TTTTTTTTGCATCTTTTGCGCGCCTGAGTCGCTCGAGTC-3′ | BstAPI |
| mshC | ||
| LL | 5′-TTTTTTTTCCATAAATTGGATTCCGGCGGGCACCGATAC-3′ | Van91I |
| LR | 5′-TTTTTTTTCCATTTCTTGGGTCGAAGAGGCGCAGCGCAG-3′ | Van91I |
| RL | 5′-TTTTTTTTCCATAGATTGGACGCGCTGTCCTACGGTGGG-3′ | Van91I |
| RR | 5′-TTTTTTTTCCATCTTTTGGTGTGCGCGCTGCTCGATCTG-3′ | Van91I |
| mshD | ||
| LL | 5′-TTTTTTTTGCATAAATTGCGCGGCTGACGAGCAGGTGAG-3′ | BstAPI |
| LR | 5′-TTTTTTTTGCATTTCTTGCTCAGCGCGCGAATCTCTGCC-3′ | BstAPI |
| RL | 5′-TTTTTTTTCCATAGATTGGACCGGAAGTTGGGGTTCGAG-3′ | Van91I |
| RR | 5′-TTTTTTTTCCATCTTTTGGCGAAGCCCTTCTCGACGTAG-3′ | Van91I |
| mca | ||
| LL | 5′-TTTTTTTTCCATAAATTGGGTGATAGCCGCCGTTCTCGC-3′ | Van91I |
| LR | 5′-TTTTTTTTCCATTTCTTGGCTTGCTGGACTCGTCGTCCG-3′ | Van91I |
| RL | 5′-TTTTTTTTCCATAGATTGGGCCGACCGAGGAGTTCGAG-3′ | Van91I |
| RR | 5′-TTTTTTTTCCATCTTTTGGTGGCGCTTGTCCTCGTCACC-3′ | Van91I |
Complementation of null deletion mutants.
Individual genes were amplified from mc2155 genomic DNA by PCR (Phusion high-fidelity DNA polymerase; NEB) using the oligonucleotide pairs listed in Table 2. Each individual mshA, mshB, mshC, or mshD gene was cloned into integrating plasmid pMV361 (31) using the restriction sites, underlined in Table 2. Each plasmid was sequenced to verify the presence of the wild-type gene. The resulting plasmids were transformed into null deletion mutants by electroporation, using kanamycin for selection. A representative transformant of the M. smegmatis mutant was verified by PCR amplification of the integrated gene to verify successful complementation. The empty pMV361 plasmid vectors were from laboratory stock and also electroporated into null mutants as controls. The constitutive hsp60 promoter drives cloned gene expression.
Table 2.
Oligonucleotides used for knockout complementation
| Primer | Sequence | Restriction enzyme used |
|---|---|---|
| mshA | 5′-GGGGGGGAATTCGTGCGTCTAGCGACAGACCT-3′ | EcoRI |
| 5′-GGGGGGAAGCTTTCACGTGCGTACTCCCCTGC-3′ | HindIII | |
| mshB | 5′-GGGGGGGAATTCATGTCATCGCATGAATCGCC-3′ | EcoRI |
| 5′-GGGGGGAAGCTTCTACTCCAGATCAAGCCCG-3′ | HindIII | |
| mshC | 5′-GGGGGGGAATTCATGCAATCGTGGTCGGCACC-3′ | EcoRI |
| 5′-GGGGGGAAGCTTTTAGAGGTCCACACCCAGCA-3′ | HindIII | |
| mshD | 5′-GGGGGGGAATTCGTGACCTCCACCGAGTGGC-3′ | EcoRI |
| 5′-GGGGGGAAGCTTGTGTTCACAGGCGCGAGGC-3′ | HindIII |
Measurement of thiol content.
