Abstract
In up to half of all cases of community-acquired pneumonia (CAP), no pathogen can be identified with conventional diagnostic methods. The most common identified causative agent is Streptococcus pneumoniae. In this study, pneumococcal antibody responses during CAP were analyzed to estimate the contribution of the pneumococcus to all cases of CAP for epidemiological purposes. Pneumococcal antibodies against 14 different serotypes were measured in serum of hospitalized CAP patients. Patients participated in one of two consecutive clinical trials in a general 600-bed teaching hospital in the Netherlands (between October 2004 and June 2009). A significant pneumococcal immune response was defined as at least a 2-fold increase in antibody concentrations against a single serotype between an early (day 1) and a late (day 30) serum sample of each patient with an end concentration above 0.35 μg/ml. A total of 349 adult CAP patients participated in two consecutive clinical trials. For 200 patients, sufficient serum samples were available to determine antibody responses: 62 pneumococcal pneumonia patients, 57 nonpneumococcal pneumonia patients, and 81 patients with an unidentified causative agent. A significant immune response was detected in 45% (28/62 patients) of pneumococcal pneumonia patients, in 5% (3/57) of nonpneumococcal pneumonia patients, and in 28% (23/81) of patients with an unidentified causative agent. The estimated contribution of pneumococci in patients with an unidentified causative agent was calculated to be 57% (95% confidence interval, 36 to 86%). A substantial fraction of pneumococcal pneumonia patients do not elicit a serotype-specific immune response.
INTRODUCTION
Streptococcus pneumoniae, the pneumococcus, is an important human pathogen causing serious diseases such as pneumonia, meningitis, and sepsis in both children and adults (5, 12, 15, 18, 20). The reported estimated mortality associated with invasive pneumococcal disease varies from 7 to 43%, depending on pneumococcal serotype, medical history of the patient, and many other factors (2, 12, 14). Community-acquired pneumonia (CAP) is one of the most common causes of death worldwide and the leading cause of death by infection in the United States (9, 13, 19). S. pneumoniae is the most common identified pathogen in CAP (1, 9, 25). It causes 13 to 48% of CAP cases requiring hospital admission. S. pneumoniae is also the most prevalent microorganism in mixed CAP (7, 9). The exact contribution of the pneumococcus to all cases of CAP is not known, as in 17 to 48% of CAP patients no definite causative agent can be identified (1, 9, 25).
S. pneumoniae is surrounded by an external polysaccharide capsule. On the basis of differences in the composition of this capsule, 92 pneumococcal serotypes have been identified. The capsular polysaccharide is the single most important trigger to the host immune response, which is serotype specific (21). Anticapsular antibodies have been proven to be protective against pneumococcal infection. Thus, capsular polysaccharides form the basis of the available pneumococcal vaccines. A 23-valent polysaccharide vaccine is in use for adults (20). For infants, a 7-valent conjugate vaccine has been widely introduced (18, 20).
The response to vaccination is generally determined by measurement of antibody concentrations by serotype-specific enzyme-linked immunosorbent assays (ELISAs). With this method, monitoring antibody responses to at least the most prevalent pneumococcal serotypes requires large sample volumes and is time-consuming. Recently, a microsphere-based flow cytometric assay has been developed for simultaneous measurements of concentrations of IgG antibodies to 14 pneumococcal serotypes from a single sample (Luminex XMAP technology) (23).
As opposed to vaccination response studies, few data exist on the immune response during pneumococcal infection (11). The studies on anticapsular antibodies in pneumococcal pneumonia that have been performed have limited impact due to the use of small patient groups, non-serotype specificity, a lack of quantitative data, and/or the use of an outdated methodology (4, 17, 21, 22, 30).
In this study we measured serotype-specific antibody concentrations at different time points after the onset of CAP. Not only pneumococcal pneumonia patients but also patients infected with another respiratory pathogen or with an unidentified causative agent were included. By analyzing antibody responses in these groups, we aimed to estimate the relative contribution of the pneumococcus to all cases of CAP.
MATERIALS AND METHODS
Study population and clinical samples.
