Abstract
Treatment of N-allylic,N′-aryl ureas with a catalytic 1:1 mixture of di-tert-butyl-o-biphenylphoshphine gold(I) chloride and silver hexafluorophosphate (1 mol %) in chloroform at room temperature led to 5-exo hydroamination to form the corresponding imidazolidin-2-ones in excellent yield. In the case of N-allylic ureas that possessed an allylic alkyl, benzyloxymethyl, or acetoxymethyl substituent, gold(I)-catalyzed 5-exo hydroamination leads to formation of the corresponding trans-3,4-disubstituted imidazolidin-2-ones in excellent yield with ≥50:1 diastereoselectivity.
Keywords: Nitrogen heterocycles, Intramolecular hydroamination, Gold, Alkenes
Introduction
Substituted imidazolidin-2-ones are components of a number of biologically active compounds[1] including NK1 and Muscarinic M3 antagonists,[2,3] HIV protease and human enterovirus 71 inhibitors,[4,5] and antiparasitic[6] and immunosuppressive agents.[7] Furthermore, chiral, non-racemic imidazolidin-2-ones have been employed as chiral auxiliaries[8,9] and as scaffolds for bis(phosphine) ligands[10,11] for use in enantioselective synthesis. A number of approaches to construction of the imidazolidin-2-one ring have been developed[12–20] including carbonylation of vicinal diamines,[13] oxidative diamination of alkenes with ureas,[14,15] and electrophilic cyclization[16] or transition metal-catalyzed carboamination of N-allylic ureas.[17,18] In contrast, transition metal-catalyzed alkene hydroamination, which represents perhaps the most conceptually simple and atom-economical approach to the cyclization of readily available N-allylic ureas, has gone largely unexplored as a route to the imidazolidin-2-one ring.
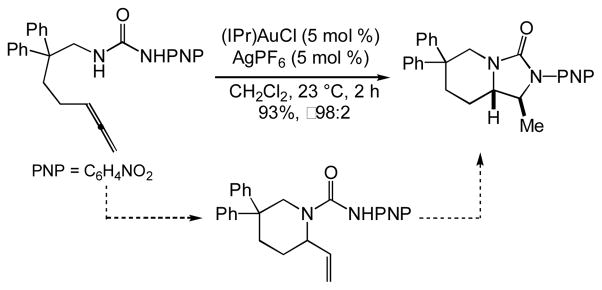
In the course of our continuing investigation of the gold(I)-catalyzed intramolecular hydroamination of allenes,[21] we recently found that treatment of N-δ-allenyl ureas with a catalytic 1:1 mixture of the gold(I) N-heterocyclic carbene complex (IPr)AuCl [IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidine] and AgPF6 (5 mol %) led to formation of bicyclic imidazolidin-2-ones in high yield and high diastereoselectivity (Scheme 1).[22] These transformations occurred via two discrete steps; initial 6-exo hydroamination of the N-δ-allenyl urea followed by 5-exo hydroamination of the resulting 1-vinyl piperidine (Scheme 1). Whereas the former step is unremarkable, the latter step represents a rare example of imidazolidin-2-one ring formation via intramolecular alkene hydroamination under remarkably mild conditions.[23–25] We therefore considered that gold(I)-catalyzed intramolecular hydroamination of acyclic N-allylic ureas might serve as an expedient route to the synthesis of substituted, monocyclic imidazolidin-2-ones. Herein we report the results of this investigation.
Scheme 1.
Gold(I)-catalyzed dihydroamination of an N-δ-allenyl urea.
Results and Discussion
Optimization and scope
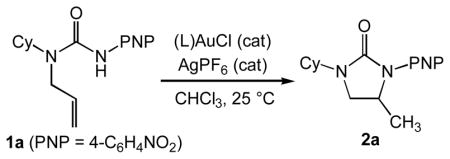
Our starting point for the gold(I)-catalyzed intramolecular hydroamination of acyclic N-allylic ureas employed the catalyst system used for the catalytic dihydroamination of N-δ-allenyl ureas with the substitution of chloroform for CH2Cl2 owing to the greater solubility of simple N-allylic ureas in the former solvent. In an initial experiment, treatment of N-allyl urea 1a with a catalytic 1:1 mixture of (IPr)AuCl (5 mol %) and AgPF6 (5 mol %) in chloroform at room temperature for 12 h led to isolation of imidazolidin-2-one 2a in 97% yield (Table 1, entry 1). Catalyst loading was lowered to 1 mol % without diminished yield, but with the anticipated increase in reaction time (Table 1, entries 2 and 3). Substitution of the sterically hindered phosphine ligand P(t-Bu)2o-biphenyl (P1) for IPr led to a ~two-fold increase in reaction rate with no diminishment of product yield (Table 1, entry 4). The effectiveness of ligand P1 in the conversion of 1a to 2a is surprising, given the marked superiority of IPr relative to P1 in the gold-catalyzed dihydroamination of N-δ-allenyl ureas.[22] The possibility that intramolecular hydroamination of N-allylic ureas is catalyzed by Ag+ or Brønsted acid generated under reaction conditions was rigorously ruled out in our investigation of allene dihydroamination.[22]
Table 1.
Effect of ligand and catalyst loading on the gold(I)-catalyzed conversion of 1a to 2a.
 | ||||
|---|---|---|---|---|
| entry | L | cat load (mol %) | time (h) | yield (%)[a] |
| 1 | IPr | 5 | 12 | 97 |
| 2 | IPr | 2 | 15 | 97 |
| 3 | IPr | 1 | 30 | 97 |
| 4 | P1 | 1 | 15 | 100 |
|
| ||||
Isolated yields of >95% purity.
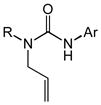
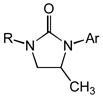
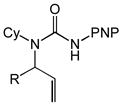
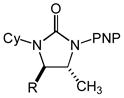
Acyclic N-allyl,N′-aryl ureas 1b-1f that possessed either an electron-rich or electron-deficient N′-aryl group in combination with an N-alkyl or N-aryl substituent underwent gold(I)-catalyzed intramolecular hydroamination to form the corresponding imidazolidin-2-ones 2b-2f in excellent yield (Table 2, entries 1–5). However, whereas the nature of the N′-aryl group had little effect on the rate of cyclization, N-methyl ureas 1b and 1d underwent intramolecular hydroamination at lower rates than those bearing an N-Cy (1a and 1f) or N-Ph (1c and 1e) group (Table 2). Acyclic N-allylic ureas that possessed an allylic methyl (3a), isopropyl (3b), benzyloxymethyl (3c), or acetoxymethyl (3d) substituent underwent gold (I)-catalyzed intramolecular hydroamination to form the corresponding trans-3,4-disubstituted imidazolidin-2-ones 4a-4d in excellent yield with ≥50:1 diastereoselectivity (Table 2, entries 6–9). In comparison, gold(I)-catalyzed intramolecular hydroamination of N-allylic urea 5 that possessed an allylic hydroxymethyl group led to predominant (dr = 3.7:1) formation of the cis-imidazolidin-2-one 6 in 97% yield (table 2, entry 10).
