Abstract
Summary: Paracoccidioidomycosis, one of the most important endemic and systemic mycoses in Latin America, presents several clinical pictures. Epidemiological studies indicate a striking rarity of disease (but not infection) in females, but only during the reproductive years. This suggested a hormonal interaction between female hormones and the etiologic dimorphic fungus Paracoccidioides brasiliensis. Many fungi have been shown to use hormonal (pheromonal) fungal molecules for intercellular communication, and there are increasing numbers of examples of interactions between mammalian hormones and fungi, including the specific binding of mammalian hormones by fungal proteins, and suggestions of mammalian hormonal modulation of fungal behavior. This suggests an evolutionary conservation of hormonal receptor systems. We recount studies showing the specific hormonal binding of mammalian estrogen to proteins in P. brasiliensis and an action of estrogen to specifically block the transition from the saprophytic form to the invasive form of the fungus in vitro. This block has been demonstrated to occur in vivo in animal studies. These unique observations are consistent with an estrogen-fungus receptor-mediated effect on pathogenesis. The fungal genes responsive to estrogen action are under study.
INTRODUCTION
Paracoccidioidomycosis (PCM) is a polymorphic entity that exhibits a variety of clinical manifestations determined in great part by the duration of the process, the sites of disease, the age and sex of the patient, and the integrity of immune functions (43, 46, 74). In humans, the characteristics of the primary infection have not been defined, as the nature of the initial encounter is obscured by a lack of knowledge of the natural habitat of the causative fungus, Paracoccidioides brasiliensis (77). However, results from experimental animal models have given certain clues concerning the initial host-fungus interaction (28, 57). At present, the stages that mark the clinical appearances of this mycosis are understood as follows.
Subclinical Infection
Subclinical infection runs a silent course and is usually demonstrated by delayed skin test reactivity to paracoccidioidin, as demonstrated repeatedly in residents of areas where the disease is endemic (34), occasionally by the presence of anti-gp43 antibodies in healthy blood donors (52), and, exceptionally, also by the demonstration of P. brasiliensis in residual lesions (3, 44). Even if silent, this infection may subsequently give rise to clinically manifested disease. The existence of reports of disease in patients diagnosed outside the recognized zones of endemicity, in good health years after departure from their countries of endemicity, indicates that the dormant fungus is capable of reviving (54, 100).
Progressive Disease
When the host is unable to contain fungal growth, the mycosis disseminates and becomes progressive, giving rise to diverse signs and symptoms. The various clinical manifestations allowed the characterization of two different clinical presentations, both progressive, namely, the acute/subacute or juvenile form and the “progressive” or chronic adult form. The most aggressive manifestations are recorded for the acute/subacute forms, whereas in patients with the chronic form, progression is slow and is accompanied by residual scarring. Patients with either progressive form usually complain of fever and constitutional symptoms such as malaise, asthenia, adynamia, anorexia, and weight loss, all of which are complemented by a wide variety of symptoms related to the afflicted organs (13, 21, 63, 65, 75).
The Acute/Subacute (Juvenile) Form
The acute/subacute (juvenile) form occurs in less than 10% of cases; it is considered a disease of the reticuloendothelial system organs. These become enlarged, producing lymph node hypertrophy, hepatomegaly, and splenomegaly. Usually, there is no clinically apparent lung involvement, but image studies have revealed pulmonary abnormalities in all cases (55, 103). As its names implies, it is a disease of the young and is diagnosed mainly in undernourished children and adults less than 30 years of age. Germane to the topic of this review, no gender differences exist between boys and girls with PCM (68).
Additionally, the juvenile form is also diagnosed in HIV-infected individuals regardless of age (61). This form tends to run a rapid course (months) and is thought to probably represent progression after recent exposure. It is considered to be the most severe of the clinical presentations of PCM, partly because of its extensive dissemination in already compromised hosts (13, 68).
Skin lesions are also observed regularly in the juvenile form (43%) and are preferentially localized in the face, the perioral regions, the neck, and the trunk, although they may also appear in the extremities as well as on the male genitalia. Such lesions are ulcerated, vegetating, or nodular; in extensively disseminated cases, skin lesions are papular or acneiform (13, 46, 68).
Other organs that may also be affected with a lower frequency are stomach, duodenum, and intestines. Bone involvement is an important component of the juvenile form and is observed for 45% of the patients, with lesions predominating in upper limbs and ribs (13, 43, 46, 68).
In patients coinfected with HIV who exhibit the acute/subacute form, there is a rapid progression of the disease accompanied by fever, lymphatic involvement, mucosal lesions, and hepatosplenomegaly (61, 66).
The Chronic Adult Form
The chronic adult form is the most common presentation, observed in approximately 90% of cases. It is observed for patients 35 to 60 years of age, with a predominance of male patients, as will be described below. In most cases, the disease appears to represent endogenous reactivation occurring years after the initial contact with the fungus, as demonstrated by cases diagnosed in Europe, the United States, and Asia in patients who had left the countries of endemicity many years previously (54, 73, 100). The reasons for the host's incapacity to control fungal proliferation in this chronic form are unknown, but smoking and drinking habits, as well as work in agriculture, have been speculated to be predisposing factors (13, 21, 43, 65, 73).
In patients with the chronic progressive adult form, signs and symptoms take months to years to become apparent; usually, there is important lung damage associated with extrapulmonary lesions. Older classifications subdivided this form into unifocal and multifocal, referring to lesions being present in one or in various organs (43, 46). Image and scan studies have revealed that the mycosis is a multiorgan disease regardless of the presence or absence of symptoms related to a particular organ (55, 75, 103).
Despite the predominance of lung lesions, respiratory symptoms may be minimal and, if present, are attributed mainly to smoking, a common habit of rural workers (1, 75). Furthermore, auscultation may reveal few abnormalities even in patients with extensive radiographic alterations (73, 75). This observation would explain why medical consultation is based mainly on the presence of mucosal and skin lesions (43, 93).
The most common respiratory symptoms are productive cough in half of the patients; blood-tinged sputum was recorded for 11% of these cases, whereas chest pain is unusual. Dyspnea is a frequent manifestation and may progress to serious respiratory failure. Auscultation regularly reveals rales, rhonchi, and diminished breath sounds, while radiographs reveal alveolar and interstitial infiltrates, often intermixed. Infiltrates are bilateral and symmetrical, with preferential distribution toward the lower and central lung fields. Disease progression results in fibronodular lesions that tend to persist despite appropriate antifungal treatment. Lung fibrosis is a prominent abnormality (60%) and is regularly accompanied by emphysema and bullae; cavities are also detected (63, 73, 75, 93, 96). High-resolution computed chest tomography reveals lesions in the lung periphery and in the posterior aspects, where lesions with ground-glass appearance are observed; nodular lesions that may be small and centrilobular, or large, and cavitating nodules may also be observed (55, 75, 103).
Oral mucosa lesions are regularly observed and localized preferentially in the gums and the hard palate but also in the oropharynx and the larynx. More infrequently, the nasal and the anal mucosa may become involved. Lesions are single or multiple and progressive and with time become destructive; they appear as tumor-like outgrowths covered by hemorrhagic dots (mulberry-like stomatitis) that may ulcerate and are accompanied by prominent edema. Dysphonia, dysphagia, and sialorrhea, as well as a loosening of teeth, are regularly observed. Lymph nodes located around the afflicted mucosal area become hypertrophied, with spontaneous rupturing resulting in fistula formation (13, 21, 43, 65, 71, 93).
The skin is another common site for the appearance of secondary lesions, as observed for 23% of the patients. Lesions are diverse, single or multiple, and localized regularly on the face but may also be seen in the lower extremities. Other sites include the neck, trunk, upper extremities, and, in males, the external genitalia. Typically, lesions are ulcerative and/or nodular. Edema of the lips is frequent. In rare disseminated cases, multiple papuloacneiform lesions may appear. Pulmonary involvement is seen in about 90% of patients with skin lesions (21, 71–73, 93).
Dissemination to the adrenal glands occurs commonly and is as frequent as 90% in autopsy cases; in vivo, however, adrenal involvement is diagnosed in 48% of the cases if cortisol is measured after adrenocorticotropic hormone (ACTH) stimulation. Adrenal function may become normal upon the completion of antifungal treatment in some patients, although damage to the glands may be permanent in others (72, 97).
Central nervous system (CNS) involvement is observed for approximately 15% of patients. In CNS disease, P. brasiliensis invasion results in granulomatous lesions of the parenchyma, preferentially located in the cerebral hemispheres, although other localities, including the spinal medullae, may also be involved (31).
Residual Form
Manifestations of the residual form are characterized by the abnormal functions of certain organs that had been the site of active fungal invasion and had healed with scarring, such as what occurs in the oropharynx and the larynx. Prominent damage occurs in the lungs due to fibrosis that may result in cor pulmonale; destruction of the adrenals is another important sequela (55, 75, 93, 97).
EPIDEMIOLOGY
Overview of the Limited Geographic Distribution of the Mycosis
One of the most peculiar characteristics of PCM is its restricted geographic distribution, with the disease being confined to Central and South America from Mexico (23° North) to Argentina (34° South); thus, the areas of endemicity are contained within the Tropics of Cancer and Capricorn (14, 16, 38, 39, 43, 46, 56, 63, 72, 74). Furthermore, the distribution of PCM is not homogenous within a particular country of endemicity but instead centers in areas where the prevailing environmental conditions appear adequate for P. brasiliensis survival and growth, thus facilitating human exposure (20, 72, 77). These favorable conditions are found in the tropical and subtropical regions of Latin America, where forests and waterways abound and where ample annual rainfall indices (>1,400 to 2,999 mm) occur throughout the year; as a result, temperatures are mild (17°C to 24°C) all year round. Soil texture also appears to be important (10, 17, 20, 30, 77). In Brazil, ecological correlates obtained through spatial analyses indicate that moisture availability plays an important role in PCM distribution (9). This methodology recently revealed the existence of a cluster of acute/subacute cases potentially linked to a climatic anomaly caused by the 1982-1983 El Niño Southern Oscillation phenomenon (8).
Brazil accounts for over 80% of all reported cases, with Colombia, Venezuela, Ecuador, and Argentina following suit but with much lower prevalence rates; other Latin American countries have reported lower numbers of cases, as have all the Central American countries (14, 16, 43, 63, 72). The annual incidence rate in Brazil is estimated to vary from 10 to 30 cases per million inhabitants; the mean mortality rate is 1.4 deaths per million inhabitants (12, 90). In contrast, Colombia has a much lower incidence that fluctuates from year to year, with the highest being 2.4 cases per million inhabitants (98).
Despite numerous important efforts, the microniche of P. brasiliensis remains uncharacterized; nonetheless, the fungus is considered to be a soil-inhabiting microorganism on the basis of its rare isolation from soil and also in connection with its recovery from internal organs of armadillos native to areas of endemicity (6, 17, 37, 77). Additionally, the high rates of reactive paracoccidioidin skin tests among rural workers engaged in agriculture tend to indicate the soil as a source of infection (79, 101). Nonetheless, the lack of information on outbreaks that could have helped to pinpoint a common source of infection, the fungus' ability to enter into prolonged periods of latency in infected individuals, and, in addition, the existence of mild clinically undetectable subclinical infections have all hindered the discovery of the habitat of P. brasiliensis (38, 43, 73, 74).
Prolonged latency has been clearly demonstrated through reports on approximately 70 nonindigenous PCM cases diagnosed in Europe, the United States, Asia, and other countries outside Latin America. Of importance, all these patients had lived in an area of endemicity for a mean of 14 years prior to overt disease (16, 63, 72).
Age and Occupational Profile of Patients with Paracoccidioidomycosis
Active PCM is diagnosed more frequently in adults than in children (16, 39, 43, 46, 56, 72). Detailed age distribution patterns, not often published at present, indicate that the disease predominates in adult patients 30 to 60 years of age (Table 1) (A. Restrepo, unpublished data) (11, 13, 22, 43, 99). Deaths were also recorded more frequently (65%) for patients 30 to 59 years of age (12), thus agreeing with morbidity data. Practically no changes in age distribution have occurred over the years, as shown by recent articles that indicated that the fourth decade of life was the most common patient age (21, 65, 75). Only a slight change has been noticed in the case of PCM-AIDS patients, who tend to be significantly younger (mean, 34 years of age) than PCM-non-AIDS patients (mean, 45 years of age) according to data in the largest series of cases published in Brazil (61). The prevalence of HIV-P. brasiliensis coinfection in that study was low, 1.4% (53 out of 3,744 patients), for patients diagnosed during the 1986-2004 period (61).
Table 1.
Distribution by age of paracoccidioidomycosis patients according to reports from various countries where the disease is endemic
| Country of report (reference) | Yr of report | No. of patients by age group (yr) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | ≥61 | Total | ||
| Colombia (99) | 1992 | 0 | 0 | 5 | 43 | 70 | 80 | 198 | |
| Colombia (22) | 1994 | 0 | 4 | 10 | 44 | 82 | 103 | 54 | 297 |
| Brazil (13) | 1999 | 16 | 51 | 61 | 120 | 153 | 113 | 70 | 584 |
| Brazil (11) | 2001 | 1 | 5 | 6 | 15 | 22 | 8 | 4 | 61 |
| Brazil (43)a | 2002 | 45 | 249 | 458 | 565 | 690 | 381 | 159 | 2,547 |
| Brazil (43)b | 2002 | 26 | 108 | 214 | 219 | 263 | 178 | 59 | 1,067 |
| Colombia (A. Restrepo et al., unpublished data) | 2010 | 2 | 4 | 21 | 32 | 69 | 50 | 37 | 215 |
| Total | 90 | 421 | 775 | 1,038 | 1,349 | 913 | 383 | 4,969 | |
| % | 1.8 | 8.5 | 15.6 | 20.9 | 27.1 | 18.4 | 7.7 | 100 | |
Data from the Medical Mycology Laboratory, School of Medicine, University of São Paulo, SP, Brazil.