Serum samples were obtained from patients above 18 years of age hospitalized with CAP who participated in two consecutive clinical trials (8, 29a). Patients were hospitalized during the periods from October 2004 to August 2006 and November 2007 to June 2009 in a general 600-bed teaching hospital in the center of the Netherlands. Inclusion and exclusion criteria are described in more detail elsewhere (8, 29a). CAP was defined as a new infiltrate on the chest X ray, evaluated by an experienced radiologist, and at least 2 out of 6 clinical signs of pneumonia (cough, sputum production, temperature of >38.0°C or <36.0°C, abnormalities on auscultation compatible with pneumonia, leukocytosis or leukopenia, C-reactive protein concentration of >15 mg/dl) (8, 29a). Patients with a history of recent hospitalization or a congenital or acquired immunodeficiency (including patients recently treated with 20 mg prednisone per day for more than 3 days in the first trial and all patients treated with corticosteroids in the second trial) were excluded. In the second clinical trial, patients were randomized to receive corticosteroids or placebo on admission. Serum samples were obtained on days 1 (day of admission), 2, 3, 5, 10, and 30. Sera were stored at −80°C. Patient data collected were age, sex, comorbid conditions (diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure, hepatic failure, renal failure, and malignancies), duration of symptoms before hospital admission, use of antibiotics before admission, duration of hospital stay, and survival. The pneumonia severity index (PSI) was calculated on admission (10).
Pathogen identification.
The following diagnostic tests were performed on materials obtained at the day of admission to identify the causative agent of CAP. An expectorated sputum sample was Gram stained and cultured, as were at least two blood samples (BacT/Alert; bioMérieux, Marcy l'Etoile, France). Sputum was considered representative if in the Gram-stained sample less than 25 epithelial cells per view (at ×100 magnification) were present in the absence of leukocytes or if less than 50 epithelial cells per view were present in the presence of leukocytes. A respiratory pathogen cultured from sputum was considered to be of etiological significance only if it was cultured in relative abundance to the commensal flora of the throat and if the Gram stain revealed the microorganism in abundance (>10 microorganisms per view at ×1,000 magnification). TaqMan real-time PCRs were performed with sputum in order to detect DNA of atypical pathogens (Mycoplasma pneumoniae, Legionella pneumophila, Coxiella burnetii, Chlamydophila pneumoniae, and C. psittaci). Urine samples were used for detection of S. pneumoniae and L. pneumophila serogroup 1 antigens (BinaxNOW S. pneumoniae and BinaxNOW Legionella; Inverness Medical). Paired serum samples (the second sample was obtained 14 to 21 days after admission) were tested for the development of antibodies to M. pneumoniae, C. burnetii, L. pneumophila, and respiratory viruses by complement fixation reactions or standard ELISA. Pharyngeal swab samples were taken for viral culture and viral PCR. The diagnostic protocol is described in more detail elsewhere (8). On the basis of the causative agent identified, patients were divided into three groups: pneumococcal CAP, nonpneumococcal CAP, and CAP with an unidentified causative agent. S. pneumoniae cultured from either sputum or blood was serotyped by the Quellung reaction.
Measurement of pneumococcal polysaccharide antibody concentrations.
Antibodies were measured in two serum samples of the CAP patients: an early sample drawn at day 1 (days 0 to 3) after hospital admission and a late sample drawn at day 30 (days 11 to 100). Patients with a duration of symptoms of more than 10 days before hospital admission were excluded, as were patients for whom the interval between the earliest and latest serum samples available was less than 10 days. Antibodies against pneumococcal polysaccharides were measured on a Luminex platform (Luminex Corporation, Austin, TX), using a quantitative multiplex immunoassay (MIA): the XMAP pneumococcal immunity panel. This assay identifies serotype-specific anti-capsular polysaccharide IgG antibodies to the following serotypes: 1, 3, 4, 8, 9, 12, 14, 19, 23, 26, 51, 56, 57, and 68, according to the American nomenclature, corresponding to serotypes 1, 3, 4, 8, 9N, 12F, 14, 19F, 23F, 6B, 7F, 18C, 19A, and 9V, respectively according to the Danish nomenclature. Samples were diluted by a factor of 1:100 with diluent solution, included in the XMAP kit, composed of phosphate-buffered saline (PBS), pH 7.3, with pneumococcal cell wall polysaccharide (C-PS) and polysaccharide 22 (PS-22) added at working concentrations to inhibit nonspecific binding of anti-cell wall polysaccharides I and II. Diluted sera were incubated with a mixture of 14 microsphere types, each coated with antigen representing 1 of 14 pneumococcal serotypes. Nonbound antibodies were washed away, and each sample was treated with phycoerythrin-conjugated goat anti-human IgG. Unbound conjugate was washed away, and the bead suspensions were analyzed on the Luminex analyzer (IS 2.3). Seven standard dilutions, included in the XMAP kit, calibrated to the FDA 89-SF reference serum, were used to generate a standard curve for quantification of antibody concentrations. Three assay controls were prepared in duplicate with the test samples in each assay.