Fifty milliliters of mc2155 and its derived strains was grown to stationary phase. In triplicate, 9 ml culture was collected by centrifugation. The pellet was resuspended with 0.5 ml monobromobimane (mBBr; Biochemika) reagent (20 mM HEPES, pH 8, 2 mM mBBr, 50% acetonitrile). Samples were heated at 60°C for 15 min for thiol group labeling and then centrifuged at 6,000 rpm for 10 min. The resulting supernatant was treated with 5 M methanesulfonic acid (MSA) to stop the reaction. Samples were frozen and stored at −70°C until quantification. Fluorescence high-pressure liquid chromatography (HPLC) was performed to quantify the MSH content using standards as previously described (22). Another 9 ml culture was treated with N-ethylmaleimide (NEM) and then with mBBr as a control.
Determination of MIC.
Mycobacterial cultures were grown to log phase. Ten microliters of 10-fold serial dilutions was spotted in duplicate on 7H10 agar supplemented with 0.5% dextrose, 0.5% glycerol, and INH (0, 0.5, 1, 2, 4, 8, 16, 32 μg/ml) or ETH (0, 2.5, 5, 10, 20, 40, 80, 160, 320 μg/ml). Plates were incubated at 37°C for 2 to 4 days. The lowest concentration of drug that caused a >99% reduction in the numbers of CFU/ml compared to the number for the drug-free control was determined as the MIC.
RESULTS
MSH synthetic genes are nonessential for M. smegmatis.
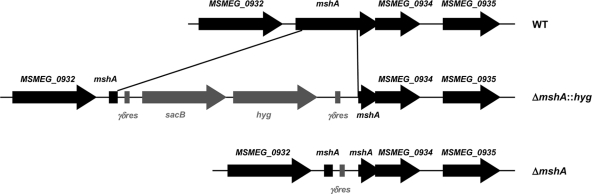
In order to investigate if MSH biosynthesis influences INH or ETH susceptibility in M. smegmatis, we first generated independent unmarked deletion mutants of mshA, mshB, mshC, and mshD using specialized transduction (Fig. 2). For each mutated gene we obtained 20 to 50 hygromycin-resistant transductants. Three to 5 independent transductants for each gene were verified to have the deletion by PCR and sequencing. A single clone was chosen for unmarking and then stocked and used for further studies (Table 3). All four genes were successfully individually deleted from the genome of M. smegmatis, demonstrating that mshA, mshB, mshC, and mshD genes are all nonessential in M. smegmatis.
Fig. 2.
Gene deletion by specialized transduction: organization of mshA gene loci in mc2155 wild type (WT) and the ΔmshA::hyg and ΔmshA unmarked mutants. The central part of target genes was deleted and replaced by the γδres-sacB-hyg-γδres cassette, which was subsequently removed utilizing the phage phAE201 expressing the γδ-resolvase. Only the first and last ∼50 bp of the target gene was maintained in the genome of desired unmarked mutants.
Table 3.
Strains used in this study
| Strain | Relevant genotype | Plasmid | How constructed |
|---|---|---|---|
| mc2155 | Wild type | None | As described previously (29) |
| mc27077 | ΔmshA (MSMEG_0933) | None | Specialized transduction of mc2155 |
| mc27083 | ΔmshA pMV361::mshA | pMV361-mshA | Transformation of mc27077 |
| mc27078 | ΔmshB (MSMEG_5129) | None | Specialized transduction of mc2155 |
| mc27085 | ΔmshB pMV361::mshB | pMV361-mshB | Transformation of mc27078 |
| mc27079 | ΔmshC (MSMEG_4189) | None | Specialized transduction of mc2155 |
| mc27087 | ΔmshC pMV361::mshC | pMV361-mshC | Transformation of mc27079 |
| mc27080 | ΔmshD (MSMEG_5783) | None | Specialized transduction of mc2155 |
| mc27089 | ΔmshD pMV361::mshD | pMV361-mshD | Transformation of mc27080 |
| mc27081 | Δmca (MSMEG_5261) | None | Specialized transduction of mc2155 |
| mc27082 | ΔmshB Δmca | None | Specialized transduction of mc27078 |
| mc27152 | ΔmshB Δmca pMV361::mshB | pMV361-mshB | Transformation of mc27082 |
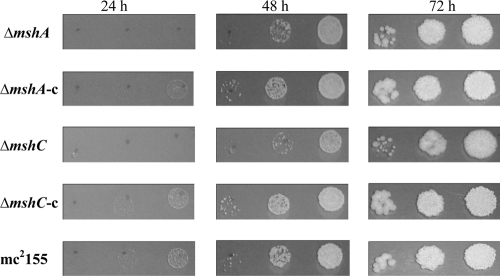
In contrast to M. tuberculosis, M. smegmatis MSH biosynthetic mutants did not require catalase to grow in vitro. Nevertheless, we observed that on solid agar, ΔmshA and ΔmshC null mutants grew slightly slower than their complementary or wild-type strains (Fig. 3). However, ΔmshB and ΔmshD null mutants did not show significant growth defects.