Data from the Hospital das Clinicas, São Paulo, SP, Brazil.
Children account for <10% of the total number of PCM patients reported in large series, with their ages ranging from 3 to 15 years, with mean ages varying from 7 to 8 years. In a compilation of data from 269 children with the mycosis, prevalence rates varied from 0.7% to 6%, differing among the Brazilian States reporting the cases (45). The prevalence cited above seems to have experienced changes in certain localities, where childhood cases have reached figures as high as 13% (35, 83). Increased numbers of childhood cases in areas where this disorder had previously been considered rare were noticed by Rios-Gonçalves et al. (83), who suggested that colonization and the gradual removal of the original native forests probably had exposed children to aerosolized fungal propagules, leading to increased disease rates (68, 83). Of importance, and contrary to the predominance of adult males with PCM, no difference was noticed between boys and girls, as shown in Table 2. Skin test studies by Rodrigues and de Resende (85) revealed that 15% of children under 10 years of age that had been skin tested with the fungal extract paracoccidioidin had a positive reaction, indicating that infections with P. brasiliensis occur early in this community. Nonetheless, no gender differences in reactivity were indicated in those studies.
Table 2.
Occupational distribution in paracoccidioidomycosis patients
| Occupation | No. of patients by report (reference), yr of publication |
Total no. (%) of patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Restrepo et al. (78), 1970 | Conti-Díaz and Calegari (30), 1979 | Marquez et al. (56), 1983 | Castillo et al. (22), 1994 | Blotta et al. (13), 1999 | Bicalho et al. (11), 2001 | Restrepo et al. (unpublished), 2010 | ||
| Agriculture | 21 | 7 | 108 | 199 | 269 | 28 | 144 | 776 (57.5) |
| Masonry | 5 | 2 | 11 | 15 | 115 | 5 | 14 | 167 (12.4) |
| Lumberjack | 5 | 32 | 7 | 44 (3.3) | ||||
| Othera | 8 | 7 | 57 | 11 | 200 | 29 | 50 | 362 (26.8) |
| Total | 39 | 48 | 176 | 225b | 584 | 62 | 215 | 1,349 (100) |
| % | 2.9 | 3.6 | 13.0 | 16.7 | 43.3 | 4.6 | 15.9 | |
Includes office work, mining, home work, drivers, school, and others.
Data available for 225 out of 333 patients.
Most PCM patients have been engaged in agriculture (over 60%) or have had soil-related occupations, mainly in connection with coffee, cotton, tobacco, and sugar cane crops (20, 43, 46, 73). In areas of endemicity these occupations expose workers to soil and dust probably infected with the fungus. In Uruguay, 67% of the patients worked as lumberjacks in indigenous forests (30). Other occupations such as masonry, bricklaying, and mining have also been considered of importance, probably because of close contact with earth materials (13, 63, 73). Few reports have detailed the occupations of the patients described, but an analysis of 5 such reports representing 1,072 patients confirmed that their predominant occupation was related to agriculture (56%), with masonry being the next most common occupation (14%) (Table 2). Similarly, rural workers accounted for 68% of all deaths, with patients who had been engaged in construction work second, at 14% (12). Rural residence is the rule for PCM patients, but as migration to urban or suburban centers is common, jobs change, and if the mycosis becomes diagnosed years later, an unrelated occupation would be registered in the medical history (12, 16, 43, 101). Of note, a change appears to be occurring in relation to agriculture, as recent practices entail the intensive use of insecticides and soil burning, such as that done in sugar cane plantations, now replacing coffee crops in several of the areas where PCM is endemic. As a consequence, the fungus in its alleged soil habitat is destroyed, and human exposure would no longer be as common (64, 70). Although new PCM cases are still being reported in agricultural areas, data on the contemporary incidence and prevalence of this disease are lacking, since the reporting of PCM is not compulsory. In the future, the epidemiologic data related to new PCM cases, especially those for the acute and subacute forms, would help to determine if this mycosis is declining in areas where sugar cane cultivation predominates (63, 64, 70).
Distribution in Adult Patients According to Gender
Gender distribution is of paramount importance in PCM, with adult males exhibiting overt disease much more often than females (43, 46, 76, 99). Seven series with large numbers of patients (over 100 each) were chosen in order to allow a clear interpretation of this difference (Table 3). A total of 5,500 patients were thus gathered and divided into 5,045 males and 455 females, for whom the male-to-female ratio was found to be 11:1. This ratio turned out to be somewhat lower than that previously reported (13:1) (76), but the more recent figure was calculated on the basis of a larger number of patients, and additionally, one of the Brazilian series presented an unusually elevated male-to-female ratio (30:1) (11).
Table 3.
Distribution of paracoccidioidomycosis by gender of patients in a large series
| Authors (reference) | Country of report, yr of publication | Total no. of patients | No. of patients reported |
Ratio of males to females | |
|---|---|---|---|---|---|
| Males | Females | ||||
| Londero and Ramos (46) | Brazil, 1990 | 260 | 245 | 15 | 16.3:1 |
| Villar et al. (99) | Colombia, 1992 | 198 | 190 | 8 | 23.7:1 |
| Castillo et al. (22) | Colombia, 1994 | 333 | 316 | 17 | 18.6:1 |
| Blotta et al. (13) | Brazil, 1999 | 584 | 492 | 92 | 5.3:1 |
| Bicalho et al. (11) | Brazil, 2001 | 62 | 60 | 2 | 30:1 |
| Lacaz et al. (43)b | Brazil, 2002 | 1,073 | 982 | 91 | 10.8:1 |
| Lacaz et al. (43)a | Brazil, 2002 | 2,837 | 2,608 | 229 | 11.3:1 |
| Restrepo et al. (unpublished) | Colombia, 2010 | 215 | 212 | 3 | 70.6:1 |
| Total | 5,562 | 5,105 | 457 | 11.1:1 | |
| % | 100 | 91.8 | 8.2 | ||
Patients with data who were diagnosed until 1964 in the Mycology Laboratory, School of Medicine, University of São Paulo, SP, Brazil.
Patients with data who were seen from 1944 to 1965 in the Hospital das Clinicas, São Paulo, SP, Brazil.
Of note, important variations become apparent both among series and among countries, with the lowest ratio of 5.3:1 reported by Blotta et al. (13) from Brazil and the highest ratio of 70.6:1 reported by Restrepo et al. (72) from Colombia. Blotta et al. commented on, but did not attempt to explain, this low ratio of males to females. Additionally, the reports from Colombia showed differences among the three studies chosen, especially in comparison with the 70.6:1 ratio referred to above. These data indicate that present knowledge is insufficient to explain the male-to-female differences according to country of residence but also intercountry variation.
If series of isolated manifestations of PCM are examined, the male predominance also shows. For example, in a series of 34 cases of gingival involvement of PCM in Brazil, 88% of the cases were men (93a).
For many years, it had been argued that men were afflicted more often than women owing to the intensive involvement of men in agriculture-related occupations (12, 38, 43, 101). Nonetheless, rural women commonly work in field-related activities, as, by necessity, they participate in the plowing and caring for of lands in areas of endemicity and thus have frequent soil exposure (39, 73, 76). As will be discussed below, and according to skin testing studies, there is evidence for an equal rate of infection in both males and females (79).
The difference in the incidences of overt PCM between adult men and women has also been attributed to the fact that the latter, due to their sex hormones, are better protected against the progression of the infection by P. brasiliensis. In a report dealing with 27 women with the mycosis, emphasis was placed on their hormonal status at the time of diagnosis (92). Results indicated that menopausal signs and symptoms were present in 19 patients (70%), with 3 additional patients having had a hysterectomy. Estradiol (E2) levels determined for five of these patients were indicative of menopause (levels below 20 pg/ml).
Distribution in Childhood Disease According to Gender
It is interesting that in 5 Brazilian childhood series that distributed their cases by gender (Table 4), no differences in the boy-to-girl ratio were found (7, 35, 45, 68, 83). The overall ratio for the 158 children with data was 1.16 to 1. Furthermore, variation among the series was rather small, with only two differences, the lower ratio of 0.6:1 in a report where there were more girls than boys (35) and a finding made by Pereira et al. (68), who, in their study of 63 patients, found differences according to age, with the 13 older children (12 to 15 years old) showing a higher boy-to-girl ratio (5.5:1) than the remaining 50 cases (1.3:1), with the difference probably being related to girls in puberty. Unfortunately, further detailed data by gender were not given in this study. Despite the latter exceptions, it is apparent that for children and adolescents, the gender ratio does not follow the trend observed for adults, for whom the male-to-female ratio reveals a strong predominance of males, as indicated above.
Table 4.
Distribution by gender of children with paracoccidioidomycosis in Brazil
| Authors, yr of publication (reference) | No. of children with data | No. of patients reported |
Ratio of boys to girls | |
|---|---|---|---|---|
| Boys | Girls | |||
| Barbosa, 1992 (7) | 30 | 17 | 13 | 1.3:1 |
| Londero et al., 1996 (45) | 36 | 20 | 16 | 1.2:1 |
| Rios-Gonçalves et al., 1998 (83) | 16 | 9 | 7 | 1.2:1 |
| Fonseca et al., 1999 (35) | 13 | 5 | 8 | 0.6:1 |
| Pereira et al., 2004 (68) | 50 up to 11 yr of age | No dataa | No dataa | 1.3:1 |
| 13 between 12 and 15 yr of age | No dataa | No dataa | 5.5:1 | |
| Grand total | 158 | |||
| Total with age data | 95 | 51 | 44 | 1.16:1 |
| % | 100 | 53.7 | 46.3 | |
The largest series, with 63 patients. The numbers of boys and girls were not stated; only the ratios were given.
Reactive Paracoccidioidin Skin Tests in Healthy Individuals of Both Genders
When discussing paracoccidioidin skin test reactivity, it is important to mention that the antigens employed have not been recognized as standard products. Researchers have utilized partially purified polysaccharide antigens obtained by different methods, e.g., directly from yeast cells obtained from different P. brasiliensis isolates or with glycopeptides obtained as dried powders by ethanol precipitation of the culture filtrates. As a consequence, products are not fully comparable.
Skin tests with paracoccidioidin began to be used in diagnosis in 1953 (51). Later, Lacaz et al. (44) undertook pioneering studies in search of subclinical infections through the application of skin tests. For 606 healthy persons attending a diagnostic center, the paracoccidioidin skin tests were positive for 5% of these persons. Additional studies with the latter group allowed the detection of a few individuals with radiographic abnormalities and/or P. brasiliensis reactive serologic tests that attested to the presence of subclinical infections; however, no gender-related data were offered. In a large study conducted in several Colombian municipalities that was comprised of almost 4,000 healthy residents, skin tests with mycelial paracoccidioidin proved reactive in 9.9% and 9.4% of the male and female populations, respectively, with no obvious gender-related differences (79). Cross-reactions with histoplasmin are to be expected due to the similarities of the causative agents, and simultaneous testing with both antigens is required. In this study, such cross-reactions occurred in 3.4% of the population tested (79). Albornoz (2) carried out a large paracoccidioidin skin test study in various Venezuelan provinces where PCM had been reported and found no gender-related differences, with the exception of the Tovar municipality, where men were 8% more reactive than women.
A skin test survey with paracoccidioidin and histoplasmin was conducted with 495 residents of Coari in the Brazilian State of Amazonas (59). Positive dermal reactions to paracoccidioidin and histoplasmin were seen in 14% and 50% of the study subjects, respectively. Specific reactions to paracoccidioidin were observed for 2% of the individuals, and specific reactions to histoplasmin were observed for 38% of the individuals. There were no significant differences in dermal reactivity to either of the two antigens in either gender or among different occupational groups. Skin test positivity in the general population of Coari reaffirmed the concept of benign, self-limited infection in paracoccidioidomycosis. It also identified the equatorial forests of the Upper Amazon Basin as zones of endemicity of the two mycoses (59).
In the Brazilian State of Goiânia, Pereira (67) applied paracoccidioidin skin tests to 966 healthy individuals, including 143 children less than 10 years of age. Positive reactions were recorded for 145 males and 43 females, but the author noted that the male and female groups were comparable in the frequency of positivity only for persons less than 10 years of age, 83 boys and 59 girls, with their skin reactivities being 6% and 5%, respectively. Persons older than 60 years of age, comprised of 38 males and 54 females, showed equal proportions of reactive skin tests (28%). The remaining groups of individuals tested were composed almost exclusively of males.