Table 2.
Substrate scope of the intramolecular hydroamination of N-allylic ureas catalyzed by a 1:1 mixture of (P1)AuCl and AgPF6 (1 mol %) in CHCl3 at room temperature (PNP = 4-C6H4NO2, PMP = 4-C6H4OMe).
| entry | substrate | product | time (h) | yield[a] | dr[b] |
|---|---|---|---|---|---|
|
|
||||
| 1[c] | 1b (R = Me; Ar = PNP) | 2b | 48 | 92 | — |
| 2[d] | 1c (R = Ph; Ar = PNP) | 2c | 15 | 93 | — |
| 3[e] | 1d (R = Me; Ar = Ph) | 2d | 72 | 86 | — |
| 4 | 1e (R = Ph; Ar = PMP) | 2e | 16 | 97 | — |
| 5 | 1f (R = Cy; Ar = Ph) | 2f | 30 | 92 | — |
|
|
||||
| 6 | 3a (R = Me) | 4a | 15 | 100 | 50:1 |
| 7 | 3b (R = i-Pr) | 4b | 16 | 93 | 50:1 |
| 8 | 3c (R = CH2OBn) | 4c | 16 | 98 | 50:1 |
| 9 | 3d (R = CH2OAc) | 4d | 16 | 98 | 50:1 |
| 10 |
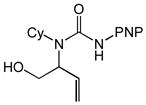 5 |
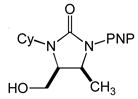 6 |
16 | 97 | 3.7:1 |
Isolated yields of >95% purity.
Determined by 1H NMR analysis of the crude reaction mixture..
Catalyst loading = 5 mol %.
Reaction temperature = 60 °C.
Catalyst loading = 10 mol %.
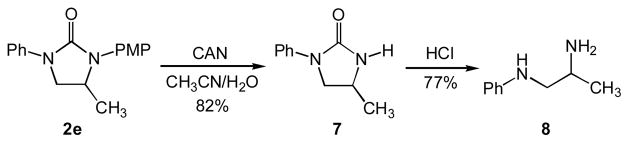
Imidazolidin-2-ones also serve as precursors to vicinal diamines and utilization of the p-methoxyphenyl (PMP) group allows for efficient dearylation to form primary amines. For example, oxidative removal of the PMP group of 2e with ceric ammonium nitrate (CAN)[26] followed by acid-catalyzed hydrolysis[20,27] of N–H imidazolidine-2-one 7 led to isolation of the differentially substituted vicinal diamine 8 in 63% yield over two steps (Scheme 2).
Scheme 2.
Dearylation and hydrolysis of imidazolidin-2-one 2e.
Proposed mechanism and stereochemical model
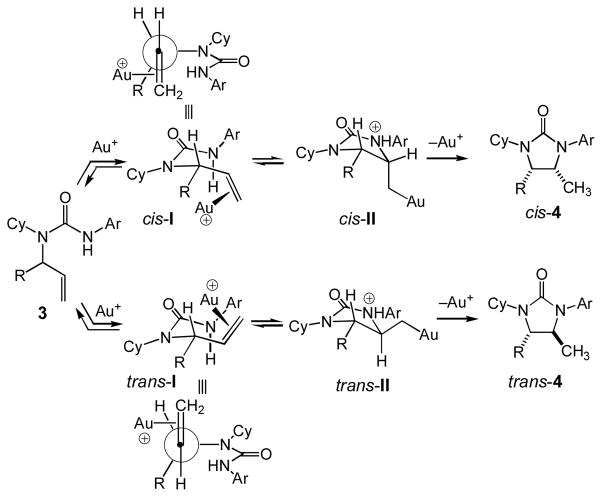
We have previously proposed a mechanism for the gold(I)-catalyzed intramolecular hydroamination of alkenes with carbamates that involves outer-sphere addition of the nucleophile on a cationic gold(I) π-alkene complex followed by protodeauration with acid released in the C–N bond forming step,[23] although little direct evidence supported this contention.[28] Since that time, we[29] and others[30] have synthesized and characterized cationic gold(I)π-alkene complexes and Toste has recently demonstrated the stoichiometric intramolecular aminoauration of N-γ-alkenyl ureas with (PPh3)AuNTf2 and triethylamine.[31] Toste also demonstrated that treatment of these (β-amino)alkyl gold complexes with TsOH led to rapid reversion to regenerate the N-γ-alkenyl ureas, which was followed by slow protodeauration to form the 1-methyl pyrrolidine.[31] Although protodeauration was slow, it appears reasonable that more electron-rich supporting ligands such as P1 might facilitate protodemetallation. In any event, these results both establish the outer-sphere aminoauration of alkenes with urea nucleophiles and also point to the potential reversibility of C–N bond formation.
From this discussion, it follows that the trans-configuration of 3,4-disubstituted imidazolidin-2-ones 4a-4d may be determined either by C–N bond formation in the case of irreversible aminoauration or by protodeauration in the case of reversible aminoauration. In the case of irreversible C–N bond formation, aminoauration of gold (π-alkene) intermediate trans-I should be favored relative to aminoauration of cis-I owing to unfavorable interaction of both the alkenyl =CH2 group (A1,3 strain) and the coordinated gold atom with the allylic substituent that is absent in the case of trans-I (Scheme 3). In the case of reversible C–N bond formation, protodeauration from intermediate trans-II should be favored relative to protodeauration of cis-II owing to the unfavorable steric interaction between the exocyclic –CH2Au(P1) group and the vicinal R group of cis-II that should be felt in the transition state for protodeauration from cis-II to form cis-4 (Scheme 3).
Scheme 3.
Proposed mechanism and stereochemical model for the gold(I)-catalyzed cyclization of N-allylic ureas 3.
Preferential formation of cis-6 in the gold(I)-catalyzed cyclization of N-allylic urea 5 is enigmatic but may result from stabilizing ligation of the allylic hydroxyl group to gold in the transition states for conversion of cis-I to cis-II and/or the conversion of cis-II to cis-4 that overrides the inherent steric destabilization of these transition states. Alternatively, recent computational analyses have pointed to the potential role of solvent, and/or counterion in the transfer of proton from the protonated nucleophile to the α-carbon atom of the gold σ-complex generated via nucleophilic addition to a gold(I) π-complex.[32] As such, it also appears feasible that the pendant hydroxyl group of 5, either in protonated form or as part of hydrogen-bonded species, may function as an intramolecular proton source for the protodeauration of cis-II leading to preferential formation of cis-6.[33]
Conclusion
We have shown that a 1:1 mixture of (P1)AuCl [P1 = P(t-Bu)2o-biphenyl] and AgPF6 catalyzes the 5-exo hydroamination of N-allylic,N′-aryl ureas to form monocyclic imidazolidin-2-ones in excellent yield under mild conditions and with low catalyst loading. Furthermore, in the case of N-allylic ureas that possessed an allylic alkyl, benzyloxymethyl, or acetoxymethyl substituent, gold(I)-catalyzed 5-exo hydroamination leads to formation of the corresponding trans-3,4-disubstituted imidazolidin-2-ones in ≥93% yield with ≥50:1 diastereoselectivity.