Fig. 3.
Growth defect of ΔmshA or ΔmshC null mutant on solid agar. The same amount of bacteria with 10-fold serial dilutions was spotted on 7H10 agar supplemented with 0.5% dextrose and 0.5% glycerol. The plates were incubated at 37°C for 24, 48, and 72 h. c, complementation.
Single deletion of mshA, mshC, or mshD or double deletion of mshB and mca blocks MSH biosynthesis.
The MSH levels for each strain were measured by fluorescence HPLC. The ΔmshA, ΔmshC, and ΔmshD null mutants produced undetectable levels of MSH, while complemented strains regained MSH production to wild-type levels (Table 4). These results were consistent with previous reports for an mshA single deletion mutant from M. tuberculosis as well as mshC chemical mutant I64 from M. smegmatis, which contained the L205P single mutation in the mshC gene (22, 34). Similarly, the M. smegmatis mshD::Tn5 mutant and M. tuberculosis mshD single deletion mutant were previously reported to produce very low levels of MSH, which varied from less than 1% to 3% of the wild-type level of MSH, depending on the growth phase (6, 11). However, the ΔmshB mutant still produced about 20% MSH (Table 4), consistent with previously published data for mshB mutants from both M. tuberculosis and M. smegmatis (7, 21). The mshB gene has a homologous gene in M. smegmatis named mca that encodes the MSH S-conjugate amidase which exhibits low N-acetylglucosaminylinositol (GlcNAc-Ins) deacetylation activity (15, 16). In this study, for the first time, the mca gene was deleted from the genome of the ΔmshB null mutant by specialized transduction, and the replacement antibiotic cassette was subsequently removed by site-specific excision (see Materials and Methods). The M. smegmatis ΔmshB Δmca double null deletion mutant produced no detectable MSH (Table 4). Complementation with M. smegmatis mshB restored MSH biosynthesis. Interestingly, the Δmca single null deletion mutant showed increased MSH levels (Table 4).
Table 4.
MICs for INH/ETH and MSH concentration
| M. smegmatis strain | MIC (μg/ml) |
MSH concn (nmol/109 cells) | |
|---|---|---|---|
| INH | ETH | ||
| mc2155 | 4 | 20 | 13 ± 5 |
| ΔmshA | 32 | 160 | <0.05 |
| ΔmshA pMV361::mshA | 4 | 20 | 16 ± 5 |
| ΔmshB | 8 | 160 | 3.2 ± 1.5 |
| ΔmshB pMV361::mshB | 4 | 20 | 10 ± 4 |
| ΔmshC | 16 | 160 | <0.05 |
| ΔmshC pMV361::mshC | 4 | 20 | 12 ± 1 |
| ΔmshDa | 4 | 20 | <0.05 |
| ΔmshD pMV361::mshD | 4 | 20 | 12 ± 1 |
| Δmca | 4 | 20 | 44 ± 0.02 |
| ΔmshB Δmca | 32 | 160 | <0.05 |
| ΔmshB Δmca pMV361::mshB | 4 | 20 | 27 ± 2 |
The quantification of fCys-GlcN-Ins and Cys-GlcN-Ins was estimated by using MSmB and Cys-mB as standards, respectively. fCys-GlcN-Ins, (1.4 ± 0.1) nmol/109cells; Cys-GlcN-Ins, (1.8 ± 0.7) nmol/109 cells.
