Abstract
Retinoblastoma protein (pRb) is a ubiquitous 928 amino acid cell cycle regulatory molecule with diverse biological activities. One critical function of pRb is control of the G1-to-S phase checkpoint of the cell cycle. In the hypophosphorylated state, pRb suppresses the activity of E2F transcription factors thereby inhibiting transcription of cell cycle promoting genes. Upon phosphorylation, primarily by cyclin dependent kinases, phosphorylated pRb dissociates from E2F and permits cell cycle progression. We previously found phosphorylated pRb to be intimately associated with hyperphosphorylated tau-containing neurofibrillary tangles of Alzheimer disease (AD), the pathogenesis of which is believed to involve dysregulation of the cell cycle and marked neuronal death. Here, we used immunohistochemistry to investigate the presence of phosphorylated pRb in other distinct neurodegenerative diseases that share the common characteristic of hyperphosphorylated tau pathology and neuronal loss with AD. We found colocalized labeling of tau pathology and phosphorylated pRb in Pick disease and progressive supranuclear palsy (3 cases each), neurodegeneration with brain iron accumulation type 1 (2 cases) and Parkinson-amyotrophic lateral sclerosis of Guam, subacute sclerosing panencephalitis, frontotemporal dementia and Parkinsonism linked to chromosome 17 and dementia pugilistica (1 case each). These observations further implicate aberrant neuronal cell cycle progression in neurodegenerative diseases, particularly tauopathies, and suggest a novel target for therapeutic intervention.
Keywords: Dementia pugilistica, Neurodegeneration with brain iron accumulation type 1, Neurofibrillary tangles, Pick disease, Progressive supranuclear palsy, Retinoblastoma protein, Subacute sclerosing panencephalitis, Tau pathology
INTRODUCTION
Retinoblastoma protein (pRb) is implicated in diverse biological functions including cellular differentiation, senescence, apoptosis, and notably, progression of the cell cycle (1). pRb is considered to be a potent tumor suppressor that exerts control of the cell cycle at the G1-to-S phase checkpoint by inhibiting the E2F family transcription factors thereby impeding the transcription of genes necessary for transition to S phase (2, 3). Importantly, the fluctuating phosphorylation status of pRb at each of its 16 potential phosphorylation sites determines its function (4); hypophosphorylated pRb intimately associates with E2F thus inhibiting cell cycle progression. The sequential phosphorylation of pRb by cyclin D-cyclin dependent kinase (CDK) 4/6 and cyclin E-CDK2 causes pRb to dissociate from E2F and advances quiescent cells to S phase (5–7). Additionally, pRb likely mediates more than G1-to-S phase checkpoint progression because sequential phosphorylation of pRb continues throughout the remainder of the cell cycle; this occurs by cyclin A-CDK2 during S phase and cyclin B-cdc2 during G2/M phases (8), before dephosphorylation occurs in late M phase by protein phosphatase 1 (9).
Inasmuch as fully differentiated mature neurons are non-proliferative, suppression of the cell cycle by hypophosphorylated pRb appears to be critical in the normal function of adult neurons. Increasing evidence suggests, however, that deteriorating neurons in neurodegenerative diseases such as Alzheimer disease (AD), abnormally initiate cell division processes (10). Specifically, aberrant expression of several cyclins, CDKs, and cyclin-dependent kinase inhibitors that may encourage expression of cell cycle promoting genes has been observed in AD (11–21). In addition, hyperphosphorylated pRb and phosphorylated minichromosome maintenance 2 (pMcm2), which is directly linked to pRb activity in cell cycle regulation, were shown to be elevated and colocalized with neurofibrillary tangles (NFTs) in AD (22, 23).
NFTs are flame-shaped intracytoplasmic inclusions composed of aggregations of hyperphosphorylated tau protein that affect proximal neurites and neuron perikarya and are hallmark pathological features of AD (24). However, tau pathology is also observed in several additional distinct neurodegenerative diseases including parkinson-amyotrophic lateral sclerosis of Guam (GP-ALS) (25), Pick disease (26, 27), progressive supranuclear palsy (PSP) (28, 29), subacute sclerosing panencephalitis (SSPE) (30, 31), frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17) (32, 33), dementia pugilistica (34), and neurodegeneration with brain iron accumulation type 1 (NBIA) (35), suggesting the possibility of a common underlying pathogenic pathway for these diseases collectively referred to as tauopathies (36).
pRb hyperphosphorylated at several sites was found to be elevated and associated with NFTs in AD (22). Because AD shares several pathological characteristics such as the accumulation of hyperphosphorylated tau in NFTs, neuronal dysfunction, and eventual neuronal death with numerous other tauopathies, we investigated the presence of pRb phosphorylated at several sites in brain samples from patients with these neurodegenerative disorders.
MATERIALS AND METHODS
Brain tissue
Using a protocol approved by the Case Western Reserve University IRB, human brain tissue samples were obtained postmortem and fixed in either routine formalin or methacarn (methanol: chloroform: acetic acid; 6:3:1 v/v/v), dehydrated through ascending ethanol, embedded in paraffin, cut into 5-μm sections, and mounted on silane-coated slides. Cases included Pick disease hippocampus (n = 3), PSP brainstem (n = 3), GP-ALS cortex (n = 1), SSPE hippocampus (n = 1), FTDP-17 hippocampus (n = 1), dementia pugilistica cortex (n = 1, gift of Raul Rudelli, MD, Bayonne, NJ), NBIA cortex (n = 2, gift of William Halliday, MD, Toronto, Canada), and non-pathological control hippocampus (n = 10) and brainstem (n = 5). All brainstem sections were cut at the level of the pons. Due to the rarity of some diseases, very limited tissue sections were available for some cases.
