Abstract
We describe the in vitro activity of macrolides and tetracycline antibiotics against Pythium insidiosum. The MICs were determined according to CLSI procedures (visual MIC) and by a colorimetric method [3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT)]. The lowest geometric mean (GM) MIC (MICs in μg/ml) (0.39 and 0.7 by visual reading and colorimetric method, respectively) and MIC ranges (0.125 to 2.0) were obtained for minocycline, while the highest MICs were shown for erythromycin (GM of 7.58 and 12.25 by visual reading and colorimetric method, respectively, and MIC ranged from 2 to 32). This significant in vitro activity makes these classes of antibiotics good candidates for experimental treatment of pythiosis.
TEXT
Pythiosis is a chronic pyogranulomatous and life-threatening disease caused by the fungus-like oomycetous Pythium insidiosum. This is the only known species of the genus able to cause infections in humans and animals, such as horses, dogs, cattle, cats, and sheep (19). The clinical presentations of pythiosis include cutaneous, gastrointestinal, vascular, and systemic forms, depending on the species affected and the site of infection. The disease has been reported in tropical and subtropical areas, progresses rapidly, and if not treated in the early stages, the infected host often dies within weeks (7, 19).
Radical surgery, amputation, and surgical debridement of lesions are the most used and effective treatments, but high rates of recurrence (45%) have been reported (7). In contrast, positive results have been observed in small and superficial lesions (19). Immunotherapy using material derived from killed mycelium from P. insidiosum is a promising noninvasive therapy and provides beneficial results, especially in early lesions (20). The efficacy of immunotherapy is substantial in horses and humans, with cure rates above 70% and 55%, respectively. However, low cure rates are described in dogs and cats (7).
Although P. insidiosum isolates show variable susceptibility to antifungal drugs in vitro (1, 2), inconsistent results of success and failure have been reported using antifungal therapies (9, 21). The inability of the genus Pythium to synthesize ergosterol explains the difficulties of antifungal therapy because ergosterol is the target of action for the majority of antifungal agents (19). Interestingly, previous studies have shown that Pythium ultimum and Pythium debaryanum are quite sensitive to the 70S ribosome-active antibiotics, such as tetracyclines and erythromycin (16, 17).
Tetracyclines and macrolides have antimicrobial activity, alone and in combination with other drugs, against other eukaryotic organisms, including algae (10), fungi (11, 14, 22), and protozoa (6, 12). Although the mechanism of action of these drugs on eukaryotic organisms is not completely understood, the main mechanisms described include the inhibition of protein synthesis (14, 17) and selective inhibition of both mitochondrial and plastid activity (12) and intracellular calcium release (22). However, there have been no susceptibility studies using these classes of antibiotics against P. insidiosum.
The aim of this study is to evaluate the in vitro susceptibility of P. insidiosum to macrolides and tetracycline antibiotics using the CLSI M38-A2 microdilution technique (5). Furthermore, to determine the MIC, the activities of antibiotic drugs were also established by a colorimetric method employing the dye 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT).
We evaluated the susceptibility of 25 P. insidiosum strains isolated from pythiosis lesions and the reference strain P. insidiosum CBS 101555. All of the isolates tested came from cases of equine pythiosis and were identified by a PCR-based assay by the method of Grooters and Gee (8).
The macrolides (erythromycin [Pharma Nostra], azithromycin [Pharma Nostra], and clarithromycin [Genix]) and the tetracyclines (minocycline [Pharma Nostra], tetracycline [Pharma Nostra], oxytetracycline [Pfizer], and doxycycline [Galena]) were obtained commercially and were diluted in dimethyl sulfoxide to generate stock solutions. The susceptibility tests were performed following the CLSI M38-A2 protocol (5), adapted for P. insidiosum by the method of Pereira et al. (15). Briefly, zoospore inoculum was obtained by the zoosporogenesis technique (20) and counted using a Neubauer chamber. The inoculum was diluted in RPMI 1640 broth (with l-glutamine and glucose and buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid [MOPS]) to obtain an inoculum with a final concentration of 2 × 103 to 3 × 103 zoospores/ml. The drugs were serially diluted in RPMI 1640 broth to obtain final concentrations ranging from 32 to 0.06 μg/ml. All of the assays were performed in duplicate.