Experimental Section
General Remarks
Catalytic reactions were performed in sealed glass tubes under an atmosphere of dry nitrogen unless noted otherwise. NMR spectra were obtained on a Varian spectrometer operating at 400 MHz for 1H NMR and 100 MHz for 13C NMR in CDCl3 unless noted otherwise. IR spectra were obtained on a Bomen MB-100 FT IR spectrometer. Gas chromatography was performed on a Hewlett-Packard 5890 gas chromatograph equipped with a 25 m polydimethylsiloxane capillary column. Flash column chromatography was performed employing 200–400 mesh silica gel (EM). Thin layer chromatography (TLC) was performed on silica gel 60 F254. Elemental analyses were performed by Complete Analysis Laboratories (Parsippany, NJ). N-Allylic ureas were synthesized employing standard procedures (see Supporting Information).
Imidazolidin-2-ones
1-Cyclohexyl-4-methyl-3-(4-nitrophenyl)imidazolidin-2-one (2a)
A suspension of 1a (30 mg, 0.10 mmol), (P1)AuCl (0.53 mg, 1.0 × 10−3 mmol), and AgPF6 (0.25 mg, 1.0 × 10−3 mmol) in CHCl3 (0.5 mL) was stirred for 15 h at room temperature. The crude reaction mixture was loaded directly onto a silica gel column and chromatographed (hexanes–EtOAc = 6:1) to give 2a (30 mg, 100%) as a yellow solid. TLC (hexanes–EtOAc = 2:1): Rf = 0.4. 1H NMR: δ = 8.14 (d, J = 9.6 Hz, 2 H), 7.66 (d, J = 9.6 Hz, 2 H), 4.36 (m, 1 H), 3.79 (m, 1 H), 3.63 (t, J = 8.8 Hz, 1 H), 3.04 (dd, J = 4.0, 8.8 Hz, 1 H), 1.80-1.64 (m, 5 H), 1.42-1.22 (m, 4 H), 1.32 (d, J = 6.0 Hz, 3 H), 1.08 (m, 1 H). 13C{1H} NMR: δ = 155.5, 145.3, 141.5, 124.8, 117.4, 51.4, 48.8, 45.1, 30.4, 29.8, 25.4, 25.3, 18.8. IR (neat, cm−1): 2938, 1684, 1503, 1323, 1252, 1111, 851, 751, 690. Anal. calcd (found) for C16H21N3O3: H, 6.98 (6.83); C, 63.35 (63.30).
Imidazolidin-2-ones 2b-2f, 4a-4d, and 6 were synthesized employing procedures similar to that used to synthesize 2a.
1,4-Dimethyl-3-(4-nitrophenyl)imidazolidin-2-one (2b)
Yellow solid, 92%. TLC (hexanes–EtOAc = 2:1): Rf = 0.2. 1H NMR: δ = 8.11 (d, J = 9.2 Hz, 2 H), 7.62 (d, J = 9.2 Hz, 2 H), 4.34 (m, 1 H), 3.61 (t, J = 8.8 Hz, 1 H), 3.06 (dd, J = 3.6, 8.8 Hz, 1 H), 2.85 (s, 3 H), 1.31 (d, J = 6.4 Hz, 3 H). 13C{1H} NMR: δ = 156.6, 145.1, 141.7, 124.8, 117.6, 51.5, 48.5, 30.8, 18.9. IR (neat, cm−1): 2927, 1697, 1496, 1312, 1267, 1111, 845, 749, 690. Anal. calcd (found) for C11H13N3O3: H, 5.57 (5.47); C, 56.16 (56.22).
4-Methyl-3-(4-nitrophenyl)-1-phenylimidazolidin-2-one (2c)
Yellow solid, 93%. TLC (hexanes–EtOAc = 3:1): Rf = 0.4. 1H NMR: δ = 8.26 (d, J = 9.0 Hz, 2 H), 7.77 (d, J = 9.5 Hz, 2 H), 7.60 (br d, J = 7.5 Hz, 2 H), 7.42 (br t, J = 7.5 Hz, 2 H), 7.17 (br t, J = 7.5 Hz, 1 H), 4.59 (m, 1 H), 4.21 (t, J = 9.0 Hz, 1 H), 3.61 (dd, J = 4.5, 9.0 Hz, 1 H), 1.50 (d, J = 6.5 Hz, 3 H). 13C{1H} NMR: = δ 153.9, 144.5, 142.5, 139.2, 129.0, 124.8, 123.8, 118.8, 118.5, 49.7, 48.3, 19.2. IR (neat, cm−1): 2924, 1701, 1505, 1405, 1282, 1108, 754, 688. Anal. calcd (found) for C16H15N3O3: H, 5.09 (4.98); C, 64.64 (64.57).
1,4-Dimethyl-3-phenylimidazolidin-2-one (2d)
Colorless oil, 86%. TLC (hexanes–EtOAc = 3:1): Rf = 0.4. 1H NMR: δ = 7.40 (m, 2 H), 7.31 (br d, J = 7.6 Hz, 2 H), 7.04 (br t, J = 7.2 Hz, 1 H), 4.28 (quintet of doublets, J = 6.4 m 8.2 Hz, 1 H), 3.56 (t, J = 8.4 Hz, 1 H), 3.01 (dd, J = 6.0, 8.4 Hz, 1 H), 2.84 (s, 3 H), 1.25 (d, J = 6.4 Hz, 3 H). 13C{1H} NMR: δ = 158.3, 138.8, 128.7, 123.3, 120.9, 52.2, 49.0, 31.0, 18.8. IR (neat, cm−1): 2927, 1694, 1494, 1431, 1367, 1261, 756, 694. Anal. calcd (found) for C11H14N2O: H, 7.42 (7.29); C, 69.45 (69.28).
3-(4-Methoxyphenyl)-4-methyl-1-phenylimidazolidin-2-one (2e)
White solid, 97%. TLC (hexanes–EtOAc = 3:1): Rf = 0.4. 1H NMR: δ = 7.58 (d, J = 8.0 Hz, 2 H), 7.34 (d, J = 7.2 Hz, 2 H), 7.30 (d, J = 8.8 Hz, 2 H), 7.04 (t, J = 7.2 Hz, 1 H), 6.91 (d, J = 7.2 Hz, 2 H), 4.27 (m, 1 H), 4.00 (t, J = 8.8 Hz, 1 H), 3.78 (s, 3 H), 3.46 (t, J = 7.6 Hz, 1 H), 1.28 (d, J = 5.6 Hz, 3 H). 13C{1H} NMR: δ = 156.9, 155.8, 140.3, 130.9, 128.8, 124.8, 122.5, 117.7, 114.3, 55.5, 50.0, 19.3. IR (neat, cm−1): 2979, 1688, 1501, 1401, 1241, 754, 688. Anal. calcd (found) for C17H18N2O2: H, 6.43 (6.33); C, 72.32 (72.15).