Immunohistochemistry
Tissue sections were deparaffinized with xylene and hydrated through descending concentrations of ethanol. Endogenous peroxidase activity was ablated by 30-minute incubation in 3% H2O2 in methanol and non-specific antibody binding was blocked with 30-minute incubation in 10% normal goat serum (NGS) in Tris-buffered saline (TBS). The sections were incubated at 4°C overnight in primary antibody diluted in 1% NGS in TBS. Primary antibodies were rabbit polyclonal antibodies against pRb (Biosource International, Camarillo, CA) phosphorylated at serine 249 and threonine 252 (ppRbser249/T252), serine 807 (ppRbser807) and serine 612 (ppRbser612), an antibody specific for pMcm2 (40/41, gift of Corrado Santocanale, PhD, Galway, Ireland) (23), mouse monoclonal antibodies to phosphorylated tau (AT8, Pierce Endogen, Rockford, IL) and glial fibrillary acidic protein (GFAP, clone GA5, Millipore, Billerica, MA). Antibody specificity was fully characterized in a previous study (22). Species-specific secondary antibodies (Millipore) and PAP complexes (Covance, Princeton, NJ) were then applied in succession. 3, 3’-diaminobenzidine (Dako, Carpinteria, CA) was used as the chromogen and imaged with a Zeiss Axiophot and Zeiss Axiocam (Carl Zeiss, Thornwood, NY).
For some experiments, tissue sections were pretreated with 70% formic acid for 5 minutes with acetate buffer and pressure cooking (Biocare Medical, Concord, CA), or treated with trypsin (400 μg/ml for 10 minutes at room temperature) to investigate effects of epitope unmasking for staining of ppRb. Further experiments were performed using double label fluorescent microscopy using AlexaFluor 488 and AlexaFluor 568 (Invitrogen, Carlsbad, CA) for detection of ppRb and AT8 or GFAP in pathological and neuritic structures and imaged using a Zeiss Axiovert microscope. Quantification of the stained structures was performed. The same fields were imaged and the structures immunostained for either ppRb alone, AT8 alone, or both were counted and expressed as the percentage of stained neurons within each group (37).
RESULTS
Phosphorylated pRb (ppRb) was frequently associated with the characteristic pathological inclusions in several neurodegenerative diseases. Labeling of NFTs and other inclusions was verified in all cases by either colocalization with hyperphosphorylated tau and/or morphological criteria.
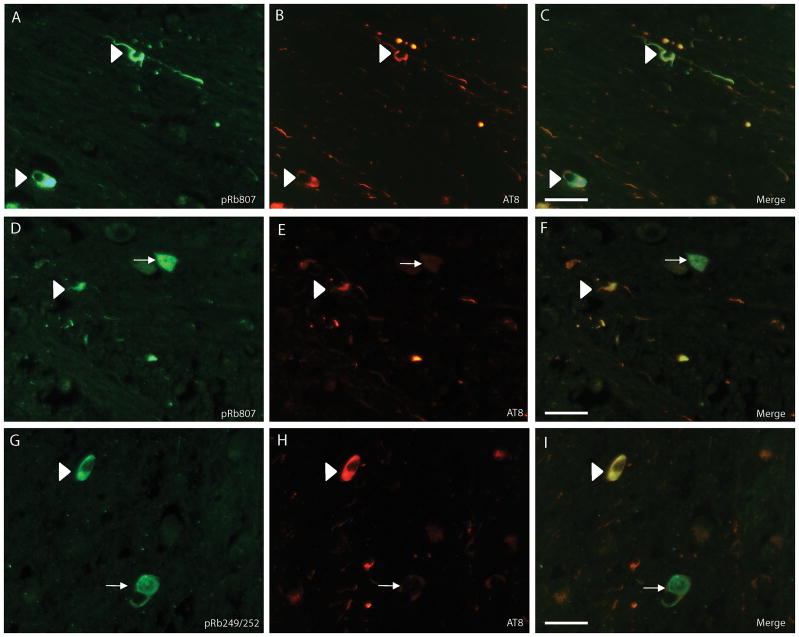
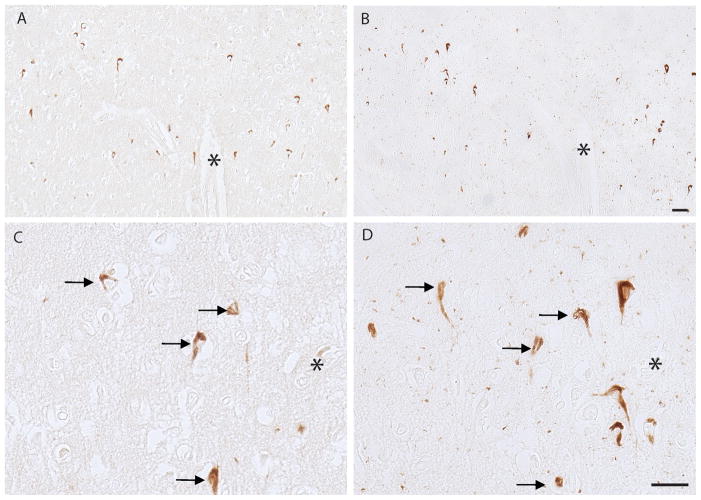
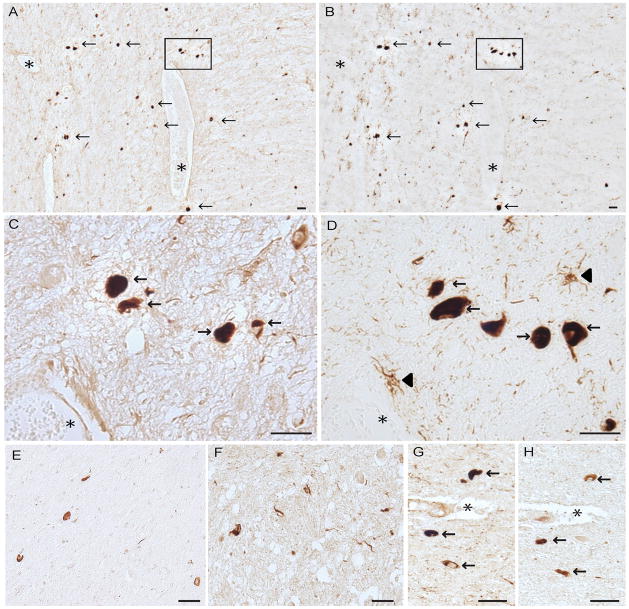
In the PSP cases, ppRbser807 was found in the majority of PSP tangles that also had accumulated phosphorylated tau; it was seen in many neuropil threads in the brainstem as well (Fig. 1). The neuritic pathology was more prominent with AT8 than with the antibody to ppRbser807 in all cases. Semiquantitative analysis demonstrated that over 50% of the stained neurons contained both ppRbser807 and phosphorylated tau; an average of approximately 20% of neurons was only ppRbser807-positive and 23% were only AT8-positive. In addition, some structures stained with AT8 displayed glial morphology (Fig. 1D, arrowheads); these were not stained for ppRbser807 (Fig. 1C).A smaller subset of PSP tangles was immunolabeled for ppRbser612 than for ppRbser807, (Fig. 1E). In addition, the antibody to pMcm2, a key cell cycle regulator that is directly modulated by pRb activity also labeled neurons and neurites in the PSP cases (Fig. 1F), supporting the activation of cell cycle by hyperphosphorylation of pRb. In serial sections of PSP brainstem using the rabbit polyclonal antibodies