M. smegmatis MSH biosynthesis mutants have various sensitivities to INH or ETH.
The MICs against INH were determined using the proportion method (see Materials and Methods), and a ≥4-fold change was considered significant and resistant. The ΔmshA and ΔmshC null mutants displayed 4- to 8-fold higher MICs than the wild-type strain for INH. In contrast, the MICs of INH for the ΔmshB and ΔmshD null mutants had no significant change (Table 4). Complementation with the M. smegmatis mshA or mshC gene restored INH susceptibility to the wild-type level (Table 4). The MIC results for INH for our MSH mutants are consistent with those published for an mshB mutant from M. tuberculosis or M. smegmatis and M. smegmatis mshC chemical mutant I64 (7, 21, 22).
In M. tuberculosis, mshA mutants were also shown to be resistant to ETH (34). Therefore, we also measured MICs of ETH for all strains. Like M. tuberculosis mshA spontaneous mutants (35), M. smegmatis ΔmshA, ΔmshB, and ΔmshC had 8-fold higher MICs for ETH. Complementation with M. smegmatis mshA, mshB, or mshC restored ETH susceptibility to that of the wild type (Table 4). Interestingly, the MIC of ETH for ΔmshD was identical to that of the wild type (Table 4).
The MICs of both INH and ETH for the ΔmshB Δmca double mutant were 8-fold higher than those for the wild type (Table 4). Complementation with the M. smegmatis mshB gene restored susceptibility for both INH and ETH (Table 4). The Δmca single deletion mutant had MICs for both INH and ETH identical to those of the wild type (Table 4), which was consistent with a previous report (23). The pMV361 control transformants of all null mutants yielded INH and ETH MICs identical to those of the parental strains, as expected (data not shown).
DISCUSSION
Our ability to successfully delete each of the 4 genes involved in MSH biosynthesis in M. smegmatis clearly shows that none of the MSH biosynthetic genes is essential for this organism under in vitro growth conditions. Unlike M. tuberculosis ΔmshA and ΔmshD, which require exogenous catalase to grow in vitro (6, 34), the M. smegmatis MSH synthetic mutants in this study grew on solid plate or liquid medium without catalase. One possible explanation might rely on the difference in the redox balance between the two microorganisms (33).
Our MSH synthetic mutants demonstrated that deletion of mshA, mshC, or mshD is sufficient to block MSH production in M. smegmatis. However, ΔmshB null mutants retained significant MSH biosynthesis. It was proposed previously that mca could partially compensate for the MSH biosynthesis pathway when mshB is deleted in M. smegmatis (21). We confirmed the hypothesis by demonstrating that deletion of both mca and mshB resulted in the complete inhibition of MSH biosynthesis in M. smegmatis. The mca gene is conserved between M. smegmatis and M. tuberculosis; therefore, it is plausible that deletion of both mca and mshB would also deplete MSH production in M. tuberculosis. Mca has deacetylation activity on GlcNAc-Ins and is also an MSH-dependent detoxification protein. Mca hydrolyzes an amide bond in the MSH-toxin conjugates, producing a mercapturic acid of the toxin which is excreted from the bacteria. The remaining product, GlcN-Ins, is reutilized as a substrate of MshC for the recycling of MSH biosynthesis (16). Our M. smegmatis Δmca null mutant had an increased MSH level, supporting the suggestion that the main function of Mca is normally not MSH biosynthesis but, rather, the MSH-dependent detoxification pathway. Compared to the amidase activity on an mBBr derivative of MSH (MSmB), Mca has low activity on MSH (4 versus 4,480 nmol min−1 mg−1) (16). Mca was proposed to be involved in the degradation of MSH (8), and the increase of the MSH level in our M. smegmatis Δmca null mutant could be partially caused by the blockage of the degradation of MSH.