1-Cyclohexyl-4-methyl-3-phenylimidazolidin-2-one (2f)
Colorless oil, 92%. TLC (hexanes–EtOAc = 2:1): Rf = 0.4. 1H NMR: δ = 7.42 (d, J = 8.0 Hz, 2 H), 7.30 (t, J = 8.0 Hz, 2 H), 7.02 (br t, J = 7.2 Hz, 1 H), 4.27 (quintet of doublets, J = 6.0, 8.8 Hz, 1 H), 3.78 (m, 1 H), 3.56 (t, J = 8.8 Hz, 1 H), 2.98 (dd, J = 5.6, 8.4 Hz, 1 H), 1.80-1.64 (m, 5 H), 1.43-1.21 (m, 4 H), 1.25 (d, J = 6.0 Hz, 3 H), 1.08 (m, 1 H). 13C{1H} NMR: δ = 157.2, 139.1, 128.7, 123.0, 120.1, 51.2, 49.3, 45.5, 30.3, 30.1, 25.6, 18.9. IR (neat, cm−1): 2930, 1691, 1419, 1255, 755, 694. Anal. calcd (found) for C16H22N2O: H, 8.58 (8.60); C, 74.38 (74.43).
trans-1-Cyclohexyl-4,5-dimethyl-3-(4-nitrophenyl)imidazolidin-2-one (4a)
Yellow solid, 100%. TLC (hexanes–EtOAc = 3:1): Rf = 0.6. 1H NMR: δ = 8.14 (d, J = 9.6 Hz, 2 H), 7.67 (d, J = 9.2 Hz, 2 H), 3.82 (dq, J = 2.8, 6.0 Hz, 1 H), 3.62 (tt, J = 3.6, 12.0 Hz, 1 H), 3.35 (dq, J = 2.4, 6.0 Hz, 1 H), 1.92-1.05 (m, 10 H), 1.29 (d, J = 6.0 Hz, 3 H), 1.28 (d, J = 6.0 Hz, 3 H). 13C{1H} NMR: δ = 154.9, 145.7, 141.4, 124.8, 117.0, 57.4, 54.5, 53.2, 32.2, 30.6, 25.9, 25.8, 25.4, 21.8, 18.2. IR (neat, cm−1): 2932, 1645, 1491, 1328, 1302, 1238, 1110, 750, 702, 639. Anal. calcd (found) for C17H23N3O3: H, 7.30 (7.41); C, 64.33 (64.32).
trans-1-Cyclohexyl-5-isopropyl-4-methyl-3-(4-nitrophenyl)imidazolidin-2-one (4b)
Yellow solid, 93%. TLC (hexanes–EtOAc = 3:1): Rf = 0.6. 1H NMR: δ = 8.14 (d, J = 9.2 Hz, 2 H), 7.72 (d, J = 9.2 Hz, 2 H), 3.96 (br q, J = 6.0 Hz, 1 H), 3.55 (m, 1 H), 3.16 (m, 1 H), 2.03-1.08 (m, 10 H), 1.28 (d, J = 6.0 Hz, 3 H), 0.95 (d, J = 6.8 Hz, 3 H), 0.75 (d, J = 6.4 Hz, 3 H). 13C{1H} NMR: δ = 155.2, 145.4, 141.2, 125.1, 116.2, 64.2, 54.0, 49.7, 31.9, 30.7, 30.4, 26.0, 25.4, 20.2, 17.9, 14.3. IR (neat, cm−1): 2935, 1698, 1495, 1297, 1246, 1107, 854, 754, 692. Anal. calcd (found) for C19H27N3O3: H, 7.88 (7.95); C, 66.06 (65.98).
trans-4-(Benzyloxymethyl)-3-cyclohexyl-5-methyl-1-(4-nitrophenyl)imidazolidin-2-one (4c)
Yellow oil, 98%. TLC (hexanes–EtOAc = 3:1): Rf = 0.5. 1H NMR: δ = 8.08 (d, J = 9.2 Hz, 2 H), 7.62 (d, J = 9.2 Hz, 2 H), 7.28-7.19 (m, 5 H), 4.45 (s, 2 H), 4.13 (dq, J = 0.8, 6.0 Hz, 1 H), 3.58 (tt, J = 3.6, 12.0 Hz, 1 H), 3.50 (m, 1 H), 3.33 (m, 1 H), 1.82-1.47 (m, 6 H), 1.35-1.18 (m, 3 H), 1.23 (d, J = 6.4 Hz, 3 H), 1.03 (tq, J = 3.2, 12.8 Hz, 1 H). 13C{1H} NMR: δ = 155.2, 145.5, 141.3, 137.3, 128.4, 127.9, 127.7, 124.9, 116.6, 73.4, 70.8, 57.9, 53.3, 53.2, 32.1, 30.5, 25.8, 25.7, 25.3, 18.7. IR (neat, cm−1): 2931, 1701, 1503, 1321, 1234, 849, 751, 697. Anal. calcd (found) for C24H29N3O4: H, 6.90 (6.92); C, 68.06 (67.95).
trans-(3-Cyclohexyl-5-methyl-1-(4-nitrophenyl)-2-oxoimidazolidin-4-yl)methyl acetate (4d)
Yellow solid, 98%. TLC (hexanes–EtOAc = 3:1): Rf = 0.4. 1H NMR: δ = 8.14 (d, J = 9.2 Hz, 2 H), 7.69 (d, J = 9.2 Hz, 2 H), 4.26 (dd, J = 3.6, 11.6 Hz, 1 H), 4.16 (dq, J = 1.6, 6.0 Hz, 1 H), 3.91 (dd, J = 7.2, 11.6 Hz, 1 H), 3.67 (tt, J = 3.6, 12.0 Hz, 1 H), 3.45 (ddd, J = 1.6, 3.6, 7.2 Hz, 1 H), 2.00 (s, 3 H), 1.93-1.54 (m, 6 H), 1.47-1.26 (m, 3 H), 1.31 (d, J = 6.0 Hz, 3 H), 1.11 (tq, J = 3.6, 12.8 Hz, 1 H). 13C{1H} NMR: δ = 170.6, 155.1, 145.2, 141.6, 125.0, 116.7, 64.4, 56.8, 53.4, 53.3, 32.2, 30.6, 25.8, 25.7, 25.3, 20.7, 18.8. IR (neat, cm−1): 2930, 1706, 1503, 1324, 1219, 1040, 858, 752, 693. Anal. calcd (found) for C19H25N3O5: H, 6.71 (6.62); C, 60.79 (60.64).