against ppRbser807 (Fig. 1G) and pMcm2 (Fig. 1H), some cells showed colocalization of both cell cycle markers. Comparisons of various pretreatments for antigen retrieval (e.g. formic acid, trypsin, and heating by pressure cooking) indicated that PSP tangles were well stained whether the tissue was left untreated or was treated using the various antigen retrieval methods (data not shown). Because the pathological structures were strongly stained with little background or non-specific staining on untreated tissue sections, most experiments were performed without pretreatments.
Figure 1.
Serial sections from the brainstem in a case of progressive supranuclear palsy (PSP). (A-D) Colocalization of ppRbser807 (A, C) and AT8 labeling of hyperphosphorylated tau (B, D) in PSP tangles. Higher magnification of boxes from (A) and (B) are shown in C and D, respectively. Glial tau pathology (arrowheads in D) is demonstrated with the AT8 antibody. (E) pRb612 is also localized to a population of PSP tangles and neurites. (F) Phosphorylated minichromosome maintenance 2 (pMcm2), another cell cycle marker, is specifically localized to PSP tangles and neurites. (G, H) Both ppRbser807 (G) and pMcm2 (H) are present in the same neurons seen in adjacent serial sections. Asterisks denote landmark vessels and arrows identify the same neurons across serial sections. Scale bars = 50 μm.
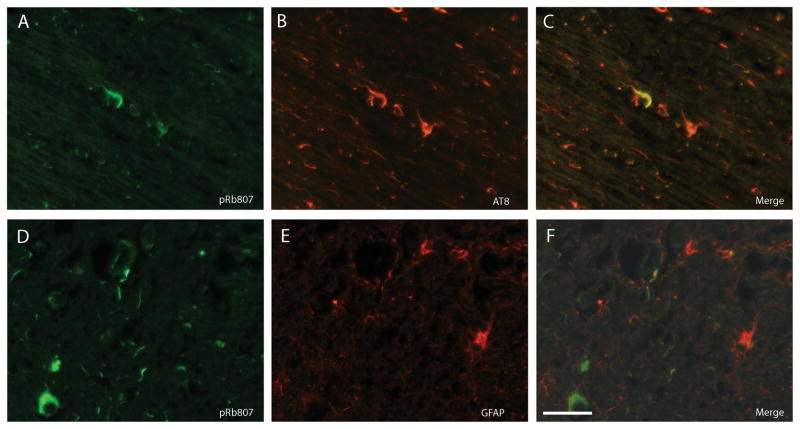
By double label fluorescence microscopy, many PSP tangles and a population of neurites were immunostained with both the antibody to ppRbser807 (Fig. 2A) and AT8 (Fig. 2B). Many more cell processes (which could be either neurites or glial processes containing tau) only stained for phosphorylated tau. Interestingly, some neurons in PSP cases showed high levels of ppRbser807 staining but no AT8-positive phosphorylated tau (Fig. 2D-F, white arrows). ppRbS249/T252 was also found in numerous PSP tangles and neurons, some of which contained nearly undetectable levels of phosphorylated tau labeled by AT8 (Fig. 2G-I, white arrows). In an area of white matter in a PSP case with many AT8-positive processes, very few cellular processes contained both ppRbser807 and AT8 (Fig. 3A-C). Double staining with anti-GFAP indicated that most glial structures did not accumulate ppRbser807 (Fig. 3D-F).
Figure 2.
Double label fluorescence microscopy in progressive supranuclear palsy (PSP). (A-C) A large number of PSP tangles show both ppRbser807 (A, green) and AT8 (B, red) marked with white arrowheads and there are also many AT8-positive neurites that do not contain ppRbser807 (C, merged image). (D-F) There are a few neurons that are strongly immunoreactive for ppRbser807 (D, green), but lack AT8 (E, red) (white arrow) and evident in merged image (F). White arrowheads indicate neurons with colocalization. (G-I) The same pattern is found using an antibody specific for ppRbS249/T252 (G, green). Many neurites only stain with antibody AT8 (H, red); some PSP tangles display only ppRbS249/T252 and not phosphorylated tau (I, merged image, white arrow); some PSP tangles show colocalization (white arrowheads). Scale bars = 50 μm.
Figure 3.
Double label immunofluorescence in another case of progressive supranuclear palsy. (A-C) Very few AT8-positive neurites (B, red) contain ppRbser807 (A, green, and merged image, C). (D-G) Even in fields with many ppRbser807-positive neurites (D, green) the vast majority do not colocalize with astrocytic processes marked using an antibody to glial fibrillary acidic protein (E, red, and merged image F). Scale bar = 50 μm.
