Abstract
Summary: Malaria remains a major cause of morbidity and mortality in the tropics, with Plasmodium falciparum responsible for the majority of the disease burden and P. vivax being the geographically most widely distributed cause of malaria. Gametocytes are the sexual-stage parasites that infect Anopheles mosquitoes and mediate the onward transmission of the disease. Gametocytes are poorly studied despite this crucial role, but with a recent resurgence of interest in malaria elimination, the study of gametocytes is in vogue. This review highlights the current state of knowledge with regard to the development and longevity of P. falciparum and P. vivax gametocytes in the human host and the factors influencing their distribution within endemic populations. The evidence for immune responses, antimalarial drugs, and drug resistance influencing infectiousness to mosquitoes is reviewed. We discuss how the application of molecular techniques has led to the identification of submicroscopic gametocyte carriage and to a reassessment of the human infectious reservoir. These components are drawn together to show how control measures that aim to reduce malaria transmission, such as mass drug administration and a transmission-blocking vaccine, might better be deployed.
INTRODUCTION
Malaria remains one of the most important causes of morbidity and mortality in the tropical regions of the world. Current estimates suggest that approximately 2.4 billion people are at risk of stable or unstable Plasmodium falciparum transmission (178), with 350 to 500 million clinical episodes and 1 million deaths annually (191). For the first time, similar global estimates are also available for P. vivax, and while there is considerably less mortality attributed to this species, its geographical reach is far greater. An estimated 2.9 billion people are at risk for vivax malaria (179), with an estimated 80 million to 300 million clinical cases annually (296). These global estimates are a direct result of an increasing ability to collate and assimilate large data sets that also allow the monitoring of trends in malaria incidence and parasite prevalence. These broad-scale estimates from countries where malaria is endemic (178), together with specific country examples (23, 24, 35, 87, 174, 227, 328, 329), have highlighted the recent trend of decreasing malaria transmission intensity in many areas of endemicity, and these observations, at least in part, have stimulated (or restimulated) the malaria elimination agenda (8b, 276). While there is considerable debate in the malaria community about the rationale and likely success of elimination programs, the ongoing discussions have led to a reevaluation of current strategies to reduce or abrogate the transmission of malaria parasites. For P. falciparum, the theoretical solution is that if one were able to clear infections in all humans and render P. falciparum noninfectious to mosquitoes for a period longer than the mosquito life span (8 weeks, for example), then transmission would stop. This panglossian ideal is of course unlikely. Nevertheless, the addition of interventions specifically aimed at reducing transmission from humans to mosquitoes would greatly enhance our ability to control and potentially eliminate malaria. Elimination of P. vivax is further complicated by the presence of hypnozoites, dormant liver-stage schizonts that can form a reservoir for transmissible parasites for many months after the initial infection.
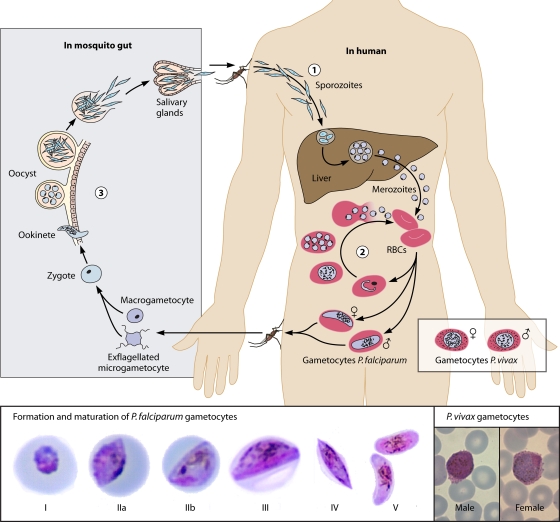
Transmission from an infected human host to a susceptible mosquito is mediated through highly specialized sexual-stage parasites, i.e., gametocytes (Fig. 1). The gametocytes of P. falciparum hold a prominent place in the history of malaria, in that it was the exflagellating male gametocyte that first led Laveran to describe malaria in the late 19th century (8a). Exflagellation is a highly active process whereby motile male microgametes free themselves from the red blood cell in order to locate and fertilize a female macrogamete. Also, on routine microscope blood films, the gametocytes of P. falciparum, with their unique crescent shape, are prominent.
Fig. 1.
(Top) Life cycle of Plasmodium falciparum and gametocyte development. Malaria parasites enter the human bloodstream in the form of sporozoites that are injected by infected female Anopheles mosquitoes taking a blood meal. The majority of sporozoites migrate to the liver, where they invade hepatocytes and multiply. Merozoites are formed that are released into the bloodstream, where they invade red blood cells, initiating the asexual multiplication cycle. A fraction of merozoites that are released from infected red blood cells form gametocytes, the transmissible parasite form. The formation and maturation of gametocytes take place in five morphologically recognizable stages. Early-stage gametocytes are sequestered, and only mature stage V gametocytes circulate in the peripheral blood, where they can be taken up by mosquitoes. Once ingested by mosquitoes, each individual gametocyte forms 1 female macrogamete or up to 8 male microgametes. In the mosquito midgut, the fusion of gametes results in the formation of a zygote that develops into a motile ookinete that can penetrate the midgut wall to form oocysts. The oocysts enlarge over time and burst to release sporozoites that migrate to the mosquito salivary gland, rendering the mosquito infectious to human beings. (Bottom) The five developmental stages of P. falciparum gametocytes and mature P. vivax gametocytes. (The P. falciparum gametocyte photographs are reprinted from reference 410 with permission; the P. vivax gametocyte photographs are courtesy of Debbie Nolder, Malaria Reference Laboratory, London School of Hygiene and Tropical Medicine, United Kingdom, reproduced with permission.)
Transitioning from the relatively protected and stable environment within the red blood cell of the human host to being an exposed parasite in the lumen of a mosquito midgut obviously requires considerably different characteristics. Not surprisingly, therefore, molecular sequencing and proteomics have identified 250 to 300 sexual stage-specific genes, 75% of which were hypothetical at the time of identification (149, 155, 239, 247). These proteins will almost certainly be involved in some of the fundamental steps of gametocyte development. What stimulates gametocyte production? Where and how do gametocytes sequester? Do they preferentially localize in the dermis? What signals allow gametes to interact at low densities? These basic biological questions are central to our understanding of how the parasite transmits itself at the population level. This issue has extra complexity related to differences in malaria exposure, transmission intensity, and age of the individual infected as well as to how treatment and control measures such as drugs might affect transmission. Ultimately, these factors combine to define the infectious reservoir of malaria within an area, i.e., the people capable of transmitting malaria to mosquitoes. This human infectious reservoir forms the part of the population that would need to be targeted with transmission-reducing interventions in order to reduce malaria or abrogate malaria transmission.
In the subsequent sections, we discuss these issues to provide a current situational assessment of our knowledge of P. falciparum gametocytes and the related gaps in our understanding. Whenever gametocytes are mentioned without specifying the malaria species, we refer to P. falciparum gametocytes. Toward the end of this review, we discuss factors associated with gametocytemia and transmission of P. vivax, whose transmission stages have been studied less well than those of P. falciparum. At present, control tools that significantly target gametocytes are limited, and those that are available have not been developed suitably for wide-scale use. Research into these gametocyte-specific knowledge gaps may yet yield a tool to augment our malaria control capabilities and go some way toward achieving malaria elimination.
GAMETOCYTE BIOLOGY AND MORPHOLOGY
P. falciparum gametocytes are markedly different from their asexual precursors. Transcriptome analyses identified 250 to 300 genes that are specifically upregulated at the mRNA level during gametocyte development (417, 528). The P. falciparum proteome revealed more than 900 proteins in gametocytes, 315 of which were found exclusively in gametocytes (239). Furthermore, a sex-specific proteome study using the rodent malaria model P. berghei indicated that a large proportion of these proteins are expressed exclusively in either male or female gametocytes (220). These findings reflect the highly specialized nature of gametocytes and the individual roles of both sexes. Some of the key antigens are summarized in Table 1 and discussed below. Gametocyte development can be divided into five morphologically recognizable stages (Fig. 1) (190), during which the gametocyte grows and elongates to gradually occupy the majority of the erythrocyte (21). The most striking morphological features of gametocytes are the presence of the subpellicular microtubule-based cytoskeleton and the surrounding double membrane, which create the characteristic crescent shape (280, 464). The differences between male and female gametocytes become morphologically most apparent from stage IV onwards, when gametocytes are characterized by an elongated shape with pointed ends. Female gametocytes are characterized by a relatively small nucleus with a nucleolus and concentrated pigment. The nucleus is larger and the pigment is more diffuse in male gametocytes, which appear to lack a nucleolus and appear in Giemsa-stained blood films as pink cells, as opposed to violet females. Sexually committed ring-stage parasites and the crescent-shaped mature gametocytes (stage V) can be found in the human peripheral blood (404). Developmental-stage parasites are thought to be sequestrated in the bone marrow, and possibly the spleen (427, 475), but can circulate in splenectomized hosts (17).
Table 1.
Key antigens in P. falciparum gametocyte development and transmission-blocking vaccines
| Antigen(s) | Localizationa | Function | Reference(s) |
|---|---|---|---|
| PfEMP-1 | RBC membrane | Sequestration | 105, 193 |
| RIFIN | Unknown | Sequestration? | 351, 505 |
| STEVOR | RBC membrane | Sequestration? | 273 |
| Pfs16 | PVM | Unknown | 21, 22 |
| Pfpeg3 and -4 | PVM | Early gametocyte development | 417 |
| PF14 744 and PF14 748 | PVM | Gametocyte development | 138 |
| Pfg377 | OB | Gametocyte maturation, osmiophilic body formation, and egression of macrogametes | 6, 108 |
| Pfg27 | GCT cytoplasm | Gametocyte formation/integrity; evidence for RNA binding | 21, 78, 253, 408 |
| Pfs48/45b | GM | Fertilization: male fertility factor attachment of male microgametes to fertile female macrogametes | 493, 494 |
| Pfs47 | GM | Fertilization: female fertility factor (not essential) | 498 |
| Pfs230b | GM | Fertilization: adherence of male gametes to red blood cells; may protect parasite from contents of blood meal; complement required for antibody function | 137, 369, 514 |
| HAP-2 | GM (male) | Fertilization: fusion of gamete surface membranes | 40, 251 |
| Pfs25 and Pfs28b | OM | Midgut penetration, ookinete survival, oocyst formation | 28, 130, 477 |
RBC, red blood cell; PVM, parasitophorous vacuole membrane; OB, osmiophilic bodies; GCT, gametocyte; GM, gametocyte/gamete membrane; OM, zygote and ookinete membrane.
Malaria transmission-blocking vaccine candidate in the most advanced stage of development.
Sequestration of Gametocytes
Immature P. falciparum gametocytes are sequestered away from the circulation, presumably to avoid immune clearance in the spleen. Once gametocytes are mature (stage V), they are released into the circulation and are thereby accessible to mosquitoes taking a blood meal. However, it may take 2 to 3 days before circulating and morphologically mature gametocytes become infectious to mosquitoes (243). Early-stage gametocytes (stages I and IIa) seem to depend on CD36 as the principal host ligand for gametocyte adherence (106, 193, 436); ICAM-1, CD49c, CD166, and CD164 receptors that are present in human bone marrow epithelial and stromal cell lines have been suggested as host ligands for later gametocyte stages (stages III and IV) (113, 390). Parasite ligands that mediate adherence are less well characterized but may include the parasite surface proteins PfEMP-1 (P. falciparum erythrocyte membrane protein 1) (105, 193, 411), RIFIN (repetitive interspersed family) (17, 505), and STEVOR (subtelomeric variable open reading frame) (273, 458). The var genes encode PfEMP-1 molecules (432), which are among the most extensively described parasite surface proteins. PfEMP-1 is the primary mechanism for cytoadherence and sequestration of asexual parasites (94, 322, 409). PfEMP-1 transcripts are also found throughout gametocyte development, with transcription profiles that appear independent of the phenotype of their asexual progenitors (411). PfEMP-1 is expressed in knob structures on erythrocytes infected with early-stage gametocytes (stages I and IIa) (105, 193), making a role in gametocyte sequestration particularly plausible in the first days of gametocyte development (193). Erythrocytes infected with late-stage gametocytes no longer have knob structures, and PfEMP-1 expression appears confined to the cytoplasm of the parasite (193). It is unclear why gametocytes switch to a different adhesion mechanism during maturation or which parasite ligands play a role in sequestration of the later stages. STEVOR transcripts are most abundant in early-stage gametocytes (273, 411), while the encoded protein is also localized at the infected erythrocyte membrane in late-stage gametocytes (273). This is consistent with a role in gametocyte sequestration, although the presence of STEVOR proteins on the surfaces of erythrocytes infected with nonsequestering stage V gametocytes makes a role in cytoadhesion speculative (351). There is recent evidence that RIFINs are transcribed in gametocytes (351, 505), but their role in gametocyte adhesion remains to be confirmed.
Preparing for Environmental Change
After approximately 6 days of gametocyte development, protein synthesis and hemoglobin digestion cease (21, 79, 192). Gametocytes appear developmentally arrested at the G0 phase of the cell cycle (423). There is no genome replication in gametocytes (370), and nucleic acid synthesis in gametocytes is restricted to RNA synthesis. Rapid genome replication and nuclear division occur only once gametocytes are activated in the mosquito midgut (21). In preparation for this activation, mRNAs encoding proteins that are required after ingestion by mosquitoes, such as the P25 and P28 proteins, are synthesized in gametocytes but translationally repressed (182, 234, 342). This translational repression depends on RNA helicases (259, 260), and the mRNAs for several sexual stage-specific proteins can be detected in gametocytes (14, 277, 403, 404, 421). Among them is pfs16 mRNA, the earliest marker of sexual stage development, present in all gametocyte stages and in sexual stage-committed asexual parasites 24 h after merozoite invasion (69, 404). Pfs16 protein is located on the parasitophorous vacuole membrane until it is lost during gametocyte activation (21, 22). Other early gametocyte proteins include Pfpeg3 and Pfpeg4 (stage II gametocytes) (417) as well as PF14 744 and PF14 748 (138). Pfg27 is transcribed at ∼30 h postinvasion (83, 408) and is hypothesized to play a role in the extended period of gametocyte development (21) that is so specific to P. falciparum. pfs25 mRNA transcription starts in mature (stage V) gametocytes (14, 15, 404). Once mature gametocytes are ingested by Anopheles mosquitoes that are taking a blood meal, they become activated and prepare for fertilization in a process called gametogenesis. Within 20 min after being taken up by a mosquito, gametocytes have differentiated into gametes in response to a variety of stimuli, including a drop in temperature of ∼5°C, a rise in pH from 7.4 to 8 to 8.2, and the presence of the mosquito-derived gametocyte-activating factor xanthurenic acid (36, 37, 200, 423). During gametogenesis, gametocytes are activated to differentiate into spherical cells and shed their erythrocyte membrane. Male gametes then undergo three rounds of DNA replication, and subsequently, up to eight motile microgametes are released during exflagellation (200, 343, 400, 420, 422, 423).
GAMETOCYTOGENESIS
Mature gametocytes are detectable in the bloodstream at days 7 to 15 after the initial wave of asexual parasites from which they are derived (105, 136). Gametocytogenesis is the process whereby male and female gametocytes develop from these asexual parasites. Our current understanding of molecular mechanisms involved in gametocytogenesis was recently reviewed by Baker, by Alano, and by Dixon et al. (5, 21, 113). Here we describe some of the key elements in gametocytogenesis and epidemiological factors that are associated with gametocyte production.
What Triggers the Sexual Pathway?
Commitment to the sexual pathway occurs in the cycle prior to the actual development of gametocytes, with all merozoites from a single schizont committed to either the sexual or the asexual pathway (68, 464). Recent data indicate that asexual parasites committed to the sexual pathway can be present as early as the first round of asexual replication after P. falciparum infection (404), and work with P. berghei indicated that some of the merozoites produced by exoerythrocytic schizonts may grow directly into gametocytes without an intervening erythrocytic schizogony (222). These initial gametocyte densities are likely to be very low, but they suggest a level of developmental flexibility important for onward transmission. Once committed to the sexual pathway, merozoites become either all male gametocytes or all female gametocytes (416, 435). Gametocyte production differs between parasite isolates (1, 133, 173), and continuous parasite culture may lead to a loss or reduction of gametocyte production related to the loss of genetic information in the right arm of chromosome 9 (89, 107). These findings indicate that there are intrinsic parasite factors related to gametocyte production and a role for a gene on chromosome 9 in gametocytogenesis. Subsequent work demonstrated that parasite strains with a disrupted Pfgig gene within this region of chromosome 9 had 5-fold less gametocyte production (154).
Limited experimentation has shown that gametocytogenesis can be induced in vitro, in parasite lines where gametocytes had not been observed during several years of continuous culture (330, 332), by addition of ammonium compounds (330) or after a short exposure to Berenil, an inhibitor of nucleic acid synthesis (332). Other studies found that gametocyte production in vitro can be induced by red blood cell lysate (86) or by the addition of mammalian hormones to culture (250, 265). Although these strategies are valuable for obtaining gametocytes from culture, they are unlikely to be the natural trigger for gametocytogenesis in natural infections. Signaling pathways may be involved in the molecular mechanism that triggers gametocytogenesis, potentially including the phorbol ester-inducing pathway (481) and the cyclic AMP (cAMP) signaling pathway (66, 377, 481). Additional findings on increased gametocytogenesis in vitro after addition of cholera toxin (132) led to the conclusion that a G-protein-dependent signaling system may mediate gametocytogenesis in response to environmental factors. These data suggest that multiple factors may contribute to the decision to form gametocytes such that the parasite is able to respond to a variety of environmental stimuli.
Gametocytogenesis is sometimes described as a stress response of the parasite that allows it to escape an increasingly unfavorable environment (21, 133). Although “stress” is unlikely to be essential for gametocyte production, as indicated by the commitment to sexual development early in infections (404) and in asymptomatic untreated infections (52, 131), gametocyte production is clearly affected by several factors during the course of an infection. In culture, gametocyte production may increase at higher parasite densities (68), in the presence of soluble factors from parasite-conditioned medium (513), and upon addition of human serum and lymphocytes (428), reticulocytes (480), or antimalarial drugs (72, 77, 202). All of these factors can be linked to increased gametocyte production in natural infections.
Host immunity, asexual parasite density, and gametocytogenesis.
Naturally acquired immunity of the human host affects asexual parasite densities and therefore the production of gametocytes from their asexual precursors. Immune responses may also influence gametocytogenesis more directly. Waves of gametocytemia typically follow, but do not necessarily coincide with, clinical episodes of uncomplicated malaria (131, 448). This suggests a potential role in gametocytogenesis for nonspecific immune effectors associated with symptomatic malaria, such as increases in tumor necrosis factor alpha (TNF-α) (236, 237), which has antiparasitic properties (236). However, this association between gametocytogenesis and nonspecific immune responses could also explain the delayed appearance of P. falciparum gametocytes as a strategy to evade potentially toxic levels of human cytokines during febrile paroxysms (218; see the section on P. vivax).
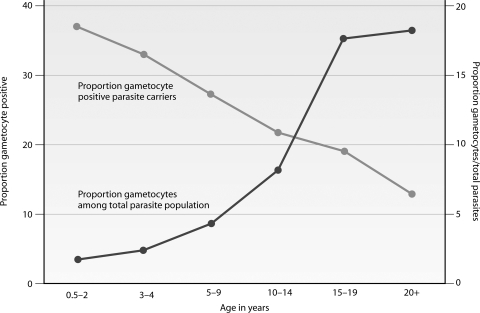
Several lines of evidence also indicate a relationship between malaria-specific immune responses and gametocytogenesis. P. falciparum gametocyte production in culture was increased after addition of lymphocytes and sera from naturally infected Gambian children but not those from malaria-naïve Europeans (428). Increased in vitro gametocyte production was also observed after addition of anti-P. falciparum antibodies produced by hybridoma cell lines (331). In animal models, gametocytogenesis in P. yoelii was induced after immunization of the rodent host with a recombinant heat shock-like protein that is expressed throughout the parasite's life cycle (295). Similarly, increased gametocyte production was observed for P. chabaudi in the early parts of infection after partial immunization of mice with infected erythrocytes that were subsequently cleared by antimalarial treatment (73). These observations are clearly indicative of some relationship between immune responses and gametocytogenesis. In naturally occurring malaria infections, this relationship is difficult to disentangle from the impact of naturally acquired immunity on asexual parasite densities. The lower gametocyte prevalence in adults than that in children (51, 120, 131) (Fig. 2) is explained at least partly by acquired immunity that allows the semi-immune adult host to control asexual parasite densities better (115, 457), resulting in lower gametocyte densities. In contrast to this overall trend, commitment to the sexual pathway may actually increase with acquired immunity. Both microscopic and submicroscopic densities of gametocytes relative to asexual parasites increase with age in cross-sectional surveys (49, 120, 334). While the proportion of gametocyte carriers among parasite carriers is highest for children, the proportion of parasites that are gametocytes may therefore be highest for those with acquired immunity (120, 334). These observations require confirmation in longitudinal studies but suggest further adaptability of gametocytogenesis in response to acquired immunity. An analogous phenomenon has been seen with P. chabaudi, where the net gametocyte production may decrease as a result of acquired immunity against asexual parasites, but with an incomplete compensatory mechanism that increases gametocytogenesis by those asexual parasites that survive (73).
Fig. 2.
Prevalence of gametocytes in different transmission settings and age groups. Gametocytes were detected by microscopy (thick blood films; screening of 100 fields at a magnification of ×1,000, using oil immersion) in cross-sectional surveys in areas of high and seasonal transmission intensity in Burkina Faso (336) and the Gambia (121), high perennial transmission in Tanzania (121), and low seasonal transmission intensity in Tanzania (413). Gametocytes are detected in a larger proportion of the population in settings of high endemicity, where gametocyte prevalence depends on age; in settings of low endemicity, gametocyte carriage is rare and more evenly distributed across age groups.
Host anemia and gametocytogenesis.
Studies from Thailand and the Gambia observed that hemoglobin concentrations were lower in gametocyte carriers (310, 360, 455, 502) and negatively correlated with peak gametocyte counts and the duration of gametocyte carriage (310). The proportion of gametocyte carriers in the population is also associated with seasonal fluctuations in the prevalence of anemia (306). These epidemiological associations do not provide evidence for a causal role of anemia in triggering gametocytogenesis. A larger proportion of gametocyte carriers among anemic individuals could merely indicate a longer duration of infection and hence a longer time to develop gametocytes (360, 383, 440, 450). However, a more direct role is suggested by in vitro and animal studies.
There are strong indications that the triggering of the gametocyte pathway is affected by the presence of reticulocytes or very young erythrocytes (479, 482), which are characterized by a relatively high RNA content, hemoglobin synthesis, and the fact that they are less dense than mature erythrocytes (479). Reticulocytes are the preferred cells for gametocyte development (157), and their formation is increased in erythropoiesis, induced by the production of erythropoietin (EPO) by anemic hosts. EPO is implicated as a stimulant for gametocyte formation (379) and also influences gametocyte sex allocation (343, 346) (see “Gametocyte Sex Ratios”). Reticulocytosis also results in increased in vitro gametocyte production in different P. falciparum strains (479) and in rodent P. berghei (291) and P. chabaudi (157). It is unclear which signal associated with reticulocytes triggers gametocytogenesis; one suggestion is that one of the proteins that are shed from maturing reticulocytes may activate the promoter of the pfs16 gene (479). Some observations from malaria transmission experiments suggest that anemia may have a role in other elements of transmission as well. A positive correlation between host hematocrit and the infectiousness of gametocytes (129) has been observed, though this was significant only at higher gametocyte densities. Conversely, a slight reduction in the host's hematocrit increases the feeding speed and quantity of blood uptake (470), thereby possibly enhancing the chance of successful mosquito feeding. The optimal hematocrit values for transmission success may therefore need to balance these associations (305).
Other factors associated with gametocytogenesis.
Several other conditions that are unfavorable for asexual growth or parasite survival have been associated with increased gametocytogenesis. As an evolutionary strategy, it would be advantageous for parasites to alter their investment in transmission stages in response to the absence or presence of mosquito vectors. This phenomenon has been demonstrated for other vector-borne parasites, such as the increased presence of microfilaria in the peripheral blood during the night, when vectors are present (148, 272). An increase in gametocyte production in infections early in the transmission season was described in some epidemiological studies (336, 492) and was hypothesized to be a result of the induction of gametocyte production by an increase in bites from uninfected mosquitoes (345). However, no direct evidence for this association exists from naturally acquired infections, and the rodent malaria parasites P. chabaudi and P. vinckei also do not increase their rates of gametocytogenesis in response to mosquito probing (415). There is also little evidence that gametocyte production is regulated in a way that ensures peak densities during peak mosquito biting hours (see “Sampling Issues: When and Where Do Mosquitoes Bite?”).
Gametocyte Sex Ratios
Similar to the molecular mechanism that triggers gametocytogenesis, factors that determine the sex of gametocytes are poorly understood. Plasmodium parasites have no sex chromosomes (82); a single parasite clone can produce both male and female gametocytes (422) and can self-fertilize. The sex of gametocytes is determined early in sexual stage commitment, and all merozoites released from one sexually committed schizont become either all male or all female gametocytes (416, 435). The resulting gametocyte sex ratios are typically female biased, which is intuitively correct, since male gametocytes can produce up to eight microgametes and therefore fertilize several female gametes, each derived from a single female gametocyte (343). Considerable levels of inbreeding in malaria parasite populations (347) could suggest that the optimal transmission strategy is to ensure a balance between male microgametes and female macrogametes in the mosquito midgut. However, there is more to gametocyte sex ratios than simply counterbalancing the differences in gamete production by gametocytes. The ratio of female to male gametocytes has an immediate impact on transmission success (284), and the optimal ratio differs in different circumstances. Ratios of 3 or 4 females to 1 male gametocyte are commonly observed in natural P. falciparum infections (215, 385, 386, 445, 464), but there are variations in the sex ratio between clones (76, 373, 378), during the course of infections (207, 343, 344, 465), between regions (215, 385, 386, 442, 445), and between years in the same region (442).
A longer duration of infection reduces hemoglobin levels as a result of asexual parasite proliferation. The related induction of erythropoiesis is linked not only by a general increase in gametocyte production (see “Host anemia and gametocytogenesis”) but also specifically, by an increase in male gametocytes (344, 386). Changes in gametocyte sex ratio may reflect a response of the parasite to overcome (sex-specific) immune responses that are mounted during the course of an infection and may influence the survival or infectivity of gametocytes, as discussed in more detail in the section on immune responses against gametocytes and transmission-blocking immunity. Gametocyte density may also define the optimal ratio for transmission success, where a more male-biased ratio becomes important at lower densities (344, 378). The presence of different parasite clones will favor shifting the female-biased sex ratio to a more even ratio to enhance the chances of transmission of an individual parasite clone (343, 378).
