Abstract
A designed mRNA consisting of 42 ribonucleotides having the cap structure was synthesized. The capped leader sequence of the brome mosaic virus (BMV) mRNA 4, m7G5'pppGUAUUAAUA (F-1), was synthesized by the phosphotriester method and followed by the capping reaction. A 32-mer consisting of an initiation codon (AUG), the coding region corresponding to a bacterial pheromone cAD1 and two stop codons, was constructed by the 18-mer (F-2) and 14-mer (F-3), which were synthesized by the phosphoramidite method. 2'-,3'-O-Methoxymethylene-guanosine 5'-phosphate was condensed with F-3 using P1-2',3'-O-methoxymethyleneguanosine-5'-yl P2-adenosine-5'-yl pyrophosphate (9) with T4 RNA ligase. The chemically synthesized RNA fragments were ligated successively with T4 RNa ligase to afford the whole RNA molecule.
Full text
PDF

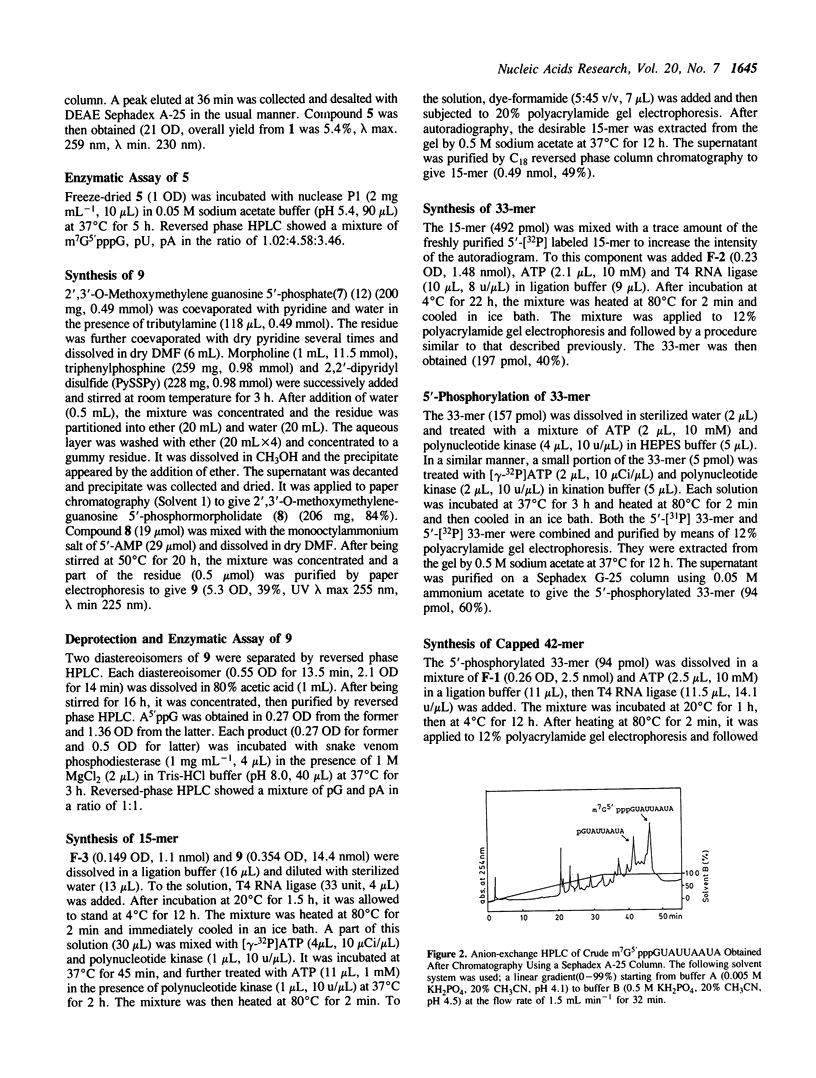
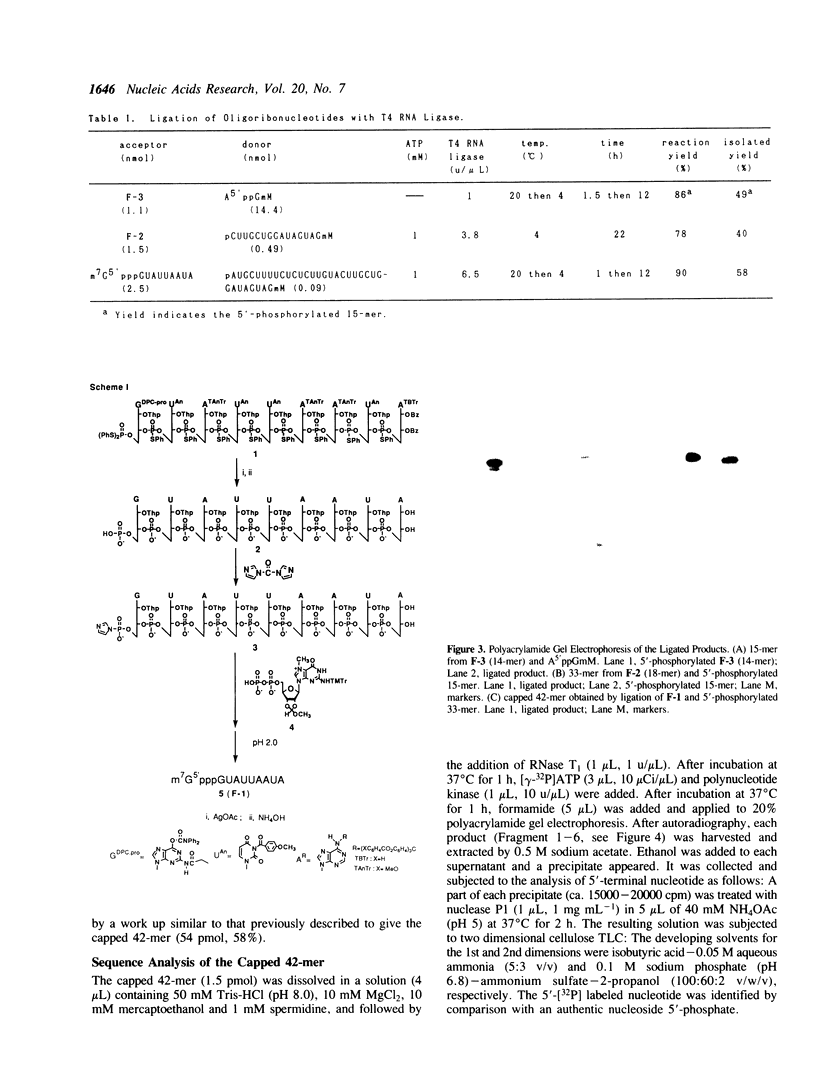


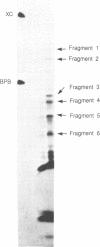
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boguski M. S., Hieter P. A., Levy C. C. Identification of a cytidine-specific ribonuclease from chicken liver. J Biol Chem. 1980 Mar 10;255(5):2160–2163. [PubMed] [Google Scholar]
- Darzynkiewicz E., Stepinski J., Ekiel I., Jin Y., Haber D., Sijuwade T., Tahara S. M. Beta-globin mRNAs capped with m7G, m2.7(2)G or m2.2.7(3)G differ in intrinsic translation efficiency. Nucleic Acids Res. 1988 Sep 26;16(18):8953–8962. doi: 10.1093/nar/16.18.8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta R., Kaesberg P. Complete nucleotide sequences of the coat protein messenger RNAs of brome mosaic virus and cowpea chlorotic mottle virus. Nucleic Acids Res. 1982 Jan 22;10(2):703–713. doi: 10.1093/nar/10.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Gumport R. I., Uhlenbeck O. C. Dinucleoside pyrophosphate are substrates for T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4839–4842. doi: 10.1073/pnas.74.11.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. Enzymatic oligoribonucleotide synthesis with T4 RNA ligase. Biochemistry. 1978 May 30;17(11):2069–2076. doi: 10.1021/bi00604a008. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Miura K. A blocked structure at the 5' terminus of mRNA from cytoplasmic polyhedrosis virus. Nature. 1975 Jan 31;253(5490):374–375. doi: 10.1038/253374a0. [DOI] [PubMed] [Google Scholar]