The hippocampus and adjacent temporal cortex in the GP-ALS case contained many neurons with NFT when stained for ppRbser807 (Fig. 4A and inset). In the FTDP-17 case there was also prominent labeling of the neuritic processes with the anti-ppRbser807 antibody (Fig. 4B).
Figure 4.
Parkinson-amyotrophic lateral sclerosis of Guam (GP-ALS) hippocampus and adjacent cortex and frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17). (A) In a case of GP-ALS there is substantial ppRbser807 in neurofibrillary tangles (NFTs). Higher magnification (inset) reveals the morphology of the NFTs containing ppRbser807. (B) In the tau mutation disorder FTDP-17, the neuritic pathology is prominently labeled with the antibody against ppRbser807. Scale bars: A, B = 50 μm; Inset = 20 μm.
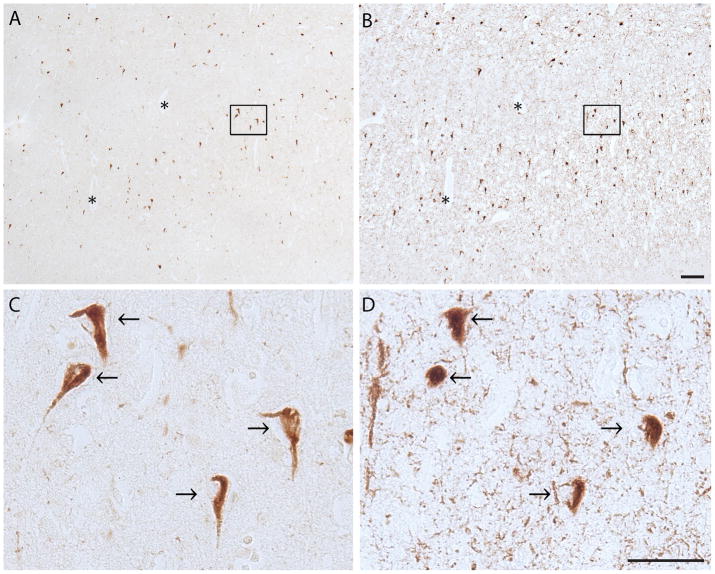
The hippocampus and adjacent temporal cortex in the SSPE case contained many NFTs with both ppRbSer807 and phosphorylated tau. Many of the same neurons contained both ppRbser807 and AT8 but the tau pathology was more extensive and also included neuritic pathology and neuropil threads that were not ppRbser807- positive (Fig. 5). Serial sections of the NBIA cases revealed many cells that showed both ppRbser807 and phosphorylated tau (Fig. 6).
Figure 5.
Serial sections from the hippocampus of a case of subacute sclerosing panencephalitis (A-D) There is colocalization of ppRbser807 (A) and hyperphosphorylated tau (B) in NFTs. Higher magnification demonstrates colocalized labeling of ppRbser807 (C) and hyperphosphorylated tau (D) in many of the same neurons across serial sections. Asterisks denote landmark vessels. Scale bars = 50 μm.
Figure 6.
Neurodegeneration with brain iron accumulation type 1. (A-D) Sections immunostained with antibody to ppRbser807 (A, C) and AT8 staining for hyperphosphorylated tau (B, D) show widespread and abundant colocalized labeling of neurofibrillary tangles. Higher magnification of boxes from A and B reveal colocalization in many of the same neurons (arrows). Asterisks denote landmark vessel. Scale bars: A, B =100 μm; C, D = 50 μm.
Semiquantitative analysis indicated that while nearly 30% of stained neurons showed colocalization, 30% displayed ppRbser807 only and over 40% were only AT8-positive. Neuropil threads, which are AT8-positive, consistently remain unlabeled with the antibody against ppRbser807 in this and most other conditions.
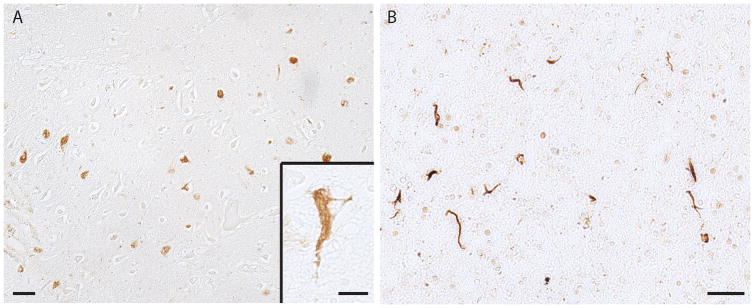
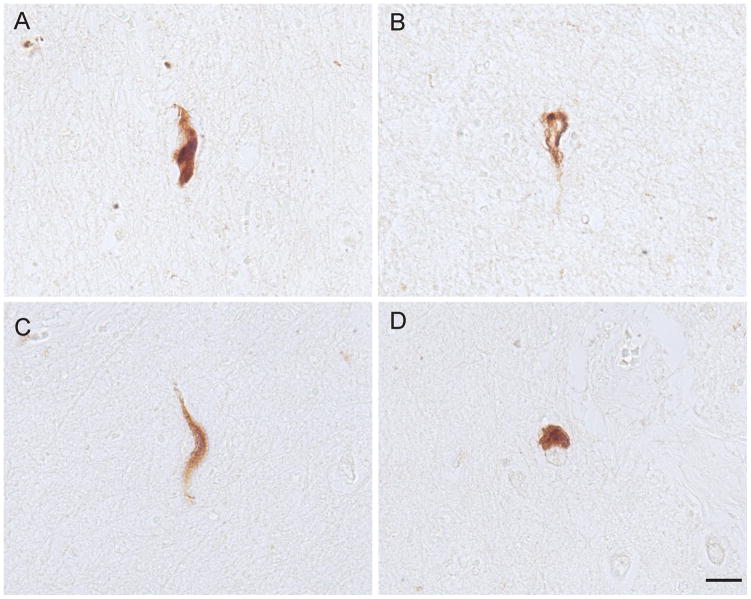
NFTs in the dementia pugilistica case also showed ppRbser807 immunoreactivity. The NFTs were present in small numbers, had variable morphologic appearances and were scattered throughout the cortical section examined (Fig. 7).