cis-1-Cyclohexyl-5-(hydroxymethyl)-4-methyl-3-(4-nitrophenyl)imidazolidin-2-one (6)
Yellow oil, 97% (cis:trans = 3.7:1). TLC (hexanes–EtOAc = 1:1): Rf = 0.45. 1H NMR: δ = [8.15 (d, J = 9.2 Hz), 8.12 (d, J = 9.6 Hz), (3.7:1), 2 H], [7.70 (d, J = 9.2 Hz), 7.56 (d, J = 9.2 Hz), (1:3.7), 2 H)], [4.45 (quintet, J = 6.9), 4.31 (dq J = 2.0, 6.4 Hz), (3.7:1), 1 H)], [3.97 (m), 3.34 (m), (3.7:1), 1 H], [3.90-3.80 (m), 3.73 (m), (3.7:1), 2 H] 3.61 (tt, J = 4.0, 12 Hz, 1 H), [2.35 (t, J = 4.5 Hz), 2.18 (t, J = 4.8 Hz), (1:3.7), 1 H], 1.87-1.46 (m, 6 H), 1.41-1.20 (m, 3 H), [1.33 (d, J = 6.4 Hz), 1.31 (d, J = 6.4 Hz), (3.7:1), 3 H], 1.11 (tq, J = 3.6, 12.8 Hz, 1 H). 13C{1H} NMR: δ = [157.1, 155.7, (3.7:1)], [145.4, 144.8, (1:3.7)], [142.1, 141.4, (3.7:1)], [124.9, 124.6 (1:3.7)], [119.3, 117.0, (3.7:1)] [63.1, 60.3, (1:3.7)], [59.6, 56.3, (1:3.7)], [53.9, 53.3 (3.7:1)], [52.6, 52.0 (1:3.7)], [32.4, 31.6 (1:3.7)], [30.6, 29.9 (1:3.7)], [26.0, 25.3 (3.7:1)], [18.9, 12.3 (1:3.7)]. IR (neat, cm−1): 2932, 1684, 1503, 1320, 1248, 1112, 850, 729, 694. HRMS calcd (found) for C17H23N3O4 (M+): 333.1689 (333.1676).
Relative configurations of 4,5-disubstituted imidazolidin-2-ones
The trans configuration of 4,5-disubstituted imidazolidin-2-ones 4a-4d was assigned on the basis of the vicinal H4-H5 coupling constants, which ranged from 3JHH ≈ 1 Hz for 4b and 4c to 3JHH = 2.4 Hz for 4a. Published values for the vicinal H4-H5 coupling constant of trans-4,5-disubstituted imidazolidin-2-ones range from 0–3 Hz,[9] while the the vicinal H4-H5 coupling constant of cis-4,5-disubstituted imidazolidin-2-ones range from 7–9 Hz.[11,15,18] In the same way, the cis and trans configurations of the major and minor diastereomers of 6 were assigned on the basis of the vicinal H4-H5 coupling constants of 3JHH ≈ 7 Hz (major) and 3JHH ≈ 2 Hz (minor).
N-(2-Aminopropyl)benzenamine (8).[34]
A solution of 2e (50 mg, 0.18 mmol) in CH3CN (2 mL) at 0 °C was treated with a solution of ceric ammonium nitrate (CAN, 0.29 g, 0.53 mmol) in water (2.5 mL) over 3 min. The solution was stirred for 25 min at 0 °C, diluted with water (10 mL), and extracted with EtOAc (3 × 10 mL). The combined organic extracts were dried (MgSO4) and concentrated under vacuum to give an oily residue that was chromatographed (EtOAc–hexanes = 2:1) to give 4-methyl-1-phenylimidazolidin-2-one (7)[19] (26 mg, 82%) as white solid. A solution of 7 (26 mg, 0.15 mmol) in concentrated HCl (4 mL) was refluxed for 30 h and then extracted with CH2Cl2 (3 × 10 mL). The aqueous layer was made basic (pH ≥ 12) with 15% NaOH and then extracted with CH2Cl2 (3 × 10 mL). The combined organic extracts were dried (MgSO4) and concentrated under vacuum to give pure 8 (16 mg, 77%) as a colorless oil. The 1H and 13C NMR spectra of 7 and 8 were identical with published data.[19,34]
Supplementary Material
Acknowledgments
Acknowledgment is made to the NIH (GM-080422) for support of this research and to the NCBC (2008-IDG-1010) for support of the Duke University NMR facility
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/adsc.200######.
References
- 1.(a) Lee CW, Hong DH, Han SB, Jung SH, Kim HC, Fine RL, Lee SH, Kim HM. Biochem Pharmacol. 2002;64:473–480. doi: 10.1016/s0006-2952(02)01105-x. [DOI] [PubMed] [Google Scholar]; (b) Thomas NJ, Carcillo JA, Herzer WA, Mi Z, Tofovic SP, Jackson EK. Eur J Pharmacol. 2003;465:133–139. doi: 10.1016/s0014-2999(03)01456-0. [DOI] [PubMed] [Google Scholar]; (c) Rotstein DM, Gabriel SD, Manser N, Filonova L, Padilla F, Sankuratri S, Ji C, deRosier A, Dioszegi M, Heilek G, Jekle A, Weller P, Berry P. Bioorg Med Chem Lett. 2010;20:3219–3222. doi: 10.1016/j.bmcl.2010.04.077. [DOI] [PubMed] [Google Scholar]; (d) Jiang B, Liu JF, Zhao SY. J Org Chem. 2003;68:2376–2384. doi: 10.1021/jo026773i. [DOI] [PubMed] [Google Scholar]; (e) Heidempergher F, Pillan A, Pinciroli V, Vaghi F, Arrigoni C, Bolis G, Caccia C, Dho L, McArthur R, Varasi M. J Med Chem. 1997;40:3369–3380. doi: 10.1021/jm970060o. [DOI] [PubMed] [Google Scholar]; (f) Eum H, Lee Y, Kim S, Baek A, Son M, Lee KW, Ko SW, Kim S, Yun SY, Lee WK, Ha HJ. Bull Korean Chem Soc. 2010;31:611–614. [Google Scholar]
- 2.(a) Shue HJ, Chen X, Schwerdt JH, Paliwal S, Blythin DJ, Lin L, Gu D, Wang C, Reichard GA, Wang H, Piwinski JJ, Duffy RA, Lachowicz JE, Coffin VL, Nomeir AA, Morgan CA, Varty GB, Shih NY. Bioorg Med Chem Lett. 2006;16:1065–1069. doi: 10.1016/j.bmcl.2005.10.072. [DOI] [PubMed] [Google Scholar]; (b) Shue HJ, Chen X, Shih NY, Blythin DJ, Paliwal S, Lin L, Gu D, Schwerdt JH, Shah S, Reichard GA, Piwinski JJ, Duffy RA, Lachowicz JE, Coffin VL, Liu F, Nomeir AA, Morgan CA, Varty GB. Bioorg Med Chem Lett. 2005;15:3896–3899. doi: 10.1016/j.bmcl.2005.05.111. [DOI] [PubMed] [Google Scholar]; (c) Reichard GA, Stengone C, Paliwal S, Mergelsberg I, Majmundar S, Wang C, Tiberi R, McPhail AT, Piwinkski JJ, Shih NY. Org Lett. 2003;5:4249–4251. doi: 10.1021/ol030104p. [DOI] [PubMed] [Google Scholar]