Reports on the impact of antimalarial treatment on gametocyte sex ratios in vivo are inconclusive. Antimalarial treatment may be followed by an increase (442–445, 447, 465) or decrease (445, 452) in the proportion of male gametocytes that may (444, 445, 452) or may not (465) be influenced by the type of treatment. The uncertainties are underlined by the fact that changes in sex allocation were sometimes reported within several days after the initiation of treatment (443, 445, 447, 452), while the environmental cues for sex determination must occur 7 to 10 days before mature gametocytes are observed (343). These findings may indicate sex-specific effects of drugs on male or female gametocytes or simply reflect the difficulty in studying parasite transmission strategies in vivo in humans. Studies using microscopy to quantify and sex gametocytes typically observe fewer than 10 gametocytes in a blood film, not all of which can be sexed reliably. These small numbers limit the precision of sex ratio estimates. The importance of this complication was shown by Robert and colleagues, who observed that as sex ratios became less female biased, the proportion of infected mosquitoes rose, as did the oocyst density in infected mosquitoes. However, this relationship was observed only for individuals for whom the sex ratio was based on sexing of ≥15 gametocytes, not for individuals for whom fewer observations were available (385).
Recent studies have begun to evaluate the use of molecular quantification of sex ratios using the sex-specific P. falciparum dyneins Pfs377 and Pf alpha-tubulin, though they have yet to provide sufficiently discriminatory data (407). The development of molecular tools that can sensitively quantify male and female gametocytes in epidemiological settings could assist in shedding light on the relevance of clonal diversity and sex ratios in malaria transmission (221, 317, 319, 385).
GAMETOCYTE LONGEVITY
For P. falciparum, the mean circulation time per gametocyte in the peripheral blood has been estimated by microscopy as 3.4 to 6.4 days (136, 429), and these estimates were recently confirmed using molecular tools to detect gametocytes (57). However, the true duration of gametocyte carriage depends greatly on the de novo production of gametocytes by asexual parasites. An untreated infection may result in asexual parasite carriage for many months (143, 314). Findings of continuous production of gametocytes for several months in natural infections (1, 7, 283) are likely to reflect this ongoing production of gametocytes by (low-density) asexual infections. After cessation of gametocyte production, i.e., after drug or immune clearance of asexual parasites, the duration of gametocyte carriage is determined by the maximum duration of gametocyte sequestration and the maximum circulation time following gametocyte release into the bloodstream. The circulation time of gametocytes in the bloodstream is then affected by the natural decay of gametocytes, antigametocyte immunity, and gametocytocidal drugs. The maximum gametocyte sequestration time was previously estimated to be 12 days (mean, 7.4 days); the maximum circulation time was estimated to be 22.2 days (mean, 6.4 days) (136). As a consequence, gametocyte carriage in individual patients may be as long as 3 to 6 weeks after asexual parasites are cleared (52, 136, 211). A recent modeling exercise based on findings with molecular gametocyte detection tools concluded that gametocytes may persist for well beyond 1 month after clearance of asexual parasites and that drugs clearing immature and mature gametocytes can lead to substantial reductions of the duration of gametocyte carriage (57). There is currently no evidence for sex-specific differences in longevity or mortality of P. falciparum gametocytes (429), although experiments with P. chabaudi indicate that female gametocytes may be lost from the circulation at a higher rate than male gametocytes (380).
EPIDEMIOLOGY OF GAMETOCYTES
Patterns in gametocyte carriage in naturally infected individuals define the contributions of different populations to the human infectious reservoir for malaria (i.e., those individuals who are infectious to mosquitoes). By microscopy, gametocytes are invariably detected in fewer than 50% of clinical (25, 55, 147, 279, 412, 455, 532) and asymptomatic (52, 121, 337) P. falciparum infections. The question “why so few gametocytes?” has been postulated in reviews by Taylor and Read (469) and by Talman et al. (464). Since these reviews, an increasing body of evidence has indicated that a large proportion of gametocyte carriers remain undetected by microscopy (reviewed in reference 16; see below), and gametocytes may actually be present in the majority of infections, albeit at low densities. However, while prevalence may be increased, the density of gametocytes relative to asexual parasites remains low. Observations from neurosyphilitic patients undergoing malaria therapy suggested that, on average, 1 gametocyte is produced per 156 asexual parasites (136); in most endemic settings, fewer than 5% of the detectable parasites are gametocytes (469). These low levels of sexual-stage parasites are hypothesized as a strategy to evade the induction and/or effects of immune responses against gametocytes or gametes (354, 392, 469) and/or to prevent damage of the mosquito midgut by developing gametes that would negatively influence transmission success (223, 258, 469). In recent years, some new evidence has emerged to suggest that human immune responses can affect the longevity of gametocytes (392), thus supporting the notion that limiting gametocyte production will reduce a stimulus for antigametocyte immune responses.
In the following sections, we discuss the variation in gametocyte carriage between different age groups, transmission settings, and seasons and the possible role for human genetic factors in determining gametocyte carriage.
Age-Dependent Carriage of Gametocytes
The dynamics of P. falciparum gametocytes have been described most extensively for symptomatic malaria cases, where waves of asexual parasitemia and accompanying fever are followed by increases in gametocyte prevalence and density (131, 268). Gametocytes can be present in up to 40% of children upon presentation at a health center with symptomatic malaria, according to microscopy results (25, 55, 147, 279, 412, 455, 532). Gametocytes can also be found in asymptomatic individuals, although at a lower rate (52, 121, 337). Broadly, the prevalence of gametocytes in asymptomatic individuals closely follows that of asexual parasites, both by microscopy (52, 121) and by molecular detection tools (337, 413). In areas of high malaria transmission intensity, gametocyte carriage is most prevalent in younger age groups (Fig. 2), who also have the highest prevalences and densities of asexual malaria parasites (44, 121, 160, 255, 334, 478). In areas of low transmission intensity, the association between gametocyte prevalence and age is weaker, and smaller proportions of all age groups carry gametocytes (1, 51, 171, 413). While gametocyte prevalence appears to follow a predictable pattern, gametocyte densities do not appear to do so. In gametocyte-positive individuals, the density of gametocytes relative to asexual parasites increases with age. A recent study reported that gametocytes make up <2% of the total parasite population in the youngest children, but this proportion gradually increases to >15% in adults (Fig. 3) (334). This finding was observed with both microscopy and quantitative molecular methods (334) and is in line with previously published patterns (120).
Fig. 3.
Age-dependent carriage of gametocytes in an area of high and seasonal malaria transmission in Burkina Faso. Gametocytes were detected by microscopy (thick blood films; screening of 100 fields at a magnification of ×1,000, using oil immersion) in cross-sectional surveys. The proportion of infections with concurrent gametocytes is highest in children and decreases with age. Among infections with gametocytes, the density of gametocytes relative to the total parasite density increases with age (334).
While in many endemic settings children have the majority of high-density parasite infections and clinical attacks, both of which are frequently accompanied by gametocytes, this does not necessarily translate into a majority contribution to the human infectious reservoir. Because of their larger representation in the population, higher likelihood of subpatent untreated infections (337) that are frequently also accompanied by gametocytes (337, 413), and potentially higher level of commitment to the sexual pathway of low-density asexual infections (120, 334), adults contribute considerably to the infectious reservoir. A study that determined the contributions of different age groups to the human infectious reservoir concluded that adults are probably responsible for 28 to 38% of mosquito infections (121), a proportion that could be greater if the larger body size and higher exposure of adults to mosquitoes (357) are taken into account.
Gametocyte Carriage in Relation to Season and Transmission Intensity
Malaria infections and symptomatic malaria episodes are most frequent in the transmission season, usually when mosquitoes are most abundant (following seasonal rains) (321, 336). Two longitudinal studies in areas of markedly different transmission characteristics in Burkina Faso indicated that patterns of gametocyte carriage followed that of asexual parasites, increasing during the peak transmission season (44). However, in both settings, there were some inconsistencies, with relatively high rates of gametocyte carriage during times when asexual parasite carriage was low or declined and stable rates of gametocyte production during the dry season (44). The absolute number of gametocyte carriers is evidently lowest in the dry season, but a larger proportion of the relatively scarce asexual infections in the dry season may produce gametocytes, and the density of gametocytes in these infections relative to that of asexual parasites may be higher in the dry season (46, 120, 264). Although these associations have been described across a range of endemic settings, there is no definitive evidence, as the data are from cross-sectional rather than more informative longitudinal studies. A study where parasite isolates were collected at different time points in the season and submitted to short-term culture to determine their gametocyte-producing capacity did not show a higher production level of gametocytes in the dry season. On the contrary, isolates collected during the months of peak transmission were more likely to produce gametocytes and produced gametocytes at higher densities (39).
Similarly, there is limited evidence for differences in gametocytogenesis between areas of different transmission intensity or after reductions in transmission intensity. Although gametocyte carriage is generally more common in areas of higher transmission intensity (120, 255, 302) (Fig. 2), the investment in transmission stages may be higher in settings of low endemicity. In Kenya, 18% of the parasite carriers living in an area exposed to 10 infectious bites per person per year (ibpy) harbored gametocytes, compared to 11% of the parasite carriers from an area exposed to 20 to 50 ibpy (120, 303). In Tanzania, gametocyte prevalence was 17% among parasite carriers exposed to ∼100 ibpy, compared to 24% among carriers exposed to ∼1 ibpy (120, 123).
In the landmark Garki project, gametocyte prevalence increased in some age groups after transmission intensity was successfully reduced through a control program (290). Similarly, interventions with insecticide spraying and mass drug administration (MDA) that reduced asexual parasite rates in Netherlands New Guinea in the 1950s were followed by increased gametocyte production in surviving infections (281). This increase in relative gametocyte production was mentioned as an explanation for the rapid recovery of P. falciparum parasite rates after an initial successful reduction (281).
Other Factors Associated with Gametocyte Carriage
Several studies have determined risk factors for gametocyte carriage. These studies were done almost exclusively with individuals visiting a health center for symptomatic malaria, and it is difficult to translate risk factors in this highly selective population to biological triggers for gametocytogenesis. In these symptomatic patients, gametocytes were observed more commonly in individuals with longer durations of infection (360, 383, 440, 450), those who presented without fever (455, 502), and those with recrudescent infections (360). The association between mixed parasite infections and P. falciparum gametocytemia remains unclear. Gametocytemia was reported to be less common in mixed infections with P. vivax (360) but more common in infections with P. malariae (50, 269).
Host genetic factors may also affect gametocyte production. Lower gametocyte densities were observed in Fulani than in Mossi tribes in Burkina Faso, an observation that may not be explained completely by differences in asexual stage immunity (340). Gametocyte carriage and malaria transmission potential were also related to human genetic variation at the β-globin locus, with a higher gametocyte density and/or higher infection rate for individuals with the HbAS, HbAC, or HbCC genotype than for those with the HbAA β-globin genotype (164, 387). Further evidence of a genetic component of gametocyte carriage was provided by Lawaly et al. (240). They demonstrated a significant heritable contribution to gametocyte prevalence in individuals from Senegal and Thailand. This effect was apparent only in asymptomatic P. falciparum infections, not in symptomatic P. falciparum cases or P. vivax infections.
Coinfections with other pathogens may also influence the epidemiology of gametocytes. Infection with HIV has been associated with an increased susceptibility to malaria infection (150, 212, 512) and with higher malaria parasite densities (496). Antimalarial treatment efficacy may also be reduced in HIV-infected individuals or HIV-infected individuals with lower CD4 counts (38, 212, 497). Although there is currently no evidence for enhanced gametocyte production in HIV-infected individuals, these associations indicate the occurrence of more-frequent, longer, and higher-density asexual parasite carriage in HIV-infected individuals. It is therefore plausible that, at population levels, HIV infection leads to increased malaria transmission (2, 495).
THE TIP OF THE ICEBERG: SUBMICROSCOPIC GAMETOCYTE DENSITIES
Gametocytes typically circulate at low densities, and microscopy is not a very sensitive tool for detecting all relevant densities of gametocytes. As early as the 1930s, it was realized that mosquitoes can become infected with P. falciparum (210, 297, 299) or P. vivax (61, 208) after taking a blood meal in which no gametocytes were observed by microscopy. In the last decade, evidence has accumulated that microscopy detects only a fraction of all gametocyte carriers.
Detecting Gametocytes by Microscopy
Longitudinal studies in regions where malaria is endemic frequently observe that microscopically detectable gametocytes are present at one time point and disappear at a follow-up visit, only to reappear later (52, 103, 126). This indicates that gametocytes often circulate at densities close to the threshold of microscopic detection. It has been estimated that routine examination of thick films (typically 100 fields at a magnification of ×100, using oil immersion) results in screening of 0.16 to 1 μl of blood (31, 176, 217), although for reliable detection of a parasite density of 1/μl, a volume of at least 3 μl has to be examined (217). Routine microscopy examining 100 microscopic fields may therefore miss gametocyte densities as high as 20 to 50 gametocytes/μl (119, 217, 292). Gametocytes of nonfalciparum malaria species lack the distinctive crescent shape and resemble other stages of the life cycle; they may therefore go undetected at even higher densities. In line with this low sensitivity of routine microscopy, gametocyte prevalence is commonly increased in studies where the efficiency of examination by microscopy is increased by either increasing the number of examined microscope fields or concentrating parasites prior to screening. Estimates of gametocyte prevalence were increased by 60% when the number of examined fields of a thick film was increased from 20 to 100 (4), by 29% when this number was doubled from 200 to 400 (289), and 3-fold when 1,000 instead of 100 high-power thick blood film fields were examined (98). Use of the quantitative buffy coat technique, in which a 55-μl blood volume is examined by use of acridine orange-coated capillary tubes (453), increased the prevalence of gametocytes from 4.6% to 19.1% (301). Magnetic deposition microscopy (MDM) is used to concentrate erythrocytic-stage malaria parasites that contain hemozoin by exploiting their paramagnetic characteristics using strong magnetic fields (529). MDM makes it possible to screen a larger blood volume, thereby improving the detection sensitivity compared to that of routine microscopy. The use of MDM increased P. falciparum gametocyte prevalence in 200 examined thick blood smear fields from 7.3% (∼0.4 μl blood examined quantitatively and up to 4 μl examined qualitatively) to 45% by MDM (∼40 μl blood examined) for symptomatic malaria cases in Papua New Guinea (216). The increasing sensitivities of these methodologies confirm that a large pool of gametocyte carriers are undetected by routine microscopy.
Molecular Gametocyte Detection Tools
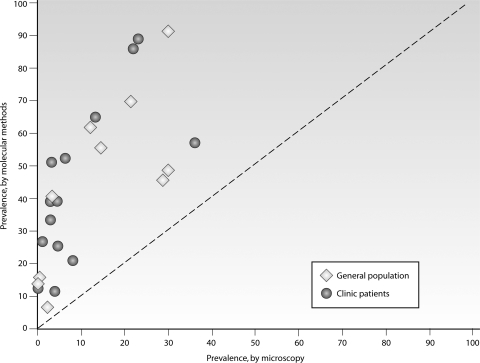
Our understanding of the prevalence and relevance of low-density gametocyte carriage has been facilitated greatly by the use of molecular detection tools that can detect, quantify, and characterize gametocytes genetically. Molecular gametocyte detection tools are based on the amplification of RNA that is expressed exclusively in gametocytes (14, 15, 277, 403, 404, 421). RNA is required specifically for gametocytes, as asexual parasites also carry the DNA encoding gametocyte-specific RNA transcripts. pfs25 mRNA has formed the basis of gametocyte detection and quantification by reverse transcriptase PCR (RT-PCR) (1, 15, 16, 286), quantitative nucleic acid sequence-based amplification (QT-NASBA) (15, 16, 404), and RT loop-mediated isothermal amplification (RT-LAMP) (70). The gametocyte-specific protein gene pfg377 is also transcribed exclusively in gametocytes (6, 239) and has a similar sensitivity for gametocyte detection to that for pfs25 (277), but it is more polymorphic than pfs25 (15). Single-nucleotide polymorphisms and size differences in the pfg377 transcript allow identification of at least 21 distinct pfg377 alleles (319) and, thereby, identification of distinct gametocyte-producing parasite clones within single infections (1, 277, 319). These molecular detection tools have sensitivities in the range of 0.02 to 0.1 gametocyte/μl (403), or perhaps as low as 0.002/μl or 2 gametocytes/ml (70). In reality, the sensitivity is limited by the volume of blood that can be collected and processed ethically and practically. Clinical and epidemiological studies that have determined gametocyte carriage by microscopy and RT-PCR or QT-NASBA are summarized in Fig. 4. In five clinical trials that used microscopy and molecular gametocyte detection tools, gametocytes were detected at enrollment in 4 to 26% of the patients by microscopy, but 3- to 10-fold more often by QT-NASBA or RT-PCR (39 to 90%) (7, 55, 279, 403, 412). Similar proportional increases in gametocyte prevalence were observed in the general asymptomatic population living in areas where malaria is endemic. In areas of very low endemicity in Tanzania and Sudan, gametocytes were detected in 0 to 6% of the population by microscopy but in 12 to 52% of the population by molecular tools (1, 314, 413). In areas of more intense seasonal transmission in the Gambia and Burkina Faso, gametocyte prevalences ranged from 3 to 30% by microscopy and from 49 to 70% by use of molecular tools (319, 337, 340). These findings indicate that gametocyte carriage is consistently underestimated by microscopy. A recent meta-analysis of microscopy and molecular methods for asexual infection showed a 2-fold higher prevalence by PCR (325), but for gametocytes, this figure may be as high as 10-fold.
Fig. 4.
Gametocyte carriage by microscopy and molecular detection tools. Gametocytes were detected by microscopy, typically screening 100 microscopic fields, and by pfs25- or pfg377-specific RT-PCR, LAMP, or QT-NASBA. Samples were derived from the general population in Burkina Faso, Tanzania, the Gambia, and Thailand (open diamonds) (70, 263, 319, 335, 337, 340, 413) and from people attending clinics in Kenya, Tanzania, Sudan, and Vietnam (closed circles) (1, 55, 140, 256, 279, 314, 412), mostly children participating in clinical trials. (Reproduced from reference 334 with permission.)
The upshot of this is that all malaria infections should be considered plausible gametocyte producers. Studies comparing Pfg377 alleles in RNA and genomic DNA extracted from the same infection tend to support this and indicate that although there is variation in gametocyte production between strains (1), most infections produce gametocytes at some time point (314, 319). In this way, chronic asymptomatic infections may also sustain gametocyte production in the absence of mosquitoes during the dry season (1, 13, 314).
The number of gametocyte-positive days and the area under the gametocyte density-time curve (455) are more informative representations incorporating the temporal element in describing the reservoir of (submicroscopic) gametocyte carriage. These findings are of considerable epidemiological relevance, as gametocytes at submicroscopic densities can infect mosquitoes (335, 402) and contribute to malaria transmission, as we discuss below.
GAMETOCYTES AND MALARIA TRANSMISSION
Gametocytes are obviously essential for malaria transmission, but their presence in the peripheral blood does not necessarily equate to infectivity. The first step in the process is that a mosquito feeds on a gametocytemic host. This is determined by global and local factors influencing mosquito abundance (433), household and behavioral characteristics that influence mosquito exposure, and the attractiveness of humans to mosquitoes. The attractiveness of humans to mosquitoes is influenced by numerous factors, including body size (357) and human odor components (368). An increased attractiveness of gametocyte carriers to mosquitoes was suggested by Koella and colleagues (238); this finding requires confirmation in other settings but, if proven, would have implications for malaria control. After a mosquito has ingested gametocytes from a human host, the chance of infection depends on a combination of gametocyte maturity, concentrations of male and female forms, and human and mosquito immune responses. The infectiousness of gametocytes can be confirmed by offering gametocytes to Anopheles mosquitoes, followed by the detection of oocysts (300, 324, 394) or sporozoites (226, 394) in mosquitoes. Transmission experiments can involve direct feeding of mosquitoes on the skin of infected individuals (393) or offering a gametocytemic blood meal to mosquitoes through a membrane (300, 490) (see “Evidence for Naturally Acquired Transmission-Blocking Activity”). The former is closer to the natural situation, may be more efficient (111), and avoids some gametocyte sampling issues that are discussed below. However, the ethical preference in most settings appears to be membrane feeding experiments.
Gametocyte Density and Mosquito Infection Rates
Despite their close representation of a real-life scenario, membrane feeding experiments have almost unequivocally shown discrepancies between the presence of gametocytes and their infectiousness (47, 101, 170, 335, 402, 423). Thus, while there is an overall positive association between gametocyte density and the mosquito infection rate (59, 335, 402), the association is not very strong and is most variable at low gametocyte concentrations (490). High densities of gametocytes do not necessarily result in mosquito infection (151, 171, 402), and conversely, low densities do not exclude infectiousness (101, 297, 335, 402). The failure of high gametocyte densities to result in mosquito infection is partly the result of immaturity of gametocytes (183, 243). As stated above, after their release into the circulation, P. falciparum gametocytes require another 2 to 3 days to become infectious to mosquitoes (243, 429). However, more puzzling is low-density infectivity and how some infections declared P. falciparum gametocyte free by routine microscopy were found to be infectious to mosquitoes in studies in the 1950s (210, 297, 299). Indeed, infectious reservoir determination by xenodiagnosis, where the infectiousness of humans is determined by mosquito experiments, was consequently considered to be more reliable than microscopic observations of gametocytes (297, 298). Despite these early observations, very few studies have since been conducted to determine the infectiousness of individuals to mosquitoes, independent of whether or not gametocytes were observed microscopically. A study in Burkina Faso indicated that the infectiousness of microscopically confirmed gametocyte carriers may be similar to that of individuals who are apparently gametocyte free by routine microscopy (45), while a study in Papua New Guinea did not find an important contribution of submicroscopic gametocytes to malaria transmission (171). Two studies that related gametocyte densities to mosquito infection rates by membrane feeding experiments using molecular detection tools indicated that mosquito infection is common below the microscopic threshold for gametocyte detection (Fig. 5). These field observations were confirmed by serial dilutions of cultured gametocytes that were offered to mosquitoes under laboratory conditions (402). In field and laboratory experiments, mosquitoes became infected after feeding on a meal containing gametocytes at a concentration below 1 gametocyte/μl. This finding highlights an important gap in our current understanding of malaria transmission. A mosquito blood meal is 2 to 3 μl, on average, and should contain at least one male and one female gametocyte to result in infection. Although the proportion of males may increase at low densities (344, 378), sex ratios are typically strongly female biased, with 3 or 4 female gametocytes for every male gametocyte (215, 385, 386, 445, 464). This makes the observed efficiency of transmission at low gametocyte concentrations even more intriguing. Unknown mechanisms may facilitate an aggregation of gametocytes that favors the encounter of males and females (352). One of these mechanisms may be a transmission-enhancing effect of antigametocyte antibodies (152, 195, 349), possibly directed against currently unknown antigens (459, 491). Other possibilities include preferential localization of gametocytes in the dermis prior to ingestion or enhanced chemotraction once in the midgut. Recently, the formation of membranous cell-to-cell connections was described for activated P. falciparum gametocytes. Up to 11 filaments were found on the surfaces of male and female gametes within minutes of gametocyte activation; no filaments were detected on nonactivated gametocytes. It was hypothesized that these “nanotubes” have adhesive properties and play a role in locating, connecting, and pooling gametocytes and gametes within a blood meal, favoring successful fertilization (391). This is a field for further research to explore factors related to tropism and gametocyte sex ratios at low gametocyte concentrations.
Fig. 5.
Gametocyte densities and mosquito infection rates. Data from two transmission studies of asymptomatic children in Burkina Faso (335) and symptomatic children 2 weeks after antimalarial treatment in Kenya (55, 402) were combined. Both studies determined gametocyte carriage by Pfs25-specific QT-NASBA and offered venous blood to mosquitoes in a membrane feeding assay (see “Evidence for Naturally Acquired Transmission-Blocking Activity”) to determine the proportion of infected mosquitoes. Circles indicate aggregated data, grouped in 10 categories of similar gametocyte densities.
Sampling Issues: When and Where Do Mosquitoes Bite?
Despite the value of membrane feeding assays, their current use has failed to answer some long-standing questions. Membrane feeding experiments are typically performed in a cross-sectional approach, sampling each individual once, either at enrollment or at a fixed time point after treatment. This approach is justifiable because of the ethical issues surrounding repeated venous bleeds on (young) gametocyte carriers. It is difficult, however, to appreciate the impact of interventions that reduce the duration of gametocyte carriage if observations are restricted to a single time point, given the potential for temporal fluctuations in infectiousness (183, 208, 270). A temporal association where peaks in infectiousness coincide with peaks in vector biting would be attractive from an evolutionary point of view, but this association remains to be proven. In transmission experiments conducted at 12- or 15-hour intervals, no significant fluctuations in the infectivity of P. falciparum gametocytes were observed (64, 161), although 48-hour fluctuations in exflagellation rates of gametocytes were described (190). A study of five Gambian infants suggested that gametocytes time their maturation in the peripheral blood according to regular daily temperature fluctuations in the host to coincide with peak vector densities (190), a hypothesis that was not supported by a study of eight Tanzanian children where gametocytes showed elevated peripheral densities between 1530 and 1930 h, well before the peak biting time of 0030 to 0330 h described for the local mosquito vector (257). Although it was suggested that due to the unknown temporal association between peaks in gametocyte densities in finger-prick samples and subdermal capillaries, gametocyte densities at mosquito sampling sites may still peak at the correct time for maximal transmission (459), the periodicity of malaria gametocytemia remains unresolved (156).
These examinations of the periodicity of gametocytemia and infectivity have highlighted another crucial hole in our knowledge, namely, the association between gametocyte densities in venous blood samples (typically used in transmission experiments), finger-prick blood samples (typically used for microscopic detection), and subdermal capillaries. The latter, where mosquitoes take their blood meal, is the real site of interest. It is conceivable that infective gametocytes actively seek subdermal sites to enhance their transmission potential. Subdermal clustering has been hypothesized to be related to the crescent shape of P. falciparum gametocyte-infected erythrocytes (304), and clustering of gametocytes in mosquito blood meals suggests some additional mechanism for aggregation (352). A unique study from 1952 is often cited in this respect and provides direct, albeit incomplete, evidence for preferential sequestration in the subdermal capillaries. In this study, 200 individuals were examined for different stages of malaria parasites by skin scarification (91), a method with a sensitivity similar to that of skin snips for detecting Onchocerca volvulus infections (315). The prevalence of gametocytes in skin samples was 3-fold higher than that in thick smears from finger-prick samples from the same area (91). Although this finding was confirmed by the same research group in a different setting (488) and corroborates findings for rodent malaria showing that the blood meal the mosquito engorges directly from the rodent contains more gametocytes than a blood sample from the animal's tail (157), evidence is incomplete.
ANTIMALARIAL DRUGS AND GAMETOCYTEMIA
All antimalarial drugs that successfully clear asexual parasitemia will limit the duration of gametocyte carriage by preventing or interrupting the production of gametocytes from asexual parasites; the speed at which asexual parasites are cleared will determine how quickly gametocyte production is disrupted after the initiation of treatment. The impact of antimalarial drugs on P. falciparum gametocytes depends on the type of antimalarial drug and the level of drug resistance.