A cross-sectional epidemiological survey for paracoccidioidomycosis and histoplasmosis, including skin tests with paracoccidioidin and histoplasmin, was conducted among three Tupi-Monde Amerindian populations from Brazilian Amazonia (29). The study followed the diagnosis of an increasing number of cases of paracoccidioidomycosis among the Surui tribe members in recent years. Rates of positivity to paracoccidioidin and histoplasmin were 44% and 79% for the Surui, 6% and 6% for the Gaviao, and 15% and 81% for the Zoro, respectively. There was no significant difference in the results for males and females, but marked differences were noted across age groups. The greater exposure of the Surui to P. brasiliensis was probably associated with their adoption of new subsistence practices. The epidemiology of this mycosis among the Tupi-Monde populations appears to be related to the environmental and socioeconomic changes taking place in Amazonia.
Mangiaterra et al. (53) conducted a skin test study in the Province of Corrientes, located at the northeastern border of Argentina in a subtropical area where important environmental modifications have been introduced as a consequence of the damming of the Parana River to build a huge hydroelectric plant. Skin tests with paracoccidioidin and histoplasmin were done for 455 persons of both sexes who were permanent residents in the area. Paracoccidioidin elicited 52 positive reactions (11%), found in 39 males and 13 females, i.e., three times higher in males than in females.
Other skin test studies reviewed did not supply sufficient information regarding sex differences in reactivity (23, 34, 36, 41, 85).
In summary, no gender differences in skin tests were noticeable, with the exception of a report by Mangiaterra et al. (53). Thus, in general, the acquisition of P. brasiliensis infection judged by skin testing procedures occurs equally in men and women and in boys and girls. This is different from the sex difference in disease, as detailed above.
OVERVIEW OF INFLUENCE OF HORMONES ON FUNGI
Hormones serve as regulatory messenger molecules, inducing the regulation of gene expression through receptor-mediated interactions and the subsequent functional response to the presence of the hormone. The findings of primitive hormonal (pheromonal) interactions in fungi and interactions of mammalian hormones with fungi with functional responses by the fungi suggest that mammalian hormone-responsive systems result from conservation throughout evolution and that hormonal interactions with fungal systems can have an important role in fungal pathogenesis as well as in the overall biology of the organisms. The subject of the interface of mycology and endocrinology has recently been reviewed in detail by us elsewhere (27) and will here be only summarized. That review, which has 106 references relevant to the subject of this interface, should be consulted for further detailed information (27).
Our understanding of the action of hormones is derived from mammalian systems, where hormones regulate a variety of functional processes in many tissues. Target cells are affected in a tissue-specific manner after the binding of the hormone to a specific receptor; the extent of the specific response is determined by the proteins, pathways, and processes with which the receptors interact as well as the concentration of hormone. Classically, hormone receptors act as transcriptional regulators in the nucleus after the hormone-receptor complex moves across the nuclear membrane. There, the complex binds to specific response elements in the DNA. However, not all receptors are located intracellularly; some are plasma membrane associated. In addition, hormones can have nongenomic actions.
Fungi also utilize endogenous hormones (sometimes called pheromones, which may be a better description for unicellular systems) as messenger molecules that regulate various activities of the organism. Primarily, these molecules are related to the control of sexual reproduction in various fungi. Fungi may also produce secondary metabolites that may have hormonal effects on mammalian cells. Fungi can also metabolize, transform, and convert mammalian steroids (including the production of steroid molecules that mammalian cells cannot produce), which has proven useful in the biotechnology production field.
Host-microbe interactions are also important in the plant kingdom, and these interactions affecting fungal infection can be mediated by host or microbial hormones.
Pathogenic Fungi and Hormones
Several pathogenic fungi are known to interact with and respond to various mammalian hormones. These interactions can influence the growth of the fungus and even the pathogenesis of an infection caused by the fungus.
Candida albicans has a protein capable of specific corticosteroid binding that exhibits a high affinity for corticosterone and progesterone. To determine the relationship of the corticosteroid-binding protein from C. albicans to the mammalian hormone receptors, the gene for the corticosteroid-binding protein was cloned and expressed. C. albicans has also been shown to have an estrogen-binding protein that has a high affinity for estradiol and estrone. In C. albicans, E2 stimulates the dimorphic transition from the yeast form to the hyphal form; this should be contrasted with our findings on P. brasiliensis, as discussed below. For C. albicans, a genome-wide inventory of up- or downregulated genes in the presence of estradiol and progesterone has been reported. C. albicans also interacts with the human peptide hormones, luteinizing hormone and human chorionic gonadotropin. In addition, a human chorionic gonadotropin-like protein in extracts of C. albicans is a potent stimulator of germ tube formation in the presence of serum. The neuroendocrine hormone serotonin has also been shown to modulate several virulence properties of C. albicans in studies in vitro.
Epidemiologic data suggest that men exhibit dermatophytic infections more frequently than women. The specific binding of progesterone was demonstrated in the cytosol of Trichophyton mentagrophytes. Furthermore, progesterone inhibited the growth of the organism. As with T. mentagrophytes, Microsporum canis and Trichophyton rubrum have a progesterone-binding protein. Trichophyton species metabolize progesterone to more polar and less growth-inhibitory compounds, leading to escape from growth inhibition.
The frequency of coccidioidal disease has been linked to gender, with men exhibiting clinical disease more frequently. However, pregnancy, especially in the third trimester, has been considered a risk factor for the development of serious life-threatening disease. Coccidioides species have proteins that bind estrogen, testosterone, and progesterone. Estrogen, at the physiological concentrations achieved during pregnancy, stimulates endosporulation and the release of endospores by the organism, which could explain its aggressive nature during pregnancy.
Saccharomyces cerevisiae has also been demonstrated to have an estrogen-binding protein. Whereas this is not homologous with the mammalian estrogen receptor, it appears that the protein essential to estrogen receptor function is conserved among eukaryotes to such an extent that the introduction of the human estrogen receptor into S. cerevisiae is sufficient for the faithful reconstitution of estrogen signaling within this fungus. This transgenic expression in fungi has proven to be very valuable in our understanding of hormone signaling and receptor function in mammalian systems. In addition to the human estrogen receptor, a number of accessory proteins are apparently required to efficiently transduce the steroid hormone signal in this fungus. A genome-wide expression profile of the human steroid progesterone response in S. cerevisiae has also been described.
In addition to the enhancement of growth by glucocorticoids, several species of Aspergillus respond to serotonin. Serotonin has antifungal properties for conidia and hyphae.
Dopamine is a neuroendocrine hormone produced by the substantia nigra of the brain. Cryptococcus is able to utilize dopamine in the production of melanin, which is considered a virulence factor for this organism and may also be related to the tropism of these fungi for the central nervous system.
Antifungal Drugs
The antifungal drug ketoconazole is a competitive stereospecific binder for the corticosteroid-binding protein in C. albicans and can antagonistically compete with mammalian glucocorticoid receptors. Inhibitory or stimulatory interactions of steroids on azoles have been reported. It has been speculated that the presence of an estrogen-like 1,2-diarylethane moiety in azoles may relate to their antifungal activity, and the synthesis of azole derivatives emphasizing this moiety produced compounds with antifungal activity.
The observation of gynecomastia in some of our patients led to our discovery that azole drugs block steroidogenesis in mammalian cells and in humans due to interference with specific P450 steroidogenic enzymes. This has led to the utility of these agents in the therapy of hypercortisolemic states and in androgen blockade in prostatic cancer.
LABORATORY STUDIES OF GENDER-SPECIFIC FUNGAL INFECTION IN PARACOCCIDIOIDOMYCOSIS
As discussed in the previous section, a variety of fungi utilize pheromones as message molecules that induce cellular responses in much the same manner as mammalian hormones act on their targets. On the basis of the epidemiology of PCM and the demonstrated resistance of adult women to the development of clinical disease, we began to investigate the interactions of P. brasiliensis with mammalian steroid hormones. Two lines of in vitro studies were initiated. The first was that of the interaction of the microbe with mammalian steroid hormones and whether any effects on growth or morphological transition could be demonstrated. The second line of study was that of determining whether P. brasiliensis produced proteins with specific binding activity similar to those demonstrated for other fungi.
In Vitro Effect of Hormones on P. brasiliensis
Because PCM is much more common in men than in women, it was suggestive that the hormonal milieu of the host might have an influence on P. brasiliensis and somehow affect its pathogenicity. To test whether there were any hormonal effects on the growth of the organism, studies were designed to examine effects on the morphological transitions of mycelia to yeast, yeast to mycelia, and conidium to yeast as well as effects on the growth and budding of the yeast form. Clinical isolates of P. brasiliensis (Mon, Gir, and Ru) were tested by using a microculture system, which allows the microscopic examination of the organisms and quantification of temporal morphological changes occurring in a culture. The fungi were grown under various conditions to allow the morphological transition or yeast growth in the presence of various steroids, including 17β-estradiol (E2), testosterone, tamoxifen (estrogen antagonist), 17α-estradiol (stereoisomer of E2), and the nonsteroidal estrogen or synthetic estrogen diethylstilbestrol (DES), at concentrations ranging from 2 × 10−10 M to 2 × 10−6 M; these concentrations span relevant physiological and pharmacological concentrations (81). Ethanol was used to solubilize and dilute the hormones, and control cultures also had the tiny amount of ethanol that would be present in the hormone-treated cultures owing to the diluent.
Mycelium-to-yeast conversion: estrogen-induced inhibition.
The morphological transition from the saprobic mycelial form into the parasitic yeast form is an important step in the pathogenesis of P. brasiliensis infection. To examine this step, mycelial fragments were exposed to hormone, and the culture was incubated to 37°C to induce transition into the yeast form. The hormones E2, testosterone, tamoxifen, 17α-estradiol, and DES were examined in detail. In the absence of added hormones, the mycelium-to-yeast transition occurred in 35 to 80% of cells in these experiments. E2 consistently inhibited the transition from the mycelium to the yeast form. The range of the data from three isolates (Mon, Gir, and Ru) showed that E2 inhibited transition to 64 to 77%, 24 to 46%, and 12 to 26% of the control value for concentrations of 2 × 10−10, 2 × 10−8, and 2 × 10−6 M, respectively. The range of data from the three isolates showed that the inhibitory effect of DES at these concentrations was less dramatic, with inhibition at 85, 54, and 37% of the control transition rates, respectively. Interestingly, the responses to the E2 inhibition of transition differed among the isolates tested, and P. brasiliensis isolate “Gir” was the least susceptible at the lower concentrations of the hormones E2 and DES. None of the other three hormones showed a significant inhibitory effect on the transition from mycelium to yeast: the inhibition for the three isolates ranged from 84 to 120% of the controls for all three hormone concentrations tested. Most importantly, there was a clear dose-response correlation with each of the three isolates with escalating E2 concentrations, and the response was a stereospecific response in that the stereoisomer 17α-E2 was not inhibitory. The dose-response was found with only one other agent, DES, the nonsteroidal estrogen (81).
Yeast-to-mycelium conversion.
Because P. brasiliensis is thermally dimorphic in both directions (mycelium to yeast, or yeast to mycelium), we examined the possibility that the inhibition of form transition by a steroid could occur in either direction. The culturing of yeast cells in the presence of a steroid and incubation at an ambient temperature to induce yeast to the mycelial transition showed that there were only small effects of the steroids tested, regardless of the steroid concentration, on any of the three isolates; the results with the three isolates were indistinguishable. At the highest steroid concentration, 2 × 10−6 M, the inhibition of transition (mean of the data from three isolates) compared with the control values was 66% for DES, 79% for testosterone, 82% for tamoxifen, 86% for E2, and 95% for 17α-estradiol. At 2 × 10−8 M, no steroid caused an inhibition of transition to <75% of the control values, and at 2 × 10−10 M, no steroid caused an inhibition of <89% of the controls (81).
Overall, the studies on the inhibition of morphological transitions showed that the presence of steroids did in fact influence the fungus. The specificity of the E2-induced inhibition of the mycelium-to-yeast transition, but not on the yeast-to-mycelium transition, was demonstrated by these studies and was suggestive that E2 at concentrations relevant to those found in adult females might exert an influence on the establishment of disease in women by blocking a crucial step in the establishment of infection.
Lack of an effect of estrogen on growth of the yeast form.
Although the hypothesis that the E2 inhibition of the mycelium-to-yeast transition explained the reduced frequency of clinical disease in adult women, it was possible that the E2 inhibition or alteration of the growth of the tissue yeast form of the organism also contributed to the reduction of clinical disease. Thus, studies were designed and performed to determine whether E2 at concentrations from 10−6 to 10−10 M in medium had an effect on the yeast form of P. brasiliensis. Spectrophotometric readings to determine growth were taken 1, 2, 3, 6, and 8 days after the initiation of the yeast cultures. Cultures grown in medium free of E2 or with E2 did not show significant growth (<99% transmission) above the threshold of detection (ca. 4 × 105 yeast cells per ml) until day 6. At that time, and again on day 8, there was no difference in growth between hormone-free medium and medium containing E2 (81). Thus, E2 was not found to be inhibitory to the growth of the yeast form.
Effect of estrogen on budding of the yeast form.
Although there was no evidence of an E2 effect on yeast growth, as assayed spectrophotometrically in vitro (a measure of mass), another way that a hormone could affect pathogenicity in vivo would be to affect the process of replication, which, for P. brasiliensis, is by multiple budding. If greater numbers of viable (even if smaller) progeny were produced from the mother cells in the presence of E2, the infection would be expected to be more difficult for host defenses to contain, or if fewer progeny were produced, one might expect that the host defense would be better able to control the infection. To assess this potential, the yeast form of P. brasiliensis was grown for 5 to 6 days at 37°C in medium with or without the presence of E2 concentrations from 10−6 to 10−9 M or in controls. Overall, there was no evidence of a significant effect on budding by E2 at any concentration (81).
