Abstract
We investigated diode laser (980 nm) evoked activation of transient receptor potential proteins (TRPV1 and TRPV2). C and A-delta (Aδ) nociceptor families are primarily responsible for pain mediation in the peripheral nervous system. TRPV1 proteins have been associated with heat evoked pain in C fibers while Aδ fibers have been associated with TRPV2. Diode laser stimulation allows a margin of safety between non-invasive activation and damage 19, 22, 34. Laser pulses (20–50 ms, 0.1–10 W, 980 nm) were used to stimulate: A) in vitro: excised patches from HEK293 cells expressing TRPV1; B) in vitro: rat DRG nociceptors expressing either TRPV1 or TRPV2; and C) in vivo: C-fibers of the rat saphenous nerve (SN) trunk. Cell currents were recorded using standard patch clamp methods. The SN was also stimulated electrically with bipolar electrodes. Stimulation (20–50 ms) of HEK and DRG cells expressing TRPV1 was highly reproducible. Activation and peak currents were achieved at estimated peak temperatures of 55°C and 70°C. Threshold activation was also observed in DRG neurons expressing TRPV2. The conduction velocity for laser-activated saphenous nerve afferents was in the C fiber range (0.5–1 m/s). Electrically stimulated nerve contained stimulation artifacts and complex neural components with conduction velocities ranging from 0.3–30 m/s. Diode laser activation of TRPV1 protein is a reproducible and effective means to probe TRP activity in both in vivo and in vitro preparations.
Keywords: Laser, Pain, Nociceptors, TRPV1, TRPV2, Optical stimulation, Compound Action Potentials, Capsazepine
1. INTRODUCTION
The development of laser devices for diagnostic and therapeutic applications has grown substantially in recent years. The means by which pain relief has been achieved is controversial. We investigated laser-evoked activation of heat gated TRPV1 and TRPV2 proteins. TRPV1 and TRPV2 are primarily responsible for activation of C and A delta nociceptors by intense heat. The proteins that form heat gated ion channels were identified and characterized in the last decade 1, 2, 3. It was recently shown4, 5 that TRPV1 proteins play an essential role in pain syndromes. TRPV1 proteins are located on peripheral endings of C nociceptors, but they can also be found within the C fiber axon itself. In inflamed or postoperative tissues, the activation threshold of heat reactive proteins is decreased and TRP density is increased6, 7. TRP protein sensitization could contribute to spontaneous firing and resting pain associated with C nociceptors. Therefore, treatments that block activation of TRPV1 may alleviate pain. Analgesics based on TRPV1 antagonists8, 9, 10 and agonists11, 12 are currently under development. Nociceptor activation and pain could be reduced if TRPV1 proteins were inhibited, down-regulated or if the nociceptors expressing these proteins were destroyed. Sources of pain may differ based upon any number of instigating factors. It is likely that TRP proteins are fundamentally involved in some pain syndromes, but not in others. In order to test for altered TRP activity, an experimental model suitable for in vitro and in vivo non-invasive activation of heat sensitive TRP proteins is highly desirable. Frequently, perfusion of heated solutions is used for in vitro activation of TRP proteins13; but this method is inadequate in technical respects and may not be useful for in vivo studies with humans and animals. Irradiation by CO214,15,16 and Tm:YAG lasers17 are less applicable for in vitro studies than for human subjects. Ideally, laser based TRP testing for laboratory experimentation on animals or diagnosis and treatment of humans could utilize the same instrument. We have previously suggested diode lasers for both in vitro and in vivo studies in both humans and rats18–22.