Effects of Drugs on Gametocytemia
Early-stage P. falciparum gametocytes are susceptible to most commonly used antimalarial drugs (77, 426, 510). Nevertheless, antimalarial treatment is not automatically followed by a decrease in gametocyte carriage. P. chabaudi and P. falciparum have been documented to increase their investment in gametocytes in response to subcurative drug therapy (71, 72), and after therapeutic doses of antimalarials, an initial increase in gametocyte carriage may also be observed. The use of sulfadoxine-pyrimethamine (SP) is associated with an increase in gametocyte carriage after treatment (55, 166, 167, 383, 440, 448, 466, 502). However, studies using molecular gametocyte detection tools suggest that this increase in microscopic gametocyte prevalence after treatment may simply reflect an increase in the density of gametocytes circulating pretreatment to a level beyond the microscopic threshold for detection (7, 403). The increase in gametocytemia after treatment is a likely result of the efflux of sequestered gametocytes rather than de novo gametocytogenesis (77). An efflux of not fully mature gametocytes may explain the finding of relatively low infectivity of gametocytes 1 week after SP treatment, despite high densities (30, 466), although this could also be related to sporontocidal effects of persisting drug concentrations (229). In general, gametocytemia after treatment is a composite of (i) the ongoing production of gametocytes after initiation of treatment, possibly enhanced by drug-induced stress by slow-acting antimalarials; (ii) the release of sequestrated gametocytes; (iii) the activity of drugs against immature gametocytes, preventing their release into the circulation; and (iv) the activity of drugs against mature gametocytes, clearing the circulating gametocyte population. It is impossible to disentangle these effects in data from field trials. A high rate of gametocyte development after initiation of treatment (i.e., samples are gametocyte negative at enrollment but positive after treatment) can be interpreted as a limited activity of a drug against immature gametocytes; a long persistence of gametocytes that were detected at enrollment can be interpreted as a limited activity against mature gametocytes. The effects of several antimalarial drugs against gametocytes are summarized in Table 2. Non-artemisinin combination therapy (ACT) drugs, especially SP and chloroquine (CQ), have partial activity against immature gametocytes. This is reflected by a large proportion of treated individuals developing mature gametocytes in the week following treatment. SP, CQ, amodiaquine (AQ), and quinine have no or very limited activity against mature gametocytes (55, 77, 225, 429, 448), resulting in a large proportion of individuals who remain gametocyte positive after treatment (Table 2). In general, mature gametocytes may persist for several weeks after successful clearance of asexual parasites (55, 136, 211, 324, 429, 446); molecular gametocyte detection tools indicate that gametocyte carriage is likely to persist for more than 1 month after successful clearance of asexual parasites (57). Drugs that actively clear gametocytes may therefore increase the impact of drug treatment on malaria transmission.
Table 2.
Impact of antimalarial drugs on development and persistence of P. falciparum gametocytes
| Drug(s)a | Median gametocyte prevalence (% [IQR]) on day 7 for those who were gametocyte free on day 0 | Median gametocyte prevalence (% [IQR]) for those who were gametocyte carriers on day 0 |
References | |
|---|---|---|---|---|
| Day 7 | Day 14 | |||
| Non-ACT drugs | ||||
| CQ | 35 (26–44) | 76 (67–88) | 60 (43–83) | 4, 7, 127, 183, 333, 367, 383, 384, 440, 466 |
| SP | 55 (47–62) | 87.1 (78.3–97.5) | 71.4 (54.0–83.0) | 7, 55, 93, 183, 287, 333, 367, 383, 440, 449, 466, 502 |
| AQ | 15 (8–23) | 69 (58–74) | 37 (22–46) | 93, 440, 442, 456 |
| SP + CQ | 39 (38–44) | 89 (58–100) | 60 (33–74) | 93, 183, 375, 461, 489 |
| SP + AQ | 10 (7–19) | 56 (33–78) | 33 (24–43) | 55, 117, 144, 146, 287, 444, 454, 526, 530 |
| ACT regimens | ||||
| SP + AS | 8 (5–18) | 38 (25–60) | 18 (17–75) | 55, 185, 287, 412, 466, 504 |
| AQ + AS | 5 (0–13) | 35 (30–40) | 13 (0–41) | 74, 103, 117, 146, 147, 185, 189, 339, 454, 526 |
| MQ + AS | 1 (1–3) | 13 (3–30) | 1 (0–9) | 11, 12, 146, 169, 205, 242, 266, 437, 451, 489, 532 |
| AL | 2 (1–4) | 20 (18–41) | 16 (4–19) | 55, 74, 117, 144, 146, 147, 205, 213, 242, 266, 279, 339, 353, 374, 444, 461, 489, 527, 530, 532 |
| DHA-PPQ | 4 (3–5) | 33 (15–49) | 23 (17–29) | 11, 12, 169, 189, 213, 279, 374, 437, 527, 530, 532 |
| ACT-PQ regimen | ||||
| SP + AS + PQ | 0 (0–0) | 20 (0–40) | 0 (0–0) | 412, 506 |
ACT, artemisinin combination therapy; CQ, chloroquine; SP, sulfadoxine-pyrimethamine; AQ, amodiaquine; AS, artesunate; MQ, mefloquine; AL, artemether-lumefantrine; DHA-PPQ, dihydroartemisinin-piperaquine; PQ, primaquine. Only drug combinations for which we were able to obtain raw data for two or more trials were included.
The 8-aminoquinolines (pamaquine, primaquine [PQ], quinocide, and tafenoquine) occupy a unique position in their effects against mature gametocytes (510). PQ is the only drug of this group that has been used extensively in patients and is currently available for wide-scale use. The exact mechanism of PQ or its effector metabolites is unknown (401, 487, 510), but PQ treatment severely disrupts the metabolic processes of plasmodial mitochondria (197). PQ clears mature gametocytes of P. falciparum (75, 211, 363, 412, 471, 510), resulting in a shorter duration of posttreatment (submicroscopic) gametocyte carriage (57, 363, 412, 438). The clinical use of PQ has been limited by its hematological toxicity, particularly in individuals who have glucose-6-phosphate dehydrogenase (G6PD) deficiency (65, 381, 412, 471, 510, 521). The consequences for public health interventions are discussed in more detail in the section on gametocytes and malaria elimination strategies. The safety and gametocytocidal activity of alternative drugs from the same family as PQ (the 8-aminoquinolines), such as bulaquine (CDRI 80/53) (162, 163) and tafenoquine (WR-238605) (99, 100, 371, 487), require further investigation. Methylene blue, which together with quinine served as a structural starting point for the development of 8-aminoquinolines (153, 406), was recently suggested as an alternative safe gametocytocidal component of drug combinations (103, 531) and may be a promising gametocytocidal component of combination therapy.
Gametocytes as an Early Marker for Spread of Drug Resistance
Antimalarial treatment failure has been associated with increased gametocyte carriage after treatment (360). CQ treatment failure is followed by an increased gametocyte prevalence and density (4, 188, 383, 440, 448, 476) and, as a consequence, increased posttreatment malaria transmission (188, 383). Similarly, posttreatment gametocytemia is also increased after treatment failure of SP alone (52, 53, 440, 476) or in combination with CQ (476). The rapid spread of CQ and SP resistance may be due to some degree to high gametocyte carriage after treatment and treatment failure (26).These associations are explained only partly by ongoing gametocyte production by asexual parasites that survive the failing antimalarial treatment. Not all genetically resistant parasites will result in treatment failure, which depends on parasite sensitivity, drug concentration, and host immunity (116, 142, 175, 474). Even if resistant parasites are cleared successfully, they appear to have a transmission advantage over fully sensitive (wild-type) parasite strains. Mutations in the Pfcrt and Pfmdr1 genes, encoding CQ resistance, were associated with a higher gametocyte density and subsequent malaria transmission after CQ monotherapy (184, 460) or SP-CQ combination treatment (183) (Fig. 6). Similarly, mutations in the dhfr and dhps genes, encoding SP resistance, were related to increased gametocyte carriage following (30, 275, 288) or even prior to (288) treatment and to a longer duration of gametocyte carriage than that for wild-type parasites (25). A study by Méndez and colleagues showed that malaria transmission was enhanced 10-fold for parasites with low levels of resistance compared to fully sensitive parasites, despite high SP cure rates in Colombia (274). This may be explained partly by a longer parasite clearance time for dhfr/dhps mutant parasites allowing asexual parasites to differentiate into gametocytes (275), although other factors may also be important (25, 275). In addition to this transmission advantage of mutant parasites in the human host, through a higher gametocyte prevalence or density (184, 275), a selective advantage in the mosquito midgut has been suggested for Pfcrt mutants (184). In an area of low endemicity in South Africa, gametocyte carriage was highest for isolates with quintuple mutations in dhfr and dhps genes, intermediate for those with fewer mutations, and lowest for wild-type infections (25). In contrast, a study in an area of intense transmission in Kenya with high levels of SP resistance indicated that there may be no increase in gametocytemia for parasite strains with ≥3 mutations in the dhfr/dhps genes compared to those with a double mutation (320).
Fig. 6.
Gametocytes as an early indicator of parasite resistance. The data presented are from a study in the Gambia where children were treated with chloroquine (184). The figure presents gametocyte prevalence by microscopy (thick blood films; screening of 100 high-power fields at a magnification of ×1,000, using oil immersion) on day 7 after treatment (left y axis; error bars indicate the upper limit of the 95% confidence interval), median gametocyte density by microscopy in gametocyte carriers on day 7 after treatment (right y axis; error bars indicate the upper limit of the interquartile range), and the mean proportion of infected mosquitoes determined by membrane feeding assays (right y axis; error bars indicate the upper limit of the 95% confidence interval). Wild type, no mutations detected at enrollment in two genes related to chloroquine resistance, P. falciparum multidrug resistance gene 1 (Pfmdr1 86Y) and P. falciparum chloroquine resistance transporter (Pfcrt 76T); single mutant, mutation detected in either Pfmdr1 or Pfcrt; double mutant, mutation detected in both Pfmdr1 and Pfcrt.
Given this relationship with resistance-associated mutations, gametocytes and posttreatment malaria transmission may serve as effective early parasitological indicators of reduced drug sensitivity (25, 183, 274). Two examples of the use of gametocyte carriage as a warning system for an increase in the prevalence of parasite resistance have been reported. In South Africa, the prevalence, density, and duration of gametocyte carriage after treatment increased between 1998 and 2002; in this period, the prevalence of dhfr/dhps mutant strains increased, although the cure rate of SP remained consistently high, with >90% successful parasite clearance (25). In Sri Lanka, the prevalence of gametocyte carriage in parasitemic individuals increased considerably after CQ resistance emerged. After control measures were implemented to contain CQ resistance, this gametocyte prevalence decreased again (188).
Combination Therapy and Gametocytes
ACT has been advocated widely as first-line therapy for treating uncomplicated malaria episodes since the early 2000s (327). Treatment with artemisinin derivatives results in rapid reductions in parasitemia (3, 363, 509), and treatment with ACT is associated with lower rates of gametocyte carriage (55, 127, 184, 324, 361, 403, 419, 461, 466) and posttreatment malaria transmission (55, 127, 184, 324, 461, 466). Artemisinins are highly active against immature gametocytes (96, 235, 363, 466, 510), destroying a substantial proportion of those sequestered in the microvasculature, resulting in a significant reduction in the release of mature gametocytes into the peripheral blood (324). As shown in Table 2, this is reflected by a low gametocyte prevalence on day 7 for individuals who were gametocyte free at enrollment. It is assumed that artemisinins have little or no activity against mature gametocytes (96, 235, 348). This resulted in persisting gametocytes on days 7 and 14 in individuals who already harbored gametocytes at enrollment (Table 2). The persistence of mature gametocytes after ACT translates to posttreatment malaria transmission (55, 461, 466).
The gametocytocidal drug PQ lacks useful activity against P. falciparum asexual parasites and, presumably, early gametocytes (363) and should therefore be combined with an effective schizonticidal drug, preferably in a drug combination that also clears early gametocytes, such as an efficacious ACT regimen (363, 412, 438, 510) (Table 2).
IMMUNE RESPONSES TO GAMETOCYTES AND TRANSMISSION-BLOCKING IMMUNITY
Immune responses against gametocytes can act in three ways. First, immune responses may interfere with gametocyte sequestration and thereby enhance gametocyte clearance before they become mature and infectious. Second, circulating gametocytes may actively be cleared, also resulting in a lower density of gametocytes in the human bloodstream. Third, responses against parasite antigens that are expressed in the gamete or postgamete stages may reduce the transmission of gametocytes. Evidence for the first two mechanisms is currently inconclusive but is discussed in the following section; the third mechanism receives attention in the two subsequent sections.
Evidence for Immune Responses Influencing Gametocyte Concentrations
Gametocyte-specific and nonspecific parasite adhesion molecules may elicit immune responses that interfere with gametocyte maturation. Since the adhesion phenotype of young gametocytes appears indistinguishable from that of trophozoites (106), it is plausible that cross-reactive asexual- and sexual-stage immunity affects early-stage gametocytes. For PfEMP-1, it has been suggested that immunity may regulate the densities of both gametocytes and trophozoites by antibody-dependent cell-mediated cytotoxicity reactions (354) and prevention of sequestration (90). The scavenger receptor CD36 was found to mediate the uptake of erythrocytes infected with early-stage gametocytes (I and IIa) by nonopsonic phagocytosis by monocytes and macrophages (436), as was previously shown in the phagocytosis of trophozoites and schizonts (267). In addition to proteins involved in erythrocyte adhesion, molecules on the surfaces of erythrocytes infected with mature gametocytes may also elicit immune responses and may be involved in the clearance of circulating gametocytes. These molecules may be involved in spatial and temporal tropism, although their existence so far is speculative (459). There is some evidence for antibodies that recognize erythrocytes infected with mature (stage V) gametocytes, and the presence of these antibodies is associated with lower gametocyte densities in naturally infected individuals (392). This is supported by earlier epidemiological observations of protection against gametocytemia among individuals whose sera showed reactivity to gametocytes in immunofluorescence assays (19). In addition, the age-dependent recognition of gametocyte-infected erythrocytes, which closely resembled that of antibodies to the surfaces of trophozoites (354), could explain the negative association between the duration of gametocyte carriage and age (52, 131). There is, however, no conclusive evidence of the immune clearance of circulating gametocytes. Most in vitro studies on clearance of parasite-infected erythrocytes by phagocytosis (48, 88, 267, 483) or by destruction via free radicals or cytokines (159, 523) have focused on asexual parasites, and several studies suggest that intraerythrocytic gametocytes may not be susceptible to phagocytosis (194, 425). If, on the other hand, gametocyte surface antigens can be identified and their role in gametocyte clearance can be established, these would have potential for vaccine development (459).
Immune Responses Influencing Gametocyte Infectivity
Independent of immunity affecting gametocyte densities, gametocyte infectiousness can also be influenced by immune responses. There is some evidence for nonspecific immune effects on infectivity; for example, cytokines such as TNF-α can inactivate gametocytes in the presence of white blood cells (218, 312) and may be related to the lower infectiousness of gametocytes during paroxysm (165). Early evidence for specific transmission-blocking immune responses came from experiments with avian malaria P. gallinaceum, where transmission was reduced 95 to 99.9% after vaccination with formalin-treated or irradiated gametes (81, 181).
Sexual-stage parasites are most vulnerable when they leave the erythrocyte after their ingestion by mosquitoes. Inside the mosquito midgut, extracellular gametes are exposed to mosquito immune responses (reviewed in references 43, 282, and 511) and to human leukocytes, antibodies, and complement. Both human antibody-mediated and cell-mediated effector mechanisms may play a role in transmission-blocking immunity (467). Human leukocytes remain active and are capable of surviving for several hours in the mosquito midgut. There, human leukocytes can phagocytize extracellular activated and exflagellating gametocytes (425), although conditions in the mosquito midgut may reduce the activity of leukocytes to a level that is unlikely to hinder malaria transmission (425). Opsonization of gametes by antibodies against sexual-stage proteins is needed for successful phagocytosis inside the mosquito midgut (246). Additionally, the presence of human leukocytes in a blood meal may increase the transmission-reducing activity (TRA) of antibodies (244, 246).
Antibodies can be directed against a large number of sexual-stage antigens and appear to play a larger role in transmission-blocking immune responses than cell-mediated responses do. Proteomic information gathered after the availability of the P. falciparum genome (155) and two rodent malaria genomes (80, 182) led to the identification in sexual-stage parasites of a large number of predicted proteins with cell-adhesive motifs (149, 239, 417) that are accessible to human antibodies. The expression, function, and transmission-blocking potential of sexual-stage proteins were recently reviewed (358). Here we concentrate on proteins that are known to be recognized by naturally infected individuals and/or are major candidates for malaria transmission-blocking vaccines (MTBV). Antigens involved in transmission-blocking immune responses can be categorized as those that act before zygote formation (prefertilization antigens) and those that affect the subsequent development of mosquito stages (postfertilization antigens) (Table 1).
Prefertilization antigens.
Two gamete-specific surface proteins have been shown to be involved in gamete-gamete interaction and male fertility: HAP2 (GCS1) (199, 251) and P48/45 (493). The HAP2 protein is present in many plant, protist, and animal genomes (294, 518) and has been shown to be essential for fertilization. Studies of rodent malaria P. berghei have indicated that HAP2 is localized on male gametocytes and microgametes (251) and that HAP2 is not necessary for adhesion of gametes but is essential for the fusion of gamete surface membranes (251). P48/45 belongs to a family of proteins that are characterized by six positionally conserved cysteines (6-Cys proteins) (494). All 6-Cys proteins contain a signal peptide, and most contain a putative glycosylphosphatidylinositol (GPI) anchor sequence, making it plausible that they are located on the surfaces of parasites or invasion-associated organelles (473, 498). P48/45 protein is detectable on the parasite membrane surface beginning in gametocyte stages and continuing up to the erythrocyte-emerged gamete and zygote stages within the mosquito midgut (233, 382, 499, 500). P48/45 is essential for the attachment of male microgametes to fertile female macrogametes in P. berghei (493). In addition to the Pfs48/45 gene, two more 6-Cys protein genes are expressed exclusively on P. falciparum gametes (Pfs47 and Pfs230 genes), suggesting a role in gamete recognition and fertilization (228, 494, 498, 499, 515). Pfs47 is a female-specific paralogue of Pfs48/45 located on the surfaces of female gametes following their emergence from erythrocytes, but it does not appear to be crucial for fertilization (498). Pfs230 is synthesized early in gametocyte development, similar to Pfs48/45 (233, 499), and is derived from proteolytic cleavage of a 360-kDa precursor molecule into 300- and 35-kDa or 307- and 47-kDa forms (67, 369, 500). The 300- and 307-kDa membrane-associated Pfs230 fragments that do not contain a GPI anchor remain on the gamete surface (500, 516) as a complex with Pfs48/45 (139, 232, 233). Pfs230 may be involved in protection of the parasite from the contents of the blood meal, in fertilization (514), or in the formation of exflagellation centers (137). Antibodies against Pfs230 can reduce oocyst development (369, 514) by lysis of gametes in a complement-dependent manner (196, 369, 376, 388). The presence of complement is essential for the transmission-blocking activities of Pfs230. Mutant parasites lacking Pfs48/45 or Pfs230 have reduced but not completely abrogated transmission potential (139, 493, 514), suggesting a possible redundancy of proteins and the presence of paralogous proteins that may compensate for their loss (498), as shown for P25 and P28 (477).
Postfertilization antigens.
P25 and P28 are expressed on the surfaces of gametes after their emergence from erythrocytes, and this expression continues until ookinete formation (358, 418, 499). There is no expression of these proteins during the parasite's life cycle in humans. This has the advantage for the parasite that the protein is not subject to immune selection pressure in the human host (84, 484) and the disadvantage for vaccine development that boosting of immune responses by natural exposure to antigen is unlikely. P25 and P28 are structurally similar proteins that have been described for P. falciparum (130, 468), P. vivax (484), and several nonhuman malaria species. Work with P. berghei has shown that the antigens play partially redundant roles in ookinete survival in the mosquito midgut, mediation of ookinete entry into midgut epithelial cells, and oocyst formation (28, 477). Double-knockout P25/P28 P. berghei parasites showed a greater reduction in oocyst formation than single-knockout parasites (477), suggesting that both target antigens may have to be included in transmission-blocking vaccines. Fusion proteins of Pfs25 and Pfs28 can induce potent transmission-blocking antibodies in mice (168). A strong association between anti-Pfs25 antibody concentration and a reduction in oocyst numbers was observed for mice, rabbits, monkeys, and humans (95). Encouragingly, antibodies against Pfs25 elicited in mice resulted in complete interference with oocyst development in four different field isolates of P. falciparum (10), and immunization of mice with protein conjugates of Pfs25 demonstrated that it is possible to elicit long-lasting transmission-blocking activity (231).
Evidence for Naturally Acquired Transmission- Blocking Activity
Naturally acquired transmission-reducing immune responses can be assessed in experiments where mosquitoes are offered gametocytes from culture or from naturally infected individuals in the presence or absence of a test serum (125, 300). In the standard membrane feeding assay (SMFA) (Fig. 7), in vitro cultured gametocytes are mixed with red blood cells and human serum and fed through membrane feeders to laboratory-reared uninfected Anopheles mosquitoes (355). The level of TRA is determined at approximately 1 week postfeeding, when the ratio of the number of oocysts developed in mosquito midguts feeding on test sera is compared to that in mosquitoes feeding on control sera (245, 490). With strict protocols to minimize the influence of day-to-day variations in transmission efficiency (490), the SMFA forms the gold standard for assessing TRA. The direct membrane feeding assay (DMFA) (Fig. 7) is an alternative for the SMFA that can be conducted in the field. In the DMFA, blood samples from naturally infected gametocyte carriers are fed to mosquitoes in the presence of autologous plasma or control serum, after a washing step (125, 300, 472). Advantages of the DMFA include the use of (i) a wide variety of parasite strains that are naturally circulating in areas of endemicity, not relying on a limited number of laboratory-adapted gametocyte-producing parasite strains; (ii) gametocyte densities found in natural infections; and (iii) locally caught and reared mosquitoes. The DMFA may more realistically represent natural infectivity, though a considerable disadvantage is that it is much less standardized than the SMFA. There is a positive but imperfect correlation between outcomes when samples are tested in both assays (125, 300). Both DMFA and SMFA are hampered by the labor-intensiveness of the assays (389), typically requiring the dissection of more than 20 mosquitoes per experiment to be statistically robust.
Fig. 7.
Three different experimental setups for assessing malaria transmission and TRA. In direct skin feeding assays, reared (malaria-free) mosquitoes are allowed to feed directly on blood through the skin of a naturally infected individual. Oocysts can be detected on the mosquito midgut after dissection of the mosquito. This is mostly done 7 to 9 days after feeding. The presence of oocysts confirms infection; TRA cannot be determined by direct skin feeding. In the direct membrane feeding assay (DMFA), a venous blood sample is taken from a naturally infected individual. This blood sample forms the source of gametocytes and test plasma. Samples are centrifuged, and the red blood cells that contain gametocytes are subsequently mixed with autologous plasma or malaria-naïve control serum. This mixture is kept at 37°C and offered to reared mosquitoes through a membrane, commonly Parafilm. Seven days after feeding, mosquitoes are dissected. The prevalence or density of infection in mosquitoes can be compared in pairwise comparisons between the batch of mosquitoes that fed on gametocytes in combination with control serum and the batch of mosquitoes that fed on gametocytes with autologous plasma. In the standard membrane feeding assay (SMFA), cultured gametocytes are offered to mosquitoes in the presence of test or control sera. The prevalence or density of infection in batches of mosquitoes feeding on gametocytes with control serum is compared to that in batches with test serum. In the SMFA, a large number of test sera can be tested simultaneously.
Setting these difficulties aside, DMFA has shown the presence of TRA in endemic populations through a combined analysis of experiments from the Gambia, Kenya, and Cameroon. In all studies, the proportion of infected mosquitoes and total oocyst burdens were higher when mosquitoes were fed in the presence of control serum than with autologous semi-immune plasma (59). Naturally acquired TRA has been associated with immune responses to both Pf230 and Pfs48/45. A strong association between TRA and anti-Pfs230 antibodies was observed in Gambian (122, 125, 195), Tanzanian (49, 58), and Papuan/Papua New Guinean (54, 172) sera, but no statistically significant association was found for sera from Cameroon (300, 491) or Sri Lanka (359). Similarly, antibodies against Pfs48/45 were positively associated with TRA in most (49, 54, 58, 122, 125, 300, 389, 491), but not all (172, 359), studies. A number of individuals show TRA but have no demonstrable antibodies to either Pfs48/45 or Pfs230 (49, 58, 122), suggesting that other antigenic targets of TRA exist. As an adjunct to this, further questions remain as to the strain or isolate specificity of TRA. One study testing a panel of sera from Cameroon and the Gambia found sera to have variable TRA against different gametocyte isolates from naturally infected individuals (128). It is unclear if this was due to antigenic variation in parasites, though sequence variation in both Pfs48/45 and Pfs230 appears limited (102, 124). Antibody concentration is clearly important. While high concentrations of sexual-stage antibodies can reduce transmission, low concentrations have been associated with transmission enhancement in P. vivax (152, 349) and P. cynomolgi (311), but not P. falciparum (195, 356, 491).
TRA is observed in young children (122, 196), in individuals exposed to low-level transmission (58, 359), and in individuals shortly after (but not before) migrating from an area where malaria is not endemic to an area where it is endemic (54). These findings suggest that TRA may be relatively short-lived and may depend on recent exposure to gametocyte antigens, a hypothesis that is further supported by observations of higher prevalences of TRA toward the end of the peak transmission season and in children with a longer recent exposure to gametocytes (59). Similarly, the observation that TRA may decrease rather than increase with increasing age of Gambian children (122) could be explained by a longer recent exposure to gametocytes in the youngest children (Fig. 2) (51).
Current Status of Transmission-Blocking Vaccines
As a consequence of the renewed interest in malaria elimination, MTBV have moved to the center stage of malaria vaccine research. MTBV candidates that are currently in the most advanced stage of development for P. falciparum are based on the prefertilization proteins Pfs48/45 and Pfs230 and the postfertilization proteins Pfs25 and Pfs28. The cysteine-rich nature of the Pfs48/45 protein and its conformational structure made the production of a correctly folded recombinant molecule a major challenge that was only recently overcome (97, 338). It was demonstrated that the correctly folded C-terminal fragment of the Pfs48/45 protein induced high antibody titers in mice and transmission-blocking immunity in 90% of immunized mice (338). Experiments with the properly folded full-length soluble recombinant Pfs48/45 protein gave equally promising results. Sera from vaccinated mice and nonhuman primates displayed >90% reductions in oocyst numbers after a single immunization, reaching nearly complete blocking after a booster dose of the vaccine (97). Antibodies induced after vaccination with the Escherichia coli-expressed Pfs230 C region decreased the number of oocysts by 70 to 90% (517). DNA vaccination with the immunogenic region C of Pfs230 with an additional GPI anchor also induced high IgG titers in mice (145). Because of its size and the difficulty in obtaining correctly folded protein, an alternative approach producing parts of the protein in tobacco plants is currently used (501). A similar approach is being pursued for Pfs48/45, Pfs28, and Pfs25.