Conidium-to-yeast conversion: estrogen-induced inhibition.
As noted above, the infectious particles of P. brasiliensis are mycelial in origin and likely most frequently are the conidia. Interestingly, when placed at 37°C, conidia can transform directly into yeast, without an intermediate step of first forming hyphae. Conversely, conidia are able to form hyphae directly when allowed to grow at ambient room temperature (82). Methods were developed to induce the formation of conidia where the mycelial fragments of an isolate were placed onto agar and incubated at 18°C for up to 6 weeks.
Four isolates of P. brasiliensis (Gra [ATCC 60855], Ara [ATCC 60835], and the clinical isolates Qtr [ATCC 60867] and Cob [ATCC 11762]) were used to obtain conidia. Similarly to the studies of the effect of a steroid on the mycelium-to-yeast transition, assays were performed with the microculture system to address the issue of whether E2 affected the conversion of conidium to yeast. The mean percent transition in control cultures was 78% (range, 65 to 84%) of the conidia converting into yeast. An inhibition of the conidium-to-yeast transition was found only in the presence of E2 and DES. 17α-Estradiol, testosterone, tamoxifen, and hydroxy-tamoxifen were inactive. The combined results from multiple experiments indicated that inhibition was significant only with E2 and DES and only at the higher concentrations (10−6 and 10−8 M) tested. The mean results from 3 to 10 experiments at 10−6 or 10−8 M showed that the transitions of E2-treated cultures were 11% ± 2% (mean ± standard error) of controls (n = 10; P < 0.0005) and 34% ± 6% of controls (n = 10; P < 0.0005), respectively. DES-treated cultures were inhibited to 16% ± 10% (n = 8; P < 0.0005) and 55% ± 9% (n = 9; P < 0.0025) of controls at concentrations of the steroid at 10−6 and 10−8 M, respectively. At lower concentrations (10−10 or 10−12 M), E2 and DES were inactive, as were the other steroids, regardless of the concentration tested (87).
Relationship of in vitro effects to physiological effects.
Concentrations as high as 1 × 10−9 to 2 × 10−9 M E2 are reached during the menstrual cycle (102). An exact concordance between physiological concentrations of hormone and hormonal effects in in vitro systems cannot, however, be expected because of unknown factors in the in vitro system which may affect hormone activity (e.g., interaction with medium components or agar or an effect of the microaerophilic environment, etc.). Other possible reasons for the difference could be that the affinity of the hormone to the binder in intact cells may be different from that in disrupted cells, other circulating estrogens and progesterone may occupy the estradiol site in vivo with an additive effect, the intact fungus may concentrate the hormone, or a function may be regulated with only a few binding sites occupied.
In a different study, Muchmore et al. (62) showed that both forms of P. brasiliensis were inhibited in their in vitro growth by the female sex hormone E2 (1.5 × 10−5 to 3 × 10−5 M), as well as by progesterone, and that stilbestrol was even more potent than E2 or progesterone. However, the concentrations of steroids used by Muchmore et al. (62) were far above the physiological range. These results are similar to results for the inhibition of growth of other fungi in response to high concentrations of steroid hormones (40, 91).
Estradiol-Binding Protein in Cultures of the Yeast and Mycelial Forms
In mammalian cells, the steroid action is via a ligand binding to a high-affinity receptor and the subsequent regulation of gene expression, as discussed above. A high-affinity binding protein for corticosterone and progesterone is present in C. albicans (47–49). However, an unequivocal phenotypic response related to the binding of the ligand has not been demonstrated. Such a response would be indicative that the binding of the steroid to the protein may be acting as a true receptor. Because of the biological response to the presence of E2 shown by P. brasiliensis, studies were performed to assess whether a specific binding protein was present, which might explain the hormonal effects of E2 on P. brasiliensis. Thus, if the organism possessed either an androgen- or an estrogen-binding protein, the binding of the appropriate host sex steroid might result in hormone responsiveness in vitro and in altered pathogenicity in vivo.
Binding studies of hormones in cytosol of the yeast form of P. brasiliensis.
A clinical isolate of P. brasiliensis from a male Colombian patient was used initially for the steroid-binding studies. Yeast cells were grown and disrupted with glass beads to produce a cytosolic extract. In order to test the hypothesis that a steroid binder was present in the organism, a single-point steroid-binding study was done using E2, progesterone, DES, dihydrotestosterone, testosterone, and corticosterone for binding to the P. brasiliensis cytosol at a steroid concentration of 130 nM. Specific binding activity was examined by addition of a 3H-labeled steroid with or without a 500-fold molar excess of the unlabeled (radioinert) steroid to the cytosol in a conventional steroid-binding assay. The bound hormone was separated from the free hormone by using a Sephadex G-50 microcolumn and centrifugation. [3H]estradiol was the most active ligand, with specific binding in the range of 200 fmol/mg of the cytosol proteins. In comparison, specific binding with [3H]progesterone showed activity at 60 fmol/mg of cytosol protein, and [3H]DES showed activity at 42 fmol/mg of cytosol protein (50). Only trace binding was detected with [3H]dihydrotestosterone or [3H]testosterone, and no binding was seen with [3H]corticosterone. The preincubation of cytosol with radioinert E2 completely eliminated [3H]progesterone binding, which was suggestive of the presence of a single estrogen-binding site that has some cross-reactivity for other steroids. However, the presence of a separate progesterone- or DES-binding site was not excluded and would require additional study.
Scatchard analysis of [3H]estradiol binding to yeast cytosol.
The equilibrium binding characteristics of the P. brasiliensis E2-binding molecule were examined by the incubation of the cytosol with multiple concentrations of [3H]estradiol at 0°C for 3 h with or without a 500-fold molar excess of radioinert E2. Bound and free E2 were separated with G-50 Sephadex columns. E2 binding was found to be saturable, and data plotted by the Scatchard method fit well to a straight line. The mean values of six such experiments were 1.7 ± 0.3 × 10−8 M for the apparent dissociation constant (Kd) and 235 ± 73 fmol/mg of protein for the binding capacity (Nmax) (50). Thus, a saturable high-affinity binding of E2 was present in the cytosol of P. brasiliensis.
Specificity of the estrogen-binding site in cytosol of yeast.
To further characterize binding, competition assays were performed to assess the specificity of the E2-binding site by the determination of the ability of a variety of radioinert ligands to compete for [3H]estradiol binding. E2 proved to be the most potent competitor in achieving a 50% inhibition of binding with a molar ratio of E2/[3H]estradiol of 2.5. Two other mammalian estrogens, estrone and estriol, were only about 25% as effective as competitors as E2. DES was a very weak competitor, which is in contrast to the mammalian estrogen receptor. Interestingly, with mammalian estrogen receptors, this synthetic compound has about the same affinity as that of E2. Progesterone was the most potent of the other nonestrogenic steroids and about equal to estrone and estriol in its affinity for the fungal steroid binder, requiring about 10-fold molar excesses to reduce specific E2 binding by 50%. Dihydrotestosterone and testosterone (androgens) were essentially inactive, being only very weak competitors (50).
Stability of the fungal binding site for enzymatic destruction.
Although E2-spcific binding occurred in the cytosolic extracts of the yeast form of P. brasiliensis, the molecule binding the steroid was undefined. Thus, studies were done to determine the chemical nature of the binding molecule. Fungal cytosol was pretreated with DNase, RNase, phospholipase A2, trypsin (100 μg/ml), or N-ethylmaleimide (20 nM) (disrupts sulfhydryl bonds) for 30 min at 37°C prior to performing E2-binding assays. The results showed that DNase, RNase, and phospholipase A2 had little effect on specific [3H]estradiol binding, indicating that DNA, RNA, or lipids were likely not responsible for the specific binding. In contrast, trypsin abolished specific E2 binding in the cytosol, and N-ethylmaleimide pretreatment caused a 50% reduction in [3H]estradiol binding. These results indicated that the fungal binder is likely, at least in part, a protein and that sulfhydryl groups in the protein are necessary for the binding of E2 (50).
Thermal stability of the fungal binding site in yeast cytosol.
The physical characteristics of the fungal E2-binding protein (EBP) were also assessed. The thermal stability of the binder in the P. brasiliensis cytosol was examined by incubation at 0°C, 37°C, and 56°C for 30 min prior to performing a binding assay and then chilling to 0°C. The [3H]estradiol-binding assay was performed by incubation at 0°C for 3 h. Binding was stable at all three temperatures, with an increased capacity of binding occurring in the cytosol pretreated by incubation at 37°C and 56°C, which was suggestive of a second, lower-affinity binding site.
An additional study of the hydrodynamic properties of the binding protein was also done. That study showed that the binding protein had a sedimentation coefficient of 4.4 s and a mass of about 60,000 Da (50).
Overall, E2 binding in P. brasiliensis was found to be specific and saturable by Scatchard analysis, which was suggestive of the presence of a single class of noninteracting binding sites, which were a 60,000-Da protein with sulfhydryl groups involved in ligand binding (50).
Additional studies were done to further characterize the specific E2 binding as well as to assess the possibility that both a high-affinity binding site and a low-affinity binding site existed in the yeast cytosol. A carefully controlled analysis by Stover et al. (95) was done on the cytosol from yeast cultures. The incubation of the cytosol with multiple concentrations of [3H]estradiol at 0°C for 3 h and analysis of specific binding showed the presence of a single class of noninteracting binding sites. However, the affinity of the single high-affinity site for E2 was approximately twice as high as that found in the study by Loose et al., with a Kd of 8.5 nM compared to about 17 nM and an Nmax of 590 fmol [3H]estradiol per mg of cytosol protein compared to about 200 fmol/mg cytosol protein (95). These differences were the result of improved techniques, including extreme care taken to ensure ice-cold conditions throughout the binding assay, to minimize interference from a low-affinity binding site (to be discussed below), and the consistent use of cytosol preparations with a high protein concentration (initial concentration, approximately 3 to 7 mg of protein per ml) to increase the total binding measured.
In an additional study, data for specific [3H]estradiol binding by yeast cytosol heated at 37°C revealed that the high-affinity binding site was stable at this temperature. However, a second binding site with a lower affinity and a higher capacity was detected, in contrast to the single binding site found in the studies using a temperature of 0°C (50, 95). Furthermore, when the yeast cytosol was preheated at 56°C, only the lower-affinity [3H]estradiol-binding site could be demonstrated. Thus, the thermal lability of the high-affinity estrogen-binding protein was shown (50, 95). The low-affinity binding site had an estimated Kd of 150 nm, and the Nmax, depending on the conditions employed, was estimated to be 1,700 to 2,600 fmol of [3H]estradiol per mg of cytosol protein (95).
Estradiol-binding sites from mycelial cytosol.
All of the binding studies were initially done with cytosols prepared from yeasts, whereas the studies done on functional responses to E2 had been done using the mycelial form. Thus, it was important to determine whether specific E2 binding also occurred with the mycelial form of the organism. Four clinical isolates of P. brasiliensis were chosen for study, and the cytosol was prepared from mycelial cultures. In single-point studies the cytosols were incubated with 26 nM [3H]estradiol with or without a 500-fold excess of radioinert E2 for 3 h at 0°C, and bound hormone was separated from free hormone. These results indicated that all the four isolates, Ber, Gir, Ru, and Zan, had specific E2 binding, with binding capacities of 38, 308, 35, and 42 fmol of [3H]estradiol per mg of cytosol protein, respectively (95).
Scatchard analysis of [3H]estradiol binding to mycelial cytosol.
The demonstration of specific E2 binding in the mycelial cytosol was further characterized. The equilibrium binding characteristics were examined by the incubation of the cytosol (from isolate “Ber”) with multiple concentrations of 6.5 to 65 nM [3H]estradiol at 0°C for 3 h. The specific binding was saturable, and data plotted by Scatchard analysis fit well to a straight line. The mean values from three experiments showed a Kd of 13 nM (range, 6 to 26 nM) and an Nmax of 78 fmol of [3H]estradiol per mg of cytosol protein (range, 45 to 136 fmol of [3H]estradiol per mg of cytosol protein), both of which were slightly lower than those found for the yeast cytosol (95).
Displacement analysis of [3H]estradiol binding to mycelial cytosol.
Competition binding studies were performed to assess the specificity of the mycelial binding site for [3H]estradiol. The competitors used were based on previous results from both binding specificity studies as well as the results of the in vitro morphological form transition experiments. E2, DES, and tamoxifen were tested for their capacities to displace [3H]estradiol from the estrogen-binding protein in the cytosol from mycelia. As expected, E2 was the most potent competitor, causing a 50% inhibition of specific [3H]estradiol binding at an E2/[3H]estradiol molar ratio of 2.5. Tamoxifen, which is a nonsteroidal estrogen antagonist in mammalian systems, did not displace [3H]estradiol from the binding site even at a 1,000-fold molar excess. Similarly to the studies done using the yeast cytosol, DES was only a moderately potent competitor, showing approximately 1 to 2% of the affinity of E2 for the estrogen-binding site (95).