The MICs were determined by visual observation after 24 h of incubation at 37°C and were designated MIC-1 and MIC-2, representing the lowest concentration of the drug that inhibits growth (100% visible-growth inhibition) and a prominent reduction in growth (50% visible-growth inhibition), respectively, compared to that of the drug-free control. The MICs were also determined by a colorimetric method using the MTT dye (Sigma Chemical, St. Louis, MO) as previously described by Meletiadis et al. (13) and were named MIC-3 and MIC-4. These parameters represent the lowest concentration of the drug that inhibits growth at 100% (MIC-3) and 50% (MIC-4), compared to that of the drug-free control. The color was assessed spectrophotometrically with a microplate reader (Bio-Rad Laboratories, Hercules, CA), based on the relative optical density at 595 nm.
All of the isolates produced detectable growth at 24 h. Table 1 summarizes the MIC ranges and the geometric means (GMs) of the MICs for the 26 strains of P. insidiosum tested. Minocycline was considered the most effective drug tested because it required the lowest concentration for the inhibition of P. insidiosum, with MIC-1 values ranging from 0.125 to 2 μg/ml and a GM of 0.39 μg/ml. For clarithromycin and doxycycline, the MIC-1 values ranged from 0.5 to 8 μg/ml, with GM values of 1.53 and 1.75 μg/ml, respectively. Similar GM values (MICs in μg/ml) were reported for azithromycin (4.57), erythromycin (7.58), oxytetracycline (7.38), and tetracycline (5.96) for which MIC-1 values ranged from 2 to 32 μg/ml.
Table 1.
In vitro susceptibility of 26 isolates of Pythium insidiosum to macrolides and tetracycline antibiotics
| Antimicrobial agent | MIC range (geometric mean) (μg/ml) by CLSI techniquea |
|||
|---|---|---|---|---|
| Visual reading |
Colorimetric reading (MTT) |
|||
| MIC-1 | MIC-2 | MIC-3 | MIC-4 | |
| Tetracyclines | ||||
| Doxycycline | 0.5–8 (1.75) | 0.125–1 (0.35) | 0.5–8 (2.75) | 0.125–0.5 (0.30) |
| Minocycline | 0.125–2 (0.39) | 0.06–0.5 (0.08) | 0.125–2 (0.70) | 0.06–0.5 (0.09) |
| Oxytetracycline | 2–32 (7.38) | 1–2 (1.57) | 2–32 (11.01) | 0.5–2 (1.41) |
| Tetracycline | 2–32 (5.96) | 0.5–2.0 (1.2) | 2–32 (8.90) | 1–2 (1.3) |
| Macrolides | ||||
| Azithromycin | 2–32 (4.57) | 0.5–2 (1.11) | 2–32 (6.29) | 0.5–2 (1.3) |
| Clarithromycin | 0.5–8 (1.53) | 0.125–1 (0.49) | 0.5–8 (2.22) | 0.25–2 (0.57) |
| Erythromycin | 2–32 (7.58) | 0.5–4 (1.61) | 2–32 (12.25) | 0.5–4 (1.5) |
MIC-1 and MIC-3 are the lowest concentrations of drug that inhibit growth (100% growth inhibition). MIC-2 and MIC-4 are the drug concentrations that result in prominent reduction in growth (50% growth inhibition) compared to growth of a the drug-free control.
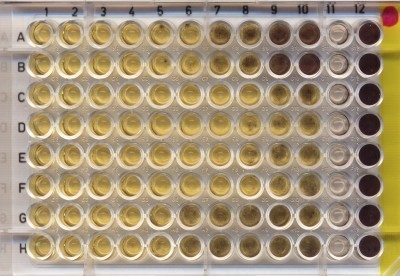
The MIC-3 based on the colorimetric MTT method allows the detection of small amounts of growth, which was easier than the visual observation method and was more accurate in the detection of microbial growth inhibition (Fig. 1). Although the ranges of the MIC-1 and MIC-3 were the same for all antibiotics tested, the MIC-3 geometric mean (μg/ml) was slightly higher for minocycline (0.7), clarithromycin (2.22), doxycycline (2.75), azithromycin (6.29), erythromycin (12.25), oxytetracycline (11.01), and tetracycline (8.90) (Table 1).
Fig. 1.