The ΔmshA and ΔmshC single mutants did not produce MSH and were shown to be resistant to INH, whereas the ΔmshB mutant, which still produced 20% MSH, was sensitive to INH, suggesting that the lack of MSH confers INH resistance in M. smegmatis. In order to further test this hypothesis, the MIC of INH was also determined for our M. smegmatis ΔmshB Δmca double mutant, which completely lacks MSH production. As we expected, the double mutant ΔmshB Δmca was resistant to INH. In contrast, ΔmshA conferred resistance to ETH but not to INH in M. tuberculosis (34). Similarly to INH, MSH-deficient mutants, such as the ΔmshA or ΔmshC null mutant, were resistant to ETH. Interestingly, M. smegmatis ΔmshB was also resistant to ETH, indicating that ETH susceptibility is modulated by the MSH level in M. smegmatis.
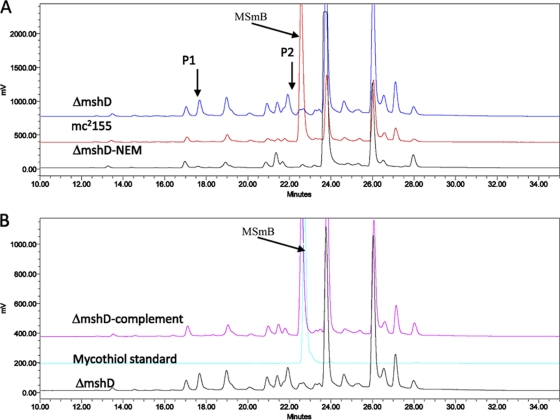
Surprisingly, M. smegmatis ΔmshD lacked MSH but was sensitive to both INH and ETH. MshD is the MSH synthase and catalyzes N-acetylation of Cys-GlcN-Ins to produce MSH in the final step of MSH biosynthesis (11). In the M. smegmatis mshD::Tn5 mutant or ΔmshD null mutant from M. tuberculosis, the most abundant reduced form of thiol is a novel thiol N-formyl-Cys-GlcN-Ins (fCys-GlcN-Ins) (Fig. 1B), a derivative of Cys-GlcN-Ins (6, 19). However, the substrate Cys-GlcN-Ins that accumulated in the mshD mutants is substantially oxidized (6, 19). Although fCys-GlcN-Ins and Cys-GlcN-Ins are the two most abundant thiols in the mshD mutant, the MSH-dependent enzymes Mca and mycothiol reductase (Mtr) have better activity on the bimane derivative of fCys-GlcN-Ins (fCySmB-GlcN-Ins) and disulfide of fCys-GlcN-Ins than on the bimane derivative of Cys-GlcN-Ins (CySmB-GlcN-Ins) and disulfide of Cys-GlcN-Ins (50-fold and ≥3-fold higher, respectively), indicating that fCys-GlcN-Ins is a better substitute for MSH than Cys-GlcN-Ins (19). Lack of MSH is associated with hypersensitivity to oxidant or alkylating agents. However, M. smegmatis mshD::Tn5 exhibited no enhanced sensitivity to peroxides compared to the parental strain, supporting the suggestion that fCys-GlcN-Ins functions as an alternative for MSH in the M. smegmatis mshD::Tn5 mutant (19). In our M. smegmatis ΔmshD null mutant, a specific extra peak (P2) eluting right before the MSH bimane (MSmB) was observed during the determination of the MSH content (Fig. 4). This specific peak was absent in the complementary strain and wild type (Fig. 4), suggesting that P2 represented the formation of fCySmB-GlcN-Ins. The P2 fraction was collected for analysis using Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS). We detected a peak showing a monoisotopic mass-to-charge ratio (m/z) of 685.1995, which is close to the calculated m/z of 685.1997 for (fCySmB-GlcN-Ins + Na)+, demonstrating that P2 is fCySmB-GlcN-Ins. Therefore, we reasoned that the sensitivity to both INH and ETH in our M. smegmatis ΔmshD null mutant could also be caused by the formation of fCys-GlcN-Ins, an analog of MSH.