2. METHODS and SUBJECTS
2.1 TRPV1 infected HEK293 cells
HEK293 cells were cultured in DMEM/F12 with 10% FBS (fetal bovine serum) and transfected using Lipofectamine Superfect or Fugene 6 according to manufacturers’ protocols. Cells were transfected with 400 ng plasmid DNA encoding rat VR1, with or without 400 ng human BK2 receptor complementary DNA. To identify transfected cells, an enhanced green fluorescence protein reporter plasmid was also transfected at one-tenth the concentration of receptor cDNAs. Cells were plated onto coverslips 1–3 days before recording and examined 4–7 days post-transfection. Voltage-clamp experiments were performed at −60 mV holding potential with 320 ms voltage ramp from −120 mV to +80 mV at 1 Hz. Data was acquired using pClamp (Axon Instruments) or Pulse-Pulsefit (HEKA GmBH) software. Recordings were filtered at 5 kHz and sampled at 1 kHz. Standard bath solution for patch-clamp experiments contained 10 mM Tris/HCl, 1 mM EGTA, 1 mM MgCl2 and 150 mM CsCl at pH 7.4. The pH 6.4 solution contained 20 mM citric acid and 1 mM MgCl2, titrated with CsOH to pH 6.4 and supplemented with CsCl to make the final Cs+ concentration 150 mM. For excised patch experiments, standard bath solution was used in both the pipette and perfusion solution. No ATP was added to the solutions. Diameters of patch pipettes were 12–15 μm for HEK293 cells and recorded as described in 23. The bath temperature was monitored with probes (Warner Instruments).
2.2 Preparation of acute cultures of rat DRG neurons
Rats were anesthetized with halothane. Following decapitation, the spinal cord was rapidly removed, and the dorsal root ganglia were dissected free. Dissected ganglia were placed in a heated bath (35°C for 70 min) containing dispase II and collagenase (2mg/ml; Sigma type 1). Following wash and trituration, recovered cells were plated on 10 polylysine coated, 35 mm Petri dishes. Cells were bathed continuously in a rat Tyrode’s solution containing (mM): 140 NaCl, 4 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH. Only one cell was used from each dish. Bath temperature was maintained at 32°C by a feedback-controlled bath heater (TCbip; Cell Microsystems).
Whole cell patch recording
Electrodes were prepared (2–4 MΩ) from glass pipettes using a Brown and Flaming type horizontal puller (Sutter model P87). Whole cell recordings were made with an Axopatch 200B (Axon Instruments). Stimuli were controlled and digital records captured with pClamp 8.2 software and Digidata 1322A A/D converter (Axon Instruments). Series resistance (Rs) was compensated 40–60% with Axopatch 200B compensation circuitry. Whole cell resistance and capacitance was determined by Clampex 8.2 software utility. A liquid junction potential of approximately 4 mV was not corrected.
Cell classification protocols
Recordings were made from cells with diameters between 25 and 45 μm. Cells were classified as type 2 or 4 according to patterns of voltage-activated currents (current signatures) that were revealed by three-classification protocols24. Cells classified in this manner have consistent anatomic, pharmacologic and histochemical properties24, 25. Cell types 2 and 4 have been identified as putative C and A delta hairy skin heat nociceptors that express TRPV1 (type 2) or TRPV2 (type 4) 26, 27.
2.3 In vivo stimulation of rat saphenous nerve
Electrophysiological experiments were performed with electrical and laser stimulation. The rats were prepared as previously described28. Rats (250–300 g) were anesthetized with sodium pentobarbital (initially 50 mg/kg, i.p., with additional doses given throughout the experiment to maintain areflexia). At the end of the experiment, the rat was euthanized by pentobarbital overdose. Recordings of compound action potentials were made from the saphenous nerve. Bipolar stimulating electrodes were placed under the nerve distal to the recording site. Laser stimuli were applied in the area between the bipolar-electrode pair. The nerve was crushed proximally to the recording site to prevent flexor reflexes during electrical stimulation of the nerve. Mineral oil was used for electrical isolation. Conduction velocities of activated fibers were calculated by dividing the distance between the stimulating and recording electrodes (~30 mm) by the latency of the electrically and laser evoked action potentials. Fibers that conducted slower than 2 m/s were classified as C-fibers. Animal use committees of UCSF, Stanford University and the University of Florida approved animal use protocols.