- 3.(a) Peretto I, Fossati C, Giardina GAM, Giardini A, Guala M, La Porta E, Petrillo P, Radaelli S, Radice L, Raveglia LF, Santoro E, Scudellaro R, Scarpitta F, Cerri A, Menegon S, Dondio GM, Rizzi A, Armani E, Amari G, Civelli M, Villetti G, Patacchini R, Bergamaschi M, Bassani F, Delcanale M, Imbimbo BP. J Med Chem. 2007;50:1693–1697. doi: 10.1021/jm061160+. [DOI] [PubMed] [Google Scholar]; (b) Peretto I, Forlani R, Fossati C, Giardina GAM, Giardini A, Guala M, La Porta E, Petrillo P, Radaelli S, Radice L, Raveglia LF, Santoro E, Scudellaro R, Scarpitta F, Bigogno C, Misiano P, Dondio GM, Rizzi A, Armani E, Amari G, Civelli M, Villetti G, Patacchini R, Bergamaschi M, Delcanale M, Salcedo C, Fernández AG, Imbimbo BP. J Med Chem. 2007;50:1571–1583. doi: 10.1021/jm061159a. [DOI] [PubMed] [Google Scholar]; (c) Peretto I, Petrillo P, Imbimbo BP. Med Res Rev. 2009;29:867–902. doi: 10.1002/med.20158. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kazmierski WM, Furfine E, Gray-Nunez Y, Spaltenstein A, Wright L. Bioorg Med Chem Lett. 2004;14:5685–5687. doi: 10.1016/j.bmcl.2004.08.038. [DOI] [PubMed] [Google Scholar]; (b) De Clerq E. Biochim Biophys Acta. 2002;1587:258–275. doi: 10.1016/s0925-4439(02)00089-3. [DOI] [PubMed] [Google Scholar]; (c) Kazmierski WM, Salituro FG, Tung RD, Wright LL. Bioorg Med Chem Lett. 2000;10:1159–1162. [PubMed] [Google Scholar]; (d) Spaltenstein A, Almond MR, Bock WJ, Cleary DG, Furfine ES, Hazen RJ, Kazmierski WM, Salituro FG, Tung RD, Wright LL. Bioorg Med Chem Lett. 2000;10:1159–1162. [PubMed] [Google Scholar]; (e) Salituro FG, Baker CT, Court JJ, Deininger DD, Kim EE, Li B, Novak PM, Rao BG, Pazhanisamy S, Porter MD, Schairer WC, Tung RD. Bioorg Med Chem Lett. 1998;8:3637–3642. doi: 10.1016/s0960-894x(98)00670-2. [DOI] [PubMed] [Google Scholar]; (f) De Lucca GV, Lam PYS. Drugs Future. 1998;23:987–994. [Google Scholar]
- 5.(a) Chern JH, Chang CS, Tai CL, Lee YC, Lee CC, Kang IJ, Lee CY, Shih SR. Bioorg Med Chem Lett. 2005;15:4206–4211. doi: 10.1016/j.bmcl.2005.06.069. [DOI] [PubMed] [Google Scholar]; (b) Shia KS, Li WT, Chang CM, Hsu MC, Chern JH, Leong MK, Tseng SN, Lee CC, Lee YC, Chen SJ, Peng KC, Tseng HY, Chang YL, Tai CL, Shih SR. J Med Chem. 2002;45:1644–1655. doi: 10.1021/jm010536a. [DOI] [PubMed] [Google Scholar]
- 6.(a) Neves JKdeAL, Botelho SPS, de Melo CML, Pereira VRA, de Lima MdoCA, Pitta IdaR, Albuquerque MCPdeA, Galdino SL. Parasitol Res. 2010;107:531–538. doi: 10.1007/s00436-010-1886-y. [DOI] [PubMed] [Google Scholar]; (b) Robert JMH, Sabourin C, Alvarez N, Robert-Piessard S, Le Baut G, Le Pape P. Eur J Med Chem. 2003;38:711–718. doi: 10.1016/s0223-5234(03)00119-3. [DOI] [PubMed] [Google Scholar]; (c) Alvarez N, Robledo S, Velez ID, Robert JM, Le Baut G, Le Pape P. J Enz Inhib Med Chem. 2002;17:443–447. doi: 10.1080/1475636021000005749. [DOI] [PubMed] [Google Scholar]
- 7.(a) Sabourin C, Robert JMH. J Enz Inhib Med Chem. 2008;23:659–667. doi: 10.1080/14756360802205455. [DOI] [PubMed] [Google Scholar]; (b) Sabourin C, Robert JMH, Robert-Piessard S, Carbonnelle D, Lang F. J Enz Inhib Med Chem. 2004;19:459–465. doi: 10.1080/14756360412331280482. [DOI] [PubMed] [Google Scholar]
- 8.(a) Lucet D, Le Gall T, Mioskowski C. Angew Chem Int Ed. 1998;37:2580–2627. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]; (b) Guillena G, Nájera C. Tetrahedron: Asymm. 1998;9:1125–1129. [Google Scholar]; (c) Parisi M, Solo A, Wulff WD, Guzei IA, Rheingold AL. Organometallics. 1998;17:3696–3700. [Google Scholar]; (d) Wulff WD. Organometallics. 1998;17:3116–3134. [Google Scholar]; (e) Palomo C, Oiarbide M, González A, García JM, Berrée F. Tetrahedron Lett. 1996;37:4565–4568. [Google Scholar]; (f) Kubota H, Kubo A, Takahashi M, Shimizu R, Da-te T, Okamura K, Nunami K-i. J Org Chem. 1995;60:6776–6784. [Google Scholar]; (g) Davies SC, Evans GB, Mortlock AA. Tetrahedron Asymm. 1994;5:585–606. [Google Scholar]; (h) Taguchi T, Shibuya A, Sasaki H, Endo J-i, Morikawa T, Shirob M. Tetrahedron Asymm. 1994;5:1423–1426. [Google Scholar]; (i) Sankhavasi W, Yamamoto M, Kohmoto S, Yamada K. Bull Chem Soc Jpn. 1991;64:1425–1427. [Google Scholar]; (j) Cardillo G, Orena M, Penna M, Sandri S, Tomasini C. Tetrahedron. 1991;47:2263–2272. [Google Scholar]; (k) Davies SG, Mortlock AA. Tetrahedron Lett. 1991;32:4191–4194. [Google Scholar]
- 9.(a) Candeias SX, Jenkins K, Ribeiro ASC, Afonso CAM, Caddick S. Synth Comm. 2001;31:3241–3254. [Google Scholar]; (b) Guillena G, Nájera C. J Org Chem. 2000;65:7310–7322. doi: 10.1021/jo000321t. [DOI] [PubMed] [Google Scholar]; (c) Cardillo G, D’Amico A, Orena M, Sandri S. J Org Chem. 1988;53:2354–2356. [Google Scholar]