Statistical methods.
Microsoft Excel software (version 2000) and SPSS software (version 17.0) were used for statistical analyses. A significant increase in antibody concentrations, or a positive immune response, was defined as at least a 2-fold increase between the early and late serum samples of each patient with an end concentration above 0.35 μg/ml. The fold increase in antibody concentrations against a single pneumococcal serotype had to be at least two times greater than the fold increase against any other serotype. It was first assumed that all patients infected with S. pneumoniae have the same probability of a positive pneumococcal immune response. A second assumption was that positive pneumococcal immune responses detected in patients in whom another pathogen was identified by conventional methods were falsely positive. The probability of mixed infections was neglected. On the basis of these assumptions, the contribution of the pneumococcus in CAP patients with an unidentified causative agent was estimated by the positive predictive value (PPV), corrected by the sensitivity of the test. The PPV of a significant increase was calculated from results in all definite pneumococcal and definite nonpneumococcal pneumonia patients. The sensitivity of the test was established from results in pneumococcal pneumonia patients. Groups were compared by using Student's t test and Fisher's exact test where appropriate. A P value of <0.05 was considered to represent a statistically significant difference.
RESULTS
Pathogens.
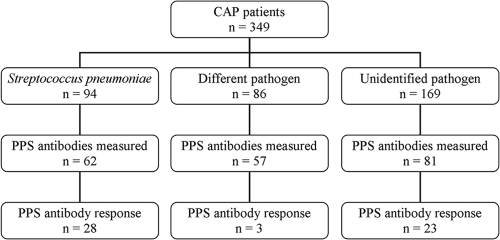
A total of 349 CAP patients participated in two clinical trials (clinical trial 1, 200 patients; clinical trial 2, 149 patients). In 94 patients (27%) S. pneumoniae was found to be the causative agent, in 86 patients (25%) a pathogen other than S. pneumoniae was identified, and in 169 patients (48%) no pathogen was identified (Fig. 1). Demographic data and disease characteristics of the patients are shown in Table 1. The most commonly identified pathogens in the nonpneumococcal pneumonia patients were C. burnetii (n = 19), Legionella species (n = 15), M. pneumoniae (n = 11), Haemophilus influenzae (n = 7), C. psittaci (n = 7), influenza A virus (n = 6), and Staphylococcus aureus (n = 4). In 29 (31%) of the pneumococcal pneumonia patients, diagnosis was based on a positive blood culture with S. pneumoniae, in 40 patients (43%) blood cultures remained negative but the urine antigen test for S. pneumoniae was positive, and in 25 patients (27%) etiologic diagnosis was based solely on a positive sputum culture with a Gram stain revealing purulence and Gram-positive diplococci in abundance. Pneumococcal strains isolated from 42 patients were available for serotyping (29 blood isolates, 13 sputum isolates). The most prevalent serogroups were serogroups 1 (n = 8), 9 (n = 8 [serotype 9V, n = 3; serotype not defined, n = 5]), 14 (n = 5), 8 (n = 4), and 3 (n = 4). Twenty-nine isolates (69%) had a serotype covered by the 14-plex panel used to measure antibodies, 7 isolates had a serogroup covered by the panel but could not be further subtyped due to technical reasons (serogroup 7 and 9), and 6 isolates were of a serotype not covered by the panel.
Fig. 1.
Flowchart of study design. A total of 349 CAP patients participated in two clinical trials. In 94 patients (27%) Streptococcus pneumoniae was found to be the causative agent by conventional microbiological techniques, in 86 patients (25%) a pathogen other than S. pneumoniae was identified, and in 169 patients (48%) no pathogen was identified. Sufficient samples for pneumococcal antibody measurements were available for 200 patients. A pneumococcal polysaccharide (PPS) antibody response was defined as at least a 2-fold increase in antibody concentrations with an end concentration above 0.35 μg/ml. The fold increase against a single pneumococcal serotype had to be at least two times greater than the fold increase against any other serotype.
Table 1.