Figure 7.
Neurofibrillary tangles (NFTs) in the cortex from a case of dementia pugilistica. (A-D) NFTs immunolabeled for ppRbser807 display the characteristic morphologies found in the disorder. Scale bar = 20 μm.
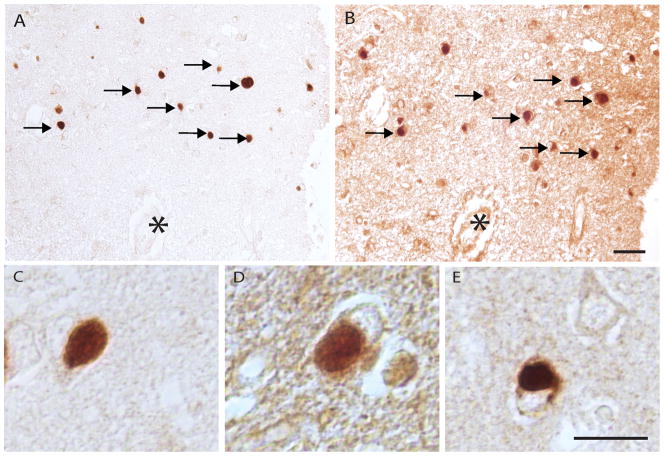
Immunostaining for ppRb was seen in many Pick bodies in neurons of the hippocampus and adjacent cortex in all cases of Pick disease examined. ppRbser807 (Fig. 8A, C) and ppRbS249/T252 (Fig. 8B, D) were observed in many of the same Pick bodies identified in adjacent serial sections. Pick bodies were also stained using the antibody against pMcm2 (Fig. 8E).
Figure 8.
Pick disease. (A, B) Pick bodies throughout the hippocampus and cortex accumulate ppRbser807 (A) and many of the same Pick bodies are also immunopositive for ppRbS249/T252 (B, arrows). Asterisks denote landmark vessel. (C-E) Higher magnification images show well-defined Pick bodies immunostained for ppRbser807 (C), ppRbS249/T252 (D), and the cell cycle marker phosphorylated minichromosome maintenance 2 (E). Scale bars: A, B = 50 μm; C-E = 20 μm.
Non-pathological control brainstem immunostained with antibodies to pRbser807 and pRbS249/T252 showed abundant healthy pigmented neurons and only light background staining (data not shown). Consistent with our previous finding (22), control hippocampal tissue from cases between the ages of 57 and 92 years demonstrated few age-associated NFTs. Younger cases between the ages of 17 and 48 years showed no specific neuronal staining.
DISCUSSION
In this study, we sought to investigate the potential role of hyperphosphorylated pRb in neurodegenerative diseases with hyperphosphorylated tau pathology and neuronal loss. We found that ppRb immunoreactivity was 1) elevated in all disease cases compared to controls, 2) often colocalized with hyperphosphorylated tau-containing NFT pathology in all diseases studied, 3) was also often present in neurons that did not contain phosphorylated tau, and 4) colocalized with hyperphosphorylated tau in Pick bodies in Pick disease cases. Because the presence of pRb indicates that cells are bypassing the G1-to-S phase checkpoint, these data suggest that vulnerable neurons in GP-ALS, Pick disease, PSP, SSPE, FTDP-17, dementia pugilistica, and NBIA aberrantly re-enter the cell cycle throughout the disease course. Importantly, pRb must be phosphorylated at several of its 16 potential phosphorylation sites in a cumulative manner for disinhibition of E2F transcription factors to occur and the cell cycle to progress (38). Evidence indicates S249/T252 and S807 only become available for phosphorylation after several other sites are phosphorylated due to conformational masking (39). Specifically, initial phosphorylation on sites T356, T373, S811, T821, and T826 must occur to invoke a conformational change in the pRb molecule that makes S249/T252 and S807 available to CDKs (38). In addition, hypophosphorylated pRb is tightly localized to the nucleus, whereas hyperphosphorylated pRb releases to a cytoplasmic distribution (40). Cytoplasmic pRb labeling was predominantly observed in the cases analyzed in this study. Therefore, our results indicate that the pRb isoforms evaluated represent hyperphosphorylated pRb that is dissociated from E2F and, as such, represents a valid indicator of cell cycle progression. Furthermore, the antibodies used in this investigation were fully characterized and shown to have high specificity in a previous study (22), lending additional support to the validity of our present findings. pRb phosphorylation by CDKs probably occurs in the nucleus after which the CDK-pRb complex translocates to the cytoplasm (22). Evidence indicates pRb phosphorylation occurs independent of, or prior to, NFT formation in AD (41), however, CDKs have also been shown to phosphorylate tau in vitro (42-44) (Fig. 9). Our finding in the present study that a number of neurons in PSP and other diseases express high levels of ppRb, yet have not accumulated phosphorylated tau supports this concept. Consequently, accumulating pRb-CDK in the cytoplasm may be necessary for NFT formation and explain the colocalization of ppRb and NFTs observed.
Figure 9.