2.4 Laser Stimuli
The Lass 10 M investigational diode laser (Lasmed LLC) was used in all studies. The Lass 10 M operates at a wavelength of 980 nm, maximum outpower of 15 W. For these studies pulse durations varied from 10–50 ms. For both patch clamp experiments and compound action potential recordings, the radiation was delivered by standard optical fiber: NA 0.22 core/cladding 0.100/0.140 mm. The diameter of laser beam at cell and nerve was varied from ~ 0.1 mm to 0.5 mm. The calibration of laser power was performed by Ophir Nova power/energy meter (Ophir Inc.). Three levels of power: 3, 4 and 5 W with pulse duration 50 ms and pulse interval 10 s were used to stimulate selected TRPV1-expressing HEK293 cells. DRG cells TRPV1 positive cells (Type 2) and TRPV2 positive cells (Type 4) were irradiated with a pulse duration of 20 ms and pulse interval ≥ 10 s. The fiber was positioned 112 um from the targeted cell at an angle of 60 degrees. The power of laser pulses was gradually increased from 0.300 W to 4.5 W with 0.5 W steps to form saturation curves.
3. EXPERIMENTAL RESULTS
3.1 Laser stimulation of HEK293 TRPV1 infected cells
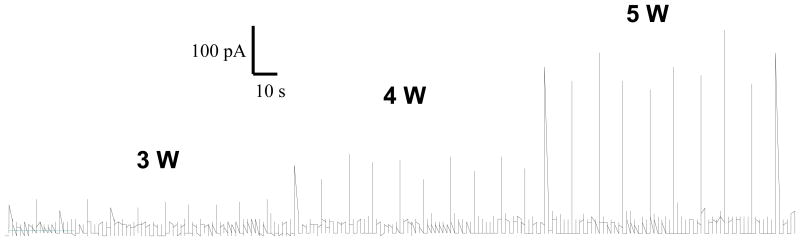
Ten excised patches of HEK293 cells were tested (TRPV1 expressing, n=9; control, n=1). The laser stimuli (3 W, 4 W, and 5 W 50 ms pulse duration) produced consistent activation of TRPV1 with relatively low variation between tests normilized STD of laser evoked current (10 stimuli with the same power) ranged from 12 – 36 %. Background noise remained constant throughout the study (<30 pA). Representative HEK cell reactivity of TRPV1 is presented in figure 1.
Fig. 1.
Currents evoked by repetitive laser stimulation of excised membrane patches HEK293 cells expressing TRPV1.
Over the range of stimulation, laser-evoked current responses were highly reproducible and stable over the timeframe of the experiments. The results confirmed our preliminary 29 data that over test periods of up to 2 hours, there was no significant cell degradation attributable to laser irradiation. Similar properties were observed in DRG neurons20, 21. High reproducibility indicates and absence of time dependent sensitization or desensitization of TRPV1 currents in HEK293 cells.
3.2 Laser stimulation of rat nociceptors expressing TRPV1 or TRPV2
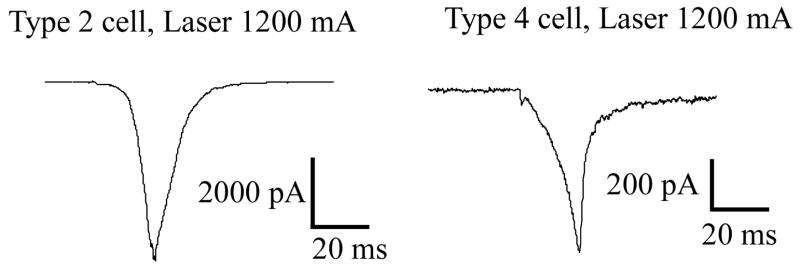
Properties of proteins expressed in neurons may differ from those artificially expressed in host cell systems (e.g. HEK293). Using the diode laser, we examined TRP protein reactivity in distinct classes of hairy skin nociceptors, which differentially express either TRPV1 (type 2) or TRPV2 (type 4). These nociceptor classes represent capsaicin sensitive (type 2) and insensitive (type 4) neurons which are both heat reactive. Fluid superfusion thresholds are 45 and 52 °C, respectively. These neurons may represent classic C-PMN and Ad-MH I class nociceptors that have been identified and characterized in vivo as afferents mediating slow burning and fast pricking pain. Laser activation produced inward currents of exceptionally fast kinetics relative to those normally observed with other heating methods (figure 2).
Fig. 2.