Demographic data and disease characteristics of 349 community-acquired pneumonia patients grouped by causative agent
| Characteristic | Streptococcus pneumoniae (n = 94) | Different pathogen (n = 86) | Unidentified pathogen (n = 169) | Total (n = 349) |
|---|---|---|---|---|
| Mean age (yr) | 60 | 56 | 68a | 63 |
| No. (%b) of patients of male sex | 48 (51) | 58 (67)c | 101 (60) | 207 (59) |
| No. (%) of patients with one or more comorbid conditionsd | 44 (47) | 32 (37) | 80 (47) | 156 (45) |
| Mean PSIe on admission | 87 | 81f | 93 | 88 |
| No. (%) of patients with antibiotic use before admission | 13 (14)g | 30 (35) | 47 (28) | 90 (26) |
| Mean duration of hospitalization (days) | 14 | 11 | 12 | 12 |
| No. (%) of patients who survived | 90 (96) | 81 (94) | 160 (95) | 331 (95) |
Significant difference (P < 0.05) by Student's t test compared to the other two pathogen groups.
Percentages are calculated as the number of patients within the pathogen group.
Significant difference (P < 0.05) by Fisher's exact test compared to the S. pneumoniae group.
Comorbid condition is one or more of the following: diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure, hepatic failure, renal failure, and malignancies.
PSI is described elsewhere (10).
Significant difference (P < 0.05) by Student's t test compared to the unidentified pathogen group.
Significant difference (P < 0.05) by Fisher's exact test compared to the other two pathogen groups.
Pneumococcal polysaccharide antibody concentrations.
Of 200 patients, sufficient serum samples were available to determine the antibody response (110 from clinical trial 1 and 90 from clinical trial 2). All 200 patients were included in the study: 62 pneumococcal pneumonia patients, of whom 20 patients had an identified serotype of the infecting strain covered by the 14-plex antibody panel; 57 nonpneumococcal pneumonia patients; and 81 patients with an unidentified causative agent (Fig. 1). No differences in immune response rates were found between patients in the two trials or between patients who did and did not receive corticosteroids in trial 2.
Antibody responses in pneumococcal CAP.
Twenty-eight (45%) of 62 pneumococcal pneumonia patients elicited a significant increase in antibody concentrations against a single pneumococcal serotype (Table 2). Of the 18 patients with a positive blood culture, 8 (44%) elicited an immune response, whereas 20 (45%) of 44 patients in whom the etiologic diagnosis was based on a positive sputum culture and/or a positive urine antigen test elicited an immune response. In patients with a positive immune response, the mean PSI at admission was lower and the duration of hospital stay was shorter than those in patients who failed to elicit an immune response. Of the 20 pneumococcal pneumonia patients infected with an identified S. pneumoniae serotype covered by the 14-plex antibody panel, 11 (55%) elicited a significant immune response, all against the infecting serotype (Table 2). Of the 14 patients in whom the strain was isolated from blood, 6 (43%) elicited a response, whereas 5 (83%) of the 6 patients in whom the strain was isolated from sputum elicited a response. End concentrations varied from 0.74 to 12.24 μg IgG/ml (Table 3). Five of the nine patients who failed to elicit an immune response were infected with S. pneumoniae serotype 1. Of the patients infected with a pneumococcal serotype not included in the 14-plex panel, none elicited a significant antibody response.
Table 2.
Clinical characteristics of pneumococcal pneumonia patients grouped by immune response
| Group and characteristic | Positive immune responsea | Negative immune responsea | Total |
|---|---|---|---|
| All pneumococcal pneumonia patients | |||
| No. of patients in group | 28 | 34 | 62 |
| Mean age (yr) | 56 | 61 | 59 |
| No. (%b) of patients of male sex | 14 (50) | 17 (50) | 31 (50) |
| No. (%) of patients with one or more comorbid conditionsc | 11 (39) | 19 (56) | 30 (48) |
| Mean PSId on admission | 74e | 91 | 83 |
| No. (%) of patients with antibiotic use before admission | 6 (21) | 2 (6) | 8 (13) |
| No. (%) of patients with etiologic diagnosis by: | |||
| Blood culture | 8 (29) | 10 (29) | 18 (29) |
| Urine antigen test | 11 (39) | 17 (50) | 28 (45) |
| Sputum culture | 9 (32) | 7 (21) | 16 (26) |
| Mean duration of hospitalization (days) | 10e | 15 | 13 |
| No. (%) of patients who survived | 28 (100) | 34 (100) | 62 (100) |
| Pneumococcal pneumonia patients with identified serotype of infecting strain within antibody panel | |||
| No. of patients in group | 11 | 9 | 20 |
| Mean age (yr) | 50 | 55 | 52 |
| No. (%) of patients of male sex | 5 (45) | 8 (89) | 13 (65) |
| No. (%) of patients with one or more comorbid conditions | 4 (36) | 2 (22) | 6 (30) |
| Mean PSI on admission | 69 | 96 | 81 |
| No. (%) of patients with antibiotic use before admission | 1 (9) | 0 (0) | 1 (5) |
| No. (%) of patients with the following source of isolate: | |||
| Blood | 6 (55) | 8 (89) | 14 (70) |
| Sputum | 5 (45) | 1 (11) | 6 (30) |
| Mean duration of hospitalization (days) | 9 | 16 | 12 |
| No. (%) of patients who survived | 11 (100) | 9 (100) | 20 (100) |
Response was defined as at least a 2-fold increase in antibody concentrations with an end concentration above 0.35 μg/ml. The fold increase against a single pneumococcal serotype had to be at least two times greater than the fold increase against any other serotype.