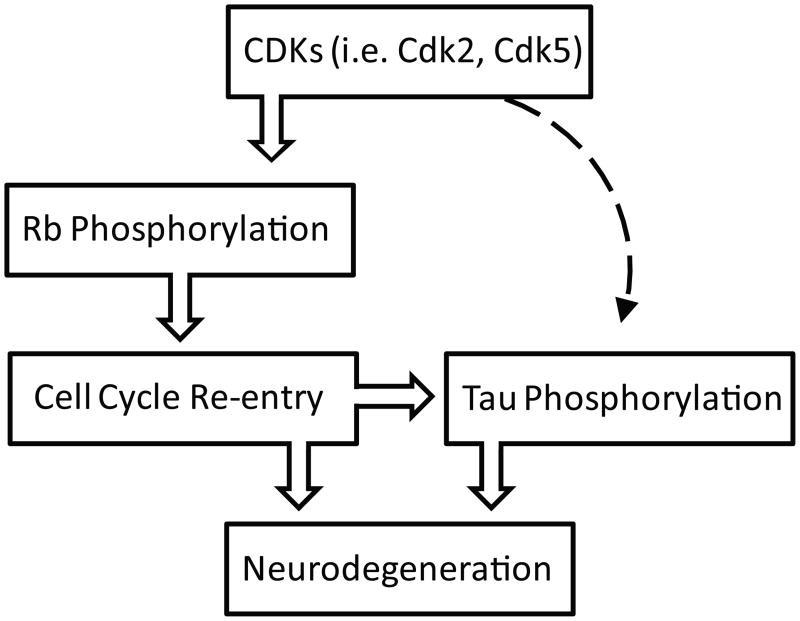
Hypothetical mechanism of pRb regulation in tauopathies. The activation of Cdks induces the hyperphosphorylation of pRb and subsequent degradation, which leads the progression of cell cycle to S-phase. The re-activation of cell cycle in post-mitotic neurons might induce hyperphosphorylation of tau and neuronal cell loss. In addition, Cdks, particularly Cdk5, might phosphorylate tau independent of cell cycle regulatory effects (broken line). Cdk5 can directly phosphorylate tau.
We also found that pMcm2 is in pathological structures in both PSP and Pick disease, and that it is often present in the same cells with ppRb (Fig. 1). CDK 2 is required for the phosphorylation of Mcm2 responsible for regulating DNA replication; this is another G1/S phase checkpoint control protein along with pRb that is tightly regulated by E2F (45, 46). Thus, this finding further supports the dysregulation of pRb and subsequent activation of cell cycle in the vulnerable neurons in tauopathies. In addition, extracellular signal-regulated kinase and p38, which are activated in AD and mediate tau phosphorylation (47–51), also phosphorylate pRb. The combined effect of CDKs, extracellular signal-regulated kinase, and p38 may, therefore, represent a link between the aberrant phosphorylation increased in tau and pRb in these tauopathies (52, 53). Further investigation into the presence of kinase(s) that mediate both tau and pRb phosphorylation in tauopathies is certainly warranted. In this regard, Cdk5 might be one candidate because multiple studies demonstrate that Cdk5 is one of the major tau kinases (54, 55) and, importantly, also phosphorylates pRb and subsequent cell cycle activation (Fig. 9) (56, 57).
Irrespective of the mechanism by which hyperphosphorylated pRb accumulates in tau pathology within the tauopathies investigated, the implications of this observation are intriguing. Although neurons were historically viewed as quiescent non-proliferating cells, our results on pRb and pMcm2 add to increasing evidence that suggests neurons may re-enter the cell cycle throughout the course of various neurodegenerative diseases. Because the various tauopathies investigated in this study and AD all involve progressive neuronal dysfunction ultimately resulting in neuronal death, re-entry into the cell cycle may be a major neurodegenerative mechanism in these diseases. In support of this notion, pRb phosphorylation occurs prior to neuronal death in animal models of ischemia (58, 59), and the forced activation of neuronal cell cycle re-entry in vivo causes severe neuronal cell loss in a transgenic mouse model (60). Although many questions remain and several future studies are justified, our finding of pRb in various tau pathologies of GP-ALS, Pick disease, PSP, SSPE, FTDP-17, dementia pugilistica, and NBIA further elucidates the role of pRb in neurodegeneration, supports the notion of a common underlying pathological pathway present in tauopathies, and suggests a novel potential target for therapeutic intervention.
Acknowledgments
Sources of support: Work in the authors’ laboratories supported by the National Institutes of Health (AG028679 to H.G.L.) and the Alzheimer’s Association (NIRG-09-132727 to H.G.L.).
References
- 1.Ma D, Zhou P, Harbour JW. Distinct mechanisms for regulating the tumor suppressor and antiapoptotic functions of Rb. J Biol Chem. 2003;278:19358–66. doi: 10.1074/jbc.M301761200. [DOI] [PubMed] [Google Scholar]
- 2.Poznic M. Retinoblastoma protein: a central processing unit. J Biosci. 2009;34:305–12. doi: 10.1007/s12038-009-0034-2. [DOI] [PubMed] [Google Scholar]
- 3.Ohtani K. Implication of transcription factor E2F in regulation of DNA replication. Front Biosci. 1999;4:D793–804. doi: 10.2741/ohtani. [DOI] [PubMed] [Google Scholar]
- 4.Ranganathan S, Scudiere S, Bowser R. Hyperphosphorylation of the retinoblastoma gene product and altered subcellular distribution of E2F-1 during Alzheimer's disease and amyotrophic lateral sclerosis. J Alzheimers Dis. 2001;3:377–85. doi: 10.3233/jad-2001-3403. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–61. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittnacht S. Control of pRB phosphorylation. Curr Opin Genet Dev. 1998;8:21–7. doi: 10.1016/s0959-437x(98)80057-9. [DOI] [PubMed] [Google Scholar]
- 7.Chew YP, Ellis M, Wilkie S, et al. pRB phosphorylation mutants reveal role of pRB in regulating S phase completion by a mechanism independent of E2F. Oncogene. 1998;17:2177–86. doi: 10.1038/sj.onc.1202443. [DOI] [PubMed] [Google Scholar]
- 8.Herwig S, Strauss M. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis. Eur J Biochem. 1997;246:581–601. doi: 10.1111/j.1432-1033.1997.t01-2-00581.x. [DOI] [PubMed] [Google Scholar]
- 9.Lukas J, Petersen BO, Holm K, et al. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol Cell Biol. 1996;16:1047–57. doi: 10.1128/mcb.16.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowser R, Smith MA. Cell cycle proteins in Alzheimer's disease: plenty of wheels but no cycle. J Alzheimers Dis. 2002;4:249–54. doi: 10.3233/jad-2002-4316. [DOI] [PubMed] [Google Scholar]
- 11.Arendt T, Rodel L, Gartner U, et al. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer's disease. Neuroreport. 1996;7:3047–9. doi: 10.1097/00001756-199611250-00050. [DOI] [PubMed] [Google Scholar]