Expression Profile of P. brasiliensis Treated with Estrogen
The binding studies, in conjunction with the in vitro demonstration that E2 inhibited the morphological transition of the organism from the mycelial (or conidial) form to the yeast form, resulted in the hypothesis that a reason for the lower incidence of clinical disease in adult women was that the presence of E2 altered a critical step in the establishment of disease (i.e., form transition) and that this regulation of morphological transition was the result of the estradiol-binding protein (EBP) in the organism acting as a receptor after the binding of E2. With this hypothesis in mind, studies were initiated to determine whether the presence of E2 during the morphological form transition resulted in an altered profile of protein expression, which might further support that the EBP was acting as a true receptor.
Temporal protein expression profile.
The temporal sequence of cytosolic protein expression during the morphological transition from the mycelial to the yeast form of P. brasiliensis (treated with E2 at a final concentration of 2.6 × 10−7, or the ethanol-treated control) was studied. Organisms were harvested at 0 h, 24 h, 72 h, and 120 h; the cytosol was prepared; and the proteins were then separated by one-dimensional SDS-PAGE. Protein patterns from cultures of the mycelial and yeast forms maintained at 25°C and 37°C, respectively, were used as controls for comparative analysis to generate an overall profile of how protein expression changed as the organism underwent the transition.
A comparison of only the mycelial and yeast protein profiles showed that 30 bands were distinctly specific to either the mycelial or yeast form: 12 bands for the mycelial form (ranging from approximately 30 kDa to 140 kDa) were not found for the yeast form, and 18 bands (ranging from approximately 22 kDa to 127 kDa) for the yeast form were not found for the mycelial form (26).
There were no notable differences in protein profiles between E2-treated and ethanol-treated cultures maintained for 24 h at 25°C. However, after shifting the cultures from 25°C to 37°C, numerous alterations in the profiles occurred during the 120-h assay period of the mycelium-to-yeast transition. Ethanol-treated controls underwent a morphological change from mycelia to yeast with a concurrent gradually increased expression of yeast bands and the concurrent disappearance of mycelial bands; thus, the protein profile became most yeastlike by 120 h of transition. For the E2-treated cultures, the morphological transition was severely inhibited, and a mycelial form was maintained through 72 h. Few bands of the yeast form were apparent after 120 h of transition. Overall, for the E2-treated cultures, bands of the mycelial form of 123, 99, 83, and 46 kDa were maintained, whereas bands of the yeast form of 102, 98, 93, 92, 78, 34, 31, 30, and 22 kDa were blocked or delayed in appearance compared to the ethanol-treated or untreated controls. Interestingly, 5 novel transition bands (50, 41, 37, 32, and 23 kDa) were observed for ethanol-treated controls by 24 h and/or 72 h but disappeared by 120 h of transition. In contrast, in E2-treated cultures these bands were absent or delayed in appearance even after 120 h of transition. In addition, fewer total bands were present in the cytosols from E2-treated cultures after 120 h of transition than from the control (26).
Methionine utilization during the transition from the mycelial form to the yeast form.
The studies cited above relied on silver staining for protein detection in the gels. An assessment of de novo-synthesized cytoplasmic proteins was done by pulsing cultures with [35S]methionine for 2 h prior to disruption and cytosol preparation. The control yeast form, maintained at 37°C, readily incorporated the label into cytosolic proteins, whereas few proteins of the mycelial form showed an incorporation of the labeled methionine. The addition of 10 μM unlabeled methionine to cultures of the mycelial form during the labeling period resulted in a substantially increased incorporation of [35S]methionine into cytosol proteins, whereas the addition of unlabeled methionine to cultures of the yeast form did not alter incorporation. The banding pattern of [35S]methionine-labeled proteins showed a good correlation with the results of silver staining for the mycelial and yeast control cultures, with 12 bands specific for the mycelial form and 13 of 18 bands specific for the yeast form still being detectable. Furthermore, the distinctive protein profiles of the mycelial and yeast forms of P. brasiliensis are suggestive of differential protein expression based on temperature (26).
The effect of E2 on the mycelial form was tested in cultures labeled in the presence or absence of excess radioinert methionine. No profile differences were apparent between control and E2-treated cultures maintained at 25°C for 24 h when labeling was done in the presence of excess methionine. However, control and E2-treated mycelial cells at 25°C labeled in the absence of excess methionine displayed a dramatic difference. After 24 h of incubation, few, if any, labeled proteins were apparent from mycelial controls, whereas E2 treatment reproducibly enhanced [35S]methionine uptake and incorporation by nontransforming mycelial cells at 25°C (26). To date, the induction of methionine uptake remains the only defined subcellular response to E2 in these cells which our laboratories have found.
The profiles of protein expression after the initiation of the form transition from mycelia to yeast by changing the culture incubation temperature to 37°C showed numerous changes. The appearance of yeast-specific proteins and the concurrent disappearance of mycelium-specific proteins were observed after 24 h of the mycelium-to-yeast transition in both control and E2-treated cultures. By 72 h and 120 h of the mycelium-to-yeast transition, the presence of E2 in the cultures had resulted in detectable differences. Most notable was the reduction and probable absence of a yeast-specific band (∼92 kDa) present in control cultures that was not observable in the protein profile of E2-treated cells. Interestingly, there was an overall difference in the number of labeled bands observed in the profiles after 120 h of transition. [35S]methionine incorporation, and presumably de novo protein synthesis, was reduced, with many fewer protein bands being observable for the E2-treated cultures, whereas the control cultures had continued de novo protein synthesis and exhibited a yeast protein profile (26). Overall, these results indicated that a switch to a utilization of exogenous methionine occurs within 24 h of a temperature increase to 37°C, that the synthesis of yeast proteins began 24 to 72 h before observable morphological changes occurred, and not only that the presence of E2 blocked the form transition from mycelia to yeast but also that de novo protein synthesis was almost totally downregulated. This downregulation of protein synthesis is likely the cause of the blocking or delaying of form transition and supports the hypothesis that the functional response of P. brasiliensis to E2 is mediated through the binding of the steroid to EBP, which acts as a receptor to further regulate gene expression.
Temporal gene expression profile.
It is of particular interest to our laboratory to elucidate the underlying molecular mechanism of how the presence of E2 results in the inhibition of the mycelium-to-yeast transition of P. brasiliensis. We are currently examining the E2-induced temporal gene expression profile during the mycelium-to-yeast transition using a random-shear genomic DNA microarray (60). Thus far, our studies indicate that E2 modulates the gene expression profile of P. brasiliensis during the initial hours of the transition from the mycelial form to the yeast form; both up- and downregulated genes have been found, compared to the control cultures. Functional categorization revealed genes related to the stress response, those specific to the mycelial form, protein synthesis machinery, regulatory elements, and transporters, among others, that have been observed to be differentially expressed in response to the presence of E2 (J. Shankar et al., unpublished data). These studies will shed light on not only gene expression events occurring during morphogenesis but also how E2 delays or blocks the morphological transition from mycelia to yeast of P. brasiliensis and will further contribute to our knowledge of the basic biology of the organism.
IN VIVO STUDIES OF GENDER DIFFERENCES DURING EXPERIMENTAL INFECTION IN MICE
Although gender-related differences in the susceptibility of humans to PCM are quite apparent, as discussed in the epidemiology section of this review, the association of gender and susceptibility in experimental infections needs to be appropriately pursued. A variety of animal species have been used for experimental infections (15, 16). Models studied with guinea pigs and hamsters have both used males.
Studies with mice and rats have had mixed results in that when using a yeast inoculum, no differences have been reported with respect to the susceptibility of male and female mice (19, 32, 84, 88, 94). In some of those studies (19, 88, 94), with some strains of mice, females were more resistant, and with other strains, there were no sex differences. Other studies have shown female mice to be more resistant than males when infected experimentally by the intratracheal route using yeast as the inoculum (18, 19). Intact female rats were shown to be more susceptible to infection after intraperitoneal challenge with yeasts than were intact males, and castrated females (CF) were more resistant (42). In other studies, the susceptibility of female mice during various stages of the estrous cycle to intravenous inoculation with yeasts was compared with that of males (88, 89). Female mice, especially those at metestrus II, developed invasive disease more frequently (88, 89). Generally, at proestrus and estrus, the blood estrogen is at its highest level, while at metestrus II, it is at its lowest (89). Those studies (18, 19, 88, 89) suggested that female hormones affect the clearance of P. brasiliensis yeasts from tissues and also that differential susceptibility might be affected by the route of infection. One aspect which should be noted is that those studies used yeasts as the inoculum rather than the natural propagules, conidia. Yeasts would not be affected by a hormonal block of the mycelium-to-yeast transition of a mycelium-derived propagule, and a mycelium-derived propagule is what would be encountered in nature, nor are the intravenous or intraperitoneal routes the natural routes of infection. However, McEwen et al. (57) used high-inoculum doses of conidia given via a pulmonary route and found that females had more severe lung pathology than did males. Thus, the variety of studies mentioned do not clearly trend toward showing that females are more resistant to pulmonary PCM.
Conidium-to-Yeast Conversion in Mice: In Vivo Inhibition in Females
Although the data from in vitro studies showed that E2 lacked an effect on the yeast form but did inhibit the mycelium-to-yeast or conidium-to-yeast transition, the in vivo studies noted above do not uniformly support the hypothesis that hormones might influence the pathogenesis of PCM in humans. We carried out further studies to examine the possible in vivo effect of the different hormonal environments in the different sexes on the pathogenesis of experimental pulmonary infection in BALB/c mice. P. brasiliensis ATCC 60855 was used to produce conidia (80), which were used as the inoculum for intranasal infection (a total of 33 male mice and 33 female mice, 4 to 6 weeks old). Three mice of each sex were euthanized at various times postinfection (1, 24, 48, 72, and 96 h) for bronchoalveolar lavage (BAL), for CFU determinations, and for histopathology at 2, 4, and 6 weeks (5).
The conidium-to-yeast transition was monitored with the BAL fluid of the mice by the staining and counting of fungal elements and noting the morphological form. At 1 h postinfection there was no difference between the sexes in the morphology of the fungus, because only conidia were observed. INT (intermediate) (conidia in transition to yeast) and yeast forms were observed as early as 24 h postinfection in the male mice. In contrast, few INT or yeast forms were observed in BAL fluids from female mice through 96 h of infection. Although the total number of fungal cells recovered in BAL fluids significantly diminished with time in males and females, significantly greater numbers of fungal cells were recovered from males through 96 h of infection, with increasing numbers of yeast forms and fewer conidial forms being apparent in the BAL fluid of males. Figure 1 shows differences in forms found in the first 4 days of observation in such experiments.
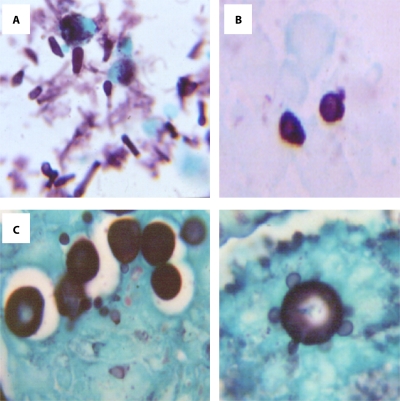
Fig. 1.
Stages in the conidium-to-yeast transition in the lungs of mice (stained with Gomori methenamine silver). (A) Conidia. An inoculum was obtained from the mycelial phase, as seen in bronchoalveolar lavage fluid 1 h postchallenge. Magnification, ×40. (B) Intermediate cells noted to be developing in males at 48 h postchallenge. Magnification, ×100. (C). Yeast cells seen in males at 48 to 96 h postchallenge. Magnification, ×100. Note that the diameters of yeast cells are twice those of intermediate cells, and note the reproduction by (multiple) budding in yeasts in the right half of the figure. (Courtesy of Beatriz Aristizabal, reproduced with permission.)
Two major factors affected the morphological transition of the organisms: gender and the length of time after infection. In males, the proportion of fungal cells remaining as conidia significantly decreased with time, while numbers of INT and yeast cells increased. Conversely, the proportion of conidia through 96 h was significantly higher for females. Multiple budding yeasts were observed for males only toward the end of the 96-h period, which is in contrast to the report by McEwen et al., who reported the appearance of budding yeasts as early as 24 h postinfection (57).
Infected males were unable to control infection, with CFU recovered at 2 to 6 weeks postinfection, whereas no CFU were recovered from the female mice (5). The histopathology of lung sections confirmed the CFU differences between male and female mice. In females, no fungal cells were found at any time. In contrast, in males, 10 to 20 fully transformed yeast cells were counted per section (5). Figure 2 shows differences in the lungs of male and female animals during this time frame. These observations by Aristizabal et al. (5), in conjunction with the finding of estrogen-binding proteins in the cytosol of P. brasiliensis, support the hypothesis that endogenous host estrogens may inhibit the critical first steps of infection, transition from the saprophytic mycelial or conidial form into the yeast form of the fungus (50, 95).
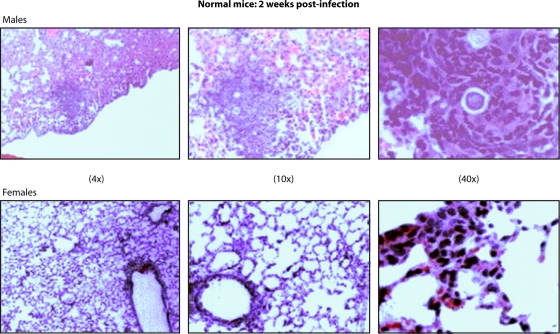
Fig. 2.