The kinetics of laser induced currents type 2 (TRPV1) and type 4 (TRPV2) expressing nociceptors. laser stimulus: 20 ms, 2.41 W (1.200 mA of laser pumping current)
Consistent with the mediation of currents by TRPV1, application of the selective TRPV1 antagonist capsazepine, dramatically reduced currents evoked by 980 nm stimuli (figure 3). Capsazepine (10 uM) was applied by close superfusion (two minute application) following evocation of a baseline response. All cells were stimulated with identical laser pulses: 20 ms and 2.41 W (1200 mA).
Fig. 3.
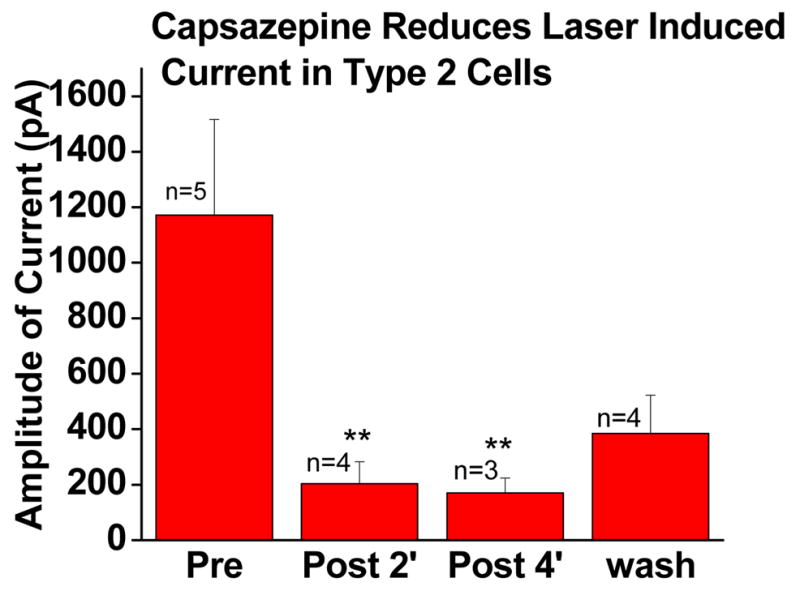
Laser induced current of TRPV1 positive (type 2) cells evoked by laser pulses: 20 ms, 2.41 W (laser current 1200 mA) before and after TRPV1 antagonist capsazepine (10 uM). Two minutes between repeated tests. Bath temperature was maintained at 32 °C between tests.
3.3 Laser induced current vs. laser power in nociceptors expressing TRPV1
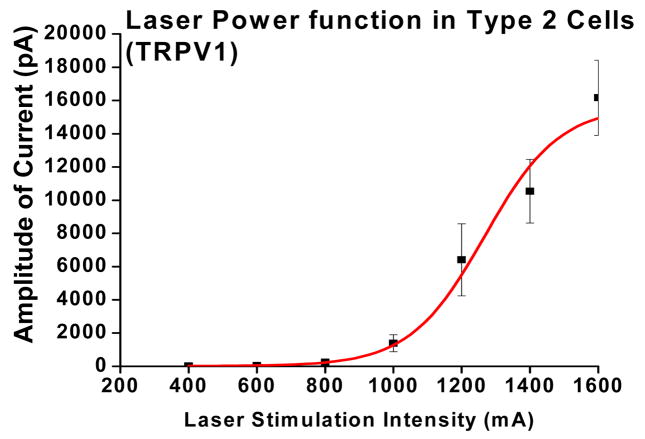
The full reactivity of TRP proteins are difficult to examine due to limitations imposed by fluid heating methods. Fluid heating is difficult to control, and may degrade membrane proteins over long exposure times required to maximize activation. We were able to drive TRPV1 currents through their full reactive range with little difficulty using 980 nm stimuli (figure 4). The threshold of laser stimulation was ~ 400 mA (~1,0 W). The saturation current/power was 1400 mA (~2.93 W). The estimated corresponding temperature threshold, extrapolated from a thermal calibration procedure30 was approximated at ~55°C. These estimated threshold temperatures are substantially higher than the thresholds (42 °C) of TRPV1-infected HEK293 and TRPV1 positive DRG cells (46°C), as determined by stimulation with heated fluids 5–20 sec2, 31. Our experiments in humans have shown that short diode laser pulses produce corresponding increases in thresholds of human pain report (57°C and 75°C for 300 and 50 ms pulses respectively19, 32.