In vitro activity of azithromycin (rows A and B), clarithromycin (rows C and D), doxycycline (rows E and F), and erythromycin (rows G and H) against Pythium insidiosum after the inclusion of MTT. The yellow wells yellow indicate fungal inhibition, while hyphae are stained purple. The drug concentrations 10-fold serially diluted decreased from columns 1 (32 μg/ml) to 10 (0.06 μg/ml); column 11 is the negative control, and column 12 is the positive control (growth of P. insidiosum without drugs).
The ranges for MIC-2 and MIC-4 (MICs shown in μg/ml), respectively, for the different antibiotics were as follows: minocycline, 0.06 to 0.5 and 0.06 to 0.5; clarithromycin, 0.125 to 1 and 0.25 to 2; doxycycline, 0.125 to 1 and 0.125 to 0.5; azithromycin, 0.5 to 2 and 0.5 to 2; erythromycin, 0.5 to 4 and 0.5 to 4; oxytetracycline, 1 to 2 and 0.5 to 2; and tetracycline, 0.5 to 2 and 1 to 2. The geometric means of these MICs are shown in Table 1.
The oral systemic doses and peak blood levels achieved in humans, respectively, were as follows: tetracycline or oxytetracycline, 500 mg and 4 to 6 μg/ml; doxycycline or minocycline, 200 mg and 2 to 4 μg/ml; erythromycin, 2 g and 2 μg/ml; clarithromycin, 500 mg and 2 to 3 μg/ml; and azithromycin, 500 mg and 0.4 μg/ml (4). Although the human blood levels achieved are directly correlated with the selection of the drug in clinical trials, other pharmacological factors must be considered. For example, while azithromycin produces relatively low concentrations in serum (0.4 μg/ml), the concentrations in tissue exceed the concentrations in serum by 10- to 100-fold (4).
In animals, the peak blood levels achieved by antibiotics are species dependent, and little information on the clinical use of newer drugs is available. For horses (10 mg/kg of body weight), peak serum oxytetracycline concentrations of 16.85 μg/ml were observed after 30 min postinjection and extended serum concentrations exceeding 0.5 μg/ml (persisting for 87 h) were observed with long-acting formulations. In dogs and cats, after the administration of 5 mg/kg, doxycycline peaks of 11.56 μg/ml and 22.89 μg/ml were observed, respectively (18).
Recent evaluations of the susceptibility of P. insidiosum to antifungal agents showed 100% growth inhibition MICs (μg/ml) ranging from 8 to 64 for caspofungin (15), 16 to 32 for itraconazole and voriconazole, 0.5 to 8 for terbinafine (1), 4 to 32 for miconazole, 16 to 64 for ketoconazole, 32 to 64 for fluconazole (2), and 8 to 32 for amphotericin B (3). Comparing these previous results with those obtained in this study, it appears that the use of these antibiotics has promising potential in the treatment of pythiosis.
This is the first description of the inhibitory action of macrolides and tetracycline antibiotics against P. insidiosum isolated from animal pythiosis cases. The mechanism of action of these classes of antibiotics against P. insidiosum should be similar to that described for Pythium ultimum, i.e., reduced incorporation of amino acids into proteins, inhibition of protein synthesis, and inhibition of amino acid transport (17).
In conclusion, based on the in vitro data reported here, macrolides and tetracycline antibiotics deserve attention as new candidates for the alternative treatment of pythiosis. The known pharmacology and safety of these antibiotics allows for their consideration as new therapeutic options for testing in animal models of disease, alone or in combination with other drugs, to determine their potential in the treatment of pythiosis. Furthermore, studies using geographically and genetically diverse P. insidiosum strains will be needed for a better understanding of the susceptibility of this species to these and other antibiotics.
Acknowledgments
We thank the Brazilian agency CAPES for their support. Érico Silva Loreto is the recipient of a PRODOC-CAPES fellowship.
Footnotes
Published ahead of print on 2 May 2011.