- 12.McShea A, Harris PL, Webster KR, et al. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer's disease. Am J Pathol. 1997;150:1933–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Nagy Z, Esiri MM, Cato AM, et al. Cell cycle markers in the hippocampus in Alzheimer's disease. Acta Neuropathol. 1997;94:6–15. doi: 10.1007/s004010050665. [DOI] [PubMed] [Google Scholar]
- 14.Nagy Z, Esiri MM, Smith AD. Expression of cell division markers in the hippocampus in Alzheimer's disease and other neurodegenerative conditions. Acta Neuropathol (Berl) 1997;93:294–300. doi: 10.1007/s004010050617. [DOI] [PubMed] [Google Scholar]
- 15.Raina AK, Monteiro MJ, McShea A, et al. The role of cell cycle-mediated events in Alzheimer's disease. Int J Exp Pathol. 1999;80:71–6. doi: 10.1046/j.1365-2613.1999.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McShea A, Wahl AF, Smith MA. Re-entry into the cell cycle: a mechanism for neurodegeneration in Alzheimer disease. Med Hypotheses. 1999;52:525–7. doi: 10.1054/mehy.1997.0680. [DOI] [PubMed] [Google Scholar]
- 17.Harris PL, Zhu X, Pamies C, et al. Neuronal polo-like kinase in Alzheimer disease indicates cell cycle changes. Neurobiol Aging. 2000;21:837–41. doi: 10.1016/s0197-4580(00)00218-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Rottkamp CA, Raina AK, et al. Neuronal CDK7 in hippocampus is related to aging and Alzheimer disease. Neurobiol Aging. 2000;21:807–13. doi: 10.1016/s0197-4580(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa O, Lee HG, Zhu X, et al. Increased p27, an essential component of cell cycle control, in Alzheimer's disease. Aging cell. 2003;2:105–10. doi: 10.1046/j.1474-9728.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa O, Zhu X, Lee HG, et al. Ectopic localization of phosphorylated histone H3 in Alzheimer's disease: a mitotic catastrophe? Acta Neuropathol (Berl) 2003;105:524–8. doi: 10.1007/s00401-003-0684-3. [DOI] [PubMed] [Google Scholar]
- 21.Previll LA, Crosby ME, Castellani RJ, et al. Increased expression of p130 in Alzheimer disease. Neurochem Res. 2007;32:639–44. doi: 10.1007/s11064-006-9146-3. [DOI] [PubMed] [Google Scholar]
- 22.Thakur A, Siedlak SL, James SL, et al. Retinoblastoma protein phosphorylation at multiple sites is associated with neurofibrillary pathology in Alzheimer disease. Int J Clin Exp Pathol. 2008;1:134–46. [PMC free article] [PubMed] [Google Scholar]
- 23.Bonda DJ, Evans TA, Santocanale C, et al. Evidence for the progression through S-phase in the ectopic cell cycle re-entry of neurons in Alzheimer disease. Aging (Milano) 2009;1:382–8. doi: 10.18632/aging.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry G, Castellani RJ, Hirai K, et al. Reactive oxygen species mediate cellular damage in Alzheimer disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- 25.Miklossy J, Steele JC, Yu S, et al. Enduring involvement of tau, beta-amyloid, alpha-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam (ALS/PDC) Acta Neuropathol. 2008;116:625–37. doi: 10.1007/s00401-008-0439-2. [DOI] [PubMed] [Google Scholar]
- 26.Perry G, Stewart D, Friedman R, et al. Filaments of Pick's bodies contain altered cytoskeletal elements. Am J Pathol. 1987;127:559–68. [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock NJ, Mirra SS, Binder LI, et al. Filamentous aggregates in Pick's disease, progressive supranuclear palsy, and Alzheimer's disease share antigenic determinants with microtubule-associated protein, tau. Lancet. 1986;2:1211. doi: 10.1016/s0140-6736(86)92212-9. [DOI] [PubMed] [Google Scholar]
- 28.Ghatak NR, Nochlin D, Hadfield MG. Neurofibrillary pathology in progressive supranuclear palsy. Acta Neuropathol. 1980;52:73–6. doi: 10.1007/BF00687231. [DOI] [PubMed] [Google Scholar]
- 29.Tabaton M, Whitehouse PJ, Perry G, et al. Alz 50 recognizes abnormal filaments in Alzheimer's disease and progressive supranuclear palsy. Ann Neurol. 1988;24:407–13. doi: 10.1002/ana.410240309. [DOI] [PubMed] [Google Scholar]
- 30.Iwatsubo T, Hasegawa M, Ihara Y. Neuronal and glial tau-positive inclusions in diverse neurologic diseases share common phosphorylation characteristics. Acta Neuropathol. 1994;88:129–36. doi: 10.1007/BF00294505. [DOI] [PubMed] [Google Scholar]
- 31.Yuksel D, Yilmaz D, Uyar NY, et al. Tau proteins in the cerebrospinal fluid of patients with subacute sclerosing panencephalitis. Brain Dev. 2010;32:467–71. doi: 10.1016/j.braindev.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Ludolph AC, Kassubek J, Landwehrmeyer BG, et al. Tauopathies with parkinsonism: clinical spectrum, neuropathologic basis, biological markers, and treatment options. Eur J Neurol. 2009;16:297–309. doi: 10.1111/j.1468-1331.2008.02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosso SM, Kamphorst W, Ravid R, et al. Coexistent tau and amyloid pathology in hereditary frontotemporal dementia with tau mutations. Ann N Y Acad Sci. 2000;920:115–9. doi: 10.1111/j.1749-6632.2000.tb06912.x. [DOI] [PubMed] [Google Scholar]
- 34.Toth C, McNeil S, Feasby T. Central nervous system injuries in sport and recreation: a systematic review. Sports Med. 2005;35:685–715. doi: 10.2165/00007256-200535080-00003. [DOI] [PubMed] [Google Scholar]
- 35.Bonelli RM, Cummings JL. Frontal-subcortical dementias. Neurologist. 2008;14:100–7. doi: 10.1097/NRL.0b013e31815b0de2. [DOI] [PubMed] [Google Scholar]