- Furusawa K., Sekine M., Hata T. Studies on pyrophosphates. Part III. A new method for the synthesis of nucleotide coenzymes by means of di-n-butylphosphiothioyl bromide. J Chem Soc Perkin 1. 1976;(16):1711–1716. [PubMed] [Google Scholar]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. Mutational analysis of the tobacco mosaic virus 5'-leader for altered ability to enhance translation. Nucleic Acids Res. 1988 Feb 11;16(3):883–893. doi: 10.1093/nar/16.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna Y., Sarkar N. Selective resistance of bacterial polyadenylate-containing RNA to hydrolysis by guanosine 3'-5'-monophosphate-sensitive nuclease of Bacillus brevis. Biochem Biophys Res Commun. 1981 Nov 30;103(2):454–460. doi: 10.1016/0006-291x(81)90474-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann P. U., McLaughlin L. W. Synthesis and reactivity of intermediates formed in the T4 RNA ligase reaction. Nucleic Acids Res. 1987 Jul 10;15(13):5289–5303. doi: 10.1093/nar/15.13.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Bruck C., Cleuter Y. Translational stability of native and deadenylylated rabbit globin mRNA injected into HeLa cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):908–911. doi: 10.1073/pnas.78.2.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Jobling S. A., Cuthbert C. M., Rogers S. G., Fraley R. T., Gehrke L. In vitro transcription and translational efficiency of chimeric SP6 messenger RNAs devoid of 5' vector nucleotides. Nucleic Acids Res. 1988 May 25;16(10):4483–4498. doi: 10.1093/nar/16.10.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G., Klein T., Littauer U. Z. T4 RNA ligase: substrate chain length requirements. FEBS Lett. 1974 Sep 15;46(1):271–275. doi: 10.1016/0014-5793(74)80385-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin L. W., Piel N., Graeser E. Donor activation in the T4 RNA ligase reaction. Biochemistry. 1985 Jan 15;24(2):267–273. doi: 10.1021/bi00323a005. [DOI] [PubMed] [Google Scholar]
- Mori M., Sakagami Y., Narita M., Isogai A., Fujino M., Kitada C., Craig R. A., Clewell D. B., Suzuki A. Isolation and structure of the bacterial sex pheromone, cAD1, that induces plasmid transfer in Streptococcus faecalis. FEBS Lett. 1984 Dec 3;178(1):97–100. doi: 10.1016/0014-5793(84)81248-x. [DOI] [PubMed] [Google Scholar]