- 10.(a) Zhang YJ, Kim KY, Park JH, Song CE, Lee K, Lah MS, Lee S-g. Adv Synth Catal. 2005;347:563–570. [Google Scholar]; (b) Zhang YJ, Park JH, Lee S-g. Tetrahedron: Asymm. 2004;15:2209–2212. [Google Scholar]; (c) Lee S-g, Zhang YJ. Org Lett. 2002;4:2429–2431. doi: 10.1021/ol0261884. [DOI] [PubMed] [Google Scholar]; (d) Lee S-g, Zhang YJ. Tetrahedron: Asymm. 2002;13:1039–1042. [Google Scholar]
- 11.Lee S-g, Zhang YJ, Song CE, Lee JK, Choi JH. Angew Chem Int Ed. 2002;41:847–849. doi: 10.1002/1521-3773(20020301)41:5<847::aid-anie847>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.(a) Kim M, Mulcahy JV, Espino CG, Du Bois J. Org Lett. 2006;8:1073–1076. doi: 10.1021/ol052920y. [DOI] [PubMed] [Google Scholar]; (b) McLaughlin M, Palucki M, Davies IW. Org Lett. 2006;8:3311–3314. doi: 10.1021/ol061233j. [DOI] [PubMed] [Google Scholar]; (c) Kim MS, Kim YW, Hahm HS, Jang JW, Lee WK, Ha HJ. Chem Commun. 2005:3062–3064. doi: 10.1039/b503750f. [DOI] [PubMed] [Google Scholar]; (d) Zhou HB, Alper H. J Org Chem. 2003;68:3439–3445. doi: 10.1021/jo020526x. [DOI] [PubMed] [Google Scholar]; (e) Benedí C, Bravo F, Uriz P, Fernandez E, Claver C, Castillon S. Tetrahedron Lett. 2003;44:6073–6077. [Google Scholar]; (f) Overman LE, Remarchuk TP. J Am Chem Soc. 2002;124:12–13. doi: 10.1021/ja017198n. [DOI] [PubMed] [Google Scholar]; (g) Bell KE, Coogan MP, Gravestock MB, Knight DW, Thornton SR. Tetrahedron Lett. 1997;38:8545–8548. [Google Scholar]; (h) Kise N, Kashiwagi K, Watanabe M, Yoshida J. J Org Chem. 1996;61:428–429. doi: 10.1021/jo951592k. [DOI] [PubMed] [Google Scholar]; (i) Park YS, Boys ML, Beak P. J Am Chem Soc. 1996;118:3757–3758. [Google Scholar]; (j) Shatzmiller S, Bercovici S. Liebigs Ann Chem. 1992:1005–1009. [Google Scholar]; (k) Ghomi S, Orr DE. Chem Ind. 1983:928–928. [Google Scholar]; (l) Parry RJ, Kunitani MG, Viele OI. J Chem Soc Chem Commun. 1975:321–322. [Google Scholar]
- 13.(a) Sartori G, Maggi R. In: Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. Ley SV, Knight JG, editors. Vol. 18. Thieme; Stuttgart: 2005. pp. 665–758. [Google Scholar]; (b) Gabriele B, Salerno G, Mancuso R, Costa M. J Org Chem. 2004;69:4741–4750. doi: 10.1021/jo0494634. [DOI] [PubMed] [Google Scholar]; (c) Qian F, McCusker JE, Zhang Y, Main AD, Chlebowski M, Kokka M, McElwee-White L. J Org Chem. 2002;67:4086–4092. doi: 10.1021/jo0109319. [DOI] [PubMed] [Google Scholar]; (d) Kim JM, Wilson TE, Norman TH, Schultz PG. Tetrahedron Lett. 1996;37:5309–5312. [Google Scholar]; (e) Shimizu CM, Kamei M, Fujisawa T. Tetrahedron Lett. 1995;36:8607–8610. [Google Scholar]
- 14.(a) Li H, Widenhoefer RA. Tetrahedron. 2010;66:4827–4831. doi: 10.1016/j.tet.2010.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Muñiz K, Hövelmann CH, Streuff J. J Am Chem Soc. 2008;130:763–773. doi: 10.1021/ja075041a. [DOI] [PubMed] [Google Scholar]; (c) Muñiz K, Streuff J, Chávez P, Hövelmann CH. Chem Asian J. 2008;3:1248–1255. doi: 10.1002/asia.200800148. [DOI] [PubMed] [Google Scholar]; (d) Hövelmann CH, Streuff J, Brelot L, Muñiz K. Chem Commun. 2008:2334–2336. doi: 10.1039/b719479j. [DOI] [PubMed] [Google Scholar]; (e) Muñiz K, Hövelmann CH, Campos-Gómez E, Barluenga J, González J, Streuff J, Nieger M. Chem Asian J. 2008;3:776–788. doi: 10.1002/asia.200700373. [DOI] [PubMed] [Google Scholar]; (f) Muñiz K. J Am Chem Soc. 2007;129:14542–14543. doi: 10.1021/ja075655f. [DOI] [PubMed] [Google Scholar]; (g) Muñiz K, Streuff J, Hövelmann CH, Núñez A. Angew Chem Int Ed. 2007;46:7125–7127. doi: 10.1002/anie.200702160. [DOI] [PubMed] [Google Scholar]; (h) Streuff J, Hövelmann CH, Nieger M, Muñiz K. J Am Chem Soc. 2005;127:14586–14587. doi: 10.1021/ja055190y. [DOI] [PubMed] [Google Scholar]; (i) Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J Am Chem Soc. 2005;127:7308–7309. doi: 10.1021/ja051181d. [DOI] [PubMed] [Google Scholar]
- 15.Hu X, Cao Z, Liu Z, Wang Y, Du H. Adv Synth Catal. 2010;352:651–655. [Google Scholar]
- 16.(a) Fujita M, Kitagawa O, Suzuki T, Taguchi T. J Org Chem. 1997;62:7330–7335. doi: 10.1021/jo970898j. [DOI] [PubMed] [Google Scholar]; (b) Balko TW, Brinkmeyer RS, Terando NH. Tetrahedron Lett. 1989;30:2045–2048. [Google Scholar]; (c) Hunt PA, May C, Moody CJ. Tetrahedron Lett. 1988;29:3001–3002. [Google Scholar]; (d) Danishefsky S, Taniyama E, Webb RR. Tetrahedron Lett. 1983;24:11–14. [Google Scholar]
- 17.(a) Harayama H, Abe A, Sakado T, Kimura M, Fugami K, Tanaka S, Tamaru Y. J Org Chem. 1997;62:2113–2122. doi: 10.1021/jo961988b. [DOI] [PubMed] [Google Scholar]; (b) Tamaru Y, Hojo M, Higashimura H, Yoshida Z-i. J Am Chem Soc. 1988;110:3994–4002. [Google Scholar]