Fig. 4.
Formation of fCySmB-GlcN-Ins in M. smegmatis ΔmshD null deletion mutant. The cells were pelleted and labeled with 2 mM fluorescence reagent mBBr (20 mM HEPES, pH 8, 50% acetonitrile) and then heated at 60°C for 15 min and spun. MSA (5 M) was added into the supernatant to stop the label reaction, and thiols were analyzed by fluorescence HPLC. P1, CySmB-GlcN-Ins, the bimane derivative of Cys-GlcN-Ins; P2, fCySmB-GlcN-Ins, the bimane derivative of fCys-GlcN-Ins; MSmB, the MSH bimane.
Mechanisms for INH or ETH resistance in M. smegmatis MSH biosynthetic mutants remain unanswered. Since previous studies has shown that coresistance to both INH and ETH in M. smegmatis and M. bovis mutants was linked to mutations in the ndh-encoded type II NADH dehydrogenase (NdhII) (36) due to the increased intracellular NADH level, we measured the NADH concentration in our mutants. None of our INH/ETH-resistant ΔmshA, ΔmshB, ΔmshC, and ΔmshB Δmca null mutants had an increased level of NADH (data not shown). Furthermore, our M. smegmatis ΔmshB null deletion mutant reveals an intriguing difference from the mshA and mshC single deletion mutants, as its resistance only to ETH indicates that MSH may not be involved in the common pathway of INH and ETH action. Therefore, we hypothesize that MSH must be involved in the activation of INH and ETH. In vitro, MSH was shown to slightly enhance the activity of M. tuberculosis EthA, suggesting that MSH is involved in the ETH activation step in M. tuberculosis (34). Our M. smegmatis MSH mutants confirmed the role of MSH in ETH susceptibility. In contrast to our M. smegmatis mshA deletion mutant, the M. tuberculosis ΔmshA mutant was sensitive to INH (34). We speculate that MSH also plays a role in the activation of INH by KatG, but since M. tuberculosis KatG is more efficient to activate INH than M. smegmatis KatG, the lack of MSH biosynthesis has no effect on INH susceptibility in TB. In contrast, in M. smegmatis, with a less efficient activation of the drug, inhibition of MSH biosynthesis results in a 4- to 8-fold increase in INH resistance.
The main finding of this study is the demonstration of the essentiality of MSH for INH or ETH susceptibility in M. smegmatis. The exception in the case of ΔmshD is probably caused by the availability of an alternative reducing molecule, fCys-GlcN-Ins. With the increasing use of ETH in the treatment of XDR-TB, screening for mutations in mshA, mshB, and mshC for clinical M. tuberculosis isolates is merited. This knowledge would facilitate the development of new diagnostics for ETH resistance using DNA analysis methodologies.
ACKNOWLEDGMENTS
We thank Derek Smith and Jun Han at the UVic-Genome BC Proteomics Centre for their assistance in the mass spectrometry sample analysis. We are grateful to David Thaler for helpful comments on the manuscript.
We acknowledge support for this work from NIH grant AI26170 and the CFAR grant AI051519. Research at the Y. Av-Gay Laboratory was supported by a British Columbia Lung Association operating grant.
Footnotes
Published ahead of print on 18 April 2011.