Recombinant Pfs25, Pfs28, Pvs25, and Pvs28 all induce antibodies in mice that significantly reduce oocyst production (27, 41, 95, 130, 219, 230). Initial vaccine studies with Pfs25 resulted in a measurable but weak immunogenicity in healthy volunteers (501). Considerable progress has since been made to enhance the immunogenicity of Pfs25 (231, 525), and several vaccine formulations based on Pfs25 and Pvs25 have been tested in phase I clinical trials in humans (262, 524). A trial of Pfs25 in combination with the Montanide ISA 51 mineral oil-based adjuvant had to be stopped after serious side effects in two volunteers that were attributed to the adjuvant. The trial showed >90% reductions in oocyst numbers in one of four individuals who completed all vaccine doses and 18 to 47% reductions in the other three (524). Other MTBV candidates include the parasite chitinase PfCHT (249) and HAP2. Promising work with rodent models demonstrated the potential of HAP2 for transmission-blocking vaccine development: anti-P. berghei HAP2 serum had no effect on gametocyte exflagellation but significantly inhibited ookinete and oocyst formation (40). In addition to these MTBV candidates that target gametocyte and gamete antigens, there are initiatives to target mosquito proteins that are important for parasite invasion. Antibodies raised in rabbits against the mosquito antigen anopheline midgut alanyl aminopeptidase (AnAPN1) blocked development of P. vivax by 98% in mosquitoes in Thailand and completely blocked P. falciparum development in mosquitoes in Cameroon (112, 501).
P. VIVAX GAMETOCYTES
P. vivax Gametocyte Biology and Gametocytogenesis
P. vivax gametocytes of both sexes are large and round or oval, filling nearly the whole enlarged and stippled host red blood cell (424). Studies on the biology of P. vivax gametocytes at the cellular and molecular levels have been complicated by the fact that the routine culture of P. vivax beyond a few weeks has yet to be established (486). This is in part because P. vivax merozoites preferentially invade reticulocytes, which are difficult to obtain routinely. The use of laboratory-produced erythroblasts may improve the possibility of culturing P. vivax in the future (341, 486), but our current understanding of P. vivax gametocytes is based largely on natural and experimental infections of humans.
Gametocyte production may start with the first generation of P. vivax merozoites, and gametocytes can be detected within 3 days after the first asexual parasites are observed (61, 62, 268, 424). Studies of individuals undergoing malaria therapy for neurosyphilis showed that the initial appearance of P. vivax gametocytes may be followed by nearly continuous gametocyte production for the first months of infection (270). There is no relevant cytoadherence of erythrocytes infected with P. vivax asexual parasites or gametocytes, and all developmental stages can be detected in the circulation. Although erythrocytes infected with P. vivax parasites increase in size, they remain flexible (463). This increased flexibility may reduce the chances of being trapped and cleared in the sinusoids of the spleen. Once gametocytes are ingested by mosquitoes, gametes emerge from the erythrocyte and undergo activation comparable to that of P. falciparum (206), with dramatic changes in protein transcript levels occurring during the process (507). Sporogony takes 8 to 10 days at 28°C and 16 days at 20°C for both parasite species, but P. vivax has a lower limit of development of 14.5°C, compared with 16°C for P. falciparum (180, 424). This is part of the explanation for the broader geographical distribution of P. vivax than that of P. falciparum.
Epidemiology of P. vivax Gametocytes
The epidemiology and control of P. vivax are greatly influenced by the presence of hypnozoites that can become activated weeks, months, or years after infection. Relapses occur in a seasonal manner (204, 296), resulting in seasonal patterns in asexual parasitemia and gametocytemia. Gametocytes are observed more commonly in P. vivax infections than in P. falciparum infections (271) and are detected by microscopy in the majority of or sometimes all infections (151, 189, 271, 350, 365, 394, 462), at densities of <10% that of asexual parasites (203, 309, 365). However, the duration of P. vivax gametocyte carriage is markedly shorter than that for P. falciparum. In the absence of treatment, P. vivax gametocytes circulate for a maximum of 3 days (82). Because of the high rate of gametocyte production by asexual parasites and their short longevity, the epidemiology of P. vivax gametocytes closely follows that of asexual parasites. In clinical malaria episodes, P. vivax gametocytemia has been associated with higher parasite densities (271, 365), younger age (151, 271, 309), lower hemoglobin levels (309), lower platelet counts (271, 309), and absence of fever (309). Similar to the case for P. falciparum, clinical episodes are most common in or shortly after the rainy season, but gametocyte carriage may be most common in the dry season (307). The mechanism behind this is poorly understood, but it was hypothesized that the investment in transmission stages is increased when fewer vectors are available, under the influence of seasonal patterns in UV radiation or of hematological or immunological characteristics (307). One study of hospitalized patients found no evidence for an association between hemoglobin E (a hemoglobinopathy with mild hemolytic anemia and mild splenomegaly in homozygotes and no symptoms in heterozygotes) and P. vivax gametocyte prevalence or the duration of gametocyte carriage (309).
P. vivax Gametocytes and Mosquito Infection Rates
Similar to the case for P. falciparum, the association between P. vivax gametocyte density and mosquito infection rates is often described as weak (34, 151, 171) or even absent (101). The limited sensitivity of microscopy for detecting all relevant gametocyte densities was demonstrated by Jeffery, who described the parasitic status and infectiousness of neurosyphilitic patients undergoing malaria therapy. These patients were infectious to mosquitoes shortly after asexual parasites were detected in the blood film but well before the appearance of microscopically detectable gametocytes (208). In all those individuals who infected mosquitoes, gametocytes were detected in the circulation in the days following the successful feeding (208). This infectivity of low or undetectable gametocyte densities has been confirmed by numerous studies (34, 61, 101, 151, 171, 208, 270, 350, 393, 394). The transmission success may depend on the duration of infection, i.e., the level of gametocyte maturation, and the proportion of male gametocytes (270). Two direct comparisons of P. vivax and P. falciparum transmission suggested that P. vivax malaria transmission may be more efficient than that of P. falciparum, with lower gametocyte concentrations resulting in mosquito infection (61, 365). Molecular gametocyte detection tools offer possibilities for a more detailed characterization of the human infectious reservoir for P. vivax. A study that used QT-NASBA to detect gametocytes based on pvs25 mRNA reported an 8-fold increased gametocyte detection rate compared to that for microscopy (33). The association between gametocyte density and the intensity of infection in mosquitoes was also improved when a molecular tool for P. vivax gametocyte detection was used instead of microscopy (34).
Antimalarial Drugs and P. vivax Gametocytemia
Gametocyte longevity is reduced with all commonly used antimalarial drugs, considered to be effective against both immature and mature sexual stages of P. vivax (18, 104, 309). The median gametocyte clearance time is 24 h (309), and gametocytes are cleared from the vast majority of patients within 4 days after successful antimalarial treatment (365). Some studies that determined the infectiousness of individuals to mosquitoes following drug treatment described that the infectiousness to mosquitoes was reduced within hours after successful treatment with CQ alone (224, 225) or in combination with PQ (224). Mosquito infection was completely prevented within 2 days after initiation of treatment (209, 224, 225). ACTs are increasingly being used for P. vivax infections in areas where both P. falciparum and P. vivax are endemic (118). Similar to the case for P. falciparum, ACTs reduce the duration of gametocyte carriage compared to that with CQ (29, 308), with differences between different ACT regimens (374). Although PQ may not have a preferential effect on P. vivax gametocytes, it plays a key role in vivax malaria control because of its value for radical clearance of liver hypnozoites (510). In addition, it may reduce the gametocyte clearance time by a synergistic effect with chloroquine against asexual parasites (365, 366). Drugs that result in a slow and incomplete clearance of asexual parasitemia result in a longer duration of gametocyte carriage. For P. vivax, treatment with SP, against which high-level P. vivax resistance is reported (362), and antibiotics results in longer gametocyte carriage than that with more efficacious antimalarial drugs (248, 362, 364, 365). Similar to the case for P. falciparum, P. vivax gametocytemia after treatment may serve as a predictor for the later reappearance of asexual parasites (189, 365).
P. vivax Transmission-Blocking Immunity and Transmission-Blocking Vaccines
The infectivity of P. vivax gametocytes is affected during paroxysms by nonspecific immune mediators such as TNF-α (218), possibly through an interaction with white blood cells (313). In addition, specific immune responses can influence the transmission of P. vivax to mosquitoes. Both transmission-enhancing and transmission-reducing immune responses have been described (152, 349, 393), as they have for P. falciparum (491). Human monoclonal and polyclonal antibodies against sexual-stage P. vivax can have this dual action, enhancing transmission at low concentrations while reducing transmission at higher concentrations (349). Transmission-blocking immune responses seem most prevalent in younger children (152), who have the lowest transmission efficiencies per gametocyte (151). Transmission-blocking immune responses appear short-lived, as individuals who have not been exposed to malaria for several months may have similar sexual-stage immune responses to those with a primary infection (152, 372), suggesting that functional transmission-blocking immunity depends on recent exposure to antigen. The functionality of transmission-blocking immune responses is associated with antibody titer, measured by indirect immunofluorescence assay (152, 349, 372), but may also depend on the intrinsic infectivity of gametocytes (152).
Molecular targets of naturally acquired P. vivax transmission-blocking immunity have not been characterized. Despite this, there has been considerable progress in developing an MTBV for P. vivax. The principal candidates are the ookinete surface antigens Pvs25 and Pvs28. Both were reported to be highly conserved in parasite isolates from different continents (484), which could reflect the lack of selective pressure exerted by human immune responses to antigens expressed only in mosquito stages. However, there may be higher degrees of polymorphism in Pvs25 and Pvs28 than in their P. falciparum homologs, especially for Pvs28 (485). Recent findings suggest a rapid progress of genetic changes in Pvs25 and Pvs28 among Korean isolates (186). Recombinant proteins for both antigens have high levels of immunogenicity, and vaccination resulted in the induction of functional transmission-blocking antibodies in rodent and primate models (10, 41, 201, 285). Antibodies induced by vaccinating mice and rabbits with recombinant Pvs25 or Pvs28 significantly reduced oocyst development of several natural Thai P. vivax isolates (285, 395). Pvs25 is the most advanced MTBV candidate for P. vivax and has undergone extensive preclinical testing and testing in phase I clinical trials. A variety of vectors, as well as several adjuvants, have been used to express Pvs25 protein (252, 262, 524). The recombinant protein requires both conformation-dependent epitopes and a strong adjuvant to induce transmission-blocking antibodies (41). Phase I trials of humans with an alum adjuvant showed that the vaccine was well tolerated and induced antibodies associated with TRA. The functional activity of antibodies strongly depended on their concentration, which was generally low in vaccinated volunteers. Five sera with the highest antibody concentrations prevented infection in 20 to 30% of mosquitoes (262). A more potent adjuvant was used in a follow-up study. Pvs25 with the Montanide ISA 51 mineral oil-based adjuvant resulted in a large proportion of severe adverse reactions that were likely the result of the adjuvant (524). An alternative approach for Pvs25 or Pvs28 vaccine development has been the use of DNA vaccines, which resulted in promising antibody titers and TRA in mice (230).
GAMETOCYTES AND MALARIA ELIMINATION STRATEGIES
Several currently used malaria control tools reduce the transmission of malaria from humans to mosquitoes. Vector control efforts such as insecticide-treated nets (ITNs) not only have an impact on malaria morbidity and parasite prevalence, because of the protection they provide against bites from sporozoite-infected mosquitoes, but also prevent mosquitoes from feeding on a gametocytemic human. This dual mode of action makes vector control measures powerful tools in malaria control and elimination strategies, although the impact of ITNs may be larger for P. falciparum than for P. vivax (42, 254, 296), possibly due to differences in biting behaviors of mosquitoes (42).
In addition to vector control measures, there are several tools to specifically prevent the transmission of gametocytes to mosquito vectors. MTBV reduce the infectiousness of gametocytes after their uptake by mosquitoes taking a blood meal from a vaccinated and gametocytemic donor. MTBV have objectives that are different from those of anti-infection or antidisease vaccines and are not designed to protect vaccinated individuals directly but to form part of an integrated malaria control program in order to reduce malaria transmission. MTBV can play a role in (i) reducing mortality and morbidity in areas of medium to high transmission intensity; (ii) eliminating malaria in areas of low endemicity; (iii) protecting against epidemics or reentry of malaria; and (iv) protecting other vaccines or drugs against the spread of resistant parasites, thereby prolonging their effective life (396–399, 434, 522). In some settings, one could consider MTBV as a standalone vaccine, for instance, for preventing reintroduction of malaria in malaria-free areas or preventing the transmission of (drug-)resistant parasite strains from an area of low transmission intensity. In most situations, MTBV are more likely to be used in combination with vaccines against other parasite stages. Depending on the achieved antibody titer and the potential for natural boosting, one would expect an MTBV to reduce malaria transmission for a certain period after completion of the vaccination schedule. This is a major advantage over interventions with antimalarial drugs that may have a similar effect in reducing the transmission from parasitemic individuals but may need repeated administration in most endemic settings. An efficacious MTBV may therefore be “an ideal public good” (522) and have an impact against both P. vivax and P. falciparum. Although several candidates are currently in clinical development, MTBV are unavailable at present and therefore are unlikely to form an important element in control or elimination programs in the coming decade.
Community interventions with antimalarial drugs and ITNs are commonly targeted to specific risk groups for (severe) disease, such as children and pregnant women (9, 198, 278), to have a maximum impact on morbidity and mortality. Control interventions that specifically aim to reduce transmission would, on the other hand, target all individuals who contribute to malaria transmission, including individuals who are apparently healthy. These types of interventions are considered by some to be altruistic in the sense that they do not protect individuals themselves but the community as a whole, and therefore they require additional ethical considerations (20) and a new degree of community awareness and engagement (109). For P. vivax elimination strategies, a major challenge in elimination efforts is the clearance of hypnozoites that form reservoirs of infection that persist in the livers of infected people. There is an urgent need for safe and efficacious drugs to clear liver schizonts and hypnozoites. Effective clearance of liver-stage P. vivax currently depends on taking a 2-week radical curative regimen of PQ (i.e., 14 days of 0.25 to 0.5 mg base/kg of body weight/day) (510); this has several logistical and clinical consequences, some of which are discussed in the section on drugs for mass drug administration. For P. falciparum, clearance of liver-stage parasites is less important in elimination strategies, while the long-lived gametocytes require specific attention. In the previous sections, we identified a number of areas related to P. falciparum gametocyte carriage, infectivity, and drug treatment in relation to transmission control. However, even with this knowledge, we are still short of knowing how best to use transmission-reducing interventions. Some of the key gaps in our current understanding are summarized in Table 3.
Table 3.
Gaps in the current knowledge of P. falciparum gametocytemia
| Category | Unanswered question or area for research |
|---|---|
| Biology | What triggers gametocytogenesis in P. falciparum? |
| What parasite adhesins are involved in gametocyte sequestration? | |
| Are there tropins that mediate the fertilization of male and female gametocytes at low gametocyte densities? | |
| Do gametocytes preferentially localize in the small capillaries? | |
| Are there circadian patterns in gametocyte concentration or infectivity? | |
| Control | What is the contribution of chronic asymptomatic parasite carriers to malaria transmission? |
| Tools | Sensitive tool for the detection of asymptomatic parasite/gametocyte carriers for public health purposes |
| Sensitive tool for sexing gametocytes at low gametocyte densities | |
| Safe and efficacious gametocytocidal component for combination therapy | |
| Sensitive parasite detection methods for field evaluation of transmission-reducing interventions in settings of low endemicity |
Who Should Be Targeted with Drug-Based Interventions?
Effective antimalarial treatment reduces transmission (510), but the impact of treatment on the level of malaria transmission experienced on a community level depends on the proportion of parasite carriers reached with antimalarial drugs, drug characteristics, and malaria epidemiology. Although the switch from ineffective malaria monotherapy to efficacious ACTs may result in or contribute to a reduction in transmission intensity (23, 24, 318), there are limitations to reducing transmission by changing the first-line antimalarial drug. The asymptomatic reservoir of parasite and gametocyte carriers plays a major role in this respect. In many regions where malaria is endemic, the majority of infections do not result in clinical symptoms (8, 32, 63, 261, 337), and even in children, long-term asymptomatic parasite carriage may occur (261). Mathematical models suggest that gametocytocidal properties of antimalarials used as first-line treatment have the most impact in low-transmission settings, while prophylactic effects have more impact in areas with high transmission levels (323). Thus, the impact of ACT in high-transmission settings (e.g., above 20% baseline slide prevalence) is smallest due to a combination of a smaller proportion of infections being treated and the different dynamics of infection prevalence (323). The prophylactic effect of a drug can also contribute considerably to the overall transmission-reducing effect, because preventing infection also reduces the potential for gametocyte development. Therefore, an ACT with the gametocytocidal effects of the artemisinin derivative and the prophylactic effect of a longer-acting partner drug is predicted to have the greatest impact on transmission across all areas (323).
MSAT of Asymptomatic Individuals
We previously argued that all parasitemic individuals can be considered potential current or future gametocyte producers. Interventions that aim to reduce malaria transmission should therefore ideally include all symptomatic and asymptomatic parasite carriers. Mathematical models unanimously indicate that this will increase the impact of interventions at all levels of transmission intensity (177, 430, 508). There are two approaches to do this by drug interventions: mass screening and treatment (MSAT) and mass drug administration (MDA). In MSAT, individuals are screened for the presence of malaria parasites, regardless of their symptoms, and are treated only if parasites are detected. The advantage of this approach is that it reduces exposure of nonparasitemic individuals to antimalarial drugs that may have disadvantageous effects in terms of side effects and an increased drug pressure in the community that may promote the spread of drug-resistant parasites (114). One of the disadvantages of this approach is that the success of identifying all parasite carriers in the population will depend on the sensitivity of the screening tool. Microscopy and rapid diagnostic tests will miss a substantial proportion of carriers. Especially in areas with low levels of endemicity, a relatively large proportion of parasite carriers will remain undetected by microscopy (325). The sensitivity of detection tools may be solved in the coming years by the development or improvement of molecular parasite detection techniques, such as PCR, LAMP, or QT-NASBA (187, 405, 439), which should become affordable and suitable for large-scale testing in the field. The use of molecular detection tools for MSAT was piloted in an elegant but laborious study in two Sudanese villages where parasite carriers were detected by PCR in the long dry season and subsequently cleared of parasites with ACTs, resulting in a significant reduction in parasite carriage in the wet season (141). An additional disadvantage of MSAT is that people who are exposed to malaria shortly after being found parasite negative during the MSAT campaign will not experience any beneficial prophylactic effect of the drug. This disadvantage can be overcome only by using an alternative (re)treatment strategy.
MDA
The logistical difficulties in detecting (submicroscopic) parasite densities prior to drug administration can be circumvented by treating individuals in MDA campaigns, regardless of parasitemia. Several MDA campaigns to reduce malaria transmission have been conducted since the 1930s (reviewed in reference 503). The success of these past MDA interventions is difficult to evaluate because of trial designs that we would currently consider inadequate and the concurrent employment of vector control measures (214). Most interventions resulted in only modest and short-term reductions in transmission intensity (503). In a review on historical MDA initiatives, it was noted that the failure to achieve long-term results could be explained by insufficient drug coverage, use of suboptimal drugs, too-high baseline levels of transmission intensity, and reintroduction of infections by movement of infected human and mosquito populations (503, 504). Studies in the Gambia using a combination of SP and a single dose of artesunate (AS) recorded only a moderate and transient difference in malaria incidence between control versus intervention villages (504). One explanation for this was the persistence of mature gametocytes unaffected by the single dose of AS. A more concerted drug regimen which included regular doses of CQ and SP, together with PQ as a gametocytocidal agent, had considerable success in a small island population in Vanuatu. From an initial prevalence of >20% in the late 1980s, P. falciparum was reported to be eradicated, and only sporadic cases of P. vivax were recorded by the year 2000 (214). Since SP and CQ are no longer viable drugs, current MDA initiatives need to use alternative drugs. In Cambodia, dramatic reductions in parasite rates, from >50% to <5%, were observed in a pilot MDA study of more than 3,000 individuals receiving two doses of artemisinin-piperaquine (AP) and adults receiving regular PQ. In villages where parasites remained, a second round of AP 1 year later reduced the parasite prevalence to 0% in children. The authors concluded that their approach was effective, safe, and affordable (441). Nevertheless, field trials that determine the impact of MDA alone or in combination with other interventions remain rare. This is perhaps not surprising given the complexity of conducting and evaluating these trials, and mathematical models could provide a convenient framework for comparing various combinations of drugs in areas with different levels of endemicity.
Predicted impact of MDA in areas with different levels of endemicity.
Okell and colleagues showed that at a high transmission level (50% parasitemia), a single round of MDA had a short-term effect, with baseline parasitemia restored within 1 year. At a low transmission level (5% parasitemia), however, the effect lasted considerably longer (326). Logically, these types of interventions are targeted at the nadir in transmission, when the parasite reservoir is at its minimum and can be enhanced with repeated MDA. In Fig. 8, we present two different epidemiological scenarios for settings with seasonal transmission and contrasting parasite rates and anopheline densities. The top panel shows transmission characteristics in the absence of interventions, and the bottom panel shows the effects of one or two rounds of MDA with a drug that has the properties of artemether-lumefantrine. Not surprisingly, the effect of MDA on the parasite rate was most pronounced in the setting with a low level of endemicity, and there was an advantage in repeating the administration. In the simulated setting of high endemicity, two rounds of MDA were highly unlikely to lead to malaria elimination. For the setting of low endemicity, the model indicates a 10% chance of elimination after two rounds of MDA in a population of 1,000 individuals and a 0.8% chance of elimination in a population of 3,000 individuals. The high basic reproduction rate of malaria (R0) (here we assume that one infected individual may potentially lead to more than 100 new infections in a susceptible population living in a setting where malaria vectors are highly prevalent [431]) warns that eliminating local transmission may not lead to long-term results if malaria can be reintroduced by parasite carriers. Thus, MDA, like other transmission-blocking interventions, is unlikely to be used alone (434).
Fig. 8.
Predicted impacts of mass drug administration in two settings of malaria endemicity, using two different transmission scenarios. (Top) Situation of seasonal transmission in an area of moderate endemicity and an area of low endemicity. In the area of moderate endemicity, parasite prevalence is ∼20% in the general population in the dry season, when people are exposed to ∼5 mosquito bites/person/week; in the wet season, parasite prevalence increases to ∼28% and mosquito exposure to 16 bites/person/week. In the setting of low endemicity, parasite prevalence is ∼5% in the dry season, when people are exposed to an average of one mosquito bite/2 months; in the wet season, parasite prevalence increases to ∼18% and mosquito exposure to 4 bites/person/week. (Bottom) Simulated impact of one or two rounds of MDA toward the end of the dry season. The timing of the MDA is indicated with an arrow; in the scenario where MDA is repeated, the gap between the two rounds of MDA is 1 month, and the correlation between coverage in the first and second rounds of MDA is 0.5. The drug used for MDA has the characteristics of artemether-lumefantrine, with an efficacy against asexual parasites of 95% and a duration of (submicroscopic) gametocyte carriage of 13 days after initiation of treatment. The coverage with the intervention is 95%.
Choice of drug for MDA.
A major reservation about MDA highlighted by several authors (114, 503, 510) is that individuals who are parasite negative receive a treatment that they do not need, and there are legitimate concerns that introducing large quantities of a drug into a population may have hastened the development of drug resistance. The choice of drug for MDA therefore requires careful consideration not just related to its likely efficacy; interventions are unlikely to be conducted with first-line treatment. At an individual level, concerns with drug use are particularly acute for PQ. As discussed above, this drug is unique in it that it has a strong gametocytocidal effect (363, 412, 438), but it also induces hemolysis that is related to G6PD deficiency (20, 92, 381, 414). Hemolysis may occur after a single dose in G6PD-deficient individuals (381, 412, 414) and may be particularly severe in individuals with the Mediterranean (B−) G6PD genotype (20, 158). Although the effect of PQ on hemoglobin levels is generally mild (412, 414) and self-limiting (110, 412), severe and life-threatening hemolysis may occur in some individuals (158, 381). Our current understanding of PQ sensitivity phenotypes is incomplete (20, 414), and Baird and Surjadjaja recently stressed that the reliable information is currently “inadequate by almost any contemporary standard of safety in chemotherapeutics, especially in the context of dosing demonstrably vulnerable populations” (20). Further safety data are particularly important for regions where malaria is endemic, where not only the prevalence of G6PD deficiency is high (316) but also mild and severe anemia can be common due to infections (e.g., malaria and helminth infections) and other genetic conditions (e.g., thalassemia and sickle-cell anemia). Further studies to determine the optimal dosage and timing (20, 241) of PQ to have a maximal impact on gametocyte persistence but minimal hemolysis and the development of practical means to screen against potentially dangerous G6PD variants in tropical settings (20) are therefore highly desirable.
Targeted Interventions
Tailored versions of both MDA and MSAT will become increasingly viable as transmission decreases and elimination becomes a possibility. At these low levels of transmission, a specific gametocytocidal therapy may be added to routine treatment, as already done in several countries, such as Sri Lanka and Mexico (20, 293).
In this later context, control will become more efficient for specifically targeting the foci of clinical and asymptomatic transmission (85, 520). These hot spots of transmission amplify transmission by transmitting malaria parasites to a large number of mosquitoes (431, 519). How one identifies these hot spots is an increasingly vibrant area of research (32, 56, 60). The recent suggestion that there are stable and less-stable hot spots of transmission (32) begs the question of how gametocyte carriage contributes. It may be that stable malaria hot spots are associated with more consistent low-level gametocyte carriage (e.g., chronically infected adults), whereas unstable hot spots are due in some part to short-term highly infectious individuals (e.g., infected children with high parasite densities). These potential hot spots of gametocytemic individuals can be targeted specifically by focal MDA/MSAT. Practical issues for this type of administration will be the size of the hot spot and how widely drugs are administered. If, for example, an index case is identified, then the issue is whether to treat individuals—all of those testing positive—in the same house, in neighboring houses, or to an arbitrary distance of 500 m. Assessing the efficacy of transmission-reducing measures will be similar to that for other approaches to control. The issue of the sensitivity of detection methods, such as those for determining parasite end points or directly sampling transmission intensity (infected mosquito end points), is applicable in all settings but will become more acute at the lowest transmission levels (293).
