Abstract
Bacterial polysaccharides (PS) are T cell-independent antigens that do not induce immunologic memory and are poor immunogens in infants. Conjugate vaccines in which the PS is covalently linked to a carrier protein have enhanced immunogenicity that resembles that of T cell-dependent antigens. The Haemophilus influenzae type b (Hib) conjugate vaccine, which uses the outer membrane protein complex (OMPC) from meningococcus as a carrier protein, elicits protective levels of anti-capsular PS antibody (Ab) after a single dose, in contrast to other conjugate vaccines, which require multiple doses. We have previously shown that OMPC robustly engages Toll-like receptor 2 (TLR2) and enhances the early anti-Hib PS Ab titer associated with an increase in TLR2-mediated induction of cytokines. We now show that the addition of OMPC to the 7-valent pneumococcal PS-CRM197 conjugate vaccine during immunization significantly increases the anti-PS IgG and IgM responses to most serotypes of pneumococcus contained in the vaccine. The addition of OMPC also increased the likelihood of anti-PS IgG3 production against serotypes 4, 6B, 9V, 18C, 19F, and 23F. Splenocytes from mice who had received OMPC with the pneumococcal conjugate vaccine produced significantly more interleukin-2 (IL-2), IL-4, IL-6, IL-10, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) than splenocytes from mice who received phosphate-buffered saline (PBS) plus the conjugate vaccine. We conclude that OMPC enhances the anti-PS Ab response to pneumococcal PS-CRM197 conjugate vaccine, an effect associated with a distinct change in cytokine profile. It may be possible to reduce the number of conjugate vaccine doses required to achieve protective Ab levels by priming with adjuvants that are TLR2 ligands.
INTRODUCTION
Antibodies (Ab) against the capsular polysaccharides (PS) of the bacteria Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae are protective against invasive infection. These bacterial PS are T cell-independent (TI) type 2 antigens, induce predominantly IgM antibody without immune memory, and are poor immunogens in infants under 24 months of age (6, 8, 18). Most bacterial PS, unlike proteins, are not processed in endosomes of antigen-presenting cells (APC), do not transit to the APC cell surface with major histocompatibility complex class II (MHC II), and do not elicit T cell help (13, 24, 30, 31, 35). Conjugate vaccines, in which PS are covalently linked to a carrier protein, induce a PS-specific Ab response that resembles a T cell-dependent (Td) protein antigen response, with a shift to IgG, immune memory, immunogenicity in young infants, and PS-specific booster responses with multiple doses (2, 3, 24, 27, 28, 29).
A variety of carrier proteins have been utilized for conjugate vaccines, including CRM197, a nontoxic diphtheria toxin mutant (34), tetanus toxoid, and the outer membrane protein complex from Neisseriae meningitidis (OMPC; 7). The mechanism by which bacterial PS linked to proteins induces a Td-type enhanced anti-PS Ab response is poorly understood. Covalent conjugation of the PS to the carrier protein and cognate B7-CD28 and CD40-CD40L interactions between PS-specific B cells and T cells and MHC II antigens and T cell receptor are critical for the Td-type improved immune response (12). It is assumed that the carrier protein is processed in the endosome and then carrier protein-specific peptides are presented with MHC II on the surface of the APC to the CD4+ T cell, which is then stimulated to make cytokines, causing clonal expansion of PS-specific B cells (12, 15, 16, 31). Unfortunately, the cost of the vaccines and the multiple doses required to obtain protective antibody levels make their use challenging in the developing world.
In the late 1990s, an outbreak of Hib infection was observed in a heavily immunized Native American population in Alaska after a change from the Hib-OMPC vaccine to one with a different carrier protein (9). Previous investigations had shown that the Hib-OMPC vaccine, unlike other Hib conjugate vaccines, elicited protective anticapsular antibody levels after a single dose, important in this population in which Hib infections occurred at an early age (11). Reinstitution of the OMPC vaccine for the initial dose of Hib immunization resulted in the termination of the Hib outbreak. Subsequent investigations revealed that the OMPC carrier protein, which contained neisserial porins, robustly engaged human Toll-like receptor 2 (TLR2), activated macrophages, and upregulated B cell proliferation and activation (1, 10, 19, 20, 37–39). In addition, the augmented anti-PS antibody levels that occurred with Hib-OMPC vaccine in a mouse model were associated with cytokines elicited by TLR-2 activation and were TLR2 dependent (10, 19). Thus, the early anti-PS antibody response to a single dose of Hib-OMPC vaccine is thought to be due to TLR2 engagement by the carrier protein.