- 18.(a) Fritz JA, Wolfe JP. Tetrahedron. 2008;64:6838–6852. doi: 10.1016/j.tet.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fritz JA, Nakhla JS, Wolfe JP. Org Lett. 2006;8:2531–2534. doi: 10.1021/ol060707b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TH, Lee GJ. J Org Chem. 1999;64:2941–2943. doi: 10.1021/jo9820061. [DOI] [PubMed] [Google Scholar]
- 20.Keyserlingk NGv, Martens J. Eur J Org Chem. 2002:301–308. [Google Scholar]
- 21.(a) Kinder RE, Zhang Z, Widenhoefer RA. Org Lett. 2008;10:3157–3159. doi: 10.1021/ol8010858. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang Z, Bender CF, Widenhoefer RA. J Am Chem Soc. 2007;129:14148–14149. doi: 10.1021/ja0760731. [DOI] [PubMed] [Google Scholar]; (c) Zhang Z, Bender CF, Widenhoefer RA. Org Lett. 2007;9:2887–2889. doi: 10.1021/ol071108n. [DOI] [PubMed] [Google Scholar]; (d) Zhang Z, Liu C, Kinder RE, Han X, Qian H, Widenhoefer RA. J Am Chem Soc. 2006;128:9066–9073. doi: 10.1021/ja062045r. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Widenhoefer RA. Org Lett. 2009;11:2671–2674. doi: 10.1021/ol900730w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.We have previously documented the utility of ureas and carbamates as nucleophiles for the gold(I)-catalyzed inter- and intramolecular hydroamination of alkenes: Han X, Widenhoefer RA. Angew Chem Int Ed. 2006;45:1747–1749. doi: 10.1002/anie.200600052.Bender CF, Widenhoefer RA. Org Lett. 2006;8:5303–5305. doi: 10.1021/ol062107i.Zhang Z, Lee SD, Widenhoefer RA. J Am Chem Soc. 2009;131:5372–5373. doi: 10.1021/ja9001162.
- 24.For examples of the gold (I)-catalyzed hydroamination of alkenes with sulfonamides see: Giner X, Najera C. Org Lett. 2008;10:2919–2922. doi: 10.1021/ol801104w.Liu XY, Li CH, Che CM. Org Lett. 2006;8:2707–2710. doi: 10.1021/ol060719x.Zhang J, Yang CG, He C. J Am Chem Soc. 2006;128:1798–1799. doi: 10.1021/ja053864z.Brouwer C, He C. Angew Chem, Int Ed. 2006;45:1744–1747. doi: 10.1002/anie.200504495.
- 25.For some recent examples of the hydroamination of electronically unactivated C=C bonds catalyzed by late transition metal complexes see: Hesp KD, Tobisch S, Stradiotto M. J Am Chem Soc. 2010;132:413–426. doi: 10.1021/ja908316n.Kashiwame Y, Kuwata S, Ikariya T. Chem Eur J. 2010;16:766–770. doi: 10.1002/chem.200902827.Shen X, Buchwald SL. Angew Chem Int Ed. 2010;122:564–567. doi: 10.1002/anie.200905402.Julian LD, Hartwig JF. J Am Chem Soc. 2010;132:13813–13822. doi: 10.1021/ja1052126.Hesp KD, Stradiotto M. Org Lett. 2009;11:1449–1452. doi: 10.1021/ol900174f.Ohmiya H, Moriya T, Sawamura M. Org Lett. 2009;11:2145–2147. doi: 10.1021/ol9007712.Cochran BM, Michael FE. J Am Chem Soc. 2008;130:2786–2792. doi: 10.1021/ja0734997.Liu Z, Hartwig JF. J Am Chem Soc. 2008;130:1570–1571. doi: 10.1021/ja710126x.Bender CF, Hudson WB, Widenhoefer RA. Organometallics. 2008;27:2356–2358.
- 26.(a) Palomo C, Cossio FP, Arrieta A, Odriozola JM, Giarbide M, Ontoria JM. J Org Chem. 1989;54:5736–5745. [Google Scholar]; (b) Sankhavasi W, Yamamoto M, Kohmoto S, Yamada K. Bull Chem Soc Jpn. 1991;64:1425–1427. [Google Scholar]
- 27.Williams OF, Bailar JC. J Am Chem Soc. 1959;81:4464–4469. [Google Scholar]
- 28.For a recent review on the mechanisms of gold(I)-catalyzed transformations see: Hashmi ASK. Angew Chem Int Ed. 2010;49:5232–5241. doi: 10.1002/anie.200907078.
- 29.(a) Brown TJ, Dickens MG, Widenhoefer RA. J Am Chem Soc. 2009;131:6350–6351. doi: 10.1021/ja9015827. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brown TJ, Dickens MG, Widenhoefer RA. Chem Commun. 2009:6451–6453. doi: 10.1039/b914632f. [DOI] [PubMed] [Google Scholar]
- 30.(a) Zuccaccia D, Belpassi L, Tarantelli F, Macchioni A. J Am Chem Soc. 2009;131:3170–3171. doi: 10.1021/ja809998y. [DOI] [PubMed] [Google Scholar]; (b) de Frémont P, Marion N, Nolan SP. J Organomet Chem. 2009;694:551–560. [Google Scholar]; (c) Hooper TN, Green M, Mcgrady JE, Patel JR, Russell CA. Chem Commun. 2009:3877–3879. doi: 10.1039/b908109g. [DOI] [PubMed] [Google Scholar]; (d) Shapiro ND, Toste FD. Proc Natl Acad Sci USA. 2008;105:2779–2782. [Google Scholar]
- 31.LaLonde RL, Brenzovich WE, Benitez D, Tkatchouk E, Kelley K, Goddard WA, Toste FD. Chem Sci. 2010:226–233. doi: 10.1039/C0SC00255K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.(a) Krauter CM, Hashmi ASK, Pernointner M. Chem Cat Chem. 2010;2:1226–1230. [Google Scholar]; (b) Zhang J, Shen W, Li L, Li M. Organometallics. 2009;28:3129–3139. [Google Scholar]; (c) Kovács G, Ujaque G, Lledós A. J Am Chem Soc. 2008;130:853–864. doi: 10.1021/ja073578i. [DOI] [PubMed] [Google Scholar]
- 33.We thank a reviewer for suggesting this pathway.
- 34.Crombie L, Hooper KC. J Chem Soc. 1955:3010–3016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.