Percentages are calculated from the number of patients within the response group.
Comorbid condition is one or more of the following: diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure, hepatic failure, renal failure, and malignancies.
PSI is described elsewhere (10).
Significant difference (P < 0.05) by Student's t test compared to negative immune response group.
Table 3.
Antibody concentrations against infecting serotype in 20 patients infected with Streptococcus pneumoniae
| Serotype of infecting strain | Positive immune responsea (n = 11) |
Negative immune responsea (n = 9) |
||||
|---|---|---|---|---|---|---|
| Concn (μg/ml) |
Fold increase | Concn (μg/ml) |
Fold increase | |||
| Early sampleb | Late samplec | Early sample | Late sample | |||
| 1 | 0.54 | 3.82 | 7.12 | 0.37 | 0.57 | 1.54d |
| 0.14 | 0.31 | 2.18d | ||||
| 0.32 | 0.30 | 0.95d | ||||
| 2.30 | 4.34 | 1.88d | ||||
| 0.02 | 1.09 | 43.94d | ||||
| 3 | 0.06 | 1.38 | 24.08d | |||
| 0.10 | 1.71 | 16.45 | ||||
| 4 | 0.01 | 1.47 | 100.95d | 0.10 | 0.14 | 1.41d |
| 8 | 0.26 | 0.74 | 2.86d | 0.09 | 0.10 | 1.21 |
| 1.22 | 12.24 | 10.04 | ||||
| 9V | 0.20 | 1.42 | 7.16 | 0.10 | 0.13 | 1.28d |
| 0.09 | 0.22 | 2.53d | ||||
| 14 | 0.02 | 1.67 | 110.89d | |||
| 0.05 | 1.15 | 22.84d | ||||
| 0.05 | 3.35 | 66.74 | ||||
| 19F | 0.11 | 6.03 | 54.76d | |||
Response was defined as at least a 2-fold increase in antibody concentrations with an end concentration above 0.35 μg/ml. The fold increase against a single pneumococcal serotype had to be at least two times greater than the fold increase against any other serotype. All positive antibody responses were against the infecting serotype.
The early serum sample was drawn at day 1 (days 0 to 3) of hospital admission.
The late serum sample was drawn at day 30 (days 11 to 100) of hospital admission.
Strain was isolated from blood.
Antibody responses in nonpneumococcal CAP.
Of the 57 CAP patients in whom a pathogen other than S. pneumoniae was found, 3 (5%) elicited a pneumococcal antibody response. In 2 of the 3 patients, L. pneumophila was identified by a positive PCR. The third patient was infected with C. psittaci, identified by a positive PCR and the development of antibodies in paired serological testing.
Antibody responses in CAP with unidentified causative agent.
Of the 81 CAP patients with an unidentified causative agent, 23 patients (28%) elicited an increase in antibody concentrations against a single pneumococcal serotype. The PPV of a significant immune response, calculated from antibody responses in definite pneumococcal and definite nonpneumococcal pneumonia patients, was 28/(28 + 3), which is equal to 0.90 (95% confidence interval [CI] = 0.74 to 0.98). Calculated sensitivity was 28/62, which is equal to 0.45 (95% CI = 0.32 to 0.58). The estimated contribution of the pneumococcus in the 81 patients with an unidentified causative agent was calculated as follows: number of patients in this group with a positive immune response × positive predictive value × inverse sensitivity = 23 × 0.90 × (1/0.45) = 46 patients (57%, 95% CI = 36 to 86%).
Serotype distribution of antibody responses.
Table 4 shows the serotype distribution of significant antibody responses. A response against serotype 14 was the most prevalent, followed by responses against serotypes 3, 7F, and 8. The serotype distribution of antibody responses in pneumococcal CAP patients was similar to that in CAP patients with an unidentified cause.