Since many pneumococcus (Pn) serotypes cause disease in children, in contrast to Hib (14), conjugate vaccines for pneumococcus consist of multiple capsular serotypes, each separately conjugated to the carrier protein. The current U.S.-licensed heptavalent pneumococcal conjugate vaccine contains seven purified serotypes of PS (types 4, 6B, 9V, 14, 18C, 19F, and 23F; this vaccine has recently expanded to include 13 serotypes) individually conjugated to CRM197. This conjugate vaccine induces protective anti-PS Ab titers to all seven serotypes in infants, effectively protecting infants from infection with homologous serotypes of pneumococcus, but only after three or four doses (2, 27, 29). Thus, like other available conjugate vaccines, utilization in the developing world is limited due to the frequency of immunizations required to achieve protection.
Activation of TLR2 on B cells has recently been shown to be important for the anti-PS antibody response after pneumococcal immunization and to increase secretion of IgM (33, 36, 39). The results of numerous investigations suggest that engaging TLR and, particularly, TLR2 is important to anti-PS antibody responses after immunization with conjugate vaccines or challenge by whole organisms. Since OMPC engages TLR2 robustly and is already utilized successfully as a carrier protein for a Hib vaccine, we hypothesized that OMPC could also be used to augment the anti-PS antibody response induced by immunization with the heptavalent pneumococcal conjugate vaccine. Our data suggest that simultaneous administration of OMPC with the pneumococcal PS-CRM197 conjugate vaccine significantly increases the anti-PS antibody response to a majority of pneumococcal serotypes contained in the vaccine.
MATERIALS AND METHODS
Antigens.
Pneumococcal PS-CRM197 conjugate vaccines against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F were provided as separate vaccines by Wyeth Vaccines LLC (Pfizer, Inc., Sanford, NC). Unconjugated pneumococcal capsular PS of the same serotypes were obtained from American Type Culture Collection (Rockville, MD). Pneumococcal cell wall polysaccharide (C-PS) was obtained from the FDA (Bethesda, MD). Purified outer membrane protein complex (OMPC) from Neisseriae meningitidis, used as the carrier protein for the Hib-OMPC conjugate vaccine, was supplied by Merck Vaccines (5.0 mg/ml 0.9% sodium chloride; Merck Vaccines, West Point, PA).
Immunization of mice.
Six-week-old female BALB/cByJ mice (Jackson Laboratories, Bar Harbor, ME) were immunized intraperitoneally on days 0 and 14 with 100 μg of OMPC mixed with serotype 4-, 6B-, 9V-, 14-, 18C-, 19F-, and 23F-CRM197 conjugate vaccines containing 5 μg of each pneumococcal PS. Control mice received pneumococcal PS-CRM197 conjugate vaccines mixed with the same sterile phosphate-buffered saline (PBS) used to dilute OMPC. Mice were bled from the tail vein weekly for 6 weeks, and the sera were screened for anti-PS antibodies via enzyme-linked immunosorbent assay (ELISA) as described below. Other mice were also immunized as described above and then euthanized at 4 weeks postimmunization, and ELISPOT was performed on purified splenocytes to measure cytokine secretion after ex vivo restimulation with either PBS or CRM197.
ELISA for serum antibodies to pneumococcal PS.
ELISA to detect antibody to pneumococcal PS in mouse sera was performed as previously described (5, 22). Briefly, 96-well PolySorp plates (Nunc, Roskilde, Denmark) were coated with 10 μg/ml of 4, 6B, 9V, 14, 18C, 19F, or 23F capsular PS (ATCC) in 50 μl of PBS at 4°C overnight. The plates were then washed (PBS–0.5% Tween 20 [PBST]) 3 times and blocked with 150 μl of 1% bovine serum albumin in PBS (BSA). Serum from each mouse was then diluted 1 to 150 with PBS after incubation with 50 μg/ml of C-PS to absorb anti-C-PS antibodies. Fifty microliters of each serum sample dilution was added to each well and incubated at room temperature for 2 h, and then plates were washed with PBST and goat anti-mouse Ig, IgG, IgM, and IgG3 conjugated to alkaline phosphatase were added (Southern Biotech). The plates were washed again with PBST and developed with p-nitrophenyl phosphate (Sigma, St. Louis, MO) as the substrate, and absorbances were read as optical densities at 410 nm in an ELISA reader (Bio-Rad, Hercules, CA).
Detection of cytokine-secreting cells by ELISPOT.
Enzyme-linked immunospot assay (ELISPOT) was used to measure splenocyte cytokine secretion as described previously (15, 16). Spleen cells were isolated from mice 4 weeks after immunization with conjugate vaccine with and without OMPC and placed in culture with PBS or 5 μg/ml of CRM197 at 1 × 107 cells/ml. Polyvinylidene difluoride (PVDF)-based microtiter plates coated with anti-mouse interleukin-1 (IL-1), IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) antibodies (5 μg/ml of each) (BD Pharmingen) were washed and blocked with 10% fetal bovine serum–PBS. Cells were transferred to the plates (5 × 105 cells/well) and incubated at 37°C. Cells were then washed off the ELISPOT plate membrane, and biotinylated anti-mouse IL-1, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IFN-γ, and TNF-α antibodies (2 μg/ml; BD Pharmingen) and streptavidin-alkaline phosphatase conjugate were added to the plates. The plates were incubated and then washed with PBST and developed with AEC (3-amino-9-ethylcarbazole; BD Pharmingen) as the substrate. Spots were enumerated using an ELISPOT reader (Cellular Technology Ltd., Cleveland, OH).
Statistics.
Student's t test was used to compare the differences between mean absorbance results in the PS-specific antibody ELISA. The Z test was utilized to compare differences between groups of mice producing anti-PS IgG3. P values of less than 0.05 were considered significant.
RESULTS
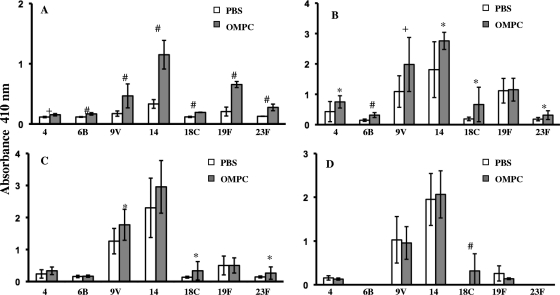
Total serotype-specific anti-pneumococcal PS Ig in sera from mice immunized with Pn PS-CRM197 conjugate vaccines mixed with 100 μg of OMPC (OMPC-Pn-CRM197) was significantly higher than in sera from mice receiving conjugate vaccine mixed with PBS at weeks 2 and 3 postimmunization for all seven serotypes (Fig. 1 A and B). The significant differences in total anti-PS Ig persisted for three serotypes at week 4 postimmunization but for only one serotype at week 6 postimmunization (Fig. 1C and D).
Fig. 1.
Total Ig response in mice immunized with pneumococcal PS-CRM197 conjugate vaccine along with PBS or 100 μg of OMPC. (A) Week 2. (B) Week 3. (C) Week 4. (D) Week 6. Comparison of results for OMPC recipients and PBS recipients is shown as follows: *, P < 0.05; +, P ≤ 0.01; and #, P ≤ 0.001. n = 5 mice per group; the experiment was repeated three times with similar results. Error bars show standard deviations.
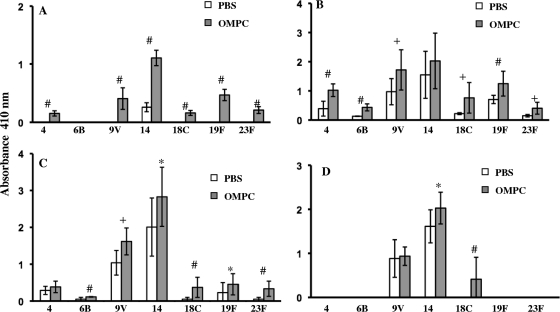
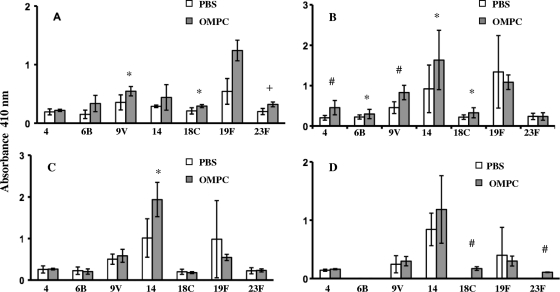
Similar results were observed for serotype-specific anti-pneumococcal PS IgG made by mice immunized with OMPC-Pn-CRM197. Mice made significantly more antibodies to six serotypes in the OMPC group at week 2 postimmunization and to all seven serotypes at week 3 postimmunization (Fig. 2 A and B). These differences persisted at week 4 postimmunization. IgG antibody, however, declined by week 6, with several serotypes having undetectable titers and only two serotypes showing differences in IgG antibody titers compared to the titers in PBS controls (Fig. 2C and D). Finally, the addition of OMPC to immunization with pneumococcal PS-CRM197 enhanced IgM anti-Pn PS antibody production for three serotypes at week 2 postimmunization and for five serotypes at week 3 but only for one serotype by week 4 and 2 serotypes by week 6 (Fig. 3 A, B, C, and D).
Fig. 2.
IgG responses in mice immunized with pneumococcal PS-CRM197 conjugate vaccine along with PBS or 100 μg of OMPC. (A) Week 2. (B) Week 3. (C) Week 4. (D) Week 6. Comparison of results for OMPC recipients and PBS recipients is shown as follows: *, P < 0.05; +, P ≤ 0.01; #, P ≤ 0.001. n = 5 mice per group; the experiment was repeated three times with similar results. Error bars show standard deviations.
Fig. 3.
IgM response in mice immunized with pneumococcal PS-CRM197 conjugate vaccine with PBS or 100 μg of OMPC. (A) Week 2. (B) Week 3. (C) Week 4. (D) Week 6. Comparison of results for OMPC recipients and PBS recipients is shown as follows: *, P < 0.05; +, P ≤ 0.01; #, P ≤ 0.001. n = 5 mice per group; the experiment was repeated three times with similar results. Error bars show standard deviations.
The numbers of mice that made anti-PS IgG3, the dominant subclass made to T cell-independent type 2 (TI-2) antigens in mice (23), were significantly greater in the OMPC-pneumococcal PS-CRM197 vaccine groups than in the groups receiving PBS-vaccine controls for all serotypes except type 14, which elicited high titers of IgG3 in both groups regardless of OMPC and which was the most immunogenic for IgG and IgM production as well (Table 1). In fact, IgG3 was not detectable in any of the mice receiving the conjugate vaccine and PBS for the remaining serotypes, while it was detected in 47 to 80% of mice receiving vaccine with OMPC (Table 1).
Table 1.
IgG3 responses in mice receiving vaccines against seven pneumococcal serotypes along with OPMC or PBS
| Pneumococcal serotype | Treatment group | No. of mice producing IgG3 | Total no. of mice | % of mice producing IgG3 | P value |
|---|---|---|---|---|---|
| 4 | PBS | 0 | 14 | 0 | 0.001 |
| OPMC | 10 | 15 | 67 | ||
| 6B | PBS | 0 | 14 | 0 | 0.012 |
| OPMC | 7 | 15 | 47 | ||
| 9V | PBS | 0 | 14 | 0 | <0.001 |
| OPMC | 12 | 15 | 80 | ||
| 14 | PBS | 12 | 14 | 86 | 0.433 |
| OPMC | 15 | 15 | 100 | ||
| 18C | PBS | 0 | 14 | 0 | 0.001 |
| OPMC | 10 | 15 | 67 | ||
| 19F | PBS | 0 | 14 | 0 | 0.001 |
| OPMC | 10 | 15 | 67 | ||
| 23F | PBS | 0 | 14 | 0 | 0.012 |
| OPMC | 7 | 15 | 47 |
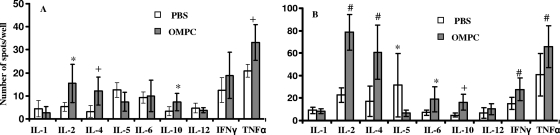
In order to determine whether different T cells were stimulated in recipients of conjugate vaccine with and without OMPC, some mice in each conjugate vaccine immunization group were sacrificed at 4 weeks postimmunization, their spleens removed, and splenocyte cytokine production measured by ELISPOT after stimulation with either PBS or CRM197. We found that OMPC-pneumococcal PS-CRM197 recipients had significant increases in IL-2-, IL-4-, IL-10-, and TNF-α-producing splenocytes compared to the levels in PBS-Pn-CRM197 vaccine controls (Fig. 4 A). Splenocytes from OMPC-pneumococcal PS-CRM197 recipients that were stimulated ex vivo with CRM197 had significant increases in IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α compared to splenocytes from PBS vaccine controls (Fig. 4B). Interestingly, significantly more IL-5-producing splenocytes were observed in mice receiving PBS than in mice receiving OMPC after re-exposure to CRM197 (Fig. 4B).
Fig. 4.
Mouse splenocyte cytokine recall responses after immunization with pneumococcal PS-CRM197 conjugate vaccine with or without 100 μg of OMPC. *, P < 0.05; +, P ≤ 0.01; #, P ≤ 0.001. (A) Spleen cells were stimulated by PBS in vitro. (B) Spleen cells were stimulated by CRM197. n = 5 mice per group; the experiment was repeated three times with similar results. Error bars show standard deviations.
DISCUSSION
Capsular PS-protein conjugate vaccines have been remarkably effective in preventing invasive pneumococcal infections in infants and children. Significant decreases in rates of pneumococcal bacteremia and meningitis in children have occurred in countries with good medical infrastructure and the ability to buy these vaccines. However, three to four doses of multivalent vaccine are required to elicit protective levels of anticapsular antibodies, making the use of the vaccine problematic in the developing world. Strategies to decrease the number of doses required to achieve protection would thus be enormously beneficial.
We previously found that the OMPC carrier protein robustly engages TLR2 and is responsible for the enhanced anti-PS antibody response observed with the Hib-OMPC vaccine (19). We thus hypothesized that stimulation of TLR2 could also increase the immune response to other pathogenic bacterial PS antigens, such as pneumococcal PS. Recently, a variety of TLR agonists were found to improve the anti-type 3 pneumococcal PS antibody titers in mice immunized with unconjugated pneumococcal PS (32, 33). In those experiments, the concomitant use of a TLR2 agonist and type 3 PS was not as effective as the use of the TLR agonist 2 days after PS immunization. However, mice were screened only 5 days after immunization in those experiments, a time point that in our experiments does not yet show increased anti-PS antibody responses (33). In contrast, we have previously shown that TLR agonists, such as CpG, can act as adjuvants when given simultaneously with the Td form of the pneumococcal PS, such as pneumococcal PS-CRM197 (5). Furthermore, to be realistic for human administration, TLR-based adjuvants would ideally be mixed with the actual vaccine to be administered, since follow-up immunization with an adjuvant, particularly in children, is not practical. Thus, coadministration of OMPC with pneumococcal PS-CRM197 vaccine was utilized in our experimental design.
TLR engagement during immunization with unconjugated pneumococcal PS was previously shown to increase the level of anti-PS IgG3 (33). We now also find that TLR2 engagement via the use of OMPC during immunization with the pneumococcal PS-CRM197 conjugate vaccine induces significantly more mice to make IgG3 anti-PS antibody than immunization with conjugate vaccine only. Since IgG3 is the dominant isotype made to bacterial PS in mice and is the isotype that is most efficient in inducing opsonophagocytosis of pneumococcus cells (23, 33), it seems possible that the use of OMPC expands isotypes that may have significant functional effects in vivo. Interestingly, mice immunized with type 14-CRM197 conjugate made IgG3 whether or not OMPC was present. This vaccine alone, without OMPC, did not stimulate HEK cells expressing human TLR2 or -4 (data not shown), but it was consistently the most immunogenic in BALB/c mice. Further studies will be required to determine how the structure of the type 14 PS, attached to the identical carrier protein as other serotypes, can enhance immunogenicity and shift the IgG isotype to IgG3.
TLR2 activation promotes B cell activation and differentiation, particularly for ligands crucial to B cell-T cell cross talk (4). It is now also recognized that endosomal TLR engagement may synergize with B cell receptors to yield enhanced B cell differentiation and antibody production (26). Although many B cells express low levels of TLR2, CD19inter germinal center cells are TLR2 positive and differentiate when exposed to TLR2 agonists (10). In addition, TLR2-activated monocytes induce IgM secretion by B cells. Thus, it seems likely that TLR2 engagement stimulates PS-specific antibody production in two ways when administered with conjugate vaccine, first, as a direct stimulus to the B cell, enhancing differentiation and isotype switching, and second, by increasing cytokine secretion by TLR2-expressing cells that enhances both B cell expansion and carrier protein-specific Th responses. Providing increased T cell help for PS-specific B cells that also present conjugate-derived peptides to T cells may explain why simultaneous administration of our TLR2 ligand was an effective way to increase anti-PS antibody levels, while being ineffective for PS alone, in which neither B cells nor other antigen-processing cells present carrier protein-derived peptides to T cells. Indeed, B and CD4+ T-cell expression of TLR2 is crucial for the antibacterial antibody response to intact S. pneumoniae cells (36).
Recent data suggest that the TACI (transmembrane activator and calcium modulator) receptor, which engages with the cytokines BAFF (B cell-activating factor) and APRIL (a proliferation-inducing ligand), plays a key role in the production and regulation of antibodies to TI antigens, such as PS. The ligands BAFF and APRIL bind to TACI, BAFF receptor (BAFFR), and BCMA (B cell maturation antigen) on B cells and regulate B cell survival, activation, and isotype switching. BAFF is expressed by monocytes and dendritic cells and binds to BAFFR, BCMA, and TACI. APRIL binds only to TACI and BCMA. A deficiency in BAFF or abnormality, blockade, or absence of the receptor TACI decreases the number of B cells and disrupts humoral immunity to TI antigens, including the encapsulated bacteria. TLR agonists increase the surface expression of BAFF receptors on B cells and act in a costimulatory fashion with BAFF to increase B cell proliferation and immunoglobulin secretion (17, 25). It thus seems possible that OMPC stimulation via TLR2 may also increase anti-PS antibody titers by directly costimulating PS-specific B cells to proliferate and secrete immunoglobulin.
Interestingly, we found that the increased anti-PS antibody levels seen after pneumococcal conjugate-plus-OMPC immunization were most pronounced in the one to two weeks immediately postimmunization and were less pronounced four to six weeks postimmunization. Similar findings were obtained for humans administered the Hib-OMPC vaccine, where protective levels of anti-PS antibody were induced by a single dose more frequently than with other conjugate vaccines, only to level off and become similar to the immunogenicity induced by the other vaccines after subsequent doses (11). Thus, the use of OMPC may provide earlier protection from invasive pneumococcal infections with fewer doses but, over the long term, does not provide higher levels of anti-PS antibody that vary significantly from those obtained with conjugate vaccine alone.
Despite being conjugated to the same carrier protein, we found that some serotypes of the heptavalent pneumococcal conjugate vaccine were poor immunogens in mice after two immunizations, while others were highly immunogenic (21). Although mouse models do not always predict the immunogenicity of vaccines in humans, there are strong parallels with data obtained for humans. For example, adult volunteers receiving a single dose of the same heptavalent pneumococcal vaccine utilized in our mouse experiments exhibited marked differences in the immunogenicity of the various pneumococcal serotypes, with some being excellent and others much less immunogenic (16). Similar results have been found in infants receiving multiple doses of the pneumococcal PS-CRM197 conjugate vaccine, although all serotypes elicited titers that were felt to be protective against invasive pneumococcal disease (27). These data reinforce the need for adjuvants that can elicit protective titers to all pneumococcal serotypes with fewer doses.
The development of effective conjugate vaccines has been instrumental in the remarkable decreases in invasive infections with Hib and, now, pneumococcus bacteria in the developed world. Effective adjuvants could allow the expansion of this success to less developed countries that have earlier ages of onset of invasive disease from encapsulated bacteria and lack the infrastructure for sequential repeated dosing of conjugate vaccines. This study demonstrates that a TLR2 agonist that is already widely and safely in use for the Hib vaccine significantly increases the antibody response to most of the serotypes of the pneumococcal conjugate vaccine. Further experiments will be required to show that the functionality of OMPC-augmented antibodies is as effective as nonadjuvant-induced antibodies. It seems likely that this finding could be exploited to induce protective antibody levels in infants after fewer doses of the pneumococcal conjugate vaccine.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI-32596 and AI-46667.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Ambrosino D. M., et al. 1992. Effect of Haemophilus influenzae polysaccharide outer membrane protein complex conjugate vaccine on macrophages. J. Immunol. 149:3978. [PubMed] [Google Scholar]
- 2. Black S., et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187–195 [DOI] [PubMed] [Google Scholar]
- 3. Black S. B., et al. 1991. Efficacy in infancy of oligosaccharide conjugate Haemophilus influenzae type b (HbOC) vaccine in a United States population of 61,080 children. Pediatr. Infect. Dis. J. 10:97–104 [DOI] [PubMed] [Google Scholar]
- 4. Borsutzky S., et al. 2005. The mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B lymphocytes via the TLR2 without the need of accessory cells. J. Immunol. 174:6308–6313 [DOI] [PubMed] [Google Scholar]
- 5. Chu R. S., et al. 2000. CpG oligodeoxynucleotides act as adjuvants for pneumococcal polysaccharide-protein conjugate vaccines and enhance antipolysaccharide immunoglobulin (IgG2a) and IgG3 antibodies. Infect. Immun. 68:1450–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowan M. J., et al. 1978. Pneumococcal polysaccharide immunization in infants and children. Pediatrics 62:721–727 [PubMed] [Google Scholar]
- 7. Donnelly J. J., Deck R. R., Liu M. A. 1990. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseriae meningitidis outer membrane protein vaccine. J. Immunol. 143:3071–3079 [PubMed] [Google Scholar]
- 8. Douglas R. M., Paton J. C., Duncan S. J., Hansman D. J. 1983. Antibody response to pneumococcal vaccination in children younger than five years of age. J. Infect. Dis. 148:131–137 [DOI] [PubMed] [Google Scholar]
- 9. Galil K., et al. 1999. Reemergence of invasive Haemophilus influenzae type b disease in a well-vaccinated population in remote Alaska. J. Infect. Dis. 179:101. [DOI] [PubMed] [Google Scholar]
- 10. Ganley-Leal L. M., Liu X., Wetzler L. M. 2006. Toll-like receptor 2-mediated human B cell differentiation. Clin. Immunol. 120:272–284 [DOI] [PubMed] [Google Scholar]
- 11. Granoff D. M., Holmes S. J. 1991. Comparative immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugate vaccines. Vaccine 9:S30. [DOI] [PubMed] [Google Scholar]
- 12. Guttormsen H. K., et al. 1999. Cognate stimulatory B-cell and T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect. Immun. 67:6375–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harding C. V., Roof R. W., Allen P. M., Unanue E. R. 1991. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc. Natl. Acad. Sci. U. S. A. 88:2740–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hausdorff W. P., Bryant J., Kloek C., Paradiso P. R., Siber G. R. 2000. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin. Infect. Dis. 30:122–140 [DOI] [PubMed] [Google Scholar]
- 15. Kamboj K. K., King C., Greenspan N. S., Kirchner H. L., Schreiber J. R. 2001. Immunization with Haemophilus influenzae type b-CRM197 conjugate vaccine elicits a mixed Th1 and Th2 CD4+ T cell cytokine response that correlates with the isotype of antipolysaccharide antibody. J. Infect. Dis. 184:931–935 [DOI] [PubMed] [Google Scholar]
- 16. Kamboj K. K., Kirchner H. L., Kimmel R., Greenspan N. S., Schreiber J. R. 2003. Significant variation in serotype-specific immunogenicity of the seven-valent Streptococcus pneumoniae capsular polysaccharide-CRM197 conjugate vaccine occurs despite vigorous T cell help induced by the carrier protein. J. Infect. Dis. 187:1629–1638 [DOI] [PubMed] [Google Scholar]
- 17. Katsenelson N., et al. 2007. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur. J. Immunol. 37:1785–1795 [DOI] [PubMed] [Google Scholar]
- 18. Kayhty H., Karanko V., Peltola H., Makela P. H. 1984. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics 74:857–865 [PubMed] [Google Scholar]
- 19. Latz E., Franko J., Golenbock D. T., Schreiber J. R. 2004. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J. Immunol. 172:2431–2438 [DOI] [PubMed] [Google Scholar]
- 20. Massari P., et al. 2002. Immune stimulation by neisserial porins is toll-like receptor 2 and Myd88 dependent. J. Immunol. 168:1533–1537 [DOI] [PubMed] [Google Scholar]
- 21. Mawas F., Feavers I. M., Corbel M. J. 2000. Serotype of Streptococcus pneumoniae capsular polysaccharide can modify the Th1/Th2 cytokine profile and IgG subclass response to pneumococcal-CRM197 conjugate vaccines in a murine model. Vaccine 19:1159–1166 [DOI] [PubMed] [Google Scholar]
- 22. McCool T. L., Harding C. V., Greenspan N. S., Schreiber J. R. 1999. B- and T-cell immune responses to pneumococcal conjugate vaccines: divergence between carrier- and polysaccharide-specific immunogenicity. Infect. Immun. 67:4862–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLay J., et al. 2002. Gamma 3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides. II. Increased susceptibility to fatal pneumococcal sepsis due to absence of anti-polysaccharide IgG3 is corrected by induction of anti-polysaccharide IgG1. J. Immunol. 168:3437–3443 [DOI] [PubMed] [Google Scholar]
- 24. Mond J. J., Lees A., Snapper C. M. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655–692 [DOI] [PubMed] [Google Scholar]
- 25. Ng L. G., et al. 2006. BAFF costimulation of Toll-like receptor activated B-1 cells. Eur. J. Immunol. 36:1837–1846 [DOI] [PubMed] [Google Scholar]
- 26. Pone E. J., et al. 2010. Toll-like receptors and B cell receptors synergize to induce immunoglobulin class switch DNA recombination: relevance to microbial antibody responses. Crit. Rev. Immunol. 30:1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rennels M. B., et al. 1998. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 101:604–611 [DOI] [PubMed] [Google Scholar]
- 28. Schneerson R., Barrera O., Sutton A., Robbins J. B. 1980. Preparation, characterization and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 152:361–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shinefield H. R., et al. 1999. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18:757–763 [DOI] [PubMed] [Google Scholar]
- 30. Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. 1973. Responses of children immunized with the capsular polysaccharide of Haemophilus influenzae, type b. Pediatrics 52:637–644 [PubMed] [Google Scholar]
- 31. Stein K. E. 1992. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 165(Suppl. 1):S49–S52 [DOI] [PubMed] [Google Scholar]
- 32. Taillardet M., et al. 2009. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood 114:4432–4440 [DOI] [PubMed] [Google Scholar]
- 33. Taillardet M., et al. 2010. Toll-like receptor agonists allow generation of long-lasting antipneumococcal humoral immunity to a plain polysaccharide vaccine. J. Infect. Dis. 202:470–479 [DOI] [PubMed] [Google Scholar]
- 34. Uchida T., Pappenheimer A. M., Jr., Harper A. A. 1972. Reconstitution of diphtheria toxin from two nontoxic cross-reacting mutant proteins. Science 175:901–903 [DOI] [PubMed] [Google Scholar]
- 35. Unanue E. R., Cerottini J. C. 1989. Antigen presentation. FASEB J. 13:2496–2502 [DOI] [PubMed] [Google Scholar]
- 36. Vasilevsky S., et al. 2008. B and CD4+ T-cell expression of TLR2 is critical for optimal induction of a T-cell-dependent humoral immune response to intact Streptococcus pneumoniae. Eur. J. Immunol. 38:3316–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wetzler L. M. 1994. Immunopotentiating ability of neisserial outer membrane proteins: use as an adjuvant for poorly immunogenic substances and potential use as vaccines. Ann. N. Y. Acad. Sci. 730:367–370 [DOI] [PubMed] [Google Scholar]
- 38. Wetzler L. M., Ho Y., Reiser H. 1996. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J. Exp. Med. 183:1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Q., et al. 2010. Bacterial lipoproteins differentially regulate human primary and memory CD4+ T and B cell responses to pneumococcal protein antigens through toll-like receptor 2. J. Infect. Dis. 201:1753–1763 [DOI] [PubMed] [Google Scholar]