- 36.Lovestone S, McLoughlin DM. Protein aggregates and dementia: is there a common toxicity? J Neurol Neurosurg Psychiatry. 2002;72:152–61. doi: 10.1136/jnnp.72.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeda A, Smith MA, Avila J, et al. In Alzheimer's disease, heme oxygenase is coincident with Alz50, an epitope of tau induced by 4-hydroxy-2-nonenal modification. J Neurochem. 2000;75:1234–41. doi: 10.1046/j.1471-4159.2000.0751234.x. [DOI] [PubMed] [Google Scholar]
- 38.Brown VD, Phillips RA, Gallie BL. Cumulative effect of phosphorylation of pRB on regulation of E2F activity. Mol Cell Biol. 1999;19:3246–56. doi: 10.1128/mcb.19.5.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittnacht S, Lees JA, Desai D, et al. Distinct sub-populations of the retinoblastoma protein show a distinct pattern of phosphorylation. EMBO J. 1994;13:118–27. doi: 10.1002/j.1460-2075.1994.tb06241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittnacht S, Weinberg RA. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell. 1991;65:381–93. doi: 10.1016/0092-8674(91)90456-9. [DOI] [PubMed] [Google Scholar]
- 41.Jordan-Sciutto KL, Malaiyandi LM, Bowser R. Altered distribution of cell cycle transcriptional regulators during Alzheimer disease. J Neuropathol Exp Neurol. 2002;61:358–67. doi: 10.1093/jnen/61.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincent I, Jicha G, Rosado M, et al. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J Neurosci. 1997;17:3588–98. doi: 10.1523/JNEUROSCI.17-10-03588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith MZ, Nagy Z, Esiri MM. Cell cycle-related protein expression in vascular dementia and Alzheimer's disease. Neurosci Lett. 1999;271:45–8. doi: 10.1016/s0304-3940(99)00509-1. [DOI] [PubMed] [Google Scholar]
- 44.Ding XL, Husseman J, Tomashevski A, et al. The cell cycle Cdc25A tyrosine phosphatase is activated in degenerating postmitotic neurons in Alzheimer's disease. Am J Pathol. 2000;157:1983–90. doi: 10.1016/S0002-9440(10)64837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chuang LC, Teixeira LK, Wohlschlegel JA, et al. Phosphorylation of Mcm2 by Cdc7 promotes pre-replication complex assembly during cell-cycle re-entry. Mol Cell. 2009;35:206–16. doi: 10.1016/j.molcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iyer NG, Xian J, Chin SF, et al. p300 is required for orderly G1/S transition in human cancer cells. Oncogene. 2007;26:21–9. doi: 10.1038/sj.onc.1209771. [DOI] [PubMed] [Google Scholar]
- 47.Perry G, Roder H, Nunomura A, et al. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. Neuroreport. 1999;10:2411–5. doi: 10.1097/00001756-199908020-00035. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X, Rottkamp CA, Boux H, et al. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:880–8. doi: 10.1093/jnen/59.10.880. [DOI] [PubMed] [Google Scholar]
- 49.Zhu X, Rottkamp CA, Hartzler A, et al. Activation of MKK6, an upstream activator of p38, in Alzheimer's disease. J Neurochem. 2001;79:311–8. doi: 10.1046/j.1471-4159.2001.00597.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhu X, Castellani RJ, Takeda A, et al. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the 'two hit' hypothesis. Mech Ageing Dev. 2001;123:39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 51.Zhu X, Sun Z, Lee HG, et al. Distribution, levels, and activation of MEK1 in Alzheimer's disease. J Neurochem. 2003;86:136–42. doi: 10.1046/j.1471-4159.2003.01820.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhu X, Lee HG, Perry G, et al. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Zhu X, Raina AK, Perry G, et al. Alzheimer's disease: the two-hit hypothesis. Lancet Neurol. 2004;3:219–26. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 54.Camins A, Verdaguer E, Folch J, et al. The role of CDK5/P25 formation/inhibition in neurodegeneration. Drug News Perspect. 2006;19:453–60. doi: 10.1358/dnp.2006.19.8.1043961. [DOI] [PubMed] [Google Scholar]
- 55.Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem. 2008;15:2321–8. doi: 10.2174/092986708785909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee KY, Helbing CC, Choi KS, et al. Neuronal Cdc2-like kinase (Nclk) binds and phosphorylates the retinoblastoma protein. J Biol Chem. 1997;272:5622–6. doi: 10.1074/jbc.272.9.5622. [DOI] [PubMed] [Google Scholar]
- 57.Hamdane M, Bretteville A, Sambo AV, et al. p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. J Cell Sci. 2005;118:1291–8. doi: 10.1242/jcs.01724. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi T, Sakai K, Sasaki C, et al. Phosphorylation of retinoblastoma protein in rat brain after transient middle cerebral artery occlusion. Neuropathol Appl Neurobiol. 2000;26:390–7. doi: 10.1046/j.1365-2990.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- 59.Katchanov J, Harms C, Gertz K, et al. Mild cerebral ischemia induces loss of cyclin-dependent kinase inhibitors and activation of cell cycle machinery before delayed neuronal cell death. J Neurosci. 2001;21:5045–53. doi: 10.1523/JNEUROSCI.21-14-05045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HG, Casadesus G, Nunomura A, et al. The neuronal expression of MYC causes a neurodegenerative phenotype in a novel transgenic mouse. Am J Pathol. 2009;174:891–7. doi: 10.2353/ajpath.2009.080583. [DOI] [PMC free article] [PubMed] [Google Scholar]