Table 4.
Serotype distribution of significant pneumococcal antibody responses in community-acquired pneumonia patients grouped by causative agent
| Serotype immune response | No. of patients |
|||
|---|---|---|---|---|
| Streptococcus pneumoniae (n = 28) | Different pathogen (n = 3) | Unidentified pathogen (n = 23) | Total (n = 54) | |
| 14 | 5 | 0 | 4 | 9 |
| 3 | 2 | 1 | 6 | 9 |
| 7F | 7 | 0 | 1 | 8 |
| 8 | 4 | 0 | 4 | 8 |
| 9V | 2 | 1 | 3 | 6 |
| 4 | 2 | 0 | 3 | 5 |
| 1 | 2 | 1 | 0 | 3 |
| 23F | 1 | 0 | 1 | 2 |
| 9N | 1 | 0 | 0 | 1 |
| 18C | 1 | 0 | 0 | 1 |
| 19F | 1 | 0 | 0 | 1 |
| 6B | 0 | 0 | 1 | 1 |
DISCUSSION
Identification of the causative agent of CAP is notoriously difficult. Because S. pneumoniae appears to be the most common pathogen causing CAP, many new diagnostic methods are being developed for the detection of this microorganism. The diagnostic yield of these, mostly PCR-based, methods is generally low (1, 16, 29). In this serology-based study, the estimated contribution of the pneumococcus in patients with an unidentified causative agent was 57%, a strikingly high proportion. This results in an estimated involvement of S. pneumoniae in 190 of all 349 (54%) CAP cases in our study: 94 patients identified by conventional methods and 96 patients estimated by a positive immune response (0.57 × 169 patients). These results are of primary importance for epidemiological reasons. As a late serum sample has to be obtained to monitor an antibody response, this serological diagnostic method is less useful for diagnosis of pneumococcal infection in the acutely ill individual patient.
The distribution of pathogens identified by conventional methods was similar to that found in earlier studies (9). The high incidence of S. pneumoniae serotype 1 was striking (14). Introduction of the 7-valent conjugate pneumococcal vaccine, which does not include serotype 1, for children in the Netherlands in 2006 may have caused the increased incidence of this serotype. A large national epidemiological study will be conducted on this subject. The high incidence of C. burnetii was due to large outbreaks of Q fever in the Netherlands from 2007 to 2009 (6).
Forty-five percent of patients in whom S. pneumoniae was found to be the causative agent elicited an immune response (Fig. 1; Table 2). Only immune responses against a limited number of serotypes could be detected, but also, in the patients known to be infected with a serotype included in the 14-plex antibody panel, the response rate was only 55% (Table 2; Table 3). Failure to elicit an antibody response could be the primary risk factor for CAP. In a follow-up study, CAP patients will therefore receive pneumococcal vaccination to monitor their ability to elicit a serotype-specific antibody response. The response rate after pneumococcal polysaccharide vaccination varies from 25 to 100% in a healthy population, depending on serotype (11).
The high mean PSI at admission and the long duration of hospital stay in pneumococcal pneumonia patients who failed to elicit an immune response compared to those for patients who did elicit a response are suggestive of more severe disease in the nonresponding patients (Table 2). Age and medical history of the patients are expected to be the most important factors contributing to these differences, but in our study, differences in age and comorbid conditions were not significant between responders and nonresponders. In earlier studies, the immune response after pneumococcal vaccination was diminished in the elderly, as was the occurrence of naturally acquired antibodies (24, 27). There was a nonsignificant difference in antibiotic use before admission between the two response groups. It could be speculated that antigen presentation was more effective in patients in whom (part of) the bacteria had been killed by antibiotics, resulting in a positive immune response. Within the group of patients with an identified serotype of the infecting strain included in the antibody panel, in most nonresponders S. pneumoniae was cultured from blood, although differences were not significant (Table 2). Failure to elicit an antibody response could have facilitated bacteremia in these patients. Most pneumococcal pneumonia patients who did not elicit an immune response were infected with S. pneumoniae serotype 1 (Table 3). In earlier studies, the immunogenicity of this serotype was intermediate (11, 28).