CONCLUDING REMARKS
In this article, we have attempted to present a current assessment of our knowledge on malaria gametocytes, with a particular emphasis on epidemiology and factors affecting their distribution within endemic populations. It remains surprising that despite their pivotal role in transmission, some of the most fundamental questions about gametocyte biology remain unknown. For P. falciparum, the within-host distribution of both immature and mature forms remains almost a complete mystery. While genomics and proteomics have made the first initial steps in confirming the considerable change in emphasis in protein transcription and production in preparing the parasite for life in the mosquito midgut, few specific targets—ligands or receptors—have been identified. Molecular amplification techniques have shown us that conventional blood sampling measures vastly underestimate the number of individuals who are potentially infectious to mosquitoes. At the level of an individual with a very low gametocyte density, how are single male and female gametes able to fuse and mate in a mosquito blood meal with approximately 107 uninfected red blood cells? At the level of a population living in a region where malaria is endemic, a reassessment of our concept of the infectious reservoir is required. Fewer than 10 attempts to measure and define the infectious reservoir have been conducted in the last 50 years, yet more will be needed if we are to optimize any existing or develop new gametocytocidal control tools. These control approaches will need to be integrated with more detailed knowledge of the underlying epidemiology concerning the spatial and temporal patterns of gametocyte carriage and infectivity. Control attempts can be enhanced further if hot spots of infection are associated with hot spots of gametocyte carriage or groups of individuals who are “super spreaders” and potentially amenable to specific targeting. Ultimately, less predictable economic and political factors may define the success of malaria control and elimination; however, improving our understanding of gametocytes will be a huge step in the right direction.
ACKNOWLEDGMENTS
We acknowledge the following people for critically reviewing parts of the manuscript: David Baker, Geoff Targett, and Colin Sutherland, London School of Hygiene & Tropical Medicine, London, United Kingdom; Ben van Schaijk and Robert Sauerwein, Radboud University Nijmegen, Nijmegen, Netherlands; and Ivo Mueller, Walter & Eliza Hall Institute, Parkville, Australia. We are grateful to Lucy Okell (Imperial College, London, United Kingdom) for her contribution to the section on mass drug administration. The following people are acknowledged for their willingness to share raw data for this review: André Lin Ouédraogo, Centre National de Recherche et de Formation sur le Paludisme, Ouagadougou, Burkina Faso; Stephan Karl and Peter Zimmerman, University of Western Australia, Crawley, Australia; Rhoel Dinglashan, Johns Hopkins Malaria Research Institute, Baltimore, MD; Abdoulaye Djimdé, Malaria Research and Training Center, Bamako, Mali; Frank Mockenhaupt, Charité, Berlin, Germany; Babacar Fayé, Université Cheikh Anta DIOP, Dakar, Senegal; Titilope Okuboyejo and Akin Sowunmi, University of Ibadan, Ibadan, Nigeria; Petra Mens, Royal Tropical Institute, Amsterdam, Netherlands; Rachel Hallett, Geoff Targett, Colin Sutherland, and Francesco Checchi, London School of Hygiene & Tropical Medicine, London, United Kingdom; Ingrid van den Broek, National Institute for Public Health and the Environment, Bilthoven, Netherlands, and Médicins Sans Frontières, London, United Kingdom; Ric Price, Menzies School of Health Research, Darwin, Australia; and Elizabeth Ashley and François Nosten, Shoklo Malaria Research Unit, Tak, Thailand.
Biographies
Teun Bousema is an epidemiologist at the London School of Hygiene & Tropical Medicine, London, United Kingdom. He attended the Radboud University Nijmegen, Nijmegen, Netherlands (M.Sc. and Ph.D.), and subsequently worked in Kenya, Tanzania, Uganda, and Mali on clinical and epidemiological projects focusing on malaria transmission-reducing interventions.
Chris Drakeley is an immunologist based at the London School of Hygiene & Tropical Medicine, London, United Kingdom. He did his Ph.D. studies in the Gambia, examining malaria infectivity. He subsequently worked in Tanzania for 10 years, at Ifakara Health Institute and the Joint Malaria Programme, Moshi, focusing on defining malaria transmission dynamics. Now based in the United Kingdom, he is involved in monitoring and evaluation of malaria control projects in a number of countries in Africa and Southeast Asia.
REFERENCES
- 1. Abdel-Wahab A., et al. 2002. Dynamics of gametocytes among Plasmodium falciparum clones in natural infections in an area of highly seasonal transmission. J. Infect. Dis. 185:1838–1842 [DOI] [PubMed] [Google Scholar]
- 2. Abu-Raddad L. J., Patnaik P., Kublin J. G. 2006. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science 314:1603–1606 [DOI] [PubMed] [Google Scholar]
- 3. Adjuik M., et al. 2004. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363:9–17 [DOI] [PubMed] [Google Scholar]
- 4. Akim N. I., et al. 2000. Dynamics of P. falciparum gametocytemia in symptomatic patients in an area of intense perennial transmission in Tanzania. Am. J. Trop. Med. Hyg. 63:199–203 [DOI] [PubMed] [Google Scholar]
- 5. Alano P. 2007. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol. Microbiol. 66:291–302 [DOI] [PubMed] [Google Scholar]
- 6. Alano P., et al. 1995. COS cell expression cloning of Pfg377, a Plasmodium falciparum gametocyte antigen associated with osmiophilic bodies. Mol. Biochem. Parasitol. 74:143–156 [DOI] [PubMed] [Google Scholar]
- 7. Ali E., et al. 2006. Increased density but not prevalence of gametocytes following drug treatment of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 100:176–183 [DOI] [PubMed] [Google Scholar]
- 8. Alves F. P., et al. 2005. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J. Med. Entomol. 42:777–779 [DOI] [PubMed] [Google Scholar]
- 8a. Anonymous. 1887. Haematozoa of malaria. Br. Med. J. 1887:960–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b. Anonymous. 2007. Is malaria eradication possible? Lancet 370:1459. [DOI] [PubMed] [Google Scholar]
- 9. Aponte J. J., et al. 2009. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet 374:1533–1542 [DOI] [PubMed] [Google Scholar]
- 10. Arakawa T., et al. 2005. Nasal immunization with a malaria transmission-blocking vaccine candidate, Pfs25, induces complete protective immunity in mice against field isolates of Plasmodium falciparum. Infect. Immun. 73:7375–7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ashley E. A., et al. 2004. Randomized, controlled dose-optimization studies of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. J. Infect. Dis. 190:1773–1782 [DOI] [PubMed] [Google Scholar]
- 12. Ashley E. A., et al. 2005. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin. Infect. Dis. 41:425–432 [DOI] [PubMed] [Google Scholar]
- 13. Babiker A., Abdel-Muhsin A. A., Ranford-Cartwright L. C., Satti G., Walliker D. 1998. Characteristics of Plasmodium falciparum parasites that survive the lengthy dry season in eastern Sudan where malaria transmission is markedly seasonal. Am. J. Trop. Med. Hyg. 59:582–590 [DOI] [PubMed] [Google Scholar]
- 14. Babiker A., et al. 1999. Detection of low level Plasmodium falciparum gametocytes using reverse transcriptase polymerase chain reaction. Mol. Biochem. Parasitol. 99:143–148 [DOI] [PubMed] [Google Scholar]
- 15. Babiker H. A., Schneider P. 2008. Application of molecular methods for monitoring transmission stages of malaria parasites. Biomed. Mater. 3:034007. [DOI] [PubMed] [Google Scholar]
- 16. Babiker H. A., Schneider P., Reece S. E. 2008. Gametocytes: insights gained during a decade of molecular monitoring. Trends Parasitol. 24:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bachmann A., et al. 2009. Absence of erythrocyte sequestration and lack of multicopy gene family expression in Plasmodium falciparum from a splenectomized malaria patient. PLoS One 4:e7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baird J. K. 2009. Resistance to therapies for infection by Plasmodium vivax. Clin. Microbiol. Rev. 22:508–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baird J. K., et al. 1991. Evidence for specific suppression of gametocytemia by Plasmodium falciparum in residents of hyperendemic Irian Jaya. Am. J. Trop. Med. Hyg. 44:183–190 [DOI] [PubMed] [Google Scholar]
- 20. Baird J. K., Surjadjaja C. 2010. Consideration of ethics in primaquine therapy against malaria transmission. Trends Parasitol. 27:11–16 [DOI] [PubMed] [Google Scholar]
- 21. Baker D. A. 2010. Malaria gametocytogenesis. Mol. Biochem. Parasitol. 172:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baker D. A., Daramola O., McCrossan M. V., Harmer J., Targett G. A. 1994. Subcellular localization of Pfs16, a Plasmodium falciparum gametocyte antigen. Parasitology 108:129–137 [DOI] [PubMed] [Google Scholar]
- 23. Barnes K. I., Chanda P., Ab Barnabas G. 2009. Impact of the large-scale deployment of artemether/lumefantrine on the malaria disease burden in Africa: case studies of South Africa, Zambia and Ethiopia. Malar. J. 8(Suppl. 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnes K. I., et al. 2005. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med. 2:e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barnes K. I., et al. 2008. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. J. Infect. Dis. 197:1605–1613 [DOI] [PubMed] [Google Scholar]
- 26. Barnes K. I., White N. J. 2005. Population biology and antimalarial resistance: the transmission of antimalarial drug resistance in Plasmodium falciparum. Acta Trop. 94:230–240 [DOI] [PubMed] [Google Scholar]
- 27. Barr P. J., et al. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med. 174:1203–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baton L. A., Ranford-Cartwright L. C. 2005. Do malaria ookinete surface proteins P25 and P28 mediate parasite entry into mosquito midgut epithelial cells? Malar. J. 4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Batty K. T., et al. 1998. A pharmacokinetic and pharmacodynamic study of artesunate for vivax malaria. Am. J. Trop. Med. Hyg. 59:823–827 [DOI] [PubMed] [Google Scholar]
- 30. Beavogui A. H., et al. 2010. Low infectivity of Plasmodium falciparum gametocytes to Anopheles gambiae following treatment with sulfadoxine-pyrimethamine in Mali. Int. J. Parasitol. 40:1213–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bejon P., et al. 2006. Thick blood film examination for Plasmodium falciparum malaria has reduced sensitivity and underestimates parasite density. Malar. J. 5:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bejon P., et al. 2010. Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med. 7:e1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beurskens M., et al. 2009. Quantitative determination of Plasmodium vivax gametocytes by real-time quantitative nucleic acid sequence-based amplification in clinical samples. Am. J. Trop. Med. Hyg. 81:366–369 [PubMed] [Google Scholar]
- 34. Bharti A. R., et al. 2006. Experimental infection of the neotropical malaria vector Anopheles darlingi by human patient-derived Plasmodium vivax in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 75:610–616 [PMC free article] [PubMed] [Google Scholar]
- 35. Bhattarai A., et al. 2007. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 4:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Billker O., et al. 1998. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392:289–292 [DOI] [PubMed] [Google Scholar]
- 37. Billker O., Shaw M. K., Margos G., Sinden R. E. 1997. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology 115:1–7 [DOI] [PubMed] [Google Scholar]
- 38. Birku Y., Mekonnen E., Bjorkman A., Wolday D. 2002. Delayed clearance of Plasmodium falciparum in patients with human immunodeficiency virus co-infection treated with artemisinin. Ethiop. Med. J. 40(Suppl. 1):17–26 [PubMed] [Google Scholar]
- 39. Biswas S. 2000. Formation of Plasmodium falciparum gametocytes in vivo and in vitro relates to transmission intensity. Ann. Trop. Med. Parasitol. 94:437–446 [DOI] [PubMed] [Google Scholar]
- 40. Blagborough A. M., Sinden R. E. 2009. Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine 27:5187–5194 [DOI] [PubMed] [Google Scholar]
- 41. Blagborough A. M., Yoshida S., Sattabongkot J., Tsuboi T., Sinden R. E. 2010. Intranasal and intramuscular immunization with baculovirus dual expression system-based Pvs25 vaccine substantially blocks Plasmodium vivax transmission. Vaccine 28:6014–6020 [DOI] [PubMed] [Google Scholar]
- 42. Bockarie M. J., Dagoro H. 2006. Are insecticide-treated bednets more protective against Plasmodium falciparum than Plasmodium vivax-infected mosquitoes? Malar. J. 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boete C. 2005. Malaria parasites in mosquitoes: laboratory models, evolutionary temptation and the real world. Trends Parasitol. 21:445–447 [DOI] [PubMed] [Google Scholar]
- 44. Boudin C., Lyannaz J., Bosseno M. F., Carnevale P., Ambroise-Thomas P. 1991. Epidemiology of Plasmodium falciparum in a rice field and a savanna area in Burkina Faso: seasonal fluctuations of gametocytaemia and malarial infectivity. Ann. Trop. Med. Parasitol. 85:377–385 [DOI] [PubMed] [Google Scholar]
- 45. Boudin C., Olivier M., Molez J. F., Chiron J. P., Ambroise-Thomas P. 1993. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am. J. Trop. Med. Hyg. 48:700–706 [DOI] [PubMed] [Google Scholar]
- 46. Boudin C., Robert V., Verhave J. P., Carnevale P., Ambroise-Thomas P. 1991. Plasmodium falciparum and P. malariae epidemiology in a West African village. Bull. World Health Organ. 69:199–205 [PMC free article] [PubMed] [Google Scholar]
- 47. Boudin C., et al. 2004. Plasmodium falciparum transmission blocking immunity under conditions of low and high endemicity in Cameroon. Parasite Immunol. 26:105–110 [DOI] [PubMed] [Google Scholar]
- 48. Bouharoun-Tayoun H., Oeuvray C., Lunel F., Druilhe P. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bousema J. T., et al. 2007. A longitudinal study of immune responses to Plasmodium falciparum sexual stage antigens in Tanzanian adults. Parasite Immunol. 29:309–317 [DOI] [PubMed] [Google Scholar]
- 50. Bousema J. T., et al. 2008. Increased Plasmodium falciparum gametocyte production in mixed infections with P. malariae. Am. J. Trop. Med. Hyg. 78:442–448 [PubMed] [Google Scholar]
- 51. Bousema J. T., Drakeley C. J., Sauerwein R. W. 2006. Sexual-stage antibody responses to P. falciparum in endemic populations. Curr. Mol. Med. 6:223–229 [DOI] [PubMed] [Google Scholar]
- 52. Bousema J. T., et al. 2004. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar. J. 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bousema J. T., et al. 2003. Treatment failure of pyrimethamine-sulphadoxine and induction of P. falciparum gametocytaemia in children in western Kenya. Trop. Med. Int. Health 8:427–430 [DOI] [PubMed] [Google Scholar]
- 54. Bousema J. T., et al. 2006. Rapid onset of transmission-reducing antibodies in Javanese migrants exposed to malaria in Papua, Indonesia. Am. J. Trop. Med. Hyg. 74:425–431 [PubMed] [Google Scholar]
- 55. Bousema J. T., et al. 2006. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J. Infect. Dis. 193:1151–1159 [DOI] [PubMed] [Google Scholar]
- 56. Bousema T., et al. 2010. Identification of hot spots of malaria transmission for targeted malaria control. J. Infect. Dis. 201:1764–1774 [DOI] [PubMed] [Google Scholar]
- 57. Bousema T., et al. 2010. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bousema T., et al. 2010. The dynamics of naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs230 & Pfs48/45 in a low endemic area in Tanzania. PLoS One 5:e14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bousema T., et al. 2011. Human immune responses that reduce the transmission of Plasmodium falciparum in African populations. Int. J. Parasitol. 41:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bousema T., et al. 2010. Serologic markers for detecting malaria in areas of low endemicity, Somalia, 2008. Emerg. Infect. Dis. 16:392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boyd M. F., Kitchen S. F. 1937. On the infectiousness of patients infected with Plasmodium vivax and Plasmodium falciparum. Am. J. Trop. Med. Hyg. 17:253–262 [Google Scholar]
- 62. Boyd M. F., Stratman-Thomas W. K., Muench H. 1935. The occurrence of gametocytes of Plasmodium vivax during the primary attack. Am. J. Trop. Med. Hyg. 16:133–138 [Google Scholar]
- 63. Branch O., et al. 2005. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar. J. 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bray R. S., McCrae A. W., Smalley M. E. 1976. Lack of a circadian rhythm in the ability of the gametocytes of Plasmodium falciparum to infect Anopheles gambiae. Int. J. Parasitol. 6:399–401 [DOI] [PubMed] [Google Scholar]
- 65. Brewer G. J., Zarafonetis C. J. 1967. The haemolytic effect of various regimens of primaquine with chloroquine in American Negroes with G6PD deficiency and the lack of an effect of various antimalarial suppressive agents on erythrocyte metabolism. Bull. World Health Organ. 36:303–308 [PMC free article] [PubMed] [Google Scholar]
- 66. Brockelman C. R. 1982. Conditions favoring gametocytogenesis in the continuous culture of Plasmodium falciparum. J. Protozool. 29:454–458 [DOI] [PubMed] [Google Scholar]
- 67. Brooks S. R., Williamson K. C. 2000. Proteolysis of Plasmodium falciparum surface antigen, Pfs230, during gametogenesis. Mol. Biochem. Parasitol. 106:77–82 [DOI] [PubMed] [Google Scholar]
- 68. Bruce M. C., Alano P., Duthie S., Carter R. 1990. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 100:191–200 [DOI] [PubMed] [Google Scholar]
- 69. Bruce M. C., Carter R. N., Nakamura K., Aikawa M., Carter R. 1994. Cellular location and temporal expression of the Plasmodium falciparum sexual stage antigen Pfs16. Mol. Biochem. Parasitol. 65:11–22 [DOI] [PubMed] [Google Scholar]
- 70. Buates S., et al. 2010. Development of a reverse transcription-loop-mediated isothermal amplification (RT-LAMP) for clinical detection of Plasmodium falciparum gametocytes. Parasitol. Int. 59:414–420 [DOI] [PubMed] [Google Scholar]
- 71. Buckling A., Crooks L., Read A. 1999. Plasmodium chabaudi: effect of antimalarial drugs on gametocytogenesis. Exp. Parasitol. 93:45–54 [DOI] [PubMed] [Google Scholar]
- 72. Buckling A., Ranford-Cartwright L. C., Miles A., Read A. F. 1999. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology 118:339–346 [DOI] [PubMed] [Google Scholar]
- 73. Buckling A., Read A. F. 2001. The effect of partial host immunity on the transmission of malaria parasites. Proc. Biol. Sci. 268:2325–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bukirwa H., et al. 2006. Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLoS Clin. Trials 1:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burgess R. W., Bray R. S. 1961. The effect of a single dose of primaquine on the gametocytes, gametogony and sporogony of Laverania falciparum. Bull. World Health Organ. 24:451–456 [PMC free article] [PubMed] [Google Scholar]
- 76. Burkot T. R., Williams J. L., Schneider I. 1984. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the same isolate. Trans. R. Soc. Trop. Med. Hyg. 78:339–441 [DOI] [PubMed] [Google Scholar]
- 77. Butcher G. A. 1997. Antimalarial drugs and the mosquito transmission of Plasmodium. Int. J. Parasitol. 27:975–987 [DOI] [PubMed] [Google Scholar]
- 78. Camarda G., et al. 2010. Regulated oligomerisation and molecular interactions of the early gametocyte protein Pfg27 in Plasmodium falciparum sexual differentiation. Int. J. Parasitol. 40:663–673 [DOI] [PubMed] [Google Scholar]
- 79. Canning E. U., Sinden R. E. 1975. Nuclear organisation in gametocytes of Plasmodium and Hepatocystis: a cytochemical study. Z. Parasitenkd. 46:297–299 [DOI] [PubMed] [Google Scholar]
- 80. Carlton J. M., et al. 2002. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature 419:512–519 [DOI] [PubMed] [Google Scholar]
- 81. Carter R., Chen D. H. 1976. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature 263:57–60 [DOI] [PubMed] [Google Scholar]
- 82. Carter R., Graves P. M. 1988. Gametocytes, p. 253–306 In Wernsdorfer W. H., McGregor I. A. (ed.), Malaria: principles and practice of malariology. Churchill Livingstone, Edinburgh, United Kingdom [Google Scholar]
- 83. Carter R., et al. 1989. Plasmodium falciparum: an abundant stage-specific protein expressed during early gametocyte development. Exp. Parasitol. 69:140–149 [DOI] [PubMed] [Google Scholar]
- 84. Carter R., Graves P. M., Quakyi I. A., Good M. F. 1989. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med. 169:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Carter R., Mendis K. N., Roberts D. 2000. Spatial targeting of interventions against malaria. Bull. World Health Organ. 78:1401–1411 [PMC free article] [PubMed] [Google Scholar]
- 86. Carter R., Miller L. H. 1979. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull. World Health Organ. 57(Suppl. 1):37–52 [PMC free article] [PubMed] [Google Scholar]
- 87. Ceesay S. J., et al. 2008. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet 372:1545–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Celada A., Cruchaud A., Perrin L. H. 1983. Phagocytosis of Plasmodium falciparum-parasitized erythrocytes by human polymorphonuclear leukocytes. J. Parasitol. 69:49–53 [PubMed] [Google Scholar]
- 89. Chaiyaroj S. C., Thompson J. K., Coppel R. L., Brown G. V. 1994. Gametocytogenesis occurs in Plasmodium falciparum isolates carrying a chromosome 9 deletion. Mol. Biochem. Parasitol. 63:163–165 [DOI] [PubMed] [Google Scholar]
- 90. Cham G. K., et al. 2009. Sequential, ordered acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 domains. J. Immunol. 183:3356–3363 [DOI] [PubMed] [Google Scholar]
- 91. Chardome M., Janssen P. J. 1952. Enquête sur l'incidence malarienne par la method dermique dans la region de Lubilash (Congo Belge). Ann. Soc. Belge Med. Trop. 32:209–211 [PubMed] [Google Scholar]
- 92. Charoenlarp P., Areekul S., Harinasuta T., Sirivorasarn P. 1972. The haemolytic effect of a single dose of 45 mg of primaquine in G-6-PD deficient Thais. J. Med. Assoc. Thai. 55:631–638 [PubMed] [Google Scholar]
- 93. Checchi F., et al. 2004. Antimalarial efficacy of sulfadoxine-pyrimethamine, amodiaquine and a combination of chloroquine plus sulfadoxine-pyrimethamine in Bundi Bugyo, western Uganda. Trop. Med. Int. Health 9:445–450 [DOI] [PubMed] [Google Scholar]
- 94. Chen Q., Schlichtherle M., Wahlgren M. 2000. Molecular aspects of severe malaria. Clin. Microbiol. Rev. 13:439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cheru L., et al. 2010. The IC(50) of anti-Pfs25 antibody in membrane-feeding assay varies among species. Vaccine 28:4423–4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chotivanich K., et al. 2006. Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrob. Agents Chemother. 50:1927–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chowdhury D. R., Angov E., Kariuki T., Kumar N. 2009. A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PLoS One 4:e6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Colbourne M. J. 1956. The effect of prolonged examination of bloodfilms on the parasite rate. West Afr. Med. J. 5:26–30 [PubMed] [Google Scholar]
- 99. Coleman R. E. 1990. Sporontocidal activity of the antimalarial WR-238605 against Plasmodium berghei ANKA in Anopheles stephensi. Am. J. Trop. Med. Hyg. 42:196–205 [DOI] [PubMed] [Google Scholar]
- 100. Coleman R. E., Clavin A. M., Milhous W. K. 1992. Gametocytocidal and sporontocidal activity of antimalarials against Plasmodium berghei ANKA in ICR mice and Anopheles stephensi mosquitoes. Am. J. Trop. Med. Hyg. 46:169–182 [DOI] [PubMed] [Google Scholar]
- 101. Coleman R. E., et al. 2004. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J. Med. Entomol. 41:201–208 [DOI] [PubMed] [Google Scholar]
- 102. Conway D. J., et al. 2001. Extreme geographical fixation of variation in the Plasmodium falciparum gamete surface protein gene Pfs48/45 compared with microsatellite loci. Mol. Biochem. Parasitol. 115:145–156 [DOI] [PubMed] [Google Scholar]
- 103. Coulibaly B., et al. 2009. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomised controlled trial. PLoS One 4:e5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Covell G., Coatney G. R., Field J. W., Singh J. 1955. Chemotherapy of malaria. Monogr. Ser. World Health Organ. 27:1–123 [PubMed] [Google Scholar]
- 105. Day K. P., Hayward R. E., Dyer M. 1998. The biology of Plasmodium falciparum transmission stages. Parasitology 116S:S95–S109 [DOI] [PubMed] [Google Scholar]
- 106. Day K. P., Hayward R. E., Smith D., Culvenor J. G. 1998. CD36-dependent adhesion and knob expression of the transmission stages of Plasmodium falciparum is stage specific. Mol. Biochem. Parasitol. 93:167–177 [DOI] [PubMed] [Google Scholar]
- 107. Day K. P., et al. 1993. Genes necessary for expression of a virulence determinant and for transmission of Plasmodium falciparum are located on a 0.3-megabase region of chromosome 9. Proc. Natl. Acad. Sci. U. S. A. 90:8292–8296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. de Koning-Ward T. F., et al. 2008. The role of osmiophilic bodies and Pfg377 expression in female gametocyte emergence and mosquito infectivity in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 67:278–290 [DOI] [PubMed] [Google Scholar]
- 109. De Martin S., et al. 2001. Community perceptions of a mass administration of an antimalarial drug combination in The Gambia. Trop. Med. Int. Health 6:442–448 [DOI] [PubMed] [Google Scholar]
- 110. Dern R. J., Beutler E., Alving A. S. 1954. The hemolytic effect of primaquine. II. The natural course of the hemolytic anemia and the mechanism of its self-limited character. J. Lab. Clin. Med. 44:171–176 [PubMed] [Google Scholar]
- 111. Diallo M., et al. 2008. Evaluation and optimization of membrane feeding compared to direct feeding as an assay for infectivity. Malar. J. 7:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dinglasan R. R., et al. 2007. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc. Natl. Acad. Sci. U. S. A. 104:13461–13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dixon M. W., Thompson J., Gardiner D. L., Trenholme K. R. 2008. Sex in Plasmodium: a sign of commitment. Trends Parasitol. 24:168–175 [DOI] [PubMed] [Google Scholar]
- 114. Dondorp A. M., et al. 2010. Artemisinin resistance: current status and scenarios for containment. Nat. Rev. Microbiol. 8:272–280 [DOI] [PubMed] [Google Scholar]
- 115. Doolan D. L., Dobano C., Baird J. K. 2009. Acquired immunity to malaria. Clin. Microbiol. Rev. 22:13–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dorsey G., et al. 2000. Predictors of chloroquine treatment failure in children and adults with falciparum malaria in Kampala, Uganda. Am. J. Trop. Med. Hyg. 62:686–692 [DOI] [PubMed] [Google Scholar]
- 117. Dorsey G., et al. 2007. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA 297:2210–2219 [DOI] [PubMed] [Google Scholar]
- 118. Douglas N. M., Anstey N. M., Angus B. J., Nosten F., Price R. N. 2010. Artemisinin combination therapy for vivax malaria. Lancet Infect. Dis. 10:405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dowling M. A., Shute G. T. 1966. A comparative study of thick and thin blood films in the diagnosis of scanty malaria parasitaemia. Bull. World Health Organ. 34:249–267 [PMC free article] [PubMed] [Google Scholar]
- 120. Drakeley C., Sutherland C., Bousema J. T., Sauerwein R. W., Targett G. A. 2006. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 22:424–430 [DOI] [PubMed] [Google Scholar]
- 121. Drakeley C. J., Akim N. I., Sauerwein R. W., Greenwood B. M., Targett G. A. 2000. Estimates of the infectious reservoir of Plasmodium falciparum malaria in The Gambia and in Tanzania. Trans. R. Soc. Trop. Med. Hyg. 94:472–476 [DOI] [PubMed] [Google Scholar]
- 122. Drakeley C. J., et al. 2006. Transmission reducing immunity is inversely related to age in P. falciparum gametocyte carriers. Parasite Immunol. 28:185–190 [DOI] [PubMed] [Google Scholar]
- 123. Drakeley C. J., et al. 2005. Altitude-dependent and -independent variations in Plasmodium falciparum prevalence in northeastern Tanzania. J. Infect. Dis. 191:1589–1598 [DOI] [PubMed] [Google Scholar]
- 124. Drakeley C. J., et al. 1996. Geographical distribution of a variant epitope of Pfs48/45, a Plasmodium falciparum transmission-blocking vaccine candidate. Mol. Biochem. Parasitol. 81:253–257 [DOI] [PubMed] [Google Scholar]
- 125. Drakeley C. J., et al. 2004. Parasite infectivity and immunity to Plasmodium falciparum gametocytes in Gambian children. Parasite Immunol. 26:159–165 [DOI] [PubMed] [Google Scholar]
- 126. Drakeley C. J., Flobbe K., Greenwood B. M., Targett G. A. 2000. Plasmodium falciparum gametocytes in Gambian adults. Ann. Trop. Med. Parasitol. 94:399–401 [DOI] [PubMed] [Google Scholar]
- 127. Drakeley C. J., et al. 2004. Addition of artesunate to chloroquine for treatment of Plasmodium falciparum malaria in Gambian children causes a significant but short-lived reduction in infectiousness for mosquitoes. Trop. Med. Int. Health 9:53–61 [DOI] [PubMed] [Google Scholar]
- 128. Drakeley C. J., et al. 1998. Transmission-blocking effects of sera from malaria-exposed individuals on Plasmodium falciparum isolates from gametocyte carriers. Parasitology 116:417–423 [DOI] [PubMed] [Google Scholar]
- 129. Drakeley C. J., Secka I., Correa S., Greenwood B. M., Targett G. A. 1999. Host haematological factors influencing the transmission of Plasmodium falciparum gametocytes to Anopheles gambiae s.s. mosquitoes. Trop. Med. Int. Health 4:131–138 [DOI] [PubMed] [Google Scholar]
- 130. Duffy P. E., Kaslow D. C. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 65:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dunyo S., et al. 2006. Gametocytaemia after drug treatment of asymptomatic Plasmodium falciparum. PloS Clin. Trials 1:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dyer M., Day K. 2000. Expression of Plasmodium falciparum trimeric G proteins and their involvement in switching to sexual development. Mol. Biochem. Parasitol. 108:67–78 [DOI] [PubMed] [Google Scholar]
- 133. Dyer M., Day K. P. 2000. Commitment to gametocytogenesis in Plasmodium falciparum. Parasitol. Today 16:102–107 [DOI] [PubMed] [Google Scholar]
- 134. Reference deleted.