Fig. 4.
TRPV1 positive (type 2) cells n=8 were activated by laser pulses: 20 ms, 1–6 W
3.5 Laser vs. electrical stimulation of rat saphenous nerve
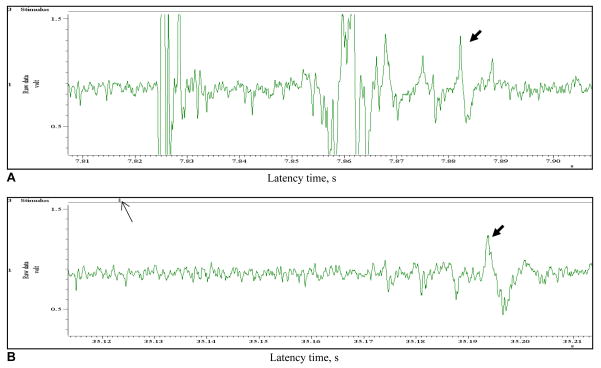
Electrical stimulation of the saphenous nerve activated a wide range of neural activity that included contributions from axons of A beta, Aδ and C fiber groups. A substantial electrical artifact was also present. In contrast, laser stimulation activated only C fiber axons. The conduction velocity of electrically activated C axons was ~ 0.46 m/s (late component of figure 5; mean latency 65 ms). The conduction velocity of laser activated axons was ~ 0.42 m/s (mean latency 70 ms). These latencies are consistent with C fibers. The activation by 980 nm irradiation was much better isolated from other nerve activity.
Fig. 5.
A, B Compound action potential (CNAP) evoked by laser and electrical stimulation of the trunk of the saphenous nerve. (A) electrical stimulation (0.25 ms); (B) laser stimulation (20 ms). Note the reduction in artifact and neural components with laser stimulation. Thick arrows indicate the probable C fiber components. Thin arrows indicate stimulus onset.
3. DISCUSSION
TRP proteins may play an important role in inflammatory and chronic pain conditions. Alterations in expression patterns, concentration of TRP proteins or their activity might contribute to increased spontaneous and evoked pain from superficial tissues (see Introduction). It is difficult to characterize the properties of these proteins using conventional methods that utilize heated fluids or agonists. In experimental settings, these methods evoke slowly developing, irregular currents that are unlike those typically associated with membrane protein function24, 27. In general, such currents are difficult to quantify because they lack consistent kinetic features normally associated with activation/deactivation/desensitization of ion channels. Such kinetic feature can themselves indicate significant changes in protein activity due to phosphorylation or modified heteromerization. Whether agonists or heated fluids are used, the slow development of current impairs attempts at quantification due to the simultaneous influence of desensitization. Laser stimulation seems better suited for quantification of TRP protein activity. Currents exhibit fast kinetics typical of membrane protein function; thereby, there will be minimal development of desensitization during study and proper evaluation of activation and desensitization kinetics. It is likely that TRP proteins have evolved to detect rapidly increasing temperatures, which are likely to be highly threatening to surface tissues. In that sense, laser activation may better represent an appropriate stimulus for the protein.
It is interesting that in human behavioral tests, brief 980 nm pulses (20 – 60 ms) primarily evoked pricking pain in humans19 and in behavioral and electrophysiology tests in rats, brief 980 nm pulses (100–200 ms) evoked only Aδ fiber activity22, 34 In the present report, 20 – 50 ms, pulses activated TRPV1 in neural cell bodies and C axons of the saphenous nerve. The absence of an Aδ component in the SN records might be due to an innervation density-spot size mismatch or higher threshold intensity, but it is not clear why C fiber activity could not be produced by similar stimuli in vivo. We hope to clarify this effect by direct test in the near future.