For the calculation of the contribution of S. pneumoniae in CAP, the first assumption made was that the probability of a positive pneumococcal immune response in patients infected with S. pneumoniae that was not detected by conventional methods was equal to the probability in patients in whom the pneumococcus was detected by conventional methods. This is a conservative approach, as it is reasonable to assume that the sensitivity of the pneumococcal antibody measurements was lower in patients in whom no causative agent could be identified. Diagnostic tests could have remained negative in these patients due to a low burden of pathogens that was below the threshold needed to trigger an immune response. Furthermore, the mean age of patients with an unidentified causative agent was significantly higher, and older age is also a well-known factor associated with impaired responses to pneumococcal polysaccharides (Table 1) (24, 27).
The second assumption made for statistical calculations regards the probability of coinfections. Of 57 patients in whom a pathogen other than S. pneumoniae was established to be the causative agent, 3 patients (5%) elicited a pneumococcal immune response (Fig. 1). CAP caused by multiple infecting microorganisms has been described to occur at incidences varying from 2 to 13% (7, 9). S. pneumoniae is the most prevalent microorganism in mixed CAP. A pneumococcal immune response in a nonpneumococcal pneumonia patient could be regarded as a sign of double infection. Indeed, a potential application of the measurement of pneumococcal antibodies during CAP could be to improve the otherwise difficult identification of S. pneumoniae in mixed infections. Nevertheless, for conservative calculations in this study, immune responses in nonpneumococcal pneumonia patients were considered false positives.
In this study, as in studies on the antibody response to vaccination, absolute values of antibody concentrations did vary considerably both between different pneumococcal serotypes and between patients infected with the same serotype (Table 3) (11). This variation complicates the application of a strict definition of a positive immune response. In this study, a significant antibody response was defined as at least a 2-fold increase in antibody concentration at least 2-fold greater than the fold increases against the other serotypes with an end concentration above 0.35 μg/ml. This definition was derived from previous studies on the antibody response to pneumococcal vaccination (11, 24). The threshold of 0.35 μg/ml is the designated protective concentration after pneumococcal conjugate vaccination in children recommended by a WHO working group (26). For the purpose of this study, 0.35 μg/ml is an arbitrary threshold and does not have any functional meaning. The 2-fold-increase cutoff was also defined rather arbitrarily. Post hoc analysis revealed that using instead a threshold of 0.17 or 0.70 μg/ml or a 3- or 4-fold increase as the cutoff for the definition of a positive immune response, response rates were equivalent and resulted in similar estimations of the contribution of the pneumococcus in all cases of CAP. Compared to the 28, 3, and 23 positive immune responses in the pneumococcal, nonpneumococcal, and unidentified pathogen groups, respectively, using the applied definition, the respective numbers would be 29, 3, and 22 using a 0.17-μg/ml threshold; 27, 3, and 22 using a 0.70-μg/ml threshold; 27, 2, and 21 using a 3-fold-increase cutoff; and 27, 2, and 20 using a 4-fold-increase cutoff. These results justify the definition of a positive immune response used in this study.
Measurement of pneumococcal anticapsular antibodies was shown to be a useful diagnostic tool in serotype-specific identification of S. pneumoniae as the causative agent of CAP. Twenty-eight percent of CAP patients in whom no causative agent could be identified with conventional diagnostic tests elicited a serotype-specific pneumococcal immune response. Taking into account the positive predictive value and sensitivity of the test, a total of 57% (95% CI = 36 to 86%) of these patients were estimated to have been infected with S. pneumoniae. A surprisingly large fraction of CAP patients infected with S. pneumoniae did not elicit a serotype-specific immune response.
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Abdeldaim G., et al. 2010. Usefulness of real-time PCR for lytA, ply, and Spn9802 on plasma samples for the diagnosis of pneumococcal pneumonia. Clin. Microbiol. Infect. 16:1135–1141 [DOI] [PubMed] [Google Scholar]
- 2. Alanee S. R., et al. 2007. Association of serotypes of Streptococcus pneumoniae with disease severity and outcome in adults: an international study. Clin. Infect. Dis. 45:46–51 [DOI] [PubMed] [Google Scholar]
- 3. Reference deleted.