- 135. Reference deleted.
- 136. Eichner M., et al. 2001. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malaria therapy data. Trans. R. Soc. Trop. Med. Hyg. 95:497–501 [DOI] [PubMed] [Google Scholar]
- 137. Eksi S., et al. 2006. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol. Microbiol. 61:991–998 [DOI] [PubMed] [Google Scholar]
- 138. Eksi S., et al. 2005. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol. Biochem. Parasitol. 143:90–99 [DOI] [PubMed] [Google Scholar]
- 139. Eksi S., et al. 2002. Targeting and sequestration of truncated Pfs230 in an intraerythrocytic compartment during Plasmodium falciparum gametocytogenesis. Mol. Microbiol. 44:1507–1516 [DOI] [PubMed] [Google Scholar]
- 140. El-Sayed B., et al. 2007. A randomized open-label trial of artesunate-sulfadoxine-pyrimethamine with or without primaquine for elimination of sub-microscopic P. falciparum parasitaemia and gametocyte carriage in eastern Sudan. PLoS One 2:e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Elzaki S. G., Gadalla N. B. H., Mansour F. A., Mukhtar E. A., El-Sayed B. B. 2006. The impact of clearance of the dry season sub-patent parasitaemia on the prevalence of Plasmodium falciparum in Eastern Sudan, abstr. 1172. Abstr. ICOPA XI Conf., Glasgow, United Kingdom [Google Scholar]
- 142. Enevold A. 2006. The influence of genetic innate resistance and acquired immunity on drug treatment outcome of uncomplicated Plasmodium falciparum malaria in Tanzania. Parassitologia 48:547–551 [PubMed] [Google Scholar]
- 143. Falk N., et al. 2006. Comparison of PCR-RFLP and Genescan-based genotyping for analyzing infection dynamics of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 74:944–950 [PubMed] [Google Scholar]
- 144. Fanello C. I., et al. 2007. A randomised trial to assess the safety and efficacy of artemether-lumefantrine (Coartem) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwanda. Trans. R. Soc. Trop. Med. Hyg. 101:344–350 [DOI] [PubMed] [Google Scholar]
- 145. Fanning S. L., et al. 2003. A glycosylphosphatidylinositol anchor signal sequence enhances the immunogenicity of a DNA vaccine encoding Plasmodium falciparum sexual-stage antigen, Pfs230. Vaccine 21:3228–3235 [DOI] [PubMed] [Google Scholar]
- 146. Faye B., et al. 2007. Efficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in Senegal. Malar. J. 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Faye B., et al. 2010. Efficacy and tolerability of artesunate-amodiaquine (Camoquin Plus) versus artemether-lumefantrine (Coartem) against uncomplicated Plasmodium falciparum malaria: multisite trial in Senegal and Ivory Coast. Trop. Med. Int. Health 15:608–613 [DOI] [PubMed] [Google Scholar]
- 148. Fischer P., Supali T., Wibowo H., Bonow I., Williams S. A. 2000. Detection of DNA of nocturnally periodic Brugia malayi in night and day blood samples by a polymerase chain reaction-ELISA-based method using an internal control DNA. Am. J. Trop. Med. Hyg. 62:291–296 [DOI] [PubMed] [Google Scholar]
- 149. Florens L., et al. 2002. A proteomic view of the Plasmodium falciparum life cycle. Nature 419:520–526 [DOI] [PubMed] [Google Scholar]
- 150. French N., et al. 2001. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS 15:899–906 [DOI] [PubMed] [Google Scholar]
- 151. Gamage-Mendis A. C., Rajakaruna J., Carter R., Mendis K. N. 1991. Infectious reservoir of Plasmodium vivax and Plasmodium falciparum malaria in an endemic region of Sri Lanka. Am. J. Trop. Med. Hyg. 45:479–487 [DOI] [PubMed] [Google Scholar]
- 152. Gamage-Mendis A. C., Rajakaruna J., Carter R., Mendis K. N. 1992. Transmission blocking immunity to human Plasmodium vivax malaria in an endemic population in Kataragama, Sri Lanka. Parasite Immunol. 14:385–396 [DOI] [PubMed] [Google Scholar]
- 153. Garavito G., et al. 2007. Blood schizontocidal activity of methylene blue in combination with antimalarials against Plasmodium falciparum. Parasite 14:135–140 [DOI] [PubMed] [Google Scholar]
- 154. Gardiner D. L., et al. 2005. Implication of a Plasmodium falciparum gene in the switch between asexual reproduction and gametocytogenesis. Mol. Biochem. Parasitol. 140:153–160 [DOI] [PubMed] [Google Scholar]
- 155. Gardner M. J., et al. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gautret P. 2001. Plasmodium falciparum gametocyte periodicity. Acta Trop. 78:1–2 [DOI] [PubMed] [Google Scholar]
- 157. Gautret P., Miltgen F., Gantier J. C., Chabaud A. G., Landau I. 1996. Enhanced gametocyte formation by Plasmodium chabaudi in immature erythrocytes: pattern of production, sequestration, and infectivity to mosquitoes. J. Parasitol. 82:900–906 [PubMed] [Google Scholar]
- 158. George J. N., Sears D. A., McCurdy P. R., Conrad M. E. 1967. Primaquine sensitivity in Caucasians: hemolytic reactions induced by primaquine in G-6-PD deficient subjects. J. Lab. Clin. Med. 70:80–93 [PubMed] [Google Scholar]
- 159. Gillman B. M., Batchelder J., Flaherty P., Weidanz W. P. 2004. Suppression of Plasmodium chabaudi parasitemia is independent of the action of reactive oxygen intermediates and/or nitric oxide. Infect. Immun. 72:6359–6366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Githeko A. K., et al. 1992. The reservoir of Plasmodium falciparum malaria in a holoendemic area of western Kenya. Trans. R. Soc. Trop. Med. Hyg. 86:355–358 [DOI] [PubMed] [Google Scholar]
- 161. Githeko A. K., et al. 1993. Confirmation that Plasmodium falciparum has aperiodic infectivity to Anopheles gambiae. Med. Vet. Entomol. 7:373–376 [DOI] [PubMed] [Google Scholar]
- 162. Gogtay N. J., et al. 2004. Preliminary report of the evaluation of the gametocytocidal action of bulaquine, in adult patients with acute, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 98:525–528 [DOI] [PubMed] [Google Scholar]
- 163. Gogtay N. J., et al. 2006. A randomized, parallel study of the safety and efficacy of 45 mg primaquine versus 75 mg bulaquine as gametocytocidal agents in adults with blood schizonticide-responsive uncomplicated falciparum malaria [ISCRTN50134587]. BMC Infect. Dis. 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Gouagna L. C., et al. 2010. Genetic variation in human HBB is associated with Plasmodium falciparum transmission. Nat. Genet. 42:328–331 [DOI] [PubMed] [Google Scholar]
- 165. Gouagna L. C., et al. 2004. Plasmodium falciparum malaria disease manifestations in humans and transmission to Anopheles gambiae: a field study in Western Kenya. Parasitology 128:235–243 [DOI] [PubMed] [Google Scholar]
- 166. Govere J. M., Durrheim D. N., Mngomezulu N. M., Barnes K., Sharp B. 2003. Infectivity of Plasmodium falciparum gametocytes to Anopheles arabiensis after treatment with sulfadoxine-pyrimethamine. Trans. R. Soc. Trop. Med. Hyg. 97:707–708 [DOI] [PubMed] [Google Scholar]
- 167. Govere J. M., et al. 1999. Sulfadoxine-pyrimethamine effectiveness against Plasmodium falciparum malaria in Mpumalanga Province, South Africa. Trans. R. Soc. Trop. Med. Hyg. 93:644. [DOI] [PubMed] [Google Scholar]
- 168. Gozar M. M., Price V. L., Kaslow D. C. 1998. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect. Immun. 66:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Grande T., et al. 2007. A randomised controlled trial to assess the efficacy of dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Peru. PLoS One 2:e1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Graves P. M. 1980. Studies on the use of a membrane feeding technique for infecting Anopheles gambiae with Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 74:738–742 [DOI] [PubMed] [Google Scholar]
- 171. Graves P. M., et al. 1988. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology 96:251–263 [DOI] [PubMed] [Google Scholar]
- 172. Graves P. M., Carter R., Burkot T. R., Quakyi I. A., Kumar N. 1988. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 10:209–218 [DOI] [PubMed] [Google Scholar]
- 173. Graves P. M., Carter R., McNeill K. M. 1984. Gametocyte production in cloned lines of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 33:1045–1050 [DOI] [PubMed] [Google Scholar]
- 174. Graves P. M., et al. 2008. Effectiveness of malaria control during changing climate conditions in Eritrea, 1998–2003. Trop. Med. Int. Health 13:218–228 [DOI] [PubMed] [Google Scholar]
- 175. Greenhouse B., et al. 2009. Decreasing efficacy of antimalarial combination therapy in Uganda is explained by decreasing host immunity rather than increasing drug resistance. J. Infect. Dis. 199:758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Greenwood B. M., Armstrong J. R. 1991. Comparison of two simple methods for determining malaria parasite density. Trans. R. Soc. Trop. Med. Hyg. 85:186–188 [DOI] [PubMed] [Google Scholar]
- 177. Griffin J. T., et al. 2010. Strategies towards Plasmodium falciparum malaria elimination in Africa using currently available tools. PLoS Med. 7:e100032420711482 [Google Scholar]
- 178. Guerra C. A., et al. 2008. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 5:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Guerra C. A., et al. 2010. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl. Trop. Dis. 4:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Guerra C. A., Snow R. W., Hay S. I. 2006. Defining the global spatial limits of malaria transmission in 2005. Adv. Parasitol. 62:157–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Gwadz R. W. 1976. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science 193:1150–1151 [DOI] [PubMed] [Google Scholar]
- 182. Hall N., et al. 2005. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307:82–86 [DOI] [PubMed] [Google Scholar]
- 183. Hallett R. L., et al. 2006. Chloroquine/sulphadoxine-pyrimethamine for Gambian children with malaria: transmission to mosquitoes of multidrug-resistant Plasmodium falciparum. PLoS Clin. Trials 1:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Hallett R. L., et al. 2004. Combination therapy counteracts the enhanced transmission of drug-resistant malaria parasites to mosquitoes. Antimicrob. Agents Chemother. 48:3940–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Hamour S., et al. 2005. Malaria in the Nuba Mountains of Sudan: baseline genotypic resistance and efficacy of the artesunate plus sulfadoxine-pyrimethamine and artesunate plus amodiaquine combinations. Trans. R. Soc. Trop. Med. Hyg. 99:548–554 [DOI] [PubMed] [Google Scholar]
- 186. Han E. T., et al. 2010. Sequence polymorphisms of Plasmodium vivax ookinete surface proteins (Pvs25 and Pvs28) from clinical isolates in Korea. Trop. Med. Int. Health 9:1072–1076 [DOI] [PubMed] [Google Scholar]
- 187. Han E. T., et al. 2007. Detection of four Plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis. J. Clin. Microbiol. 45:2521–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Handunnetti S. M., et al. 1996. Features of recrudescent chloroquine-resistant Plasmodium falciparum infections confer a survival advantage on parasites and have implications for disease control. Trans. R. Soc. Trop. Med. Hyg. 90:563–567 [DOI] [PubMed] [Google Scholar]
- 189. Hasugian A. R., et al. 2007. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin. Infect. Dis. 44:1067–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Hawking F., Wilson M. E., Gammage K. 1971. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 65:549–559 [DOI] [PubMed] [Google Scholar]
- 191. Hay S. I., et al. 2010. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 7:e1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Hayward R. E. 2000. Plasmodium falciparum phosphoenolpyruvate carboxykinase is developmentally regulated in gametocytes. Mol. Biochem. Parasitol. 107:227–240 [DOI] [PubMed] [Google Scholar]
- 193. Hayward R. E., Tiwari B., Piper K. P., Baruch D. I., Day K. P. 1999. Virulence and transmission success of the malarial parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 96:4563–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Healer J., Graszynski A., Riley E. 1999. Phagocytosis does not play a major role in naturally acquired transmission-blocking immunity to Plasmodium falciparum malaria. Infect. Immun. 67:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Healer J., McGuinness D., Carter R., Riley E. 1999. Transmission-blocking immunity to Plasmodium falciparum in malaria-immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology 119:425–433 [DOI] [PubMed] [Google Scholar]
- 196. Healer J., et al. 1997. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect. Immun. 65:3017–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hill D. R., et al. 2006. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis. Am. J. Trop. Med. Hyg. 75:402–415 [PubMed] [Google Scholar]
- 198. Hill J., Lines J., Rowland M. 2006. Insecticide-treated nets. Adv. Parasitol. 61:77–128 [DOI] [PubMed] [Google Scholar]
- 199. Hirai M., et al. 2008. Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr. Biol. 18:607–613 [DOI] [PubMed] [Google Scholar]
- 200. Hirai M., Mori T. 2010. Fertilization is a novel attacking site for the transmission blocking of malaria parasites. Acta Trop. 114:157–161 [DOI] [PubMed] [Google Scholar]
- 201. Hisaeda H., et al. 2000. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 68:6618–6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hogh B., et al. 1998. The differing impact of chloroquine and pyrimethamine/sulfadoxine upon the infectivity of malaria species to the mosquito vector. Am. J. Trop. Med. Hyg. 58:176–182 [DOI] [PubMed] [Google Scholar]
- 203. Huh A. J., et al. 2011. Parasitemia characteristics of Plasmodium vivax malaria patients in the Republic of Korea. J. Korean Med. Sci. 26:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Hulden L., Heliovaara K. 2008. Natural relapses in vivax malaria induced by Anopheles mosquitoes. Malar. J. 7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Hutagalung R., et al. 2005. A randomized trial of artemether-lumefantrine versus mefloquine-artesunate for the treatment of uncomplicated multi-drug resistant Plasmodium falciparum on the western border of Thailand. Malar. J. 4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ihalamulla R. L., Mendis K. N. 1987. Plasmodium vivax: isolation of mature asexual stages and gametocytes from infected human blood by colloidal silica (Percoll) gradient centrifugation. Trans. R. Soc. Trop. Med. Hyg. 81:25–28 [DOI] [PubMed] [Google Scholar]
- 207. James S. P. 1931. Some general results of a study of induced malaria in England. Trans. R. Soc. Trop. Med. Hyg. 24:477–525 [Google Scholar]
- 208. Jeffery G. M. 1952. The infection of mosquitoes by Plasmodium vivax (Chesson strain) during the early primary parasitemias. Am. J. Trop. Med. Hyg. 1:612–617 [DOI] [PubMed] [Google Scholar]
- 209. Jeffery G. M. 1958. Infectivity to mosquitoes of Plasmodium vivax following treatment with chloroquine and other antimalarials. Am. J. Trop. Med. Hyg. 7:207–211 [DOI] [PubMed] [Google Scholar]
- 210. Jeffery G. M., Eyles D. E. 1955. Infectivity to mosquitoes of Plasmodium falciparum as related to gametocyte density and duration of infection. Am. J. Trop. Med. Hyg. 4:781–789 [DOI] [PubMed] [Google Scholar]
- 211. Jeffery G. M., Young M. D., Eyles D. E. 1956. The treatment of Plasmodium falciparum infection with chloroquine, with a note on infectivity to mosquitoes of primaquine- and pyrimethamine-treated cases. Am. J. Hyg. (London) 64:1–11 [DOI] [PubMed] [Google Scholar]
- 212. Kamya M. R., et al. 2006. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J. Infect. Dis. 193:9–15 [DOI] [PubMed] [Google Scholar]
- 213. Kamya M. R., et al. 2007. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin. Trials 2:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kaneko A., et al. 2000. Malaria eradication on islands. Lancet 356:1560–1564 [DOI] [PubMed] [Google Scholar]
- 215. Kar P. K., Dua V. K., Gupta N. C., Gupta A., Dash A. P. 2009. Plasmodium falciparum gametocytaemia with chloroquine chemotherapy in persistent malaria in an endemic area of India. Indian J. Med. Res. 129:299–304 [PubMed] [Google Scholar]
- 216. Karl S., et al. 2008. Enhanced detection of gametocytes by magnetic deposition microscopy predicts higher potential for Plasmodium falciparum transmission. Malar. J. 7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Karl S., Davis T. M., St. Pierre T. G. 2009. A comparison of the sensitivities of detection of Plasmodium falciparum gametocytes by magnetic fractionation, thick blood film microscopy, and RT-PCR. Malar. J. 8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Karunaweera N. D., et al. 1992. Tumour necrosis factor-dependent parasite-killing effects during paroxysms in non-immune Plasmodium vivax malaria patients. Clin. Exp. Immunol. 88:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kaslow D. C., et al. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74–76 [DOI] [PubMed] [Google Scholar]
- 220. Khan S. M., et al. 2005. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121:675–687 [DOI] [PubMed] [Google Scholar]
- 221. Kheir A., et al. 2010. Transmission and cross-mating of high-level resistance Plasmodium falciparum dihydrofolate reductase haplotypes in the Gambia. Am. J. Trop. Med. Hyg. 82:535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Killick-Kendrick R., Warren M. 1968. Primary exoerythrocytic schizonts of a mammalian Plasmodium as a source of gametocytes. Nature 220:191–192 [DOI] [PubMed] [Google Scholar]
- 223. Klein T. A., Harrison B. A., Grove J. S., Dixon S. V., Andre R. G. 1986. Correlation of survival rates of Anopheles dirus A (Diptera: Culicidae) with different infection densities of Plasmodium cynomolgi. Bull. World Health Organ. 64:901–907 [PMC free article] [PubMed] [Google Scholar]
- 224. Klein T. A., Lima J. B., Toda Tang A. 1992. Vector incrimination and effects of antimalarial drugs on malaria transmission and control in the Amazon basin of Brazil. Mem. Inst. Oswaldo Cruz 87(Suppl. 3):393–397 [DOI] [PubMed] [Google Scholar]
- 225. Klein T. A., Tada M. S., Lima J. B. 1991. Infection of Anopheles darlingi fed on patients with Plasmodium falciparum before and after treatment with quinine or quinine plus tetracycline. Am. J. Trop. Med. Hyg. 44:604–608 [DOI] [PubMed] [Google Scholar]
- 226. Klein T. A., Tada M. S., Lima J. B., Katsuragawa T. H. 1991. Infection of Anopheles darlingi fed on patients infected with Plasmodium vivax before and during treatment with chloroquine in Costa Marques, Rondonia, Brazil. Am. J. Trop. Med. Hyg. 45:471–478 [DOI] [PubMed] [Google Scholar]
- 227. Kleinschmidt I., et al. 2009. Marked increase in child survival after four years of intensive malaria control. Am. J. Trop. Med. Hyg. 80:882–888 [PMC free article] [PubMed] [Google Scholar]
- 228. Kocken C. H., et al. 1993. Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol. Biochem. Parasitol. 61:59–68 [DOI] [PubMed] [Google Scholar]
- 229. Kone A., et al. 2010. Sulfadoxine-pyrimethamine impairs Plasmodium falciparum gametocyte infectivity and Anopheles mosquito survival. Int. J. Parasitol. 40:1221–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Kongkasuriyachai D., et al. 2004. Potent immunogenicity of DNA vaccines encoding Plasmodium vivax transmission-blocking vaccine candidates Pvs25 and Pvs28—evaluation of homologous and heterologous antigen-delivery prime-boost strategy. Vaccine 22:3205–3213 [DOI] [PubMed] [Google Scholar]
- 231. Kubler-Kielb J., et al. 2007. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc. Natl. Acad. Sci. U. S. A. 104:293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Kumar N. 1987. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 9:321–335 [DOI] [PubMed] [Google Scholar]
- 233. Kumar N., Carter R. 1984. Biosynthesis of the target antigens of antibodies blocking transmission of Plasmodium falciparum. Mol. Biochem. Parasitol. 13:333–342 [DOI] [PubMed] [Google Scholar]
- 234. Kumar N., Carter R. 1985. Biosynthesis of two stage-specific membrane proteins during transformation of Plasmodium gallinaceum zygotes into ookinetes. Mol. Biochem. Parasitol. 14:127–139 [DOI] [PubMed] [Google Scholar]
- 235. Kumar N., Zheng H. 1990. Stage-specific gametocytocidal effect in vitro of the antimalaria drug qinghaosu on Plasmodium falciparum. Parasitol. Res. 76:214–218 [DOI] [PubMed] [Google Scholar]
- 236. Kwiatkowski D. 1995. Malarial toxins and the regulation of parasite density. Parasitol. Today 11:206–212 [DOI] [PubMed] [Google Scholar]
- 237. Kwiatkowski D., et al. 1990. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336:1201–1204 [DOI] [PubMed] [Google Scholar]
- 238. Lacroix R., Mukabana W. R., Gouagna L. C., Koella J. C. 2005. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 3:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Lasonder E., et al. 2002. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 419:537–542 [DOI] [PubMed] [Google Scholar]
- 240. Lawaly Y. R., et al. 2010. Heritability of the human infectious reservoir of malaria parasites. PLoS One 5:e11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Lawpoolsri S., et al. 2009. Optimally timing primaquine treatment to reduce Plasmodium falciparum transmission in low endemicity Thai-Myanmar border populations. Malar. J. 8:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Lefevre G., et al. 2001. A clinical and pharmacokinetic trial of six doses of artemether-lumefantrine for multidrug-resistant Plasmodium falciparum malaria in Thailand. Am. J. Trop. Med. Hyg. 64:247–256 [DOI] [PubMed] [Google Scholar]
- 243. Lensen A., et al. 1999. Plasmodium falciparum: infectivity of cultured, synchronized gametocytes to mosquitoes. Exp. Parasitol. 91:101–103 [DOI] [PubMed] [Google Scholar]
- 244. Lensen A., et al. 1998. Mechanisms that reduce transmission of Plasmodium falciparum malaria in semiimmune and nonimmune persons. J. Infect. Dis. 177:1358–1363 [DOI] [PubMed] [Google Scholar]
- 245. Lensen A., et al. 1996. Measurement by membrane feeding of reduction in Plasmodium falciparum transmission induced by endemic sera. Trans. R. Soc. Trop. Med. Hyg. 90:20–22 [DOI] [PubMed] [Google Scholar]
- 246. Lensen A. H., Bolmer-Van de Vegte M., van Gemert G. J., Eling W. M., Sauerwein R. W. 1997. Leukocytes in a Plasmodium falciparum-infected blood meal reduce transmission of malaria to Anopheles mosquitoes. Infect. Immun. 65:3834–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Le Roch K. G., et al. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503–1508 [DOI] [PubMed] [Google Scholar]
- 248. Leslie T., et al. 2007. Sulfadoxine-pyrimethamine, chlorproguanil-dapsone, or chloroquine for the treatment of Plasmodium vivax malaria in Afghanistan and Pakistan: a randomized controlled trial. JAMA 297:2201–2209 [DOI] [PubMed] [Google Scholar]
- 249. Li F., Patra K. P., Vinetz J. M. 2005. An anti-chitinase malaria transmission-blocking single-chain antibody as an effector molecule for creating a Plasmodium falciparum-refractory mosquito. J. Infect. Dis. 192:878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Lingnau A., Margos G., Maier W. A., Seitz H. M. 1993. The effects of hormones on the gametocytogenesis of Plasmodium falciparum in vitro. Appl. Parasitol. 34:153–160 [PubMed] [Google Scholar]
- 251. Liu Y., et al. 2008. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 22:1051–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Lobo C. A., Dhar R., Kumar N. 1999. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect. Immun. 67:1688–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Lobo C. A., Fujioka H., Aikawa M., Kumar N. 1999. Disruption of the Pfg27 locus by homologous recombination leads to loss of the sexual phenotype in P. falciparum. Mol. Cell 3:793–798 [DOI] [PubMed] [Google Scholar]
- 254. Luxemburger C., et al. 1994. Permethrin-impregnated bed nets for the prevention of malaria in schoolchildren on the Thai-Burmese border. Trans. R. Soc. Trop. Med. Hyg. 88:155–159 [DOI] [PubMed] [Google Scholar]
- 255. Mabunda S., Casimiro S., Quinto L., Alonso P. 2008. A country-wide malaria survey in Mozambique. I. Plasmodium falciparum infection in children in different epidemiological settings. Malar. J. 7:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Maeno Y., et al. 2008. A dried blood sample on filter paper is suitable for detecting Plasmodium falciparum gametocytes by reverse transcription polymerase chain reaction. Acta Trop. 107:121–127 [DOI] [PubMed] [Google Scholar]
- 257. Magesa S. M., Mdira Y. K., Akida J. A., Bygbjerg I. C., Jakobsen P. H. 2000. Observations on the periodicity of Plasmodium falciparum gametocytes in natural human infections. Acta Trop. 76:239–246 [DOI] [PubMed] [Google Scholar]
- 258. Maier W. A., Becker-Feldman H., Seitz H. M. 1987. Pathology of malaria-infected mosquitoes. Parasitol. Today 3:216–218 [DOI] [PubMed] [Google Scholar]
- 259. Mair G. R., et al. 2006. Regulation of sexual development of Plasmodium by translational repression. Science 313:667–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Mair G. R., et al. 2010. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 6:e1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Males S., Gaye O., Garcia A. 2008. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin. Infect. Dis. 46:516–522 [DOI] [PubMed] [Google Scholar]
- 262. Malkin E. M., et al. 2005. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23:3131–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Marangi M., et al. 2009. Prevalence of Plasmodium spp. in malaria asymptomatic African migrants assessed by nucleic acid sequence based amplification. Malar. J. 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Marques P. X., et al. 2005. Plasmodium species mixed infections in two areas of Manhica District, Mozambique. Int. J. Biol. Sci. 1:96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Maswoswe S. M., Peters W., Warhurst D. C. 1985. Corticosteroid stimulation of the growth of Plasmodium falciparum gametocytes in vitro. Ann. Trop. Med. Parasitol. 79:607–616 [DOI] [PubMed] [Google Scholar]