- Mukaiyama T., Hashimoto M. Synthesis of oligothymidylates and nucleoside cyclic phosphates by oxidation-reduction condensation. J Am Chem Soc. 1972 Nov 29;94(24):8528–8532. doi: 10.1021/ja00779a039. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Miyake T., Nagao K., Uemura H., Nishikawa S., Sugiura M., Ikehara M. Elongation of oligonucleotides in the 3'-direction with activated mononucleotides and their analogs using RNA ligase. Nucleic Acids Res. 1980 Feb 11;8(3):601–610. doi: 10.1093/nar/8.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Nishikawa S., Markham A. F., Tanaka S., Miyake T., Wakabayashi T., Ikehara M., Sugiura M. Joining of 3'-modified oligonucleotides by T4 RNA ligase. Synthesis of a heptadecanucleotide corresponding to the bases 61--77 from Escherichia coli tRNAfMet. Biochemistry. 1978 Nov 14;17(23):4894–4899. doi: 10.1021/bi00616a006. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Nishikawa S., Sugiura M., Ikehara M. Joining of ribooligonucleotides with T4 RNA ligase and identification of the oligonucleotide-adenylate intermediate. Nucleic Acids Res. 1976 Jun;3(6):1613–1623. doi: 10.1093/nar/3.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Tanaka S., Tanaka T., Miyake T., Markham A. F., Nakagawa E., Wakabayashi T., Taniyama Y., Nishikawa S., Fukumoto R. Total synthesis of a RNA molecule with sequence identical to that of Escherichia coli formylmethionine tRNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5493–5497. doi: 10.1073/pnas.78.9.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Uemura H., Doi T., Miyake T., Nishikawa S., Ikehara M. A new method for 3'-labelling of polyribonucleotides by phosphorylation with RNA ligase and its application to the 3'-modification for joining reactions. Nucleic Acids Res. 1979 Feb;6(2):443–454. doi: 10.1093/nar/6.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk E., McLaughlin L. W., Neilson T., Romaniuk P. J. The effect of acceptor oligoribonucleotide sequence on the T4 RNA ligase reaction. Eur J Biochem. 1982 Jul;125(3):639–643. doi: 10.1111/j.1432-1033.1982.tb06730.x. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Kodama Y., Hashimoto J., Miura K. I. Importance of 5'-terminal blocking structure to stabilize mRNA in eukaryotic protein synthesis. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2734–2738. doi: 10.1073/pnas.74.7.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sninsky J. J., Last J. A., Gilham P. T. The use of terminal blocking groups for the specific joining of oligonucleotides in RNA ligase reactions containing equimolar concentrations of acceptor and donor molecules. Nucleic Acids Res. 1976 Nov;3(11):3157–3166. doi: 10.1093/nar/3.11.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Snoper T. J., Cozzarelli N. R. Bacteriophage T4 RNA ligase. Reaction intermediates and interaction of substrates. J Biol Chem. 1977 Mar 10;252(5):1732–1738. [PubMed] [Google Scholar]
- Tanaka T., Tamatsukuri S., Ikehara M. Solid phase synthesis of oligoribonucleotides using the o-nitrobenzyl group for 2'-hydroxyl protection and H-phosphonate chemistry. Nucleic Acids Res. 1987 Sep 25;15(18):7235–7248. doi: 10.1093/nar/15.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura H., Maeda M., Fukazawa T., Sekine M., Hata T. Chemical synthesis of the 24 RNA fragments corresponding to hop stunt viroid. Nucleic Acids Res. 1989 Oct 25;17(20):8135–8147. doi: 10.1093/nar/17.20.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumoto Y., Iwase R., Sekine M., Hata T., Ohshima Y. Pre-mRNA with a trimethylguanosine cap structure can be spliced efficiently in vitro. Biochem Biophys Res Commun. 1989 Aug 15;162(3):977–983. doi: 10.1016/0006-291x(89)90769-9. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Cameron V. Equimolar addition of oligoribonucleotides with T4 RNA ligase. Nucleic Acids Res. 1977 Jan;4(1):85–98. doi: 10.1093/nar/4.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Hidaka S., Miura K. Relationship between structure of the 5' noncoding region of viral mRNA and efficiency in the initiation step of protein synthesis in a eukaryotic system. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1012–1016. doi: 10.1073/pnas.79.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Nakagawa I., Sekine M., Hata T., Shimotohno K., Hiruta M., Miura K. Chemical synthesis of the 5'-terminal part bearing cap structure of messenger RNA of cytoplasmic polyhedrosis virus (CPV): m7G5'pppAmpG and m7G5'pppAmpGpU. Nucleic Acids Res. 1984 Mar 26;12(6):2939–2954. doi: 10.1093/nar/12.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]