Histological observation of the lungs of mice 2 weeks after intranasal infection with P. brasiliensis conidia. A granulomatous reaction in a male mouse and a lack of inflammatory reaction in a female mouse are contrasted. (hematoxylin and eosin staining; magnifications are shown). (Courtesy of Beatriz Aristizabal, reproduced with permission.)
As noted above, McEwen et al. (57), using a conidial inoculum given intranasally, demonstrated that severe pulmonary PCM occurred at the same rate in female and male mice. However, there were several differences from the study by Aristizabal et al. (5), which might explain the contrasting results of the two studies. McEwen, et al. used mice that were 12 weeks old (57), a 4-fold-lower inoculum, and diethyl ether as an anesthetic, which is known to be more destructive to the alveolar lining than methoxyflurane (5). Furthermore, the sources of the BALB/c mice used were different. Finally, the experiment durations were different, up to 6 months (57), versus 6 weeks (5).
Influence of Hormonal Status on the Mouse Model of Paracoccidioidomycosis
The possible role played by E2 in the pathogenesis of PCM was further examined by comparing the histological responses of BALB/c mice infected with the conidia of P. brasiliensis. This was done to assess how the hormonal status of the animals might affect this response and how the hormonal status of the host affects the longer-term course of infection. Again, P. brasiliensis (isolate ATCC 60855) was used to produce conidia for the intranasal infection of 4- to 6-week-old male and female mice, and the responses were assessed for each of six groups: normal males (NM), normal females (NF), castrated males (CM), castrated females (CF), castrated males reconstituted with E2 (CM+E), and castrated females reconstituted with testosterone (CF+T). Gonadectomies were performed at the age of 2 weeks. Hormonal reconstitution using daily high doses of E2 or testosterone given in sesame oil began 48 h prior to infection by subcutaneous injection and continued for 6 weeks. Castrated males were given 17β-estradiol valerate, and castrated females received testosterone propionate (4). Inverse hormone reconstitution was done to determine whether the presence of E2 at high concentrations in the tissues was able to alter the pathogenesis or inhibit morphological transitions.
Normal male and female mice.
Lung sections were stained with a GMS (Gomori methenamine silver) stain technique to recognize the three types of P. brasiliensis propagules: conidia and INT and yeast cells. After 2 weeks of infection, yeast cells were found in NM, whereas NF had no observable yeasts in the lung tissue (P < 0.05). Later in the course of infection (4 and 6 weeks postchallenge), well-developed yeast cells were seen in sections taken from NM, and no yeast cells were observed in sections from NF. These histological observations of the presence (NM) or absence (NF) of yeast cells in the lungs were corroborated by the determination of CFU of P. brasiliensis: no organisms were recovered from NF and >2 log10 CFU were recovered from NM at the same time points (4).
Castrated and inversely hormone-reconstituted male and female mice.
The histological and CFU recovery studies with castrated and inversely hormone-reconstituted male and female mice gave confusing results. By histological assessments, both CM+E and CF+T mice had yeast cells present in their lungs even at 2 weeks postinfection, and observation at 4 and 6 weeks postinfection also showed the presence of yeast cells in both. The histological observations were corroborated by the determination of the number of CFU of P. brasiliensis recovered (4). Interestingly, the CM+E and CF+T groups both showed progressive infection, with increasing numbers of CFU in the lungs with time. However, the numbers of CFU in CM+E mice were significantly lower than those in CM mice at the 2-week time point. Later observations did not give such clear patterns.
Gender-related differences in the host response to paracoccidioidomycosis.
Histological studies were also performed in the study by Aristizabal et al. (4), using NM, NF, CM, CF, CM+E, and CF+T mice to observe the host's cellular response. The types of host cells present in the tissue section were determined by using hematoxylin and eosin (H&E) staining and a quantitative scoring system. The total area of 10 lung fields selected, once the empty spaces represented by major bronchi and arteries had been excluded, was determined, and the area of all inflammatory foci was calculated. The cellular response was differentiated into a chronic inflammatory response, including a granulomatous response, and an acute inflammatory response for purposes of scoring. A chronic inflammatory response was defined as consisting primarily of mononuclear cells, whereas the granulomatous response showed a distinctive pattern of chronic inflammation in which activated macrophages were the predominant cell type, characterized by an epithelioid appearance (giant cells could be present).
NM showed a chronic inflammatory reaction with a predominance of lymphocytes and macrophages, with a few plasmacytes also observed. Granuloma formation was also apparent, and some contained yeast cells in the middle of the granuloma. As was expected, granulomas were composed of macrophages with an eosinophilic cytoplasm, which is indicative of their epithelioid transformation. In contrast, a chronic inflammatory reaction was scarce in NF, although some small mononuclear cells (lymphocytes) were regularly observed. There were few chronic inflammatory foci or granulomas, and yeast cells were absent in NF (4).
The gonadectomized mice showed responses similar to those of normal animals. In comparison with the NM mice, CM mice had a less severe, mild-to-moderate chronic inflammatory reaction. Increased numbers of lymphocytes and macrophages as well as some plasmacytes were seen. However, granulomas were absent, and there were no yeast cells apparent, as opposed to NM, where granulomas and yeasts were observed. Similarly to NF, CF showed no chronic inflammatory reaction, nor were granulomas or yeasts observed (4).
Overall, the results of the in vivo studies demonstrated that gender influences the establishment of PCM. Not only did intact female mice inhibit the conidium-to-yeast transition in a quantitative manner, they also showed fewer foci of infection histologically than did intact male mice. The finding that the treatment of castrated male mice with E2 could result in significantly fewer CFU than in control CM or NM mice supports our hypothesis that E2 is the factor responsible for influencing the gender bias seen in PCM by inhibiting the transition of infecting mycelial or conidial propagules into yeasts. However, these data also indicate that E2 alone is not sufficient to prevent infection in females and that it likely inhibits the development of severe disease. The studies with castrated or castrated and reconstituted animals may be more difficult to interpret, as sex hormones may be produced in glands (adrenals) other than gonads, and reconstitution with exogenous hormones may, without monitoring of the resultant hormone levels, not achieve an accurate hormonal reconstitution.
These results are likely indicative of differences in the efficacies of the immune systems between genders. Sex hormones are known to have immunomodulatory effects, sometimes stimulating the immune response or sometimes suppressing the immune response. In general, estrogens appear to stimulate a proinflammatory Th1 response, while androgens appear more immunosuppressive and stimulate anti-inflammatory Th2 responses. Low concentrations of E2 can be stimulatory, and it could well be that in females the hormonal environment facilitates the synthesis and release of cytokines by both T and B cells, whereby physiological levels of E2 contribute to the activation of macrophages (24, 25, 33, 58, 86). In PCM, the progression of severe disease seen most often in males has been associated with a Th2 response. Thus, E2 could influence not only the inhibition of transition from conidia to yeast in P. brasiliensis but also the immune response of the host by activating macrophages and increasing their fungicidal activity. If, as we believe the evidence supports, female hormones retard conidium-to-yeast and mycelium-to-yeast conversion in vivo, then male and female hosts would encounter different mixes of the fungal elements over time, as the figures in the present paper illustrate. Since the different fungal forms, as may be expected, would engender different types of immune responses and inflammatory activities (28), in turn affecting the destruction and clearance of the forms, and since the immune responses initiated will also be affected differently by the hormonal milieu of the host, it would follow that the overall host immune responses will be different in males and females. Thus, this whole picture of events would explain the male-female differences in susceptibility.
More recent studies have examined the potential for differences in the host response to PCM based on gender. Pinzan et al. (69) further examined gender bias in the immune response to experimental infection. Those authors studied a lectin, paracoccin, derived from P. brasiliensis, demonstrating that in response to paracoccin, spleen cells of male and female mice are polarized toward a Th2 cytokine response and a Th1 cytokine response, respectively. Moreover, following the castration-reconstitution approach used by Aristizabal et al. (4), the cells of castrated and inversely hormone-reconstituted animals produced the cytokine pattern reflecting the reconstituting hormone and not the gender of the cells of the castrated animals. The NO production of macrophages in response to P. brasiliensis was said to be greater for females, but this was not obviously significantly different in the figures shown, where the standard deviations overlapped. A possible correlate of this was that the macrophage inhibition of the fungus was lower by male cells, but the differences appear to be ≤2-fold, which alone would not clearly seem to explain the much greater sex differences noted for natural infection, as presented in our epidemiological summary. The hypothesis of those authors is that E2 increases the glycosylation of membrane proteins, leading to increased levels of glycan at the cell surface of macrophages, resulting in the increased binding of a fungal lectin and increased NO production in females. However, most of the male-female differences are dependent on the paracoccin effect, and it is possible that studying one P. brasiliensis component is artificial and that other P. brasiliensis components may show opposing effects.
CONCLUSIONS AND FUTURE DIRECTIONS
PCM shows a very strong gender bias for the development of clinical disease. Studies that we have performed have linked this bias to the effects that 17β-estradiol has on the organism in that E2 blocks or delays a crucial initial step in the establishment of disease, that of the morphological transition from a mycelial or conidial form into the parasitic yeast form observed in tissues. This function effect is thought to be occurring through the binding of E2 to a stereospecific fungal protein, acting as a true steroid receptor modulating gene expression. The E2 mediation of the inhibition of form transition can be demonstrated in vitro. However, there are also differences in host responses by males and females, which have been shown to be related to specific cytokine expression. Thus, the gender bias for the development of infection may be related to both direct effects of E2 on the organism and differences in host immune responses that are under the regulation of sex hormones.
Much remains to be done to fully characterize the nature of the observed gender bias in this disease. In our own laboratory we have begun to examine gene expression through the use of a microarray. To date, our results have shown a form-specific expression of genes and temporal differences in expression (60). In ongoing studies we are finding changes in gene expression during form transition and that the presence of E2 not only blocks the morphological change but also alters the subsequent expression of several hundred, if not more, genes in the fungus. However, the cascade and order of events induced by the presence of E2 that are reflected by the changes in gene expression remain to be elucidated. Additional studies on the gender differences in host responses to infection are also needed to determine exactly how or why males seem to respond with a Th2 response. A careful delineation of these differences may well allow improved treatments or the prevention of disease in the future. If a vaccine effort could be mounted to prevent the disease, an immunization effort directed at males or at the male response to immunization and infection might be considered.
ACKNOWLEDGMENTS
We thank all the many collaborators in our own prior work cited, and we express particular appreciation to Beatriz Helena Aristizabal, whose contributions to the experimental work led to an elucidation of many of the findings discussed in this review.
Biographies
Jata Shankar, Ph.D., received his doctoral award in Bioscience from Jamia Millia Islamia (Central University) and the Institute of Genomics and Integrative Biology (Council of Scientific and Industrial Research), Delhi, India. He is currently a Postdoctoral Fellow at the California Institute for Medical Research, Santa Clara Valley Medical Center, and Division of Infectious and Geographic Medicine, School of Medicine, Stanford University, and has 9 years of experience from doctoral and postdoctoral work. His work includes the generation of expressed sequence tags and the identification of several genes of Aspergillus fumigatus as well as deducing the effect of the antifungal drug amphotericin B on A. fumigatus using microarray technology. Transcriptional profiling of phase-specific genes for a dimorphic fungus, P. brasiliensis, and the temporal gene expression profile of P. brasiliensis treated with estrogen have been a major part of recent studies undertaken. His research areas include molecular biology, cell biology of fungi, genomics, and bioinformatics.
Angela Restrepo, a Colombian medical laboratory trainee, received her M.S. and doctoral degrees in Microbiology from Tulane University, New Orleans, LA. She occupied academic positions at the School of Medicine, University of Antioquia, Medellín, Colombia, and then, as a cofounder of the Corporacion para Investigaciones Biologicas (CIB), moved to the newly established facilities. She then initiated research lines focused on endemic mycoses with an emphasis on paracoccidioidomycosis and established a laboratory for the diagnosis of the mycoses that also participated in clinical studies with antifungal compounds. The affiliation of the CIB with several Colombian Universities allows training of graduate students to undertake careers in science. She has received many awards, including Honoris Causa doctorates from three Colombian Universities and honorary memberships in the Medical Mycology Society of the Americas (MMSA) and the International Society of Human and Animal Mycology (ISHAM), and has published >350 articles, including chapters in prominent medical textbooks. Presently, she is the CIB's Scientific Director.
Karl V. Clemons is a Senior Research Scientist at the California Institute for Medical Research (CIMR) and Senior Lecturer in Medicine at Stanford University. He is the Director of the Laboratory Animal Facility, Institutional Biosafety Officer, and Responsible Official (Select Agents) for the CIMR. His research on mycoses has included the development of animal models for studies of pathogenesis, host resistance, preclinical drug trials, fungal molecular epidemiology, genetics of pathogenesis, and cell biology. He has published extensively in peer-reviewed papers, book chapters, and published abstracts. He has spoken at numerous meetings and served on the organizing committees for international meetings on aspergillosis and coccidioidomycosis. He is a past Chair and Division Advisor of Division F of the American Society for Microbiology. He is an Associate Editor for Medical Mycology, is on the Editorial Board of Antimicrobial Agents and Chemotherapy, and serves as an ad hoc reviewer for other scientific journals.