- 4. Berntsson E., Broholm K. A., Kaijser B. 1978. Serological diagnosis of pneumococcal disease with enzyme-linked immunosorbent assay (ELISA). Scand. J. Infect. Dis. 10:177–181 [DOI] [PubMed] [Google Scholar]
- 5. Bogaert D., De Groot R., Hermans P. W. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144–154 [DOI] [PubMed] [Google Scholar]
- 6. Delsing C. E., Kullberg B. J. 2008. Q fever in the Netherlands: a concise overview and implications of the largest ongoing outbreak. Neth. J. Med. 66:365–367 [PubMed] [Google Scholar]
- 7. de Roux A., et al. 2006. Mixed community-acquired pneumonia in hospitalised patients. Eur. Respir. J. 27:795–800 [DOI] [PubMed] [Google Scholar]
- 8. Endeman H., et al. 2008. Clinical features predicting failure of pathogen identification in patients with community acquired pneumonia. Scand. J. Infect. Dis. 40:715–720 [DOI] [PubMed] [Google Scholar]
- 9. File T. M. 2003. Community-acquired pneumonia. Lancet 362:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fine M. J., et al. 1997. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 336:243–250 [DOI] [PubMed] [Google Scholar]
- 11. Go E. S., Ballas Z. K. 1996. Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J. Allergy Clin. Immunol. 98:205–215 [DOI] [PubMed] [Google Scholar]
- 12. Harboe Z. B., et al. 2009. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 6:e1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heron M., et al. 2009. Deaths: final data for 2006. Natl. Vital Stat. Rep. 57:1–134 [PubMed] [Google Scholar]
- 14. Jansen A. G., et al. 2009. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, and outcome. Clin. Infect. Dis. 49:e23–e29 [DOI] [PubMed] [Google Scholar]
- 15. Jefferson T., Ferroni E., Curtale F., Giorgi Rossi P., Borgia P. 2006. Streptococcus pneumoniae in western Europe: serotype distribution and incidence in children less than 2 years old. Lancet Infect. Dis. 6:405–410 [DOI] [PubMed] [Google Scholar]
- 16. Johansson N., Kalin M., Tiveljung-Lindell A., Giske C. G., Hedlund J. 2010. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 50:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalin M., Lindberg A. A. 1985. Antibody response against the type specific capsular polysaccharide in pneumococcal pneumonia measured by enzyme linked immunosorbent assay. Scand. J. Infect. Dis. 17:25–32 [DOI] [PubMed] [Google Scholar]
- 18. Lucero M. G., et al. 2009. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst. Rev. (4):CD004977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mandell L. A. 2004. Epidemiology and etiology of community-acquired pneumonia. Infect. Dis. Clin. North Am. 18:761–776,vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moberley S. A., Holden J., Tatham D. P., Andrews R. M. 2008. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. (1):CD000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Musher D. M., et al. 1993. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin. Infect. Dis. 17:66–73 [DOI] [PubMed] [Google Scholar]
- 22. Musher D. M., et al. 1997. Genetic regulation of the capacity to make immunoglobulin G to pneumococcal capsular polysaccharides. J. Invest. Med. 45:57–68 [PubMed] [Google Scholar]
- 23. Pickering J. W., et al. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589–596 [DOI] [PubMed] [Google Scholar]
- 24. Ridda I., et al. 2009. Immunological responses to pneumococcal vaccine in frail older people. Vaccine 27:1628–1636 [DOI] [PubMed] [Google Scholar]
- 25. Ruiz-Gonzalez A., Falguera M., Nogues A., Rubio-Caballero M. 1999. Is Streptococcus pneumoniae the leading cause of pneumonia of unknown etiology? A microbiologic study of lung aspirates in consecutive patients with community-acquired pneumonia. Am. J. Med. 106:385–390 [DOI] [PubMed] [Google Scholar]
- 26. Siber G. R., et al. 2007. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 25:3816–3826 [DOI] [PubMed] [Google Scholar]
- 27. Simell B., Lahdenkari M., Reunanen A., Kayhty H., Vakevainen M. 2008. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 15:1391–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soininen A., et al. 2009. IgG antibody concentrations after immunization with 11-valent mixed-carrier pneumococcal conjugate vaccine in efficacy trial against pneumonia among Filipino infants. Vaccine 27:2680–2688 [DOI] [PubMed] [Google Scholar]
- 29. Werno A. M., Murdoch D. R. 2008. Medical microbiology: laboratory diagnosis of invasive pneumococcal disease. Clin. Infect. Dis. 46:926–932 [DOI] [PubMed] [Google Scholar]
- 29a. World Health Organization International clinical trials registry platform ID NCT00471640. http://apps.who.int/trialsearch/Trial.aspx?TrialID=NCT00471640
- 30. Zysk G., et al. 2003. Immune response to capsular polysaccharide and surface proteins of Streptococcus pneumoniae in patients with invasive pneumococcal disease. J. Infect. Dis. 187:330–333 [DOI] [PubMed] [Google Scholar]