Using laser stimuli, we were able to drive protein function through its full range, and construct a sigmoidal dose-response’ curve of the form that is frequently used to characterize protein activity. While construction of such curves is typical in the evaluation of voltage and ligand gated proteins, it has not been possible to apply such techniques to the analysis of TRP protein function. Due to the rapid activation and consistent kinetics of currents evoked by laser stimulation, we were able to readily form curves that characterized the full range of TRP protein activity in a specific class of nociceptive cells. Presumably, such methods are applicable to quantitative assessment of protein activity that may exhibit shifts following neurotrauma, or in the development of antagonists suitable to the treatment of applicable pain conditions. Using 980 nm stimuli were able to evoke consistent, low noise, TRP protein activity in both neurons and axons. The latter is likely to have diagnostic value as TRP protein trafficking in axons may represent altered peripheral function and distribution of particular significance for chronic pain.
4. CONCLUSION
The similar optical and thermal properties of tissue, water and mineral oil for 980 nm diode laser radiation permitted non-invasive laser characterization of TRPV1 in both in vivo and in vitro preparations. These methods, combined with behavioral19–22 and electro-physiology protocols are promising tools for evaluation and development of TRP related pain treatment and diagnosis.
Acknowledgments
Authors are grateful: Drs H.H. Chuang, D. Julius and X. Cheng, J. Levine, UCSF for studies conducted in HEK cells and in vivo electrophysiology studies. The study was partially supported by NIH grant 2R44NS046951-02.
References
- 1.Caterina MJ, Shumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 3.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 4.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 5.Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2003;33(5–6):479–87. doi: 10.1016/s0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 6.Gopinath P, Wan E, Holdcroft A, Facer P, Davis J, Smith G, Bountra C, Anand P. Increased capsaicin receptor TRPV1 in skin nerve fibers and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Women’s Health. 2005:5. doi: 10.1186/1472-6874-5-2. 1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115(1–2):37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;23;25(12):3126–31. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ognyanov VI, Balan C, Bannon AW, Bo Y, Dominguez C, Fotsch C, Gore VK, Klionsky L, Ma VV, Qian YX, Tamir R, Wang X, Xi N, Xu S, Zhu D, Gavva NR, Treanor JJ. Norman Design of potent, orally available antagonists of the transient receptor potential vanilloid 1. Structure-activity relationships of 2-piperazin-1-yl-1H-benzimidazoles. J Med Chem. 2006;49(12):3719–42. doi: 10.1021/jm060065y. [DOI] [PubMed] [Google Scholar]
- 10.Rami HK, Thompson M, Stemp G, Fell S, Jerman JC, Stevens AJ, Smart D, Sargent B, Sanderson D, Randall AD, Gunthorpe MJ, Davis JB. Discovery of SB-705498: a potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg Med Chem Lett. 2006;16(12):3287–91. doi: 10.1016/j.bmcl.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. J Clin Invest. 2004;113(9):1344–52. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tender GC, Walbridge S, Olah Z, Karai L, Iadarola M, Oldfield EH, Lonser LL. Selective ablation of nociceptive neurons for elimination of Hyper-algesia and neurogenic inflammation. J Neurosurg. 2005;102:522–525. doi: 10.3171/jns.2005.102.3.0522. [DOI] [PubMed] [Google Scholar]
- 13.Dittert I, Benedikt J, Vyklický L, Zimmermann K, Reeh PW, Vlachová V. Improved superfusion technique for rapid cooling or heatingof cultured cells under patch-clamp conditions. Journal of Neuroscience Methods. 2006;151:178–85. doi: 10.1016/j.jneumeth.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Mor J, Carmon A. Laser emitted radiant heat for pain research. Pain. 1975;1(3):233–7. doi: 10.1016/0304-3959(75)90040-8. [DOI] [PubMed] [Google Scholar]
- 15.Meyer RA, Walker RE, Mountcastle VB. A Laser stimulator for the study of cutaneous thermal and pain sensations. IEEE Trans On Biomedical Eng. 1976;23:54–60. doi: 10.1109/tbme.1976.324616. [DOI] [PubMed] [Google Scholar]
- 16.Bromm B, Treede R-D. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol. 1984;3:33–40. [PubMed] [Google Scholar]
- 17.Spiegel J, Hansen C, Treede RD. Laser-evoked potentials after painful hand and foot stimulation in humans: evidence for generation of the middle-latency component in the secondary somatosensory cortex. Neurosci Lett. 1996;216(3):179–82. doi: 10.1016/0304-3940(96)13025-1. [DOI] [PubMed] [Google Scholar]
- 18.Nemenov MI, Gladusheva LG, Tsirulnikov EM, Andreeva IG. Thermal and Skin Pain Sensations Due to Laser Irradiation. SPIE Proceeding. 1994;2323:537–538. [Google Scholar]
- 19.Nemenov MI, Mikkelsen J. Limitations of laser application in pain research. Abstracts: 9th World Congress on Pain; Vienna. 1999. p. 94. [Google Scholar]
- 20.Greffrath W, Nemenov MI, Schwarz S, Baumgartner U, Vogel H, Arendt-Nielsen L, Treede R-D. Inward currents in primary nociceptive neurons of the rat in vitro and pain sensations in humans in vivo elicited by laser radiant heat pulses. Pflugers Arch-Eur J Physiol. 2001;41(Suppl 6):R141. [Google Scholar]
- 21.Greffrath W, Nemenov MI, Schwarz S, Baumgartner U, Vogel H, Arendt-Nielsen L, Treede R-D. Inward currents in primary nociceptive neurons of the rat and pain sensations in humans elicited by infrared diode laser pulses. Pain. 2002;99:145–155. doi: 10.1016/s0304-3959(02)00071-4. [DOI] [PubMed] [Google Scholar]
- 22.Tzabazis A, Klyukinov M, Manering N, Nemenov MI, Shafer SL, Yeomans DC. Differential activation of trigeminal C or A delta nociceptors by infrared diode laser in rats: behavioral evidence. Brain Res. 2005;1037(1–2):148–56. doi: 10.1016/j.brainres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Chuang HH, Prescott ED, Kong H, Shields S, Jordt S-E, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–62. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 24.Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified Acutely Dissociated Cells of Rat DRG: Histochemistry and Patterns of Capsaicin-, Proton-, and ATP-Activated Currents. J Neurophysiol. 2000;84:2365–2379. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- 25.Petruska JC, Napaporn J, Johnson RD, Cooper BY. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neuroscience. 2002;115:15–30. doi: 10.1016/s0306-4522(02)00409-8. [DOI] [PubMed] [Google Scholar]
- 26.Jiang N, Rau KK, Johnson RD, Cooper BY. The proton sensitivity Ca2+ permeability and molecular basis, of ASIC channels expressed in glabrous and hairy skin afferents. J Neurophysiology. 2006;95:2466–2478. doi: 10.1152/jn.00861.2005. [DOI] [PubMed] [Google Scholar]
- 27.Nene D, Jiang N, Rau KK, Cooper BY. Nociceptor Activation and Damage by Pulsed E-Fields. Proceedings of SPIE; 2006. in press. [Google Scholar]
- 28.Chen X, Levine JD. Altered temporal pattern of mechanically evoked C-fiber activity in a model of diabetic neuropathy in the rat. Neuroscience. 2003;121(4):1007–15. doi: 10.1016/s0306-4522(03)00486-x. [DOI] [PubMed] [Google Scholar]
- 29.Nemenov MI, Chuang HH, Julius D. 2003 unpublished. [Google Scholar]
- 30.Cooper BY. 2005 unpublished. [Google Scholar]
- 31.Pataputian A, Pier AM, Story GM, Viswanath V. Thermo TRP channels and beyond: mechanisms of temperature sensation. Nature Review/Neuroscience. 2003;8:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 32.Nemenov MI, Mikkelson J. 1999 unpublished. [Google Scholar]
- 33.Magerl W, Ali Z, Ellrich J, Meyer RA, Treede RD. C- and Aδ-fiber components of heat-evoked cerebral potentials in healthy human subjects. Pain. 1999;82:127–37. doi: 10.1016/S0304-3959(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 34.Nemenov MI, Mitchell K, Keller J, Yaskovich R, Iadarola MJ. Evaluation of C-fiber and A-delta stimulus parameters for tissue injury using an infrared laser diode thermal stimulator. Proceeding of American Pain Association annual conference; May 2007; in press. [Google Scholar]
- 35.Cuellar JM, Nemenov MI, Yeomans DC. 2006 unpublished. [Google Scholar]