David A. Stevens has studied fungi for >35 years in vitro, in animal models and clinical trials, involving therapy, host defenses, molecular epidemiology, and biology, and has authored >600 full articles. He is Professor, Medicine, Stanford University; Chief, Infectious Diseases Division, Santa Clara Valley Medical Center; and President, California Institute for Medical Research, San Jose, CA. In 1999 he was the recipient of the Rhoda Benham Medal from the MMSA, and 2006 he was the recipient of the Lucille Georg Medal, ISHAM, and Smith Memorial Award from the Coccidioidomycosis Study Group. The bacterium named Halomonas stevensii recognizes his contributions toward the discovery of species in this genus. He was a Project Leader in the NIAID Mycoses Study Group from 1990 to 2000. His former trainees include 8 current Professors. He was Chair, Mycology Division, American Society for Microbiology, and 3 of his former Fellows were elected Chair. He is a member of the American Society for Clinical Investigation and a Fellow of the American Academy of Microbiology. He is a Co-Organizer of Advances Against Aspergillosis international meetings.
REFERENCES
- 1. Aguiar W. S., Silva B. M., Passos E. D., Zandonade E., Falqueto A. 2003. Association between smoking and paracoccidioidomycosis: a case-control study in the State of Espírito Santo, Brazil. Cad. Saude Publica 19:245–253(In Portugese.) [DOI] [PubMed] [Google Scholar]
- 2. Albornoz M. C. 1975. Resultados de las encuestas epidemiológicas realizadas con paracoccidioidina en Venezuela. Castellania 3:37–42 [Google Scholar]
- 3. Angulo-Ortega A. 1972. Calcifications in paracoccidioidomycosis: are they the morphological manifestation of subclinical infections?, p. 129–132In Proceedings of the First Pan American Symposium on Paracoccidioidomycosis. PAHO scientific publication no. 254. Pan American Health Organization, Washington, DC [Google Scholar]
- 4. Aristizabal B. H., Clemons K. V., Cock A. M., Restrepo A., Stevens D. A. 2002. Experimental Paracoccidioides brasiliensis infection in mice: influence of the hormonal status of the host on tissue responses. Med. Mycol. 40:169–178 [DOI] [PubMed] [Google Scholar]
- 5. Aristizabal B. H., Clemons K. V., Stevens D. A., Restrepo A. 1998. Morphological transition of Paracoccidioides brasiliensis conidia to yeast cells: in vivo inhibition in females. Infect. Immun. 66:5587–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bagagli E., et al. 2003. High frequency of Paracoccidioides brasiliensis infection in armadillos (Dasypus novemcinctus): an ecological study. Med. Mycol. 41:217–223 [DOI] [PubMed] [Google Scholar]
- 7. Barbosa G. L. 1992. Paracoccidioidomicose na criança. Rev. Pat. Trop. 21:269–383 [Google Scholar]
- 8. Barrozo L. V., et al. 2010. First description of a cluster of acute/subacute paracoccidioidomycosis cases and its association with a climatic anomaly. PLoS Negl. Trop. Dis. 4:e643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrozo L. V., et al. 2009. Climate and acute/subacute paracoccidioidomycosis in a hyper-endemic area in Brazil. Int. J. Epidemiol. 38:1642–1649 [DOI] [PubMed] [Google Scholar]
- 10. Barrozo-Simoes L., Alencar-Marques S., Bagagli E. 2004. Distribution of paracoccidioidomycosis: determination of ecologic correlates through spatial analyses. Med. Mycol. 42:517–523 [DOI] [PubMed] [Google Scholar]
- 11. Bicalho R. N., Santo M. F., de Aguiar M. C., Santos V. R. 2001. Oral paracoccidioidomycosis: a retrospective study of 62 Brazilian patients. Oral Dis. 7:56–60 [PubMed] [Google Scholar]
- 12. Bittencourt J. I., de Oliveira R. M., Coutinho Z. F. 2005. Paracoccidioidomycosis mortality in the State of Parana, Brazil, 1980/1998. Cad. Saude Publica 21:1856–1864(In Portugese.) [DOI] [PubMed] [Google Scholar]
- 13. Blotta M. H., et al. 1999. Endemic regions of paracoccidioidomycosis in Brazil: a clinical and epidemiologic study of 584 cases in the southeast region. Am. J. Trop. Med. Hyg. 61:390–394 [DOI] [PubMed] [Google Scholar]
- 14. Bonifaz A. 2010. Micología média básica. McGraw Hill Interamericana Editores, Mexico City, Mexico [Google Scholar]
- 15. Brummer E., Clemons K. V. 1987. Animal models of systemic mycoses, p. 79–100In Miyaji M. (ed.), Animal models in medical mycology. CRC Press, Boca Raton, FL [Google Scholar]
- 16. Brummer E., Castaneda E., Restrepo A. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 6:89–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cadavid D., Restrepo A. 1993. Factors associated with Paracoccidiodes brasiliensis infection among permanent residents of three endemic areas in Colombia. Epidemiol. Infect. 111:121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calich V. L., Burger E., Kashino S. S., Fazioli R. A., Singer-Vermes L. M. 1987. Resistance to Paracoccidioides brasiliensis in mice is controlled by a single dominant autosomal gene. Infect. Immun. 55:1919–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calich V. L., Singer-Vermes L. M., Siqueira A. M., Burger E. 1985. Susceptibility and resistance of inbred mice to Paracoccidioides brasiliensis. Br. J. Exp. Pathol. 66:585–594 [PMC free article] [PubMed] [Google Scholar]
- 20. Calle D., et al. 2001. Paracoccidioidomycosis in Colombia: an ecological study. Epidemiol. Infect. 126:309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campos M. V., et al. 2008. Paracoccidioidomycosis at Brasilias university hospital. Rev. Soc. Bras. Med. Trop. 41:169–172 [DOI] [PubMed] [Google Scholar]
- 22. Castillo J., Ordóñez N., López S., Castañeda E. 1994. Paracoccidioidomicosis: diagnóstico por el laboratorio de 333 casos. Biomedica 14:230–239 [Google Scholar]
- 23. Cermeno J., et al. 2009. Epidemiological study of paracoccidioidomycosis and histoplasmosis in a suburb of San Felix city, Bolivar state, Venezuela. Invest. Clin. 50:213–220 [PubMed] [Google Scholar]
- 24. Chao T. C., Van Alten P. J., Greager J. A., Walter R. J. 1995. Steroid sex hormones regulate the release of tumor necrosis factor by macrophages. Cell. Immunol. 160:43–49 [DOI] [PubMed] [Google Scholar]
- 25. Chao T. C., Van Alten P. J., Walter R. J. 1994. Steroid sex hormones and macrophage function: modulation of reactive oxygen intermediates and nitrite release. Am. J. Reprod. Immunol. 32:43–52 [DOI] [PubMed] [Google Scholar]
- 26. Clemons K. V., Feldman D., Stevens D. A. 1989. Influence of oestradiol on protein expression and methionine utilization during morphogenesis of Paracoccidioides brasiliensis. J. Gen. Microbiol. 135:1607–1617 [DOI] [PubMed] [Google Scholar]
- 27. Clemons K. V., Shankar J., Stevens D. A. 2010. Mycologic endocrinology, p. 269–290In Freestone P. P. E., Lyte M. (ed.), Microbial endocrinology: interkingdom signaling in infectious diseases and health. Springer, Berlin, Germany [Google Scholar]
- 28. Cock A. M., et al. 2000. Fibrotic sequelae in pulmonary paracoccidioidomycosis: histopathological aspects in BALB/c mice infected with viable and non-viable Paracoccidioides brasiliensis propagules. Rev. Inst. Med. Trop. Sao Paulo 42:59–66 [DOI] [PubMed] [Google Scholar]
- 29. Coimbra C. E., Jr., et al. 1994. Paracoccidioidin and histoplasmin sensitivity in Tupi-Monde Amerindian populations from Brazilian Amazonia. Ann. Trop. Med. Parasitol. 88:197–207 [DOI] [PubMed] [Google Scholar]
- 30. Conti-Díaz I. A., Calegari L. F. 1979. Paracoccidioidomycosis in Uruguay; its status and current problems. Bol. Oficina Sanit. Panam. 86:219–229 [PubMed] [Google Scholar]
- 31. de Almeida S. M., Queiroz-Telles F., Teive H. A., Ribeiro C. E., Werneck L. C. 2004. Central nervous system paracoccidioidomycosis: clinical features and laboratorial findings. J. Infect. 48:193–198 [DOI] [PubMed] [Google Scholar]
- 32. Defaveri J., Rezkallah-Iwasso M. T., de Franco M. F. 1982. Experimental pulmonary paracoccidioidomycosis in mice: morphology and correlation of lesions with humoral and cellular immune response. Mycopathologia 77:3–11 [DOI] [PubMed] [Google Scholar]
- 33. Deshpande R., Khalili H., Pergolizzi R. G., Michael S. D., Chang M. D. 1997. Estradiol down-regulates LPS-induced cytokine production and NFkB activation in murine macrophages. Am. J. Reprod. Immunol. 38:46–54 [DOI] [PubMed] [Google Scholar]
- 34. Fava S. D. C., Fava-Netto C. 1998. Epidemiologic surveys of histoplasmin and paracoccidioidin sensitivity in Brazil. Rev. Inst. Med. Trop. Sao Paulo 40:1–14 [DOI] [PubMed] [Google Scholar]
- 35. Fonseca E. R., Pardal P. P., Severo L. C. 1999. Paracoccidioidomycosis in children in Belem, Para. Rev. Soc. Bras. Med. Trop. 32:31–33(In Portugese.) [DOI] [PubMed] [Google Scholar]
- 36. Fornajeiro N., Maluf M. L., Takahachi G., Svidzinski T. I. 2005. Paracoccidioidomycosis epidemiological survey using gp43, in two cities of northwestern region of Parana, Brazil. Rev. Soc. Bras. Med. Trop. 38:191–193 [DOI] [PubMed] [Google Scholar]
- 37. Franco M., Bagagli E., Scapolio S., da Silva Lacaz C. 2000. A critical analysis of isolation of Paracoccidioides brasiliensis from soil. Med. Mycol. 38:185–191 [DOI] [PubMed] [Google Scholar]
- 38. Franco M., Mendes R. P., Moscardi-Bacchi M., Montenegro M. R. 1989. Paracoccidioidomycosis. Baillieres Clin. Trop. Med. Commun. Dis. 4:185–220 [Google Scholar]
- 39. Greer D. L., Restrepo A. 1975. The epidemiology of paracoccidioidomycosis, p. 117–141In Al-Doory Y. (ed.), The epidemiology of human mycotic diseases, vol. III Charles C. Thomas, Springfield, IL [Google Scholar]
- 40. Jeraj N., Lenasi H., Breskvar K. 2005. The involvement of cAMP in the growth inhibition of filamentous fungus Rhizopus nigricans by steroids. FEMS Microbiol. Lett. 242:147–154 [DOI] [PubMed] [Google Scholar]
- 41. Kalmar E. M., et al. 2004. Paracoccidioidomycosis: an epidemiologic survey in a pediatric population from the Brazilian Amazon using skin tests. Am. J. Trop. Med. Hyg. 71:82–86 [PubMed] [Google Scholar]
- 42. Kerr I. B., Schaeffer G. V., Miranda D. S. 1984. Sex hormones and susceptibility of the rat to paracoccidioidomycosis. Mycopathologia 88:149–154 [DOI] [PubMed] [Google Scholar]
- 43. Lacaz C. S., Porto E., Martins J. E. C., Heins-Vaccari Melo N. T. 2002. Paracoccidioidomicose, p. 639–729In Lacaz C. S., Porto E., Martins J. E. C., Heins-Vaccari Melo N. T. (ed.), Tratado de micologia medica Lacaz, vol. 9 Sarvier, Sao Paulo, Brazil [Google Scholar]
- 44. Lacaz C. S., Passos-Filho M. C. R., Fava-Netto C., Macarron B. 1959. Contribução para o estudo da Blastomicose-Infecção. Inquérito com a paracoccidioidina. Estudo serológio e clínico-radiológico dos paraccocidioidino-positivos. Rev. Inst. Med. Trop. Sao Paulo 1:254–259 [Google Scholar]
- 45. Londero A. T., Rios-Gonçalves A. J., Terra G. M. F., Nogueira S. A. 1996. Paracoccidioidomycosis in Brazilian children. A critical review (1911-1994). Arq. Bras. Med. 70:197–203 [Google Scholar]
- 46. Londero A. T., Ramos C. D. 1990. Paracoccidioidomicose: estudo clinico-micologico de 260 casos observados no interior do Estado do Rio Grande do Sul. J. Pneumol. 16:129–132 [Google Scholar]
- 47. Loose D. S., Feldman D. 1982. Characterization of a unique corticosterone-binding protein in Candida albicans. J. Biol. Chem. 257:4925–4930 [PubMed] [Google Scholar]
- 48. Loose D. S., Schurman D. J., Feldman D. 1981. A corticosteroid binding protein and endogenous ligand in C. albicans indicating a possible steroid-receptor system. Nature 293:477–479 [DOI] [PubMed] [Google Scholar]
- 49. Loose D. S., Stevens D. A., Schurman D. J., Feldman D. 1983. Distribution of a corticosteroid-binding protein in Candida and other fungal genera. J. Gen. Microbiol. 129:2379–2385 [DOI] [PubMed] [Google Scholar]
