Abstract
Blastomycosis is a severe, commonly fatal infection caused by the dimorphic fungus Blastomyces dermatitidis in dogs that live in the United States, Canada, and parts of Africa. The cost of treating an infection can be expensive, and no vaccine against this infection is commercially available. A genetically engineered live-attenuated strain of B. dermatitidis lacking the major virulence factor BAD-1 successfully vaccinates against lethal experimental infection in mice. Here we studied the safety, toxicity, and immunogenicity of this strain as a vaccine in dogs, using 25 beagles at a teaching laboratory and 78 foxhounds in a field trial. In the beagles, escalating doses of live vaccine ranging from 2 × 104 to 2 × 107 yeast cells given subcutaneously were safe and did not disseminate to the lung or induce systemic illness, but a dose of <2 × 106 yeast cells induced less fever and local inflammation. A vaccine dose of 105 yeast cells was also well tolerated in vaccinated foxhounds who had never had blastomycosis; however, vaccinated dogs with prior infection had more local reactions at the vaccine site. The draining lymph node cells and peripheral blood lymphocytes from vaccinated dogs demonstrated gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF) specifically in response to stimulation with Blastomyces antigens. Thus, the live-attenuated vaccine against blastomycosis studied here proved safe, well tolerated, and immunogenic in dogs and merits further studies of vaccine efficacy.
INTRODUCTION
Blastomyces dermatitidis is the causative fungus of blastomycosis, which is a potentially life-threatening systemic infection in humans and other mammals, most often dogs. In the United States, blastomycosis is restricted to locations in the southeastern, south-central, and Upper Midwest states, where environmental conditions favor the growth of B. dermatitidis in the soil. In areas of high endemicity, annual incidence rates of canine blastomycosis of 1 to 2% have been reported (2). The fungus is presumably inhaled from infected soil into the lungs of a susceptible animal, initially causing a pneumonia. In dogs, the fungus often disseminates from the lungs to the skin, eyes, lymph nodes, and bones. The mortality rate exceeds 90% for dogs that do not receive prompt antifungal treatment (9). Blastomycosis is most commonly seen in large dogs, where the cost of treatment can exceed $1,000. Vaccine prevention would offer a cost-effective alternative for those dogs at risk in areas of high endemicity, but no commercial vaccine is available.
Blastomyces dermatitidis is a dimorphic fungus that grows in the soil as a mold and, after the inhalation of infectious particles or spores, switches to the pathogenic yeast form in the lung. Yeast cells display a surface adhesin termed BAD-1 (Blastomyces adhesin 1). Gene targeting and deletion of BAD-1 attenuate pathogenicity of the yeast in a murine model of lethal pulmonary infection, defining BAD-1 as a virulence factor (3). This genetically modified, attenuated strain of B. dermatitidis given subcutaneously as a vaccine protects mice from lethal infection with wild-type virulent B. dermatitidis (for strains that are genetically related and unrelated to the vaccine strain) and produces sterilizing immunity (10). Thus, this recombinant attenuated strain holds promise as a potential vaccine against blastomycosis. In this report, we describe studies that were done to assess the safety and immunogenicity of this recombinant attenuated strain of B. dermatitidis yeast administered to dogs as a candidate vaccine against blastomycosis.
MATERIALS AND METHODS
Attenuated vaccine strain.
An attenuated, genetically engineered mutant strain of B. dermatitidis ATCC 26199 (5), lacking BAD-1 and designated strain 55 (3), was used as a vaccine for this study. This strain of B. dermatitidis was maintained in yeast form on Middlebrook 7H10 agar with oleic acid-albumin complex (Sigma Chemical Co., St. Louis, MO) at 39°C. The vaccine was prepared fresh for each experiment. Yeast cells were grown on 7H10 slants for 3 to 4 days, washed twice in sterile phosphate-buffered saline (PBS), counted, and adjusted to the desired vaccine dose (yeast cells per ml). The endotoxin level of the vaccine preparation was ≤0.1 U/ml, as described previously (12).
Vaccination of laboratory beagles.
Twenty adult beagles of both sexes were used in a preliminary study of the vaccine's safety. All dogs were housed and cared for according to guidelines of the University of Tennessee Animal Care Committee, who approved all aspects of this work. They were fed dry food and given water ad libitum. After a 2-week acclimation period, four dogs per group were injected under the skin in the cervical area within 6 cm of the right superficial cervical lymph node with 1 ml of PBS, pH 7.4, as a control or with 2 × 104, 2 × 105, 2 × 106, or 2 × 107 vaccine yeast cells (3, 10, 12) in 1 ml of PBS. Yeast cells were injected superficially in the subcutaneous space, and the site was marked with a permanent marking pen. Swelling or nodules in the area were evaluated by picking up a skin fold that contained the injection site.
Temperature and samples taken to assess reaction to the vaccine.
Rectal temperature, size of the draining lymph node, and swelling of the injection site were evaluated by a veterinarian blinded to the identities of the treatment groups. Evaluations were done 2, 5, 7, 9, 12, 14, 16, 19, 24, and 26 days after the first injection of vaccine. Swelling or nodules in the area were evaluated by picking up a skin fold that contained the injection site. A temperature of ≥103.0°F was defined as a fever, and one of ≥104.0°F was defined as a high fever. The dogs were euthanized 28 days after the initial dose of saline or yeast with pentobarbital given intravenously after the dogs were used for a nonsurvival, surgery-teaching laboratory.
Skin from the injection sites, draining lymph nodes (cervical lymph nodes), and pieces of the lung were removed aseptically, homogenized, and plated on Sabouraud's agar for CFU. Tissue samples were also fixed in 10% formalin for histological examination. If a mass was noted at the injection site, it was harvested. If a mass could not be palpated, the site that had been marked was harvested. Parts of the superficial cervical lymph nodes and peripheral blood were collected to assess immunogenicity of the vaccine.
Histology of skin draining lymph nodes and injection sites.
Sections of the left and right superficial cervical lymph nodes were stained with hematoxylin and eosin (H&E) and with Gomori's methenamine silver (GMS) stain. The pathologist was blinded to the treatment groups. The response of the lymph node was graded on a seven-point scale. For the purposes of this study, a “reactive” lymph node was defined as one in which secondary lymphoid follicles were present. The degree of lymph node reactivity was measured initially on a scale of 0 to 3: 0 = a node in which no secondary follicles are present, 1 = a node containing 1 or 2 follicles, 2 = a node containing 3 or 4 follicles, and 3 = a node containing >4 follicles. In addition, more subjective measures of reactivity of each node were assessed by the degree of hypercellularity. Nodes were rated on a scale of 0 to 4 for this parameter, with a score of 0 indicating no increase in cellularity and a score of 4 indicating severe hypercellularity. The two assigned scores (number of secondary follicles and degree of hypercellularity) for each node were combined, resulting in a final score of 0 to 7 to indicate the node's reactivity. The number of B. dermatitidis yeast cells seen in a histologic section was quantified as none, rare, few, or many.
The inflammatory response at the injection site was also rated subjectively, as no reaction or a mild, moderate, or severe inflammatory cell infiltrate. The number of B. dermatitidis yeast cells within the injection site was quantified as none, rare, few, or many.
Proliferation of T cells.
To assess in vitro proliferation of T cells, peripheral blood mononuclear cells (PBMC) and lymph node cells (LNC) were cultured in the presence and absence of antigen or the mitogen phytohemagglutinin (PHA). PBMC from 20 ml of heparinized blood from vaccinated and control dogs were isolated over Ficoll. Cells (1 × 106) were cultured in 96-well plates with 5 μg/ml PHA, 12.5 μg/ml Blastomyces cell wall membrane (CWM) antigen (10) containing ≤0.1 endotoxin unit/ml as described previously (12), or medium alone. After 96 h of incubation at 37°C in 5% CO2, 1 μCi of [3H]thymidine was added to each well. After 18 to 24 h, cells were harvested and counts per minute (cpm) of label measured in a beta counter. Data were expressed as stimulation indexes, determined as follows: cpm of sample cultured with antigen/cpm of sample cultured with medium. Values of >5 were defined as indicating significant antigen-specific stimulation.
Quantification of cytokine transcripts.
Real-time PCR was used to quantify canine mRNA expression in PBMC. Total RNA was isolated from 1 × 106 to 5 × 106 PBMC cultured in vitro for 48 h with CWM antigen, PHA, or medium, using an RNeasy minikit (Qiagen, Valencia, CA). RNA was purified twice over RNeasy minicolumns and treated on column with an RNase-free DNase set (Qiagen) to remove genomic DNA. RNA (0.5 to 1 μg) in a final volume of 20 μl was reverse transcribed using random hexamers and a TaqMan RT-PCR kit (Applied Biosystems). Five microliters of a 1:10 dilution of cDNA was amplified in a final volume of 25 μl, using SYBR Green Supermix (Bio-Rad Laboratories). The following primers were used at a final concentration of 100 nM: for gamma interferon (IFN-γ), GGAGCATGGATACCATCAAGGA (forward) and CCTGCAGATCGTTCACAGGAA (reverse); for tumor necrosis factor alpha (TNF-α), GTGCCGTCAGATGGGTTGTA (forward) and GAGGAGCACATGGGTGGAA (reverse); for granulocyte-macrophage colony-stimulating factor (GM-CSF), CCTGGAGACCCGCCTAGA (forward) and CTGGGTTGCACAGGGAGATT (reverse); for interleukin-4 (IL-4), GACTCGTGCATGGAGCTGACT (forward) and GATCTGCCGCAGTACAGTAGCA (reverse); and for 18S rRNA as an endogenous control gene, CGCCGCTAGAGGTGAAATTCT (forward) and CGAACCTCCGACTTTCGTTCT (reverse). Amplification was performed in an iCycler iQ real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA) and assayed under the same conditions for all targets: 5 min at 95°C and 45 cycles of 15 s at 95°C and 45 s at 60°C. Transcript quantity was calculated using the comparative threshold cycle (CT) method (6) and reported as the n-fold difference relative to a calibrator cDNA (i.e., sample from medium-stimulated cells).
Evaluation of the vaccine in a field study.
After preliminary safety studies of the vaccine were done with laboratory beagles, a kennel of American and English foxhounds in Lexington, KY, was selected to evaluate vaccine safety and immunogenicity in a field trial. This kennel was chosen because it had had 5 to 10 cases of blastomycosis a year among approximately 100 hounds. Approximately 55% of the dogs were female, and 45% were males. There were seven puppies, and the rest of the hounds were adults. The adult dogs ranged in age from 1 to 10 years. Half of the dogs were vaccinated subcutaneously with 105 vaccine yeast cells in 1 ml of PBS, and the other half received 1 ml of PBS subcutaneously as a control. The vaccination sites were examined on days 2, 4, 6, 8, and 10 postinjection for inflammatory reactions. We chose a lower vaccine dose to minimize inflammation at the vaccine site as well as to minimize fever. To increase immunogenicity, dogs were boosted 21 days after the initial vaccine dose and monitored for reactions as described for the first injection. A veterinary technician blinded to which dogs were given the vaccine or PBS graded the skin reactions as follows: 0 = no swelling or inflammation; 1 = a small mass or swelling palpated (less than 1 cm); 2 = a mass of 1 to 2 cm, without redness; 3 = a mass of >1 cm, with redness; and 4 = a mass of >1 cm, with redness and drainage.
To assess how dogs previously infected with B. dermatitidis would react to the vaccine, we included in the trial an additional 25 dogs that had previously been infected with B. dermatitidis and treated for blastomycosis and assessed their inflammatory skin reactions at the site of vaccination after the first dose of the vaccine. All hounds were monitored closely for lethargy and depression.
Two to three weeks after the 2nd vaccine dose, peripheral blood was taken from the foxhounds with no history of blastomycosis (half vaccinated and the other half not vaccinated) and tested for immunogenicity (proliferation and cytokine production by antigen-stimulated T cells) as described above. Sera from these dogs were tested by radioimmunoassay (RIA) for antibody to B. dermatitidis BAD-1 antigen, using previously described methods (7, 8), to assess any evidence of prior subclinical infection in these vaccine recipients. Richmond Road Veterinary Clinic in Lexington, KY, provided care for the foxhounds.
Statistical analysis.
The relative changes in cytokine transcripts and proliferation in CWM antigen- versus medium control-stimulated samples were analyzed using the Wilcoxon rank test for nonparametric data (4). Differences in cytokine transcripts and rectal temperatures were compared among groups by use of analysis of variance (ANOVA) models (4). A P value of <0.05 was considered statistically significant.
RESULTS
Pilot study of escalating vaccine doses to test safety and toxicity.
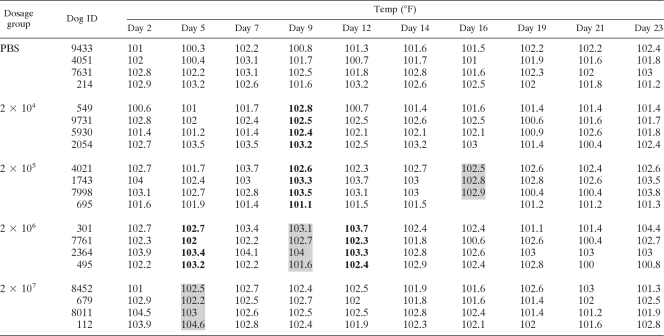
Twenty adult laboratory beagles were divided into five groups, with four dogs in each group. Beagles received 2 × 104 to 2 × 107 vaccine yeast cells or PBS alone as a control, as described in Materials and Methods. The injections of B. dermatitidis did not cause any lethargy or loss of appetite in the dogs. Dogs in all groups had increases in rectal temperature (Table 1). Temperatures of 104.0°F or greater were seen only in the dogs injected with the vaccine. These high temperatures were seen more often at higher vaccine doses and were usually short-lived, resolving by 72 h.
Table 1.
Rectal temperatures of laboratory beagles after vaccinationa
Yeast cells were injected on days 0 and 14. Shaded boxes, P < 0.05 versus PBS-treated dogs; data in bold, P < 0.1 versus PBS-treated dogs.
Inflammation at the injection sites was more severe at doses of >106 yeast cells (Table 2). The persistence of yeast at the injection site was also more likely as larger vaccine doses were injected. In contrast, only one of the four dogs given a dose of 2 × 104 yeast cells had moderate swelling at the injection site, and only one of the dogs given 2 × 105 yeast cells had severe inflammation at the site. Thus, the lower vaccine doses of 2 × 104 and 2 × 105 were better tolerated than the two higher vaccine doses.
Table 2.
Inflammation and presence of yeast cells at injection sites of vaccinated dogs
| Dosage group and dog ID | Inflammation at injection sitea (no. of dogs with score/no. of dogs in group) |
Presence of yeast cells at injection site (no. of dogs/no. of dogs in group) |
||||
|---|---|---|---|---|---|---|
| 1+ | 2+ | 3+ | Few yeast cells (histology) | Many yeast cells (histology) | Culture | |
| Saline | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 9433 | ||||||
| 4051 | ||||||
| 7631 | ||||||
| 0214 | ||||||
| 2 × 104 | 1/4 | 1/4 | 0/4 | 0/4 | 0/4 | 1/4 |
| 1549 | + | Many | ||||
| 9731 | + | |||||
| 5930 | ||||||
| 2054 | ||||||
| 2 × 105 | 1/4 | 1/4 | 1/4 | 1/4 | 0/4 | 1/4 |
| 4021 | ||||||
| 1743 | + | + | Few | |||
| 7988 | + | |||||
| 0695 | + | |||||
| 2 × 106 | 0/4 | 0/4 | 2/4 | 2/4 | 0/4 | 2/4 |
| 0301 | + | + | Few | |||
| 7761 | ||||||
| 2364 | ||||||
| 0495 | + | + | 1 colony | |||
| 2 × 107 | 0/4 | 1/4 | 3/4 | 1/4 | 3/4 | 2/4 |
| 8452 | + | + | ||||
| 0679 | + | + | 1 colony | |||
| 8011 | + | + | Few | |||
| 0112 | + | + | ||||
1+, mild inflammation; 2+, moderate inflammation; 3+, severe inflammation. A + symbol denotes the individual dog involved.
Dissemination of B. dermatitidis vaccine yeast from the injection sites of beagles.
The lymph nodes of all five groups (vaccine and placebo control groups) were generally reactive, with few apparent differences among the groups (Table 3). Reactions in PBS-treated groups were typically described as follows. “The node is hypercellular with numerous secondary follicles in the cortical area. The subcapsular areas contain sheets of well-differentiated lymphocytes. There are increased numbers of macrophages/antigen presenting cells.” In contrast, for groups given vaccine yeast, the histology of inflammatory reactions at the injection site was typically described as follows. There was a “focally extensive area of marked pyogranulomatous and lymphoplasmacytic inflammation with accompanying fibroplasias present in the deep dermis and subcutis. Within this inflamed area there is a central, cell-poor space lined by flattened fibroblasts consistent with a fluid-filled cavity. Many macrophages contain round, thick walled, 5 to 20 micrometer in diameter yeast consistent in appearance with yeast: a few also contain small amounts of gray/yellow, amorphous material. Most of the yeast are present within macrophages.”
Table 3.
Evaluation of cervical lymph nodes after injection of saline or vaccine yeasta
| Dosage group and dog ID | Right draining lymph node injection site at 28 days |
Left draining lymph node injection site at 14 daysb |
|||
|---|---|---|---|---|---|
| Reaction score | Yeast visualized | Yeast cultured | Reaction score | Yeast cultured | |
| Saline | |||||
| 9433 | 6 | N | N | 7 | N |
| 4051 | 6 | N | N | 6 | N |
| 7631 | 5 | N | N | 5 | N |
| 0214 | 5 | N | N | 5 | N |
| 2 × 104 | |||||
| 1549 | 4 | N | N | 6 | N |
| 9731 | 7* | N | N | 6* | N |
| 5930 | 7 | N | N | 4 | N |
| 2054 | 6 | N | N | 5 | N |
| 2 × 105 | |||||
| 4021 | 5 | N | N | 6 | N |
| 1743 | 6* | + | N | 6* | N |
| 7988 | 4 | N | N | 6 | N |
| 0695 | 6 | N | N | 4 | N |
| 2 × 106 | |||||
| 0301 | 7* | N | N | 7* | N |
| 7761 | 7 | N | N | 7 | N |
| 2364 | 7 | N | N | 7 | N |
| 0495 | 5* | N | N | 3* | N |
| 2 × 107 | |||||
| 8452 | 3* | N | N | 2* | N |
| 0679 | 0* | N | + | 2* | + |
| 8011 | 5* | N | N | 2* | N |
| 0112 | 7* | + | N | 6* | N |
Reaction scores, from 0 (no reaction) to 7 (most reactive), are defined in Materials and Methods. N, no organisms seen or cultured; +, yeast seen on histopathology or culture; *, yeast seen or cultured from injection sites that drained the respective lymph nodes.
No yeast cells were visualized in the left draining lymph node at 14 days for any of the dogs.
Vaccine yeasts were seen on histopathology or fungal culture from the right superficial cervical lymph node for one dog given 2 × 105 yeast cells and two dogs given 2 × 107 yeast cells (Table 3). These animals were given vaccine subcutaneously on the right side near the lymph nodes, and their lymphoid tissues were analyzed 28 days after vaccination was administered on that side.
There was no evidence of lung dissemination of B. dermatitidis yeast on histological examination of the lung tissues stained with GMS and on fungal culture of the lungs. There was also no evidence of an inflammatory cell infiltrate in the lungs of 15 dogs. Five dogs did have a minimal area of lung infiltrate with lymphocytes and macrophages: these minimal infiltrates were seen in one dog from the saline group, one from the 2 × 104 vaccine group, two from the 2 × 105 vaccine group, and one from the 2 × 107 vaccine group.
Proliferation and cytokine responses in a pilot study with laboratory beagles.
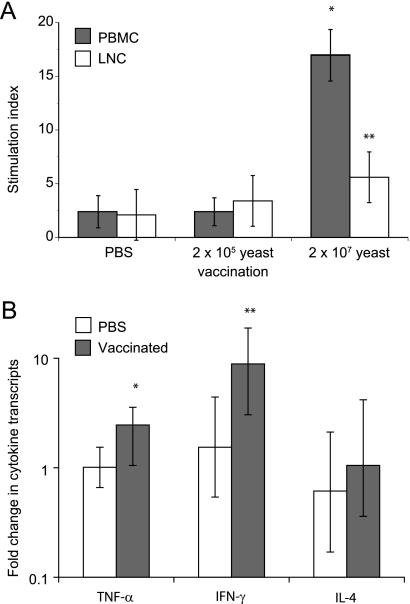
To see if the vaccine was immunogenic, we tested samples from two groups that received vaccine yeast (doses of 2 × 105 and 2 × 107) and from the placebo control group. LNC and PBMC from dogs immunized once with 2 × 107 yeast cells proliferated in vitro specifically in response to the protective cell wall antigen of B. dermatitidis (CWM antigen) (10) (Fig. 1A). Cells from dogs immunized with 2 × 105 yeast cells or PBS did not proliferate in an antigen-specific manner. Stimulation of PBMC with CWM antigen induced trends toward significant transcription of IFN-γ and TNF-α (Fig. 1B), but not IL-4, in dogs that received vaccine yeast. In contrast, T helper 1 cytokine transcripts (IFN-γ and TNF-α) were not induced in control dogs vaccinated with PBS alone. These results indicate that vaccination with attenuated yeast was immunogenic and capable of inducing antigen-specific immune responses in the laboratory beagles.
Fig. 1.
Immune responses in vaccinated laboratory beagles. Dogs were vaccinated once with 2 × 105 or 2 × 107 vaccine yeast cells or with PBS. At 23 days postvaccination, the skin draining lymph nodes and peripheral blood were collected. LNC and PBMC were stimulated with CWM antigen or medium alone. (A) T cell responses are shown as stimulation indices, defined as proliferation in the presence of CWM antigen/proliferation in the presence of medium alone. *, P < 0.05 versus all other groups; **, P = 0.1 versus PBS-treated dogs. (B) Levels of IFN-γ, TNF-α, and IL-4 transcripts in PBMC stimulated with CWM. Results are mean (± standard error of the mean [SEM]) fold increases over the levels in unstimulated PBMC. Transcripts in stimulated cells were assessed by the comparative CT method, using unstimulated cells as the calibrator. *, P = 0.12; **, P = 0.17 versus PBS-treated dogs.
Administration of a safe vaccine dose in a field trial with foxhounds.
To further evaluate the vaccine in the field, we immunized foxhounds with a dose of vaccine yeast that was well tolerated (105 yeast cells) and also boosted the vaccinated dogs after 3 weeks. Throughout the vaccination period, vaccine site reactions and the general health of the hounds were monitored. There was no noticeable depression, lethargy, or loss of appetite in any of the hounds that received the live-attenuated vaccine strain.
Among the vaccinated dogs, 25 hounds that had previously been diagnosed and treated for blastomycosis received the yeast vaccine (n = 11) or a saline control (n = 14). Of the 11 dogs that had had blastomycosis and received live vaccine, 5 showed grade 2 or higher inflammatory reactions at the injection site. Table 4 depicts the severity and time course of the reactions. None of the 14 dogs with a history of blastomycosis that were given saline had clinically relevant reactions.
Table 4.
Clinically relevant inflammatory reactions at vaccine yeast injection sites
| Dog IDa | Inflammatory reaction at injection siteb |
||
|---|---|---|---|
| Day 6 | Day 8 | Day 10 | |
| Gl | 3 | ||
| Co | 3 | 2 | 2 |
| Fi | 3 | 3 | 4 |
| Ar | 3 | 3 | 3 |
| Ra | 3 | 3 | 3 |
The first two letters of the dogs' names were used to label the subjects.
The reactions were graded as follows: 0 = no swelling or inflammation; 1 = small mass or swelling palpated (less than 1 cm); 2 = mass of 1 to 2 cm, without redness; 3 = mass of >1 cm, with redness; and 4 = mass of >1 cm, with redness and drainage. Reactions of grade 2 and higher were considered clinically relevant. There were no reactions observed on days 1, 2, and 4.
The reactions in vaccinated dogs with a history of blastomycosis required about 6 days to become prominent, and one site abscessed and drained by day 10. The initial date of diagnosis did not appear to influence the chance of reacting to the vaccine among previously infected dogs. The dogs that had clinically relevant reactions to injection of the vaccine had had blastomycosis in 1996, 1996, 2001, 2001, and 2003, whereas the dogs that did not develop reactions had had blastomycosis in 1996, 1996, 1996, 1996, 2002, and 2002. No dog had had more than one bout of blastomycosis. Thus, there was no statistically significant difference in the time since diagnosis of blastomycosis between reactors and nonreactors.
A total of 57 foxhounds in the kennel that did not have a history of blastomycosis were evaluated after two doses of live vaccine yeast or saline. There were a total of 12 that showed inflammatory reactions at the vaccine site. Seven reactions occurred among the 28 dogs given vaccine yeast, and five were among the 29 dogs given placebo control vaccine. Of the seven reactions in the live yeast vaccine group, there were four reactions graded 2+ or higher after the first vaccine and three reactions that were graded 2+ after the second vaccine. Of the five reactions in the saline group, three reactions were graded 2+ after the first injection and two reactions of 2+ occurred after the second saline injection. None of the hounds, either with or without a history of blastomycosis, had injection site reactions that produced systemic signs of illness or lethargy.
Vaccine-induced antigen-specific immune responses in the field trial.
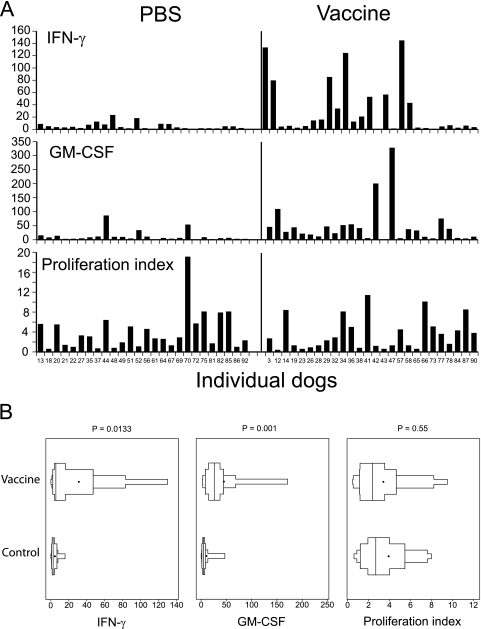
Two weeks after the booster, the foxhounds were analyzed for evidence of immunity. Among 27 dogs that received vaccine yeast, PBMC from 17 dogs (63%) produced significant (>5-fold) elevations of IFN-γ transcripts in cells stimulated with B. dermatitidis CWM antigen versus cells stimulated with medium alone, and 22 dogs (81%) produced significant elevations in GM-CSF transcripts (Fig. 2A). In contrast, among the 26 dogs that did not have a history of blastomycosis and received PBS as a vaccine control, stimulation of PBMC with CWM antigen produced significantly elevated levels of IFN-γ transcripts in 8 (31%) dogs and of GM-CSF transcripts in 11 (42%) dogs. On average, antigen-stimulated PBMC from dogs that received vaccine yeast produced IFN-γ transcript levels that were 11-fold higher than those produced by stimulation with medium alone and GM-CSF transcript levels that were 22-fold higher than those produced by stimulation with medium alone (Fig. 2A and B). The antigen-stimulated PBMC from PBS control-vaccinated dogs showed significantly smaller IFN-γ and GM-CSF transcript increases (3- and 5-fold, respectively) than those produced by stimulation with medium alone. Cytokine production in both vaccinated and PBS-treated dogs that had detectable anti-BAD-1 antibodies prior to immunization, as measured by RIA (8), was not elevated compared to that in antibody-negative dogs (data not shown). Thus, cytokine production was linked to experimental immunization but not to prior exposure to B. dermatitidis. In contrast to cytokine responses, the proliferative responses of PBMC to CWM antigen did not differ significantly between the vaccine and control groups (Fig. 2A and B).
Fig. 2.
Immunogenicity of the vaccine in a field trial. Foxhounds were vaccinated with 105 yeast cells and boosted 3 weeks later. Two to 3 weeks after the booster, the dogs were bled and PBMC isolated and stimulated with CWM antigen or medium alone. Forty-eight hours later, cells were lysed and RNA was isolated and analyzed for cytokine expression. After 96 h of culture, PBMC were pulsed with [3H]thymidine and analyzed for proliferation. (A) Fold increases in cytokine transcripts and proliferation of PBMC stimulated with CWM antigen (right) versus medium (left) for individual dog samples. (B) Analysis of immune responses in vaccinated foxhounds, using box-percentile plots. Data show the distribution and variability of fold increases in cytokine transcripts and antigen-stimulated proliferation among foxhounds that received vaccine yeast or PBS as a control. In each plot, the black dot and middle line represent the mean and median, respectively. The next lines to the left and right represent the 25th and 75th percentiles, respectively, and the two lines at the far left and far right represent the 10th and 90th percentiles, respectively.
Because of the possible immune response of some PBS control-vaccinated dogs as measured by cytokine responses, we subjected these data to a more careful statistical analysis. A ranking analysis and the accompanying distribution graphic for these data demonstrated that the dogs that received vaccine yeast produced significantly elevated levels of CWM antigen-specific IFN-γ and GM-CSF transcripts upon in vitro stimulation compared to those of the PBS-treated dogs (Fig. 2B). As before, dogs vaccinated with yeast versus PBS did not differ significantly in CWM antigen-induced proliferation of PBMC, showing that vaccination in this field trial did not induce antigen-specific lymphocyte proliferation. Nevertheless, these results indicated that over 80% of the dogs vaccinated with attenuated yeast showed evidence of vaccine-induced immunity, as assessed by transcript analysis upon restimulation in vitro. In contrast, only 1 of about 8 to 10 PBS-treated dogs showed evidence of immunity.
DISCUSSION
In the initial study with laboratory beagles, injecting up to 2 × 107 yeast cells subcutaneously did not cause signs of systemic disease such as lethargy or anorexia, although a few dogs had a mildly elevated temperature. The dogs receiving larger doses of yeast were more likely to have temperatures of ≥104.0°F, but dogs from all groups had temperatures of ≥103.0°F attributed to the excitement of being examined and having temperatures taken (Table 1). The lack of systemic signs was likely due to little or no spread of yeast from the injection site. There was no evidence that vaccine yeast spread to the lungs in any dog, as evidenced by culture or histopathological examination of the lungs. A small number of vaccine yeast cells could be identified in the draining lymph nodes in only 3 dogs, in spite of organisms being present at the injection site in 8 dogs.
Among laboratory beagles, the injection sites for those that received 2 × 107 yeast cells had the most prominent inflammatory responses. These sites were also more likely to contain residual yeast than sites where smaller vaccine doses were injected. There was, however, considerable individual variation in the presence of yeast and the severity of inflammation between dogs receiving the same dose of yeast (Table 2). For example, dog 1549, which received only 2 × 104 yeast cells, had moderate tissue inflammation, with many yeast cells found on culture, while dogs 7761 and 2364, which received 2 × 106 yeast cells, had no reaction and no yeast found on histology or culture. An injection of 2 × 104 vaccine yeast cells was well tolerated and produced an acceptable degree of inflammation in even the most severely reactive dog. There was no evidence of regional lymph node involvement in beagles that received a dose of 2 × 104 yeast cells, and there was no evidence of lung infection in any dog. Based on the findings with the laboratory beagles, a dose of 105 yeast cells of the vaccine strain was selected for further study in the foxhounds.
None of the foxhounds had any clinical signs of systemic illness after vaccination with the attenuated vaccine. A concern was that the dogs that had recovered from blastomycosis might be overly reactive to the vaccine. Twenty-five dogs that had recovered from blastomycosis with itraconazole treatment were included in the study. Five of 11 of these dogs had a strong reaction to the vaccine (Table 4), while none of the 14 dogs given a saline placebo had a severe reaction. In the dogs that reacted, there was no difference in time since blastomycosis treatment between dogs that reacted and those that did not react. This suggests that some dogs had a more robust immune response than others. One of the five previously infected dogs developed an abscess at the vaccine injection site (Table 4).
Fifty-seven dogs in the kennel without a previous episode of blastomycosis received two doses of vaccine yeast or a saline placebo control 3 weeks apart. Four of 28 dogs receiving the vaccine strain had a significant reaction to the first dose, and 3 of 28 dogs had a significant reaction to the second dose. The three that reacted significantly to the second dose also reacted to the first dose. Overall, there was a low incidence of adverse reactions to the vaccine in dogs without a history of blastomycosis. Strong reactions in dogs without a history of infection could also have been related to prior exposure to B. dermatitidis, as these dogs live in an area where blastomycosis is highly endemic and could have been infected asymptomatically.
It is noteworthy that in the placebo control group receiving saline, 3 of 29 dogs had a significant reaction (grade 2+) to the first injection and 2 of 29 dogs had a significant reaction to the second injection. The two dogs in the placebo group that reacted to the second dose of saline had reacted to the first dose. There is no good explanation for the response to the injection of saline.
In addition to being safe, the vaccine was immunogenic in both laboratory beagles and foxhounds. We tested responses in only two of the beagle groups that received vaccine yeast: those that received doses of 2 × 105 and 2 × 107 yeast cells. Only the group that received the higher dose showed proliferative responses in an antigen-specific manner. In view of those responses, we studied the cytokine transcript levels in PBMC from dogs that received 2 × 107 vaccine yeast cells. These animals evinced substantial elevations in both IFN-γ and TNF-α compared to the PBS control group, whereas IL-4 was not elevated. These cytokine elevations in vaccinated beagles showed a trend toward statistical significance but were not significant (P values of <0.05 were considered significant), likely due to type 2 error in view of the small number of dogs per group. IFN-γ, TNF-α, and GM-CSF have been linked functionally to protective immunity with this vaccine (11, 12) and to protective immunity against other related systemic mycoses, such as histoplasmosis (1), whereas IL-4 has instead been shown to undermine this immunity. In this regard, we were encouraged by the quality of the immune response in dogs that received the attenuated vaccine. We did not test the laboratory beagles that received lower doses of vaccine for cytokine levels, but the responses among the foxhounds indicated that the vaccine was immunogenic at the lower doses (see below).
Only laboratory beagles that received the higher dose of vaccine exhibited Blastomyces antigen-induced proliferation in samples of PBMC or LNC. The reasons for the lack of response in dogs that received the lower vaccine doses are not clear but could be related to the timing of collection with respect to evolution of the immune response or to issues of sample transport. The beagles and foxhounds were from Tennessee, which required sample shipping to Wisconsin, where the assays of antigen-induced proliferation were performed. Despite sending the lymphocytes in whole heparinized blood by overnight express mail, the unavoidable delay before cell isolation and stimulation could have resulted in nonoptimal responses. Despite these potential technical issues, the cells from nearly all subjects were viable, as assessed by trypan blue staining, and responded to the mitogen control PHA (data not shown), indicating some viability in the cell population. The antigen-specific cytokine responses in these responding animals corroborated that the immunological responses were likely induced by vaccination.
Foxhounds that were vaccinated with 105 yeast cells in an open field trial confirmed that the vaccine was well tolerated and immunogenic even at lower doses than those analyzed in detail in laboratory beagles. Foxhounds vaccinated with yeast were significantly more likely to show type 1 cytokine transcript responses to Blastomyces antigen, particularly GM-CSF responses, than those in the placebo control group. Moreover, the height of the vaccine immune response was significantly greater in dogs that received the vaccine than in the placebo control group. While not all dogs that received vaccine yeast gave an immune response, we looked at only a limited number of type 1 cytokine transcripts. We did not investigate type 17 immune responses in vaccinated or control dogs, although more recent studies have shown that Th17 cells and IL-17 cytokines may have an important role in adaptive immunity to fungi, including B. dermatitidis (13).
We concluded that the live-attenuated vaccine described here is safe and immunogenic. The foxhounds were monitored to see if the vaccine protected them from infection, but there have not been cases of blastomycosis in either the vaccinated or placebo group over the last few years. Nevertheless, we propose that based on the preliminary results here showing safety and immunogenicity, the live vaccine should now be examined in a separate field trial investigating vaccine efficacy.
ACKNOWLEDGMENTS
We thank Gary Clark and Kirk Snyder for their collaboration on this study.
This work was supported by grant funds to B.S.K. from the USPHS.
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Allendorfer R., Brunner G. D., Deepe G. S., Jr 1999. Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J. Immunol. 162:7389–7396 [PubMed] [Google Scholar]
- 2. Baumgardner D. J., Paretsky D. P., Yopp A. C. 1995. The epidemiology of blastomycosis in dogs: north central Wisconsin, U. S. A. J. Med. Vet. Mycol. 33:171–176 [DOI] [PubMed] [Google Scholar]
- 3. Brandhorst T. T., Wüthrich M., Warner T., Klein B. 1999. Targeted gene disruption reveals an adhesin indispensable for pathogenicity of Blastomyces dermatitidis. J. Exp. Med. 189:1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher L. D., van Belle G. 1993. Biostatistics: a methodology for the health sciences, p. 611–613 John Wiley & Sons, New York, NY [Google Scholar]
- 5. Harvey R. P., Schmid E. S., Carrington C. C., Stevens D. A. 1978. Mouse model of pulmonary blastomycosis: utility, simplicity, and quantitative parameters. Am. Rev. Respir. Dis. 117:695–703 [DOI] [PubMed] [Google Scholar]
- 6. Johnson M. R., Wang K., Smith J. B., Heslin M. J., Diasio R. B. 2000. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal. Biochem. 278:175–184 [DOI] [PubMed] [Google Scholar]
- 7. Klein B. S., Jones J. M. 1990. Isolation, purification, and radiolabeling of a novel 120-kD surface protein on Blastomyces dermatitidis yeasts to detect antibody in infected patients. J. Clin. Invest. 85:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein B. S., Squires R. A., Lloyd J. K., Ruge D. R., Legendre A. M. 2000. Canine antibody response to Blastomyces dermatitidis WI-1 antigen. Am. J. Vet. Res. 61:554–558 [DOI] [PubMed] [Google Scholar]
- 9. Legendre A. M. 2006. Blastomycosis. Saunders Elsevier, St. Louis, MO [Google Scholar]
- 10. Wüthrich M., Filutowicz H. I., Klein B. S. 2000. Mutation of the WI-1 gene yields an attenuated Blastomyces dermatitidis strain that induces host resistance. J. Clin. Invest. 106:1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wüthrich M., Filutowicz H. I., Warner T., Deepe G. S., Jr., Klein B. S. 2003. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J. Exp. Med. 197:1405–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wüthrich M., Filutowicz H. I., Warner T., Klein B. S. 2002. Requisite elements in vaccine immunity to Blastomyces dermatitidis: plasticity uncovers vaccine potential in immune-deficient hosts. J. Immunol. 169:6969–6976 [DOI] [PubMed] [Google Scholar]
- 13. Wüthrich M., et al. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest. 121:554–568 [DOI] [PMC free article] [PubMed] [Google Scholar]