- 266. Mayxay M., et al. 2004. Randomized comparison of chloroquine plus sulfadoxine-pyrimethamine versus artesunate plus mefloquine versus artemether-lumefantrine in the treatment of uncomplicated falciparum malaria in the Lao People's Democratic Republic. Clin. Infect. Dis. 39:1139–1147 [DOI] [PubMed] [Google Scholar]
- 267. McGilvray I. D., Serghides L., Kapus A., Rotstein O. D., Kain K. C. 2000. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 96:3231–3240 [PubMed] [Google Scholar]
- 268. McKenzie F. E., Jeffery G. M., Collins W. E. 2007. Gametocytemia and fever in human malaria infections. J. Parasitol. 93:627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. McKenzie F. E., Jeffery G. M., Collins W. E. 2002. Plasmodium malariae infection boosts Plasmodium falciparum gametocyte production. Am. J. Trop. Med. Hyg. 67:411–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. McKenzie F. E., Jeffery G. M., Collins W. E. 2002. Plasmodium vivax blood-stage dynamics. J. Parasitol. 88:521–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. McKenzie F. E., et al. 2006. Gametocytemia in Plasmodium vivax and Plasmodium falciparum infections. J. Parasitol. 92:1281–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. McMahon J. E., Marshall T. F., Vaughan J. P., Kolstrup N. 1979. Tanzania Filariasis Project: a provocative day test with diethylcarbamazine for the detection of microfilariae of nocturnally periodic Wuchereria bancrofti in the blood. Bull. World Health Organ. 57:759–765 [PMC free article] [PubMed] [Google Scholar]
- 273. McRobert L., et al. 2004. Distinct trafficking and localization of STEVOR proteins in three stages of the Plasmodium falciparum life cycle. Infect. Immun. 72:6597–6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Mendez F., et al. 2007. Selection of antifolate-resistant Plasmodium falciparum by sulfadoxine-pyrimethamine treatment and infectivity to Anopheles mosquitoes. Am. J. Trop. Med. Hyg. 77:438–443 [PubMed] [Google Scholar]
- 275. Mendez F., et al. 2002. Determinants of treatment response to sulfadoxine-pyrimethamine and subsequent transmission potential in falciparum malaria. Am. J. Epidemiol. 156:230–238 [DOI] [PubMed] [Google Scholar]
- 276. Mendis K., et al. 2009. From malaria control to eradication: the WHO perspective. Trop. Med. Int. Health 14:802–809 [DOI] [PubMed] [Google Scholar]
- 277. Menegon M., et al. 2000. Genotyping of Plasmodium falciparum gametocytes by reverse transcriptase polymerase chain reaction. Mol. Biochem. Parasitol. 111:153–161 [DOI] [PubMed] [Google Scholar]
- 278. Menendez C., D'Alessandro U., ter Kuile F. O. 2007. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect. Dis. 7:126–135 [DOI] [PubMed] [Google Scholar]
- 279. Mens P. F., et al. 2008. A randomized trial to monitor the efficacy and effectiveness by QT-NASBA of artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment and transmission control of uncomplicated Plasmodium falciparum malaria in western Kenya. Malar. J. 7:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Meszoely C. A., Erbe E. F., Steere R. L., Trosper J., Beaudoin R. L. 1987. Plasmodium falciparum: freeze-fracture of the gametocyte pellicular complex. Exp. Parasitol. 64:300–309 [DOI] [PubMed] [Google Scholar]
- 281. Metselaar D. 1960. Relative increase in the prevalence of Plasmodium falciparum some years after the beginning of a house-spraying campaign in Netherlands New Guinea. Trans. R. Soc. Trop. Med. Hyg. 54:523–528 [DOI] [PubMed] [Google Scholar]
- 282. Michel K., Kafatos F. C. 2005. Mosquito immunity against Plasmodium. Insect Biochem. Mol. Biol. 35:677–689 [DOI] [PubMed] [Google Scholar]
- 283. Miller M. J. 1958. Observations on the natural history of malaria in the semi-resistant West African. Trans. R. Soc. Trop. Med. Hyg. 52:152–168 [DOI] [PubMed] [Google Scholar]
- 284. Mitri C., Thiery I., Bourgouin C., Paul R. E. 2009. Density-dependent impact of the human malaria parasite Plasmodium falciparum gametocyte sex ratio on mosquito infection rates. Proc. Biol. Sci. 276:3721–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Miyata T., et al. 2010. Plasmodium vivax ookinete surface protein Pvs25 linked to cholera toxin B subunit induces potent transmission-blocking immunity by intranasal as well as subcutaneous immunization. Infect. Immun. 78:3773–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Mlambo G., Vasquez Y., LeBlanc R., Sullivan D., Kumar N. 2008. A filter paper method for the detection of Plasmodium falciparum gametocytes by reverse transcription polymerase chain reaction. Am. J. Trop. Med. Hyg. 78:114–116 [PubMed] [Google Scholar]
- 287. Mockenhaupt F. P., et al. 2005. A randomised, placebo-controlled, and double-blind trial on sulfadoxine-pyrimethamine alone or combined with artesunate or amodiaquine in uncomplicated malaria. Trop. Med. Int. Health 10:512–520 [DOI] [PubMed] [Google Scholar]
- 288. Mockenhaupt F. P., et al. 2005. Plasmodium falciparum dhfr but not dhps mutations associated with sulphadoxine-pyrimethamine treatment failure and gametocyte carriage in northern Ghana. Trop. Med. Int. Health 10:901–908 [DOI] [PubMed] [Google Scholar]
- 289. Molineaux L., Gramiccia G. 1980. The Garki Project. Research on the epidemiology and control of malaria in the Sudan savannah of West Africa. World Health Organization, Geneva, Switzerland [Google Scholar]
- 290. Molineaux L., Storey J., Cohen J. E., Thomas A. 1980. A longitudinal study of human malaria in the West African savanna in the absence of control measures: relationships between different Plasmodium species, in particular P. falciparum and P. malariae. Am. J. Trop. Med. Hyg. 29:725–737 [DOI] [PubMed] [Google Scholar]
- 291. Mons B. 1986. Intra erythrocytic differentiation of Plasmodium berghei. Acta Leiden. 54:1–124 [PubMed] [Google Scholar]
- 292. Moody A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15:66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Moonen B., et al. 2010. Operational strategies to achieve and maintain malaria elimination. Lancet 376:1592–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Mori T., Kuroiwa H., Higashiyama T., Kuroiwa T. 2006. Generative cell specific 1 is essential for angiosperm fertilization. Nat. Cell Biol. 8:64–71 [DOI] [PubMed] [Google Scholar]
- 295. Motard A., et al. 1995. Immunization with the malaria heat shock like protein hsp70-1 enhances transmission to the mosquito. Int. Immunol. 7:147–150 [DOI] [PubMed] [Google Scholar]
- 296. Mueller I., et al. 2009. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect. Dis. 9:555–566 [DOI] [PubMed] [Google Scholar]
- 297. Muirhead-Thomson R. C. 1954. Factors determining the true reservoir of infection of Plasmodium falciparum and Wuchereria bancrofti in a West African village. Trans. R. Soc. Trop. Med. Hyg. 48:208–225 [DOI] [PubMed] [Google Scholar]
- 298. Muirhead-Thomson R. C. 1957. The malarial infectivity of an African village population to mosquitoes (Anopheles gambiae); a random xenodiagnostic survey. Am. J. Trop. Med. Hyg. 6:971–979 [DOI] [PubMed] [Google Scholar]
- 299. Muirhead-Thomson R. C. 1998. Where do most mosquitoes acquire their malarial (Plasmodium falciparum) infection? From adults or from children? Ann. Trop. Med. Parasitol. 92:891–893 [DOI] [PubMed] [Google Scholar]
- 300. Mulder B., et al. 1999. Plasmodium falciparum: membrane feeding assays and competition ELISAs for the measurement of transmission reduction in sera from Cameroon. Exp. Parasitol. 92:81–86 [DOI] [PubMed] [Google Scholar]
- 301. Mulder B., van der Ligt W., Sauerwein R., Verhave J. P. 1998. Detection of Plasmodium falciparum gametocytes with the OBC test and Giemsa-stained thick blood films for malaria transmission studies in Cameroon. Trans. R. Soc. Trop. Med. Hyg. 92:395–396 [DOI] [PubMed] [Google Scholar]
- 302. Muturi E. J., et al. 2006. Concomitant infections of Plasmodium falciparum and Wuchereria bancrofti on the Kenyan coast. Filaria J. 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Mwangi T. W., Ross A., Snow R. W., Marsh K. 2005. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J. Infect. Dis. 191:1932–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Nacher M. 2004. Does the shape of Plasmodium falciparum gametocytes have a function? Med. Hypotheses 62:618–619 [DOI] [PubMed] [Google Scholar]
- 305. Nacher M. 2002. Malarial anaemia: a crossroad? Med. Hypotheses 59:363–365 [DOI] [PubMed] [Google Scholar]
- 306. Nacher M., et al. 2004. Seasonal variation in hyperparasitaemia and gametocyte carriage in patients with Plasmodium falciparum malaria on the Thai-Burmese border. Trans. R. Soc. Trop. Med. Hyg. 98:322–328 [DOI] [PubMed] [Google Scholar]
- 307. Nacher M., et al. 2004. Seasonal fluctuations in the carriage of Plasmodium vivax gametocytes in Thailand. Ann. Trop. Med. Parasitol. 98:115–120 [DOI] [PubMed] [Google Scholar]
- 308. Nacher M., et al. 2004. Comparison of artesunate and chloroquine activities against Plasmodium vivax gametocytes. Antimicrob. Agents Chemother. 48:2751–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Nacher M., et al. 2004. Risk factors for Plasmodium vivax gametocyte carriage in Thailand. Am. J. Trop. Med. Hyg. 71:693–695 [PubMed] [Google Scholar]
- 310. Nacher M., et al. 2002. Decreased hemoglobin concentrations, hyperparasitemia, and severe malaria are associated with increased Plasmodium falciparum gametocyte carriage. J. Parasitol. 88:97–101 [DOI] [PubMed] [Google Scholar]
- 311. Naotunne T. D., Rathnayake K. D., Jayasinghe A., Carter R., Mendis K. N. 1990. Plasmodium cynomolgi: serum-mediated blocking and enhancement of infectivity to mosquitoes during infections in the natural host, Macaca sinica. Exp. Parasitol. 71:305–313 [DOI] [PubMed] [Google Scholar]
- 312. Naotunne T. S., et al. 1991. Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J. Exp. Med. 173:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Naotunne T. S., Karunaweera N. D., Mendis K. N., Carter R. 1993. Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology 78:555–562 [PMC free article] [PubMed] [Google Scholar]
- 314. Nassir E., et al. 2005. Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. Int. J. Parasitol. 35:49–55 [DOI] [PubMed] [Google Scholar]
- 315. Newell E. D. 1997. Comparison of the use of skin scarification and skin biopsies to determine the prevalence and intensity of Onchocerca volvulus infection. Ann. Trop. Med. Parasitol. 91:633–642 [DOI] [PubMed] [Google Scholar]
- 316. Nkhoma E. T., Poole C., Vannappagari V., Hall S. A., Beutler E. 2009. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol. Dis. 42:267–278 [DOI] [PubMed] [Google Scholar]
- 317. Noden B. H., et al. 1994. Plasmodium falciparum: the population structure of mature gametocyte cultures has little effect on their innate fertility. Acta Trop. 58:13–19 [DOI] [PubMed] [Google Scholar]
- 318. Nosten F., et al. 2000. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356:297–302 [DOI] [PubMed] [Google Scholar]
- 319. Nwakanma D., et al. 2008. High gametocyte complexity and mosquito infectivity of Plasmodium falciparum in the Gambia. Int. J. Parasitol. 38:219–227 [DOI] [PubMed] [Google Scholar]
- 320. Oesterholt M. J., et al. 2009. Submicroscopic gametocytes and the transmission of antifolate-resistant Plasmodium falciparum in Western Kenya. PLoS One 4:e4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Oesterholt M. J., et al. 2006. Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar. J. 5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Oh S. S., Chishti A. H., Palek J., Liu S. C. 1997. Erythrocyte membrane alterations in Plasmodium falciparum malaria sequestration. Curr. Opin. Hematol. 4:148–154 [DOI] [PubMed] [Google Scholar]
- 323. Okell L. C., Drakeley C. J., Bousema T., Whitty C. J., Ghani A. C. 2008. Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med. 5:e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Okell L. C., Drakeley C. J., Ghani A. C., Bousema T., Sutherland C. J. 2008. Reduction of transmission from malaria patients by artemisinin combination therapies: a pooled analysis of six randomized trials. Malar. J. 7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Okell L. C., Ghani A. C., Lyons E., Drakeley C. J. 2009. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J. Infect. Dis. 200:1509–1517 [DOI] [PubMed] [Google Scholar]
- 326. Okell L. C. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS One, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Olliaro P., Taylor W. R., Rigal J. 2001. Controlling malaria: challenges and solutions. Trop. Med. Int. Health 6:922–927 [DOI] [PubMed] [Google Scholar]
- 328. O'Meara W. P., et al. 2008. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet 372:1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. O'Meara W. P., Mangeni J. N., Steketee R., Greenwood B. 2010. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect. Dis. 10:545–555 [DOI] [PubMed] [Google Scholar]
- 330. Ono T., Nakabayashi T. 1990. Gametocytogenesis induction by ammonium compounds in cultured Plasmodium falciparum. Int. J. Parasitol. 20:615–618 [DOI] [PubMed] [Google Scholar]
- 331. Ono T., Nakai T., Nakabayashi T. 1986. Induction of gametocytogenesis in Plasmodium falciparum by the culture supernatant of hybridoma cells producing anti-P. falciparum antibody. Biken J. 29:77–81 [PubMed] [Google Scholar]
- 332. Ono T., Ohnishi Y., Nagamune K., Kano M. 1993. Gametocytogenesis induction by Berenil in cultured Plasmodium falciparum. Exp. Parasitol. 77:74–78 [DOI] [PubMed] [Google Scholar]
- 333. Osorio L., Ferro B. E., Castillo C. M. 2002. Effects of chloroquine and sulfadoxine/pyrimethamine on gametocytes in patients with uncomplicated Plasmodium falciparum malaria in Colombia. Mem. Inst. Oswaldo Cruz 97:1221–1223 [DOI] [PubMed] [Google Scholar]
- 334. Ouedraogo A. L., et al. 2010. The plasticity of Plasmodium falciparum gametocytaemia in relation to age in Burkina Faso. Malar. J. 9:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Ouedraogo A. L., et al. 2009. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One 4:e8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Ouedraogo A. L., et al. 2008. Seasonal patterns of Plasmodium falciparum gametocyte prevalence and density in a rural population of Burkina Faso. Acta Trop. 105:28–34 [DOI] [PubMed] [Google Scholar]
- 337. Ouedraogo A. L., et al. 2007. Age-dependent distribution of Plasmodium falciparum gametocytes quantified by Pfs25 real-time QT-NASBA in a cross-sectional study in Burkina Faso. Am. J. Trop. Med. Hyg. 76:626–630 [PubMed] [Google Scholar]
- 338. Outchkourov N. S., et al. 2008. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc. Natl. Acad. Sci. U. S. A. 105:4301–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Owusu-Agyei S., et al. 2008. An open label, randomised trial of artesunate+amodiaquine, artesunate+chlorproguanil-dapsone and artemether-lumefantrine for the treatment of uncomplicated malaria. PLoS One 3:e2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Paganotti G. M., et al. 2006. Genetic complexity and gametocyte production of Plasmodium falciparum in Fulani and Mossi communities in Burkina Faso. Parasitology 132:607–614 [DOI] [PubMed] [Google Scholar]
- 341. Panichakul T., et al. 2007. Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. Int. J. Parasitol. 37:1551–1557 [DOI] [PubMed] [Google Scholar]
- 342. Paton M. G., et al. 1993. Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol. Biochem. Parasitol. 59:263–275 [DOI] [PubMed] [Google Scholar]
- 343. Paul R. E., Brey P. T., Robert V. 2002. Plasmodium sex determination and transmission to mosquitoes. Trends Parasitol. 18:32–38 [DOI] [PubMed] [Google Scholar]
- 344. Paul R. E., Coulson T. N., Raibaud A., Brey P. T. 2000. Sex determination in malaria parasites. Science 287:128–131 [DOI] [PubMed] [Google Scholar]
- 345. Paul R. E., Diallo M., Brey P. T. 2004. Mosquitoes and transmission of malaria parasites—not just vectors. Malar. J. 3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Paul R. E., Doerig C., Brey P. T. 2000. Erythropoiesis and molecular mechanisms for sexual determination in malaria parasites. IUBMB Life 49:245–248 [DOI] [PubMed] [Google Scholar]
- 347. Paul R. E., et al. 1995. Mating patterns in malaria parasite populations of Papua New Guinea. Science 269:1709–1711 [DOI] [PubMed] [Google Scholar]
- 348. Peatey C. L., et al. 2009. Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J. Infect. Dis. 200:1518–1521 [DOI] [PubMed] [Google Scholar]
- 349. Peiris J. S., et al. 1988. Monoclonal and polyclonal antibodies both block and enhance transmission of human Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 39:26–32 [DOI] [PubMed] [Google Scholar]
- 350. Pethleart A., et al. 2004. Infectious reservoir of Plasmodium infection in Mae Hong Son Province, north-west Thailand. Malar. J. 3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Petter M., Bonow I., Klinkert M. Q. 2008. Diverse expression patterns of subgroups of the rif multigene family during Plasmodium falciparum gametocytogenesis. PLoS One 3:e3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Pichon G., Awono-Ambene H. P., Robert V. 2000. High heterogeneity in the number of Plasmodium falciparum gametocytes in the bloodmeal of mosquitoes fed on the same host. Parasitology 121:115–120 [DOI] [PubMed] [Google Scholar]
- 353. Piola P., et al. 2005. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet 365:1467–1473 [DOI] [PubMed] [Google Scholar]
- 354. Piper K. P., Hayward R. E., Cox M. J., Day K. P. 1999. Malaria transmission and naturally acquired immunity to PfEMP-1. Infect. Immun. 67:6369–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Ponnudurai T., et al. 1989. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98:165–173 [DOI] [PubMed] [Google Scholar]
- 356. Ponnudurai T., Van Gemert G. J., Bensink T., Lensen A. H., Meuwissen J. H. 1987. Transmission blockade of Plasmodium falciparum: its variability with gametocyte numbers and concentration of antibody. Trans. R. Soc. Trop. Med. Hyg. 81:491–493 [DOI] [PubMed] [Google Scholar]
- 357. Port G. R., Boreham P. F. L., Bryan J. H. 1980. The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera: Culicidae). Bull. Entomol. Res. 70:133–144 [Google Scholar]
- 358. Pradel G. 2007. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology 134:1911–1929 [DOI] [PubMed] [Google Scholar]
- 359. Premawansa S., et al. 1994. Plasmodium falciparum malaria transmission-blocking immunity under conditions of low endemicity as in Sri Lanka. Parasite Immunol. 16:35–42 [DOI] [PubMed] [Google Scholar]
- 360. Price R., et al. 1999. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 60:1019–1023 [DOI] [PubMed] [Google Scholar]
- 361. Price R. N., et al. 1996. Effects of artemisinin derivatives on malaria transmissibility. Lancet 347:1654–1658 [DOI] [PubMed] [Google Scholar]
- 362. Pukrittayakamee S., et al. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Pukrittayakamee S., et al. 2004. Activities of artesunate and primaquine against asexual- and sexual-stage parasites in falciparum malaria. Antimicrob. Agents Chemother. 48:1329–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Pukrittayakamee S., et al. 2001. Therapeutic responses to antibacterial drugs in vivax malaria. Trans. R. Soc. Trop. Med. Hyg. 95:524–528 [DOI] [PubMed] [Google Scholar]
- 365. Pukrittayakamee S., et al. 2008. Effects of different antimalarial drugs on gametocyte carriage in P. vivax malaria. Am. J. Trop. Med. Hyg. 79:378–384 [PubMed] [Google Scholar]
- 366. Pukrittayakamee S., Vanijanonta S., Chantra A., Clemens R., White N. J. 1994. Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J. Infect. Dis. 169:932–935 [DOI] [PubMed] [Google Scholar]
- 367. Puta C., Manyando C. 1997. Enhanced gametocyte production in Fansidar-treated Plasmodium falciparum malaria patients: implications for malaria transmission control programmes. Trop. Med. Int. Health 2:227–229 [DOI] [PubMed] [Google Scholar]
- 368. Qiu Y. T., Smallegange R. C., Van Loon J. J., ter Braak C. J., Takken W. 2006. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s.s. Med. Vet. Entomol. 20:280–287 [DOI] [PubMed] [Google Scholar]
- 369. Quakyi I. A., et al. 1987. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J. Immunol. 139:4213–4217 [PubMed] [Google Scholar]
- 370. Raabe A. C., Billker O., Vial H. J., Wengelnik K. 2009. Quantitative assessment of DNA replication to monitor microgametogenesis in Plasmodium berghei. Mol. Biochem. Parasitol. 168:172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Ramharter M., et al. 2002. In vitro activity of tafenoquine alone and in combination with artemisinin against Plasmodium falciparum. Am. J. Trop. Med. Hyg. 67:39–43 [DOI] [PubMed] [Google Scholar]
- 372. Ranawaka M. B., Munesinghe Y. D., de Silva D. M., Carter R., Mendis K. N. 1988. Boosting of transmission-blocking immunity during natural Plasmodium vivax infections in humans depends upon frequent reinfection. Infect. Immun. 56:1820–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Ranford-Cartwright L. C., Balfe P., Carter R., Walliker D. 1993. Frequency of cross-fertilization in the human malaria parasite Plasmodium falciparum. Parasitology 107:11–18 [DOI] [PubMed] [Google Scholar]
- 374. Ratcliff A., et al. 2007. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet 369:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Ratcliff A., et al. 2007. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 101:351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Read D., et al. 1994. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 16:511–519 [DOI] [PubMed] [Google Scholar]
- 377. Read L. K., Mikkelsen R. B. 1991. Comparison of adenylate cyclase and cAMP-dependent protein kinase in gametocytogenic and nongametocytogenic clones of Plasmodium falciparum. J. Parasitol. 77:346–352 [PubMed] [Google Scholar]
- 378. Reece S. E., Drew D. R., Gardner A. 2008. Sex ratio adjustment and kin discrimination in malaria parasites. Nature 453:609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Reece S. E., Duncan A. B., West S. A., Read A. F. 2005. Host cell preference and variable transmission strategies in malaria parasites. Proc. Biol. Sci. 272:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Reece S. E., Duncan A. B., West S. A., Read A. F. 2003. Sex ratios in the rodent malaria parasite, Plasmodium chabaudi. Parasitology 127:419–425 [DOI] [PubMed] [Google Scholar]
- 381. Reeve P. A., Toaliu H., Kaneko A., Hall J. J., Ganczakowski M. 1992. Acute intravascular haemolysis in Vanuatu following a single dose of primaquine in individuals with glucose-6-phosphate dehydrogenase deficiency. J. Trop. Med. Hyg. 95:349–351 [PubMed] [Google Scholar]
- 382. Rener J., Graves P. M., Carter R., Williams J. L., Burkot T. R. 1983. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J. Exp. Med. 158:976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Robert V., Awono-Ambene H. P., Le Hesran J. Y., Trape J. F. 2000. Gametocytemia and infectivity to mosquitoes of patients with uncomplicated Plasmodium falciparum malaria attacks treated with chloroquine or sulfadoxine plus pyrimethamine. Am. J. Trop. Med. Hyg. 62:210–216 [DOI] [PubMed] [Google Scholar]
- 384. Robert V., Molez J. F., Trape J. F. 1996. Short report: gametocytes, chloroquine pressure, and the relative parasite survival advantage of resistant strains of falciparum malaria in west Africa. Am. J. Trop. Med. Hyg. 55:350–351 [DOI] [PubMed] [Google Scholar]
- 385. Robert V., et al. 1996. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans. R. Soc. Trop. Med. Hyg. 90:621–624 [DOI] [PubMed] [Google Scholar]
- 386. Robert V., Sokhna C. S., Rogier C., Ariey F., Trape J. F. 2003. Sex ratio of Plasmodium falciparum gametocytes in inhabitants of Dielmo, Senegal. Parasitology 127:1–8 [DOI] [PubMed] [Google Scholar]
- 387. Robert V., et al. 1996. Effect of the sickle cell trait status of gametocyte carriers of Plasmodium falciparum on infectivity to anophelines. Am. J. Trop. Med. Hyg. 54:111–113 [DOI] [PubMed] [Google Scholar]
- 388. Roeffen W., et al. 1995. Plasmodium falciparum: a comparison of the activity of Pfs230-specific antibodies in an assay of transmission-blocking immunity and specific competition ELISAs. Exp. Parasitol. 80:15–26 [DOI] [PubMed] [Google Scholar]
- 389. Roeffen W., et al. 1996. Association between anti-Pfs48/45 reactivity and P. falciparum transmission-blocking activity in sera from Cameroon. Parasite Immunol. 18:103–109 [DOI] [PubMed] [Google Scholar]
- 390. Rogers N. J., Hall B. S., Obiero J., Targett G. A., Sutherland C. J. 2000. A model for sequestration of the transmission stages of Plasmodium falciparum: adhesion of gametocyte-infected erythrocytes to human bone marrow cells. Infect. Immun. 68:3455–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Rupp I. Malaria parasites form filamentous cell-to-cell connections during reproduction in the mosquito midgut. Cell Res., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Saeed M., et al. 2008. Plasmodium falciparum antigens on the surface of the gametocyte-infected erythrocyte. PLoS One 3:e2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Sattabongkot J., et al. 2003. Comparison of artificial membrane feeding with direct skin feeding to estimate the infectiousness of Plasmodium vivax gametocyte carriers to mosquitoes. Am. J. Trop. Med. Hyg. 69:529–535 [PubMed] [Google Scholar]
- 394. Sattabongkot J., Maneechai N., Rosenberg R. 1991. Plasmodium vivax: gametocyte infectivity of naturally infected Thai adults. Parasitology 102:27–31 [DOI] [PubMed] [Google Scholar]