- 50. Loose D. S., Stover E. P., Restrepo A., Stevens D. A., Feldman D. 1983. Estradiol binds to a receptor-like cytosol binding protein and initiates a biological response in Paracoccidioides brasiliensis. Proc. Natl. Acad. Sci. U. S. A. 80:7659–7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mackinnon J. E., Artagaveytia-Allende R. C., Arroyo L. 1953. Specificity of cutaneous reaction with paracoccidioidin. An. Fac. Med. Univ. Repub. Montev. Urug. 38:363–382 [PubMed] [Google Scholar]
- 52. Maluf M. L., Pereira S. R., Takahachi G., Svidzinski T. I. 2003. Prevalence of paracoccidioidomycosis infection determined by serologic test in donors' blood in the Northwest of Parana, Brazil. Rev. Soc. Bras. Med. Trop. 36:11–16 [DOI] [PubMed] [Google Scholar]
- 53. Mangiaterra M. L., Giusiano G. E., Alonso J. M., Gorodner J. O. 1999. Paracoccidioides brasiliensis infection in a subtropical region with important environmental changes. Bull. Soc. Pathol. Exot. 92:173–176 [PubMed] [Google Scholar]
- 54. Manns B. J., Baylis B. W., Urbanski S. J., Gibb A. P., Rabin H. R. 1996. Paracoccidioidomycosis: case report and review. Clin. Infect. Dis. 23:1026–1032 [DOI] [PubMed] [Google Scholar]
- 55. Marchiori E., et al. 2011. Paracoccidioidomycosis: high-resolution computed tomography-pathologic correlation. Eur. J. Radiol. 77:80–84 [DOI] [PubMed] [Google Scholar]
- 56. Marques S. A., et al. 1983. Epidemiologic aspects of paracoccidioidomycosis in the endemic area of Botucatu (Sao Paulo-Brazil). Rev. Inst. Med. Trop. Sao Paulo 25:87–92 [PubMed] [Google Scholar]
- 57. McEwen J. G., Bedoya V., Patino M. M., Salazar M. E., Restrepo A. 1987. Experimental murine paracoccidiodomycosis induced by the inhalation of conidia. J. Med. Vet. Mycol. 25:165–175 [DOI] [PubMed] [Google Scholar]
- 58. Mock B. A., Nacy C. A. 1988. Hormonal modulation of sex differences in resistance to Leishmania major systemic infections. Infect. Immun. 56:3316–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mok W. Y., Netto C. F. 1978. Paracoccidioidin and histoplasmin sensitivity in Coari (state of Amazonas), Brazil. Am. J. Trop. Med. Hyg. 27:808–814 [DOI] [PubMed] [Google Scholar]
- 60. Monteiro J. P., et al. 2009. Genomic DNA microarray comparison of gene expression patterns in Paracoccidioides brasiliensis mycelia and yeasts in vitro. Microbiology 155:2795–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morejon K. M., Machado A. A., Martinez R. 2009. Paracoccidioidomycosis in patients infected with and not infected with human immunodeficiency virus: a case-control study. Am. J. Trop. Med. Hyg. 80:359–366 [PubMed] [Google Scholar]
- 62. Muchmore H. G., McKown B. A., Mohr J. A. 1972. Effect of steroid hormones on the growth of Paracoccidioides brasiliensis, p. 300–304 In Proceedings of the First Pan American Symposium on Paracoccidioides. PAHO scientific publication no. 254. Pan American Health Organization, Washington, DC [Google Scholar]
- 63. Nucci M., Colombo A. L., Queiroz-Telles F. 2009. Paracoccidioidomycosis. Curr. Fungal Infect. Rep. 3:15–20 [Google Scholar]
- 64. Ono M. A., Itano E. N., Mizuno L. T., Mizuno E. H., Camargo Z. P. 2002. Inhibition of Paracoccidioides brasiliensis by pesticides: is this a partial explanation for the difficulty in isolating this fungus from the soil? Med. Mycol. 40:493–499 [DOI] [PubMed] [Google Scholar]
- 65. Paniago A. M., et al. 2003. Paracoccidioidomycosis: a clinical and epidemiological study of 422 cases observed in Mato Grosso do Sul. Rev. Soc. Bras. Med. Trop. 36:455–459 [DOI] [PubMed] [Google Scholar]
- 66. Paniago A. M., et al. 2005. Paracoccidioidomycosis in patients with human immunodeficiency virus: review of 12 cases observed in an endemic region in Brazil. J. Infect. 51:248–252 [DOI] [PubMed] [Google Scholar]
- 67. Pereira A. J. C. S. 1988. Inquerito intradérmico para paracoccidioidomicose en Goiânia. Rev. Pat. Trop. 17:157–186 [Google Scholar]
- 68. Pereira R. M., Bucaretchi F., de Melo Barison E., Hessel G., Tresoldi A. T. 2004. Paracoccidioidomycosis in children: clinical presentation, follow-up and outcome. Rev. Inst. Med. Trop. Sao Paulo 46:127–131 [DOI] [PubMed] [Google Scholar]
- 69. Pinzan C. F., Ruas L. P., Casabona-Fortunato A. S., Carvalho F. C., Roque-Barreira M. C. 2010. Immunological basis for the gender differences in murine Paracoccidioides brasiliensis infection. PLoS One 5:e10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Queiroz-Telles F. 2008. Influence of alternating coffee and sugar cane agriculture in the incidence of paracoccidioidomycosis in Brazil. Biomedica 28(Suppl. 1):129 [Google Scholar]
- 71. Ramos E., Silva M., Saraiva L. E. 2009. Paracoccidioidomycosis. Dermatol. Clin. 26:257–269 [DOI] [PubMed] [Google Scholar]
- 72. Restrepo A., González A., Agudelo C. A. Paracoccidioidomycosis. In Dismukes W., Kauffman C., Sobel J. (ed.), Clinical mycology, 2nd ed., in press Springer, Berlin, Germany [Google Scholar]
- 73. Restrepo A., Tobón A., Agudelo C. 2008. Paracoccidioidomycosis, p. 331–342In Hospenthal D., Rinaldi M. (ed.), Diagnosis and treatment of human mycoses. Humana Press, Totowa, NJ [Google Scholar]
- 74. Restrepo A., Tobón A. M. 2009. Paracoccidioides brasiliensis, p. 3357–3363In Mandell G. L., Bennett J. E., Dolin R. (ed.) Mandell, Douglas and Bennett's principles and practice of infectious diseases, vol. 7 Elsevier, Philadelphia, PA [Google Scholar]
- 75. Restrepo A., Benard G., de Castro C. C., Agudelo C. A., Tobon A. M. 2008. Pulmonary paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 29:182–197 [DOI] [PubMed] [Google Scholar]
- 76. Restrepo A., Salazar M. E., Clemons K. V., Feldman D., Stevens D. A. 1997. Hormonal influences in the host-interplay with Paracoccidioides brasiliensis, p. 125–133In Stevens D. A., Vanden-Bosche H., Odds F.(ed.), Topics in fungal infections. National Foundation for Infectious Diseases, Bethesda, MD [Google Scholar]
- 77. Restrepo A., McEwen J. G., Castaneda E. 2001. The habitat of Paracoccidioides brasiliensis: how far from solving the riddle? Med. Mycol. 39:233–241 [DOI] [PubMed] [Google Scholar]
- 78. Restrepo A., et al. 1970. Paracoccidioidomycosis (South American blastomycosis). A study of 39 cases observed in Medellin, Colombia. Am. J. Trop. Med. Hyg. 19:68–76 [PubMed] [Google Scholar]
- 79. Restrepo A., Robledo M., Ospina S., Restrepo M., Correa A. 1968. Distribution of paracoccidioidin sensitivity in Colombia. Am. J. Trop. Med. Hyg. 17:25–37 [PubMed] [Google Scholar]
- 80. Restrepo A., Salazar M. E., Cano L. E., Patino M. M. 1986. A technique to collect and dislodge conidia produced by Paracoccidioides brasiliensis mycelial form. J. Med. Vet. Mycol. 24:247–250 [PubMed] [Google Scholar]
- 81. Restrepo A., et al. 1984. Estrogens inhibit mycelium-to-yeast transformation in the fungus Paracoccidioides brasiliensis: implications for resistance of females to paracoccidioidomycosis. Infect. Immun. 46:346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Restrepo B. I., McEwen J. G., Salazar M. E., Restrepo A. 1986. Morphological development of the conidia produced by Paracoccidioides brasiliensis mycelial form. J. Med. Vet. Mycol. 24:337–339 [PubMed] [Google Scholar]
- 83. Rios-Gonçalves A. J., et al. 1998. Paracoccidioidomycosis in children in the state of Rio de Janeiro (Brazil). Geographic distribution and the study of a “reservarea.” Rev. Inst. Med. Trop. Sao Paulo 40:11–13 [DOI] [PubMed] [Google Scholar]
- 84. Robledo M. A., et al. 1982. Host defense against experimental paracoccidioidomycosis. Am. Rev. Respir. Dis. 125:563–567 [DOI] [PubMed] [Google Scholar]
- 85. Rodrigues M. T., de Resende M. A. 1996. Epidemiologic skin test survey of sensitivity to paracoccidioidin, histoplasmin and sporotrichin among gold mine workers of Morro Velho Mining, Brazil. Mycopathologia 135:89–98 [DOI] [PubMed] [Google Scholar]
- 86. Ruh M. F., Bi Y., D'Alonzo R., Bellone C. J. 1998. Effect of estrogens on IL-1beta promoter activity. J. Steroid Biochem. Mol. Biol. 66:203–210 [DOI] [PubMed] [Google Scholar]
- 87. Salazar M. E., Restrepo A., Stevens D. A. 1988. Inhibition by estrogens of conidium-to-yeast conversion in the fungus Paracoccidioides brasiliensis. Infect. Immun. 56:711–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sano A., Miyaji M., Nishimura K. 1991. Studies on the relationship between paracoccidioidomycosis in ddY mice and their estrous cycle. Mycopathologia 115:73–81 [DOI] [PubMed] [Google Scholar]
- 89. Sano A., Miyaji M., Nishimura K. 1992. Studies on the relationship between the estrous cycle of BALB/c mice and their resistance to Paracoccidioides brasiliensis infection. Mycopathologia 119:141–145 [DOI] [PubMed] [Google Scholar]
- 90. Santo A. H. 2008. Paracoccidioidomycosis-related mortality trend, state of Sao Paulo, Brazil: a study using multiple causes of death. Rev. Panam. Salud Publica 23:313–324 [DOI] [PubMed] [Google Scholar]
- 91. Schar G., Stover E. P., Clemons K. V., Feldman D., Stevens D. A. 1986. Progesterone binding and inhibition of growth in Trichophyton mentagrophytes. Infect. Immun. 52:763–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Severo L. C., Roesch E. W., Oliveira E. A., Rocha M. M., Londero A. T. 1998. Paracoccidioidomycosis in women. Rev. Iberoam. Micol. 15:88–89 [PubMed] [Google Scholar]
- 93. Shikanai-Yasuda M. A., et al. 2006. Guidelines in paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 39:297–310 [DOI] [PubMed] [Google Scholar]
- 93a. Silva C. O., et al. 2007. Gingival involvement in oral paracoccidioidomycosis. J. Periodontol. 78:1229–1234 [DOI] [PubMed] [Google Scholar]
- 94. Singer-Vermes L. M., Sakamoto T. N., Vaz C. A., Calich V. L. 1995. Influence of the genetic pattern and sex of mice in experimental paracoccidioidomycosis. Clin. Exp. Immunol. 101:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stover E. P., Schar G., Clemons K. V., Stevens D. A., Feldman D. 1986. Estradiol-binding proteins from mycelial and yeast-form cultures of Paracoccidioides brasiliensis. Infect. Immun. 51:199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tobon A. M., et al. 2003. Residual pulmonary abnormalities in adult patients with chronic paracoccidioidomycosis: prolonged follow-up after itraconazole therapy. Clin. Infect. Dis. 37:898–904 [DOI] [PubMed] [Google Scholar]
- 97. Tobón A. M., et al. 2010. Adrenal function status in patients with paracoccidioidomycosis after prolonged post-therapy follow-up. Am. J. Trop. Med. Hyg. 83:111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Torrado E., Castañeda E., de la Hoz F., Restrepo A. 2000. Paracoccidioidomicosis: definición de las áreas endémicas de Colombia. Biomedica 20:327–334 [Google Scholar]
- 99. Villar L. A. C., et al. 1992. Paracoccidioidomicosis en Santander: aspectos clínico-epidemiólogicos (1953-1992). Medicas UIS 16:182–188 [Google Scholar]
- 100. Walker S. L., Pembroke A. C., Lucas S. B., Vega-Lopez F. 2008. Paracoccidioidomycosis presenting in the UK. Br. J. Dermatol. 158:624–626 [DOI] [PubMed] [Google Scholar]
- 101. Wanke B., Londero A. T. 1994. Epidemiology and paracoccidioidomycosis infection, p. 109–117In Franco M., Lacaz C. S., Restrepo-Moreno A. (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, FL [Google Scholar]
- 102. Williams R. H. 1981. Textbook of endocrinology, 6th ed W. B. Saunders Co., Philadelphia, PA [Google Scholar]
- 103. Yamaga L. Y. I., et al. 2003. The role of gallium-67 scan in defining the extent of disease in an endemic deep mycosis, paracoccidioidomycosis: a predominantly multifocal disease. Eur. J. Nucl. Med. Mol. Imaging 30:888–894 [DOI] [PubMed] [Google Scholar]