REFERENCES
- 1. Argenta J. S., et al. 2008. In vitro activities of voriconazole, itraconazole, and terbinafine alone or in combination against Pythium insidiosum isolates from Brazil. Antimicrob. Agents Chemother. 52:767–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cavalheiro A. S., et al. 2009. In vitro activity of terbinafine combined with caspofungin and azoles against Pythium insidiosum. Antimicrob. Agents Chemother. 53:2136–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cavalheiro A. S., et al. 2009. In vitro activity of terbinafine associated to amphotericin B, fluvastatin, rifampicin, metronidazole and ibuprofen against Pythium insidiosum. Vet. Microbiol. 137:408–411 [DOI] [PubMed] [Google Scholar]
- 4. Chambers H. F., Deck D. H. 2009. Tetracyclines, macrolides, clindamycin, chloramphenicol, streptogramins, and oxazolidinones, p. 795–806 In Katzung B. G., Masters S. B., Trevor A. J. (ed.), Basic and clinical pharmacology, 11th ed. McGraw-Hill Medical, New York, NY [Google Scholar]
- 5. Clinical Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard, 2nd ed. M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6. Derouin F., Caroff B., Chau F., Prokocimer P., Pocidalo J. J. 1992. Synergistic activity of clarithromycin and minocycline in an animal model of acute experimental toxoplasmosis. Antimicrob. Agents Chemother. 36:2852–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaastra W., et al. 2010. Pythium insidiosum: an overview. Vet. Microbiol. 146:1–16 [DOI] [PubMed] [Google Scholar]
- 8. Grooters A. M., Gee M. K. 2002. Development of a nested polymerase chain reaction assay for the detection and identification of Pythium insidiosum. J. Vet. Intern. Med. 16:147–152 [DOI] [PubMed] [Google Scholar]
- 9. Krajaejun T., et al. 2006. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin. Infect. Dis. 43:569–576 [DOI] [PubMed] [Google Scholar]
- 10. Lass-Florl C., Mayr A. 2007. Human protothecosis. Clin. Microbiol. Rev. 20:230–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lew M. A., Beckett K. M., Levin M. J. 1977. Antifungal activity of four tetracycline analogues against Candida albicans in vitro: potentiation by amphotericin B. J. Infect. Dis. 136:263–270 [DOI] [PubMed] [Google Scholar]
- 12. Lin Q. H., Katakura K., Suzuki M. 2002. Inhibition of mitochondrial and plastid activity of Plasmodium falciparum by minocycline. FEBS Lett. 515:71–74 [DOI] [PubMed] [Google Scholar]
- 13. Meletiadis J., Meis J. F. G. M., Mouton J. W., Donnelly J. P., Verweij P. E. 2000. Comparison of NCCLS and 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J. Clin. Microbiol. 38:2949–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen M. H., Clancy C. J., Yu Y. C., Lewin A. S. 1997. Potentiation of antifungal activity of amphotericin B by azithromycin against Aspergillus species. Eur. J. Clin. Microbiol. 16:846–848 [DOI] [PubMed] [Google Scholar]
- 15. Pereira D. I. B., et al. 2007. Caspofungin in vitro and in vivo activity against Brazilian Pythium insidiosum strains isolated from animals. J. Antimicrob. Chemother. 60:1168–1171 [DOI] [PubMed] [Google Scholar]
- 16. Rawn C. D., Schwarz M. 1987. Protection of Pythium species against antibacterial antibiotics by cholesterol. Phytopathology 77:319–323 [Google Scholar]
- 17. Rawn C. D., Vanetten J. L. 1978. Mechanism of antibacterial antibiotic sensitivity in Pythium ultimum. J. Gen. Microbiol. 108:133–139 [Google Scholar]
- 18. Riviere J. E. 2009. Tetracycline antibiotics, p. 895–914 In Riviere J. E., Papich M. G.(ed.), Veterinary pharmacology and therapeutics, 9th ed. Wiley-Blackwell, Ames, IA [Google Scholar]
- 19. Santurio J. M., Alves S. H., Pereira D. B., Argenta J. S. 2006. Pythiosis: an emergent mycosis. Acta Sci. Vet. 34:1–14 [Google Scholar]
- 20. Santurio J. M., et al. 2003. Three types of immunotherapics against pythiosis insidiosi developed and evaluated. Vaccine 21:2535–2540 [DOI] [PubMed] [Google Scholar]
- 21. Shenep J. L., et al. 1998. Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin. Infect. Dis. 27:1388–1393 [DOI] [PubMed] [Google Scholar]
- 22. Shi W. N., et al. 2010. The combination of minocycline and fluconazole causes synergistic growth inhibition against Candida albicans: an in vitro interaction of antifungal and antibacterial agents. FEMS Yeast Res. 10:885–893 [DOI] [PubMed] [Google Scholar]