REFERENCES
- 1. Banerjee A., et al. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227–230 [DOI] [PubMed] [Google Scholar]
- 2. Bardarov S., et al. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007–3017 [DOI] [PubMed] [Google Scholar]
- 3. Bardou F., Raynaud C., Ramos C., Laneelle M. A., Laneelle G. 1998. Mechanism of isoniazid uptake in Mycobacterium tuberculosis. Microbiology 144(Pt 9):2539–2544 [DOI] [PubMed] [Google Scholar]
- 4. Baulard A. R., et al. 2000. Activation of the pro-drug ethionamide is regulated in mycobacteria. J. Biol. Chem. 275:28326–28331 [DOI] [PubMed] [Google Scholar]
- 5. Bernstein J., Lott W. A., Steinberg B. A., Yale H. L. 1952. Chemotherapy of experimental tuberculosis. V. Isonicotinic acid hydrazide (nydrazid) and related compounds. Am. Rev. Tuberc. 65:357–364 [DOI] [PubMed] [Google Scholar]
- 6. Buchmeier N. A., Newton G. L., Fahey R. C. 2006. A mycothiol synthase mutant of Mycobacterium tuberculosis has an altered thiol-disulfide content and limited tolerance to stress. J. Bacteriol. 188:6245–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchmeier N. A., Newton G. L., Koledin T., Fahey R. C. 2003. Association of mycothiol with protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics. Mol. Microbiol. 47:1723–1732 [DOI] [PubMed] [Google Scholar]
- 8. Bzymek K. P., Newton G. L., Ta P., Fahey R. C. 2007. Mycothiol import by Mycobacterium smegmatis and function as a resource for metabolic precursors and energy production. J. Bacteriol. 189:6796–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fox H. H. 1952. The chemical approach to the control of tuberculosis. Science 116:129–134 [DOI] [PubMed] [Google Scholar]
- 10. Hazbon M. H., et al. 2006. Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 50:2640–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koledin T., Newton G. L., Fahey R. C. 2002. Identification of the mycothiol synthase gene (mshD) encoding the acetyltransferase producing mycothiol in actinomycetes. Arch. Microbiol. 178:331–337 [DOI] [PubMed] [Google Scholar]
- 12. Morlock G. P., Metchock B., Sikes D., Crawford J. T., Cooksey R. C. 2003. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 47:3799–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mukherjee J. S., et al. 2004. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet 363:474–481 [DOI] [PubMed] [Google Scholar]
- 14. Newton G. L., et al. 1996. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 178:1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newton G. L., Av-Gay Y., Fahey R. C. 2000. N-Acetyl-1-d-myo-inosityl-2-amino-2-deoxy-alpha-d-glucopyranoside deacetylase (MshB) is a key enzyme in mycothiol biosynthesis. J. Bacteriol. 182:6958–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newton G. L., Av-Gay Y., Fahey R. C. 2000. A novel mycothiol-dependent detoxification pathway in mycobacteria involving mycothiol S-conjugate amidase. Biochemistry 39:10739–10746 [DOI] [PubMed] [Google Scholar]
- 17. Newton G. L., Buchmeier N., Fahey R. C. 2008. Biosynthesis and functions of mycothiol, the unique protective thiol of actinobacteria. Microbiol. Mol. Biol. Rev. 72:471–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newton G. L., et al. 2003. The glycosyltransferase gene encoding the enzyme catalyzing the first step of mycothiol biosynthesis (mshA). J. Bacteriol. 185:3476–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newton G. L., Ta P., Fahey R. C. 2005. A mycothiol synthase mutant of Mycobacterium smegmatis produces novel thiols and has an altered thiol redox status. J. Bacteriol. 187:7309–7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quemard A., et al. 1995. Enzymatic characterization of the target for isoniazid in Mycobacterium tuberculosis. Biochemistry 34:8235–8241 [DOI] [PubMed] [Google Scholar]
- 21. Rawat M., Kovacevic S., Billman-Jacobe H., Av-Gay Y. 2003. Inactivation of mshB, a key gene in the mycothiol biosynthesis pathway in Mycobacterium smegmatis. Microbiology 149:1341–1349 [DOI] [PubMed] [Google Scholar]