- 395. Sattabongkot J., et al. 2003. Blocking of transmission to mosquitoes by antibody to Plasmodium vivax malaria vaccine candidates Pvs25 and Pvs28 despite antigenic polymorphism in field isolates. Am. J. Trop. Med. Hyg. 69:536–541 [PubMed] [Google Scholar]
- 396. Sattler M. A., et al. 2005. Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar es Salaam (Tanzania) during an extended dry period. Malar. J. 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Sauerwein R. W. 2007. Malaria transmission-blocking vaccines: the bonus of effective malaria control. Microbes Infect. 9:792–795 [DOI] [PubMed] [Google Scholar]
- 398. Saul A. 1993. Minimal efficacy requirements for malarial vaccines to significantly lower transmission in epidemic or seasonal malaria. Acta Trop. 52:283–296 [DOI] [PubMed] [Google Scholar]
- 399. Saul A. 2007. Mosquito stage, transmission blocking vaccines for malaria. Curr. Opin. Infect. Dis. 20:476–481 [DOI] [PubMed] [Google Scholar]
- 400. Schall J. J. 2000. Transmission success of the malaria parasite Plasmodium mexicanum into its vector: role of gametocyte density and sex ratio. Parasitology 121:575–580 [DOI] [PubMed] [Google Scholar]
- 401. Schlesinger P. H., Krogstad D. J., Herwaldt B. L. 1988. Antimalarial agents: mechanisms of action. Antimicrob. Agents Chemother. 32:793–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Schneider P., et al. 2007. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am. J. Trop. Med. Hyg. 76:470–474 [PubMed] [Google Scholar]
- 403. Schneider P., et al. 2006. (Sub)microscopic Plasmodium falciparum gametocytaemia in Kenyan children after treatment with sulphadoxine-pyrimethamine monotherapy or in combination with artesunate. Int. J. Parasitol. 36:403–408 [DOI] [PubMed] [Google Scholar]
- 404. Schneider P., et al. 2004. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol. Biochem. Parasitol. 137:35–41 [DOI] [PubMed] [Google Scholar]
- 405. Schoone G. J., Oskam L., Kroon N. C., Schallig H. D., Omar S. A. 2000. Detection and quantification of Plasmodium falciparum in blood samples using quantitative nucleic acid sequence-based amplification. J. Clin. Microbiol. 38:4072–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Schulemann W. 1932. Synthetic anti-malarial preparations. Proc. R. Soc. Med. 25:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Schwank S. 2010. Ph.D. thesis. University of London, London, United Kingdom [Google Scholar]
- 408. Sharma A., Sharma I., Kogkasuriyachai D., Kumar N. 2003. Structure of a gametocyte protein essential for sexual development in Plasmodium falciparum. Nat. Struct. Biol. 10:197–203 [DOI] [PubMed] [Google Scholar]
- 409. Sharma Y. D. 1991. Knobs, knob proteins and cytoadherence in falciparum malaria. Int. J. Biochem. 23:775–789 [DOI] [PubMed] [Google Scholar]
- 410. Sharp S. 2007. Ph.D. thesis. University of London, London, United Kingdom [Google Scholar]
- 411. Sharp S., et al. 2006. Programmed transcription of the var gene family, but not of stevor, in Plasmodium falciparum gametocytes. Eukaryot. Cell 5:1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Shekalaghe S., et al. 2007. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine- pyrimethamine and artesunate. PLoS One 2:e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Shekalaghe S. A., et al. 2007. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop. Med. Int. Health 12:547–553 [DOI] [PubMed] [Google Scholar]
- 414. Shekalaghe S. A., et al. 2010. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A−) individuals. Antimicrob. Agents Chemother. 54:1762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Shutler D., Reece S. E., Mullie A., Billingsley P. F., Read A. F. 2005. Rodent malaria parasites Plasmodium chabaudi and P. vinckei do not increase their rates of gametocytogenesis in response to mosquito probing. Proc. Biol. Sci. 272:2397–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Silvestrini F., Alano P., Williams J. L. 2000. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology 121:465–471 [DOI] [PubMed] [Google Scholar]
- 417. Silvestrini F., et al. 2005. Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Mol. Biochem. Parasitol. 143:100–110 [DOI] [PubMed] [Google Scholar]
- 418. Simonetti A. B., Billingsley P. F., Winger L. A., Sinden R. E. 1993. Kinetics of expression of two major Plasmodium berghei antigens in the mosquito vector, Anopheles stephensi. J. Eukaryot. Microbiol. 40:569–576 [DOI] [PubMed] [Google Scholar]
- 419. Sinclair D., Zani B., Donegan S., Olliaro P., Garner P. 2009. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst. Rev. 2009:CD007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Sinden R. E. 1983. The cell biology of sexual development in Plasmodium. Parasitology 86:7–28 [DOI] [PubMed] [Google Scholar]
- 421. Sinden R. E. 2004. A proteomic analysis of malaria biology: integration of old literature and new technologies. Int. J. Parasitol. 34:1441–1450 [DOI] [PubMed] [Google Scholar]
- 422. Sinden R. E. 1983. Sexual development of malarial parasites. Adv. Parasitol. 22:153–216 [DOI] [PubMed] [Google Scholar]
- 423. Sinden R. E., Butcher G. A., Billker O., Fleck S. L. 1996. Regulation of infectivity of Plasmodium to the mosquito vector. Adv. Parasitol. 38:53–117 [DOI] [PubMed] [Google Scholar]
- 424. Sinden R. E., Gilles H. M. 2002. The malaria parasites, p. 8–35 In Warrel D. A., Gilles H. M. (ed.), Essential malariology, 4th ed. Hodder Arnold, London, United Kingdom [Google Scholar]
- 425. Sinden R. E., Smalley M. E. 1976. Gametocytes of Plasmodium falciparum: phagocytosis by leucocytes in vivo and in vitro. Trans. R. Soc. Trop. Med. Hyg. 70:344–345 [DOI] [PubMed] [Google Scholar]
- 426. Smalley M. E. 1977. Plasmodium falciparum gametocytes: the effect of chloroquine on their development. Trans. R. Soc. Trop. Med. Hyg. 71:526–529 [DOI] [PubMed] [Google Scholar]
- 427. Smalley M. E., Abdalla S., Brown J. 1981. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans. R. Soc. Trop. Med. Hyg. 75:103–105 [DOI] [PubMed] [Google Scholar]
- 428. Smalley M. E., Brown J. 1981. Plasmodium falciparum gametocytogenesis stimulated by lymphocytes and serum from infected Gambian children. Trans. R. Soc. Trop. Med. Hyg. 75:316–317 [DOI] [PubMed] [Google Scholar]
- 429. Smalley M. E., Sinden R. E. 1977. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology 74:1–8 [DOI] [PubMed] [Google Scholar]
- 430. Smith D. L., Hay S. I. 2009. Endemicity response timelines for Plasmodium falciparum elimination. Malar. J. 8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Smith D. L., McKenzie F. E., Snow R. W., Hay S. I. 2007. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 5:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Smith J. D., et al. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Smith T., Charlwood J. D., Takken W., Tanner M., Spiegelhalter D. J. 1995. Mapping the densities of malaria vectors within a single village. Acta Trop. 59:1–18 [DOI] [PubMed] [Google Scholar]
- 434. Smith T. A., Chitnis N., Briet O. J., Tanner M. Uses of mosquito-stage transmission-blocking vaccines against Plasmodium falciparum. Trends Parasitol., in press [DOI] [PubMed] [Google Scholar]
- 435. Smith T. G., Lourenco P., Carter R., Walliker D., Ranford-Cartwright L. C. 2000. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology 121:127–133 [DOI] [PubMed] [Google Scholar]
- 436. Smith T. G., et al. 2003. CD36-mediated nonopsonic phagocytosis of erythrocytes infected with stage I and IIA gametocytes of Plasmodium falciparum. Infect. Immun. 71:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Smithuis F., et al. 2006. Efficacy and effectiveness of dihydroartemisinin-piperaquine versus artesunate-mefloquine in falciparum malaria: an open-label randomised comparison. Lancet 367:2075–2085 [DOI] [PubMed] [Google Scholar]
- 438. Smithuis F., et al. 2010. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect. Dis. 10:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Snounou G., et al. 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61:315–320 [DOI] [PubMed] [Google Scholar]
- 440. Sokhna C. S., Trape J. F., Robert V. 2001. Gametocytaemia in Senegalese children with uncomplicated falciparum malaria treated with chloroquine, amodiaquine or sulfadoxine + pyrimethamine. Parasite 8:243–250 [DOI] [PubMed] [Google Scholar]
- 441. Song J., et al. 2010. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar. J. 9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Sowunmi A., Balogun S. T., Gbotosho G. O., Happi C. T. 2008. Plasmodium falciparum gametocyte sex ratios in children with acute, symptomatic, uncomplicated infections treated with amodiaquine. Malar. J. 7:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Sowunmi A., Balogun S. T., Gbotosho G. O., Happi C. T. 2008. Some features of primary and recrudescent amodiaquine-resistant Plasmodium falciparum infections in Nigerian children. Mem. Inst. Oswaldo Cruz 103:754–759 [DOI] [PubMed] [Google Scholar]
- 444. Sowunmi A., et al. 2008. Activities of artemether-lumefantrine and amodiaquine-sulfalene-pyrimethamine against sexual-stage parasites in falciparum malaria in children. Chemotherapy 54:201–208 [DOI] [PubMed] [Google Scholar]
- 445. Sowunmi A., et al. 2007. Activities of amodiaquine, artesunate, and artesunate-amodiaquine against asexual- and sexual-stage parasites in falciparum malaria in children. Antimicrob. Agents Chemother. 51:1694–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Sowunmi A., Fateye B. A. 2003. Changes in Plasmodium falciparum gametocytaemia in children with chloroquine-sensitive asexual infections. Parasite 10:363–369 [DOI] [PubMed] [Google Scholar]
- 447. Sowunmi A., Fateye B. A. 2003. Gametocyte sex ratios in children with asymptomatic, recrudescent, pyrimethamine-sulfadoxine-resistant, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 97:671–682 [DOI] [PubMed] [Google Scholar]
- 448. Sowunmi A., Fateye B. A. 2003. Plasmodium falciparum gametocytaemia in Nigerian children: before, during and after treatment with antimalarial drugs. Trop. Med. Int. Health 8:783–792 [DOI] [PubMed] [Google Scholar]
- 449. Sowunmi A., et al. 2005. Effects of antifolates—co-trimoxazole and pyrimethamine-sulfadoxine—on gametocytes in children with acute, symptomatic, uncomplicated, Plasmodium falciparum malaria. Mem. Inst. Oswaldo Cruz 100:451–455 [DOI] [PubMed] [Google Scholar]
- 450. Sowunmi A., Fateye B. A., Adedeji A. A., Fehintola F. A., Happi T. C. 2004. Risk factors for gametocyte carriage in uncomplicated falciparum malaria in children. Parasitology 129:255–262 [DOI] [PubMed] [Google Scholar]
- 451. Sowunmi A., et al. 2009. Therapeutic efficacy and effects of artesunate-mefloquine and mefloquine alone on malaria-associated anemia in children with uncomplicated Plasmodium falciparum malaria in southwest Nigeria. Am. J. Trop. Med. Hyg. 81:979–986 [DOI] [PubMed] [Google Scholar]
- 452. Sowunmi A., et al. 2009. Effects of mefloquine and artesunate mefloquine on the emergence, clearance and sex ratio of Plasmodium falciparum gametocytes in malarious children. Malar. J. 8:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Spielman A., et al. 1988. Malaria diagnosis by direct observation of centrifuged samples of blood. Am. J. Trop. Med. Hyg. 39:337–342 [DOI] [PubMed] [Google Scholar]
- 454. Staedke S. G., et al. 2004. Combination treatments for uncomplicated falciparum malaria in Kampala, Uganda: randomised clinical trial. Lancet 364:1950–1957 [DOI] [PubMed] [Google Scholar]
- 455. Stepniewska K., et al. 2008. Plasmodium falciparum gametocyte dynamics in areas of different malaria endemicity. Malar. J. 7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Strickland G. T., Fox E., Sarwar M., Khaliq A. A., Macdonald M. 1986. Effects of chloroquine, amodiaquine and pyrimethamine-sulfadoxine on Plasmodium falciparum gametocytemia. Am. J. Trop. Med. Hyg. 35:259–262 [DOI] [PubMed] [Google Scholar]
- 457. Struik S. S., Riley E. M. 2004. Does malaria suffer from lack of memory? Immunol. Rev. 201:268–290 [DOI] [PubMed] [Google Scholar]
- 458. Sutherland C. J. 2001. Stevor transcripts from Plasmodium falciparum gametocytes encode truncated polypeptides. Mol. Biochem. Parasitol. 113:331–335 [DOI] [PubMed] [Google Scholar]
- 459. Sutherland C. J. 2009. Surface antigens of Plasmodium falciparum gametocytes—a new class of transmission-blocking vaccine targets? Mol. Biochem. Parasitol. 166:93–98 [DOI] [PubMed] [Google Scholar]
- 460. Sutherland C. J., et al. 2002. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am. J. Trop. Med. Hyg. 67:578–585 [DOI] [PubMed] [Google Scholar]
- 461. Sutherland C. J., et al. 2005. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Suwanabun N., et al. 2001. Development of a method for the in vitro production of Plasmodium vivax ookinetes. J. Parasitol. 87:928–930 [DOI] [PubMed] [Google Scholar]
- 463. Suwanarusk R., et al. 2004. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J. Infect. Dis. 189:190–194 [DOI] [PubMed] [Google Scholar]
- 464. Talman A. M., Domarle O., McKenzie F. E., Ariey F., Robert V. 2004. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar. J. 3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Talman A. M., et al. 2004. Influence of chemotherapy on the Plasmodium gametocyte sex ratio of mice and humans. Am. J. Trop. Med. Hyg. 71:739–744 [PubMed] [Google Scholar]
- 466. Targett G., et al. 2001. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 183:1254–1259 [DOI] [PubMed] [Google Scholar]
- 467. Targett G. A. 1988. Plasmodium falciparum: natural and experimental transmission-blocking immunity. Immunol. Lett. 19:235–240 [DOI] [PubMed] [Google Scholar]
- 468. Taylor D., Cloonan N., Mann V., Cheng Q., Saul A. 2000. Sequence diversity in rodent malaria of the Pfs28 ookinete surface antigen homologs. Mol. Biochem. Parasitol. 110:429–434 [DOI] [PubMed] [Google Scholar]
- 469. Taylor L. H., Read A. F. 1997. Why so few transmission stages? Reproductive restraint by malaria parasites. Parasitol. Today 13:135–140 [DOI] [PubMed] [Google Scholar]
- 470. Taylor P. J., Hurd H. 2001. The influence of host haematocrit on the blood feeding success of Anopheles stephensi: implications for enhanced malaria transmission. Parasitology 122:491–496 [DOI] [PubMed] [Google Scholar]
- 471. Taylor W. R., White N. J. 2004. Antimalarial drug toxicity: a review. Drug Saf. 27:25–61 [DOI] [PubMed] [Google Scholar]
- 472. Tchuinkam T., et al. 1993. Experimental infections of Anopheles gambiae with Plasmodium falciparum of naturally infected gametocyte carriers in Cameroon: factors influencing the infectivity to mosquitoes. Trop. Med. Parasitol. 44:271–276 [PubMed] [Google Scholar]
- 473. Templeton T. J., Kaslow D. C. 1999. Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol. Biochem. Parasitol. 101:223–227 [DOI] [PubMed] [Google Scholar]
- 474. Terlouw D. J., et al. 2003. Treatment history and treatment dose are important determinants of sulfadoxine-pyrimethamine efficacy in children with uncomplicated malaria in Western Kenya. J. Infect. Dis. 187:467–476 [DOI] [PubMed] [Google Scholar]
- 475. Thomson J. G., Robertson A. 1935. The structure and development of Plasmodium falciparum gametocytes in the internal organs and peripheral circulation. Trans. R. Soc. Trop. Med. Hyg. 29:31–40 [Google Scholar]
- 476. Tjitra E., Suprianto S., Anstey N. M. 2002. Higher gametocyte prevalence following failure of treatment of Plasmodium falciparum malaria with sulfadoxine-pyrimethamine and the combination of chloroquine plus sulfadoxine-pyrimethamine: implications for progression of anti-folate resistance. Trans. R. Soc. Trop. Med. Hyg. 96:434–437 [DOI] [PubMed] [Google Scholar]
- 477. Tomas A. M., et al. 2001. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 20:3975–3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Toure Y. T., et al. 1998. Gametocyte infectivity by direct mosquito feeds in an area of seasonal malaria transmission: implications for Bancoumana, Mali as a transmission-blocking vaccine site. Am. J. Trop. Med. Hyg. 59:481–486 [DOI] [PubMed] [Google Scholar]
- 479. Trager W. 2005. What triggers the gametocyte pathway in Plasmodium falciparum? Trends Parasitol. 21:262–264 [DOI] [PubMed] [Google Scholar]
- 480. Trager W., Gill G. S. 1992. Enhanced gametocyte formation in young erythrocytes by Plasmodium falciparum in vitro. J. Protozool. 39:429–432 [DOI] [PubMed] [Google Scholar]
- 481. Trager W., Gill G. S. 1989. Plasmodium falciparum gametocyte formation in vitro: its stimulation by phorbol diesters and by 8-bromo cyclic adenosine monophosphate. J. Protozool. 36:451–454 [DOI] [PubMed] [Google Scholar]
- 482. Trager W., Gill G. S., Lawrence C., Nagel R. L. 1999. Plasmodium falciparum: enhanced gametocyte formation in vitro in reticulocyte-rich blood. Exp. Parasitol. 91:115–118 [DOI] [PubMed] [Google Scholar]
- 483. Trubowitz S., Masek B. 1968. Plasmodium falciparum: phagocytosis by polymorphonuclear leukocytes. Science 162:273–274 [DOI] [PubMed] [Google Scholar]
- 484. Tsuboi T., et al. 1998. Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol. Med. 4:772–782 [PMC free article] [PubMed] [Google Scholar]
- 485. Tsuboi T., Tachibana M., Kaneko O., Torii M. 2003. Transmission-blocking vaccine of vivax malaria. Parasitol. Int. 52:1–11 [DOI] [PubMed] [Google Scholar]
- 486. Udomsangpetch R., Kaneko O., Chotivanich K., Sattabongkot J. 2008. Cultivation of Plasmodium vivax. Trends Parasitol. 24:85–88 [DOI] [PubMed] [Google Scholar]
- 487. Vale N., Moreira R., Gomes P. 2009. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 44:937–953 [DOI] [PubMed] [Google Scholar]
- 488. van den Berghe L., Chardome M., Peel E. 1952. Supériorité des préparations de scarification du derme sur les préparations de sang périphérique pour le diagnostic de malaria. An. Inst. Med. Trop. 9:553–562 [PubMed] [Google Scholar]
- 489. van den Broek I. V., et al. 2005. Efficacy of chloroquine + sulfadoxine-pyrimethamine, mefloquine + artesunate and artemether + lumefantrine combination therapies to treat Plasmodium falciparum malaria in the Chittagong Hill Tracts, Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 99:727–735 [DOI] [PubMed] [Google Scholar]
- 490. van der Kolk M., et al. 2004. Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission reducing activity using empirical data. Parasitology 130:13–22 [DOI] [PubMed] [Google Scholar]
- 491. van der Kolk M., de Vlas S. J., Sauerwein R. W. 2006. Reduction and enhancement of Plasmodium falciparum transmission by endemic human sera. Int. J. Parasitol. 36:1091–1095 [DOI] [PubMed] [Google Scholar]
- 492. van der Kolk M., et al. 2003. Transmission of Plasmodium falciparum in urban Yaoundé, Cameroon, is seasonal and age-dependent. Trans. R. Soc. Trop. Med. Hyg. 97:375–379 [DOI] [PubMed] [Google Scholar]
- 493. van Dijk M. R., et al. 2001. A central role for P48/45 in malaria parasite male gamete fertility. Cell 104:153–164 [DOI] [PubMed] [Google Scholar]
- 494. van Dijk M. R., et al. 2010. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog. 6:e1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495. Van Geertruyden J. P., D'Alessandro U. 2007. Malaria and HIV: a silent alliance. Trends Parasitol. 23:465–467 [DOI] [PubMed] [Google Scholar]
- 496. Van Geertruyden J. P., Menten J., Colebunders R., Korenromp E., D'Alessandro U. 2008. The impact of HIV-1 on the malaria parasite biomass in adults in sub-Saharan Africa contributes to the emergence of antimalarial drug resistance. Malar. J. 7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Van Geertruyden J. P., et al. 2006. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J. Infect. Dis. 194:917–925 [DOI] [PubMed] [Google Scholar]
- 498. van Schaijk B. C., et al. 2006. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 149:216–222 [DOI] [PubMed] [Google Scholar]
- 499. Vermeulen A. N., et al. 1985. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 162:1460–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Vermeulen A. N., et al. 1986. Characterization of Plasmodium falciparum sexual stage antigens and their biosynthesis in synchronised gametocyte cultures. Mol. Biochem. Parasitol. 20:155–163 [DOI] [PubMed] [Google Scholar]
- 501. Vogel G. 2010. The ‘do unto others’ malaria vaccine. Nature 238:847–848 [DOI] [PubMed] [Google Scholar]
- 502. von Seidlein L., Drakeley C., Greenwood B., Walraven G., Targett G. 2001. Risk factors for gametocyte carriage in Gambian children. Am. J. Trop. Med. Hyg. 65:523–527 [DOI] [PubMed] [Google Scholar]
- 503. von Seidlein L., Greenwood B. M. 2003. Mass administrations of antimalarial drugs. Trends Parasitol. 19:452–460 [DOI] [PubMed] [Google Scholar]
- 504. von Seidlein L., et al. 2000. Efficacy of artesunate plus pyrimethamine-sulphadoxine for uncomplicated malaria in Gambian children: a double-blind, randomised, controlled trial. Lancet 355:352–357 [DOI] [PubMed] [Google Scholar]
- 505. Wang C. W., et al. 2010. Identification of a major rif transcript common to gametocytes and sporozoites of Plasmodium falciparum. Malar. J. 9:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Weerasinghe K. L., et al. 2002. A safety and efficacy trial of artesunate, sulphadoxine-pyrimethamine and primaquine in P falciparum malaria. Ceylon Med. J. 47:83–85 [DOI] [PubMed] [Google Scholar]
- 507. Westenberger S. J., et al. 2010. A systems-based analysis of Plasmodium vivax lifecycle transcription from human to mosquito. PLoS Negl. Trop. Dis. 4:e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. White L. J., et al. 2009. The role of simple mathematical models in malaria elimination strategy design. Malar. J. 8:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. White N. J. 2008. Qinghaosu (artemisinin): the price of success. Science 320:330–334 [DOI] [PubMed] [Google Scholar]
- 510. White N. J. 2008. The role of anti-malarial drugs in eliminating malaria. Malar. J. 7(Suppl. 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Whitten M. M., Shiao S. H., Levashina E. A. 2006. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol. 28:121–130 [DOI] [PubMed] [Google Scholar]
- 512. Whitworth J., et al. 2000. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet 356:1051–1056 [DOI] [PubMed] [Google Scholar]
- 513. Williams J. L. 1999. Stimulation of Plasmodium falciparum gametocytogenesis by conditioned medium from parasite cultures. Am. J. Trop. Med. Hyg. 60:7–13 [DOI] [PubMed] [Google Scholar]
- 514. Williamson K. C. 2003. Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol. 25:351–359 [DOI] [PubMed] [Google Scholar]
- 515. Williamson K. C., Criscio M. D., Kaslow D. C. 1993. Cloning and expression of the gene for Plasmodium falciparum transmission-blocking target antigen, Pfs230. Mol. Biochem. Parasitol. 58:355–358 [DOI] [PubMed] [Google Scholar]
- 516. Williamson K. C., Fujioka H., Aikawa M., Kaslow D. C. 1996. Stage-specific processing of Pfs230, a Plasmodium falciparum transmission-blocking vaccine candidate. Mol. Biochem. Parasitol. 78:161–169 [DOI] [PubMed] [Google Scholar]
- 517. Williamson K. C., Keister D. B., Muratova O., Kaslow D. C. 1995. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol. Biochem. Parasitol. 75:33–42 [DOI] [PubMed] [Google Scholar]
- 518. Wong J. L., Leydon A. R., Johnson M. A. 2010. HAP2(GCS1)-dependent gamete fusion requires a positively charged carboxy-terminal domain. PLoS Genet. 6:e1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Woolhouse M. E., et al. 1997. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl. Acad. Sci. U. S. A. 94:338–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. World Health Organization 2008. Global malaria control and elimination: report of a technical review. WHO, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241596756 [Google Scholar]
- 521. World Health Organization 2006. Guidelines for the treatment of malaria. WHO/HTM/MAL/2006.1108. WHO, Geneva, Switzerland [Google Scholar]
- 522. World Health Organization 2000. Malaria transmission blocking vaccines: an ideal public good. WHO document number TDR/RBM/MAL/VAC/2000.1. WHO, Geneva, Switzerland [Google Scholar]
- 523. Wozencraft A. O., Dockrell H. M., Taverne J., Targett G. A., Playfair J. H. 1984. Killing of human malaria parasites by macrophage secretory products. Infect. Immun. 43:664–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Wu Y., et al. 2008. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 3:e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Wu Y., et al. 2006. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. U. S. A. 103:18243–18248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Yeka A., et al. 2005. Artemisinin versus nonartemisinin combination therapy for uncomplicated malaria: randomized clinical trials from four sites in Uganda. PLoS Med. 2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Yeka A., et al. 2008. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda. PLoS One 3:e2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528. Young J. A., et al. 2005. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol. Biochem. Parasitol. 143:67–79 [DOI] [PubMed] [Google Scholar]
- 529. Zimmerman P. A., Thomson J. M., Fujioka H., Collins W. E., Zborowski M. 2006. Diagnosis of malaria by magnetic deposition microscopy. Am. J. Trop. Med. Hyg. 74:568–572 [PMC free article] [PubMed] [Google Scholar]
- 530. Zongo I., et al. 2007. Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin. Infect. Dis. 45:1453–1461 [DOI] [PubMed] [Google Scholar]
- 531. Zoungrana A., et al. 2008. Safety and efficacy of methylene blue combined with artesunate or amodiaquine for uncomplicated falciparum malaria: a randomized controlled trial from Burkina Faso. PLoS One 3:e1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Zwang J., et al. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4:e6358. [DOI] [PMC free article] [PubMed] [Google Scholar]