Abstract
Individuals with defects in T cell-mediated immunity (CMI) are highly susceptible to infection with Cryptococcus neoformans. The purpose of these studies was to determine if protection against experimental pulmonary cryptococcosis can be generated in T cell-deficient hosts. BALB/c mice were depleted of CD4+ and/or CD8+ T cells or given an isotype control antibody prior to vaccination with a C. neoformans strain, designated H99γ, previously shown to induce protection against C. neoformans infection in immunocompetent mice. Mice depleted of CD4+ or CD8+ T cells, but not both subsets, survived an acute pulmonary infection with C. neoformans strain H99γ and a subsequent second challenge with wild-type C. neoformans strain H99. We observed a significant increase in the percentage of CD4+ and CD8+ T cells expressing the activation marker CD69 in the lungs of mice immunized with C. neoformans strain H99γ prior to a secondary challenge with wild-type cryptococci. CD4+ T cells within the lungs of immunized mice also appeared to acquire a predominantly activated effector memory cell phenotype (CD69+ CD44+ CCR7− CD45RB− CD62L−) following a second pulmonary challenge with wild-type C. neoformans, compared to CD4+ T cells from naïve mice. Lastly, immunization of immunocompetent mice with C. neoformans strain H99γ prior to depletion of CD4+ and/or CD8+ T cells resulted in significant protection against a second challenge with wild-type C. neoformans. Our studies demonstrate that protective immunity against pulmonary cryptococcosis can be generated in immunosuppressed hosts, thus supporting the development of cryptococcal vaccines.
INTRODUCTION
Cryptococcus neoformans, the causative agent of cryptococcosis, is an opportunistic fungal pathogen that primarily causes life-threatening meningoencephalitis in individuals with impaired T cell function (26). Global estimates suggest that one million cases of cryptococcal meningitis occur each year resulting in approximately 620,000 deaths within 3 months of infection (28). Furthermore, one-third of patients with cryptococcal meningitis who receive appropriate therapy go on to experience mycologic and/or clinical failure (30, 34). The clinical relevance of disseminated cryptococcal disease is projected to increase due to an increasing population of immunocompromised patients (i.e., HIV-infected individuals [3, 9, 11, 12, 21], individuals receiving corticosteroid therapy [8, 10, 14], individuals with lymphoproliferative disorders [4, 13, 20, 22], and organ transplant recipients [19]). Therefore, there remains an urgent need for the development of new immunotherapies, antifungal drugs, and/or vaccines to combat C. neoformans infections.
Currently, there are no standardized vaccines available for the prevention of fungal diseases in humans. The induction of T helper 1 (Th1)-type cell-mediated immunity (CMI) is critical for optimal protection against primary and opportunistic fungal pathogens (5). CD4+ and CD8+ T cell subsets are each important for the elimination of fungal pathogens, although the necessity for CD4+ T cells appears to be greater. Consequently, it may seem counterintuitive to suggest that vaccination regimens designed to prevent fungal infections in patients with T cell deficiencies is possible. However, studies using experimental models of Blastomyces dermatitidis and Histoplasma capsulatum, two invasive fungal pathogens, indicated that immunodeficient hosts can develop protective immune responses following vaccination against these two fungal pathogens (39). Previous studies from our laboratory have demonstrated that mice given a primary infection with a C. neoformans strain engineered to express gamma interferon (IFN-γ) developed Th1-type cell-mediated immune responses resulting in the resolution of infection and protection against a secondary infection with a fully pathogenic C. neoformans strain (37, 38). The goal of the present study was to evaluate the generation of protective immunity against C. neoformans infection in mice depleted of CD4+ and/or CD8+ T cells prior to or following immunization with C. neoformans strain H99γ. Altogether, our results support the feasibility of developing vaccines to combat C. neoformans infection in patients with severe immunodeficiencies.
MATERIALS AND METHODS
Mice.
Female BALB/c (H-2d) mice (National Cancer Institute/Charles River Laboratories) with an average weight of 20 to 25 g were used throughout these studies. Mice were housed at The University of Texas at San Antonio Small Animal Laboratory Vivarium and handled according to guidelines approved by The University of Texas at San Antonio Institutional Animal Care and Use Committee.
Strains and media.