- 22. Rawat M., et al. 2002. Mycothiol-deficient Mycobacterium smegmatis mutants are hypersensitive to alkylating agents, free radicals, and antibiotics. Antimicrob. Agents Chemother. 46:3348–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rawat M., et al. 2004. Targeted mutagenesis of the Mycobacterium smegmatis mca gene, encoding a mycothiol-dependent detoxification protein. J. Bacteriol. 186:6050–6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rawat R., Whitty A., Tonge P. J. 2003. The isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance. Proc. Natl. Acad. Sci. U. S. A. 100:13881–13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rozwarski D. A., Grant G. A., Barton D. H., Jacobs W. R., Jr., Sacchettini J. C. 1998. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279:98–102 [DOI] [PubMed] [Google Scholar]
- 26. Sambandamurthy V. K., et al. 2002. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 8:1171–1174 [DOI] [PubMed] [Google Scholar]
- 27. Sareen D., Steffek M., Newton G. L., Fahey R. C. 2002. ATP-dependent l-cysteine:1D-myo-inosityl 2-amino-2-deoxy-alpha-d-glucopyranoside ligase, mycothiol biosynthesis enzyme MshC, is related to class I cysteinyl-tRNA synthetases. Biochemistry 41:6885–6890 [DOI] [PubMed] [Google Scholar]
- 28. Shoeb H. A., Bowman B. U., Jr., Ottolenghi A. C., Merola A. J. 1985. Peroxidase-mediated oxidation of isoniazid. Antimicrob. Agents Chemother. 27:399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snapper S. B., Melton R. E., Mustafa S., Kieser T., Jacobs W. R., Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911–1919 [DOI] [PubMed] [Google Scholar]
- 30. Spies H. S., Steenkamp D. J. 1994. Thiols of intracellular pathogens. Identification of ovothiol A in Leishmania donovani and structural analysis of a novel thiol from Mycobacterium bovis. Eur. J. Biochem. 224:203–213 [DOI] [PubMed] [Google Scholar]
- 31. Stover C. K., et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460 [DOI] [PubMed] [Google Scholar]
- 32. Takayama K., Wang L., David H. L. 1972. Effect of isoniazid on the in vivo mycolic acid synthesis, cell growth, and viability of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ung K. S., Av-Gay Y. 2006. Mycothiol-dependent mycobacterial response to oxidative stress. FEBS Lett. 580:2712–2716 [DOI] [PubMed] [Google Scholar]
- 34. Vilcheze C., et al. 2008. Mycothiol biosynthesis is essential for ethionamide susceptibility in Mycobacterium tuberculosis. Mol. Microbiol. 69:1316–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vilcheze C., et al. 2000. Inactivation of the inhA-encoded fatty acid synthase II (FASII) enoyl-acyl carrier protein reductase induces accumulation of the FASI end products and cell lysis of Mycobacterium smegmatis. J. Bacteriol. 182:4059–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vilcheze C., et al. 2005. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob. Agents Chemother. 49:708–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang F., et al. 2007. Mechanism of thioamide drug action against tuberculosis and leprosy. J. Exp. Med. 204:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilming M., Johnsson K. 1999. Spontaneous formation of the bioactive form of the tuberculosis drug isoniazid. Angew. Chem. Int. ed. Engl. 38:2588–2590 [DOI] [PubMed] [Google Scholar]
- 39. World Health Organization 2009. Epidemiology. In Global tuberculosis control—epidemiology, strategy, financing. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/2009/pdf/chapter1.pdf [Google Scholar]
- 40. Zhang Y., Garbe T., Young D. 1993. Transformation with katG restores isoniazid-sensitivity in Mycobacterium tuberculosis isolates resistant to a range of drug concentrations. Mol. Microbiol. 8:521–524 [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y., Heym B., Allen B., Young D., Cole S. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591–593 [DOI] [PubMed] [Google Scholar]