C. neoformans strains H99 (serotype A, Matα) and H99γ, an IFN-γ-producing strain derived from H99 (37), were recovered from 15% glycerol stocks stored at −80°C prior to use in the experiments described here. The strains were maintained on yeast extract-peptone-dextrose (YPD) medium (1% yeast extract, 2% peptone, 2% dextrose, 2% Bacto agar). Yeast cells were grown for 18 to 20 h at 30°C with shaking in YPD broth (Becton Dickinson and Company, Sparks, MD), collected by centrifugation, and washed three times with sterile phosphate-buffered saline (PBS), and viable yeast cells were quantified using trypan blue dye exclusion in a hemacytometer.
Pulmonary infections.
Pulmonary C. neoformans infections were initiated by nasal inhalation as previously described (38). Briefly, BALB/c mice were anesthetized with 2% isoflurane using a rodent anesthesia device (Eagle Eye Anesthesia, Jacksonville, FL), given a yeast inoculum of 1 × 104 CFU of either C. neoformans strain H99γ or heat-killed C. neoformans strain H99 (HK C. neoformans) in 50 μl of sterile PBS, and allowed 70 days to resolve the infection. Subsequently, the immunized mice received a second experimental pulmonary inoculation with 1 × 104 CFU of wild-type C. neoformans strain H99 in 50 μl of sterile PBS. The inocula used for immunizations and challenge were verified by quantitative culture on YPD agar. The mice were fed ad libitum and monitored by inspection twice daily. Mice were euthanized on predetermined days postinoculation, and lung tissues were excised using an aseptic technique. Tissues were homogenized in 1 ml of sterile PBS, followed by culture of 10-fold dilutions of each tissue on YPD agar supplemented with chloramphenicol (Mediatech, Inc., Herndon, VA). CFU were enumerated following incubation at 30°C for 48 h. Alternatively, mice intended for survival analysis were monitored by inspection twice daily and euthanized if they appeared to be in pain or moribund. Mice were euthanized using CO2 inhalation.
Pulmonary leukocyte isolation.
Lungs were excised at specific time points postinoculation and digested enzymatically at 37°C for 30 min in 10 ml of digestion buffer (RPMI 1640 and 1 mg/ml of collagenase type IV [Sigma-Aldrich, St. Louis, MO]) with intermittent (every 10 min) stomacher homogenizations. The enzymatically digested tissues were then successively filtered through sterile nylon filters with various pore sizes (70 and 40 μm; BD Biosciences) and washed with sterile Hanks balanced salt solution to enrich for leukocytes. Erythrocytes were lysed by incubation in NH4Cl buffer (0.859% NH4Cl, 0.1% KHCO3, 0.0372% Na2EDTA [pH 7.4]; Sigma-Aldrich) for 3 min on ice, followed by the addition of a 10-fold excess of PBS. The resulting leukocyte population was then collected by centrifugation (800 × g) for 5 min, washed twice with sterile PBS, resuspended in sterile PBS plus 2% heat-inactivated fetal bovine serum (FACS buffer), and enumerated in a hemacytometer using trypan blue dye exclusion. Flow cytometric analysis was used to determine the percentage of each leukocyte (CD45+) population within the lung cell suspension for standardization of hemacytometer counts.
T cell depletion.
Mice were depleted of CD4+ and/or CD8+ T cell subsets via intraperitoneal administration of anti-CD4 (GK1.5, rat IgG2b) and anti-CD8a (2.43, rat IgG2b) antibodies (each from National Cell Culture Center, Minneapolis, MN). Each mouse received 200 μg of GK1.5 and/or 2.43 or control rat IgG2b (eBioscience Inc., San Diego, CA) antibodies in a volume of 200 μl PBS 48 h prior to infection and weekly thereafter during the observation period. The efficiency of T cell depletion in the lungs and spleens was assessed by flow cytometric analysis using anti-CD4 and anti-CD8 antibodies that bind epitopes of the CD4 and CD8 protein at locations distinct from GK1.5 and 2.43 (data not shown). Efficiency was determined to be >98% at each anatomic location for each depletion via comparison of T cell subsets in treated mice with those in controls.
Antibodies.
For flow cytometry experiments, cells were incubated with CD16/CD32 (Fc Block; BD Biosciences, San Diego, CA) and the following antibodies conjugated to phycoerythrin (PE), allophycocyanin, Alexa 647, or PECy7 were added: CD3, CD4, CD8α, CD45 (all from BD Biosciences), CD25, CD69, CD44, CD62L, CD45RB, and CCR7 (all from eBioscience Inc., San Diego, CA).
Flow cytometry.
Standard methodology was employed for the direct immunofluorescence of pulmonary leukocytes. Briefly, in 96-well U-bottom plates, 100 μl containing 1 × 106 leukocyte-enriched lung cells in FACS buffer were incubated with 50 μl of Fc Block (BD Pharmingen) diluted in FACS buffer for 5 min to block the nonspecific binding of antibodies to cellular Fc receptors. Subsequently, an optimal concentration of fluorochrome-conjugated antibodies (0.06 to 0.5 μg/1 × 106 cells in 50 μl of FACS buffer) was added in various combinations to allow for dual- or triple-staining experiments and plates were incubated for 30 min on ice. Following incubation, the cells were washed three times with FACS buffer and cells were fixed in 200 μl of 2% ultrapure formaldehyde (Polysciences, Inc., Warrington, PA). Cells incubated with either FACS buffer alone or single fluorochrome-conjugated antibodies were used to determine the calculations used for positive staining and spillover/compensation, and the flow cytometer determined background fluorescence. The samples were analyzed using BD FACSArray software on a BD FACSArray flow cytometer (BD Biosciences). Dead cells were excluded on the basis of forward angle and 90° light scatter. For data analyses, 30,000 events (cells) were evaluated from a predominantly leukocytic population identified by backgating from CD45+-stained cells.
Statistical analysis.
One-way analysis of variance with the Tukey-Kramer multiple-comparison test was used to compare fungal burdens and pulmonary cell populations using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). Survival data were analyzed using the log-rank test (GraphPad Software). Significant differences were defined as P < 0.05.
RESULTS AND DISCUSSION
C. neoformans infections among HIV-infected individuals in the United States occur at a prevalence rate of 5 to 10% and are a leading mycological cause of morbidity and mortality among AIDS patients (26). Studies have shown that 2.8% of organ transplant recipients can develop cryptococcal infections, resulting in an overall death rate of 42% (19). Thus, there is an urgent need for the development of anticryptococcal vaccines that can be effective in immunosuppressed patients who would undoubtedly benefit the most. Given that the predominant mechanism of host defense against C. neoformans infections is T cell mediated (15–18, 37, 38), uncertainty remains as to the efficacy that a vaccine against C. neoformans will have in inducing protection in severely immunocompromised populations.
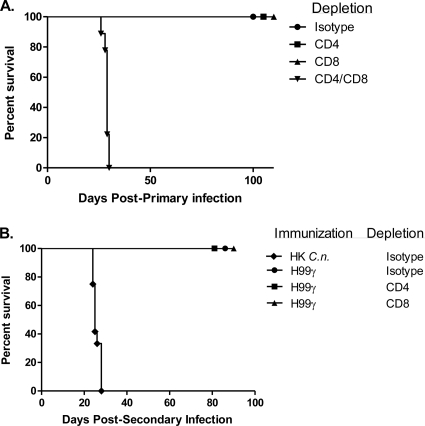
We have shown that an experimental pulmonary infection with an IFN-γ-producing C. neoformans strain, designated H99γ, in mice results in the induction of Th1-type CMI responses and resolution of the acute infection (37). Furthermore, we have demonstrated that prior immunization with C. neoformans strain H99γ results in complete protection against a second pulmonary challenge with a pathogenic C. neoformans strain. The induction of protective immunity following a pulmonary challenge with C. neoformans strain H99γ, the afferent phase of the vaccination response, was shown to be T cell dependent (38); however, what remains to be determined is the requirement of CD4+ or CD8+ T cells for the induction of protection. Consequently, BALB/c mice were treated with an isotype control antibody or depleted of CD4+ and/or CD8+ T cells using a complement-fixing antibody and subsequently given a pulmonary challenge with C. neoformans strain H99γ. Depletion of CD4+ and/or CD8+ T cells or inoculation with the isotype control antibody was continued throughout the observation period. Figure 1A shows that mice depleted of CD4+ or CD8+ T cells retained the capacity to survive a pulmonary infection with C. neoformans strain H99γ at day 100 postinoculation. In contrast, 100% mortality with a median survival time of 29 days was observed in mice depleted of both CD4+ and CD8+ T cells, similar to previous studies using nude mice that are also T cell deficient (38). Cultures of lung and brain tissues excised from surviving mice upon the termination of the survival experiment at day 100 did not have detectable viable cryptococci. We also confirmed the depletion of CD4+ or CD8+ T cells in pulmonary and spleen leukocytes of protected mice to each be greater than 98% (data not shown). Thus, the afferent phase of the vaccination response requires the presence of either CD4+ or CD8+ T cells and is completely abrogated in the absence of both populations.
Fig. 1.
Role of CD4+ T cells and CD8+ T cells during the induction of protective immunity by C. neoformans strain H99γ. (A) BALB/c mice were treated with isotype control antibodies or depleted of CD4+ and/or CD8+ T cells prior to inoculation with C. neoformans strain H99γ. Depletion was maintained throughout the observation period. The survival data shown are from one experiment using 10 mice per group. (B) Alternatively, BALB/c mice were treated with isotype control antibodies or depleted of CD4+ or CD8+ T cells prior to immunization with C. neoformans strain H99γ or HK C. neoformans (C.n.) and allowed 70 days to resolve the infection. Depletion was ceased at day 70, and mice were subsequently given a second intranasal challenge with wild-type C. neoformans strain H99. The survival data shown are from one experiment using 10 mice per group.
Subsequently, we examined if the absence of CD4+ or CD8+ T cells during the afferent phase of vaccination using C. neoformans strain H99γ impacted protection against a subsequent pulmonary challenge with wild-type C. neoformans, the efferent phase of infection. Mice were given isotype control, anti-CD4, or anti-CD8 complement-fixing antibodies, followed by a pulmonary immunization with C. neoformans strain H99γ. Separate immunocompetent untreated mice were concurrently given an intranasal immunization with HK C. neoformans as a control. The antibody treatment continued until day 70 postinoculation, at which time all mice received a second pulmonary challenge with wild-type C. neoformans strain H99. Figure 1B shows that mice depleted of either CD4+ or CD8+ T cells during the afferent phase of vaccination were capable of mounting a protective response against a secondary challenge with wild-type C. neoformans. Cultures of lung and brain tissues of the challenged mice did not have detectable viable cryptococci at the termination of the survival experiment (day 80). Mice immunized with HK C. neoformans succumbed to a challenge with wild-type C. neoformans strain H99, with a median survival time of 25 days (Fig. 1B), similar to previous observations (37, 38). These results support the putative efficacy of clinically safe anticryptococcal vaccines given to immunocompromised patients (i.e., HIV-infected individuals) to induce protective anamnestic responses against a subsequent C. neoformans infection following immune reconstitution of the patient (i.e., effective highly active antiretroviral therapy). These vaccines could, theoretically, further reduce the need for life-long maintenance antifungal therapy for patients who are successfully treated for AIDS-associated cryptococcal meningitis due to a high relapse rate (2, 35).
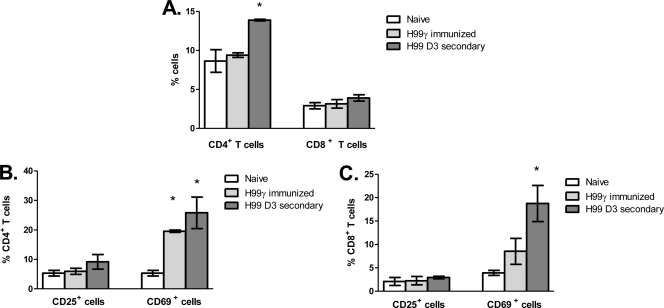
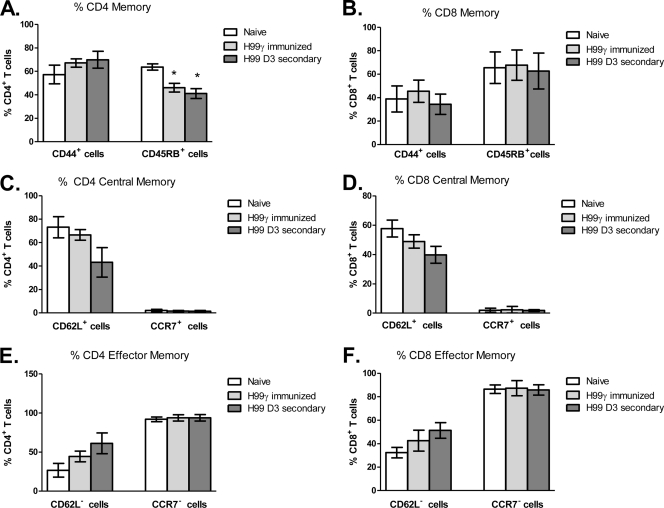
We subsequently phenotyped T cell activation and memory statuses in the lungs of naïve mice and mice immunized with C. neoformans strain H99γ and then challenged with wild-type cryptococci. To determine the T cell activation and memory profile (central or effector) during the protective immune response, BALB/c mice were given an intranasal immunization with C. neoformans strain H99γ or HK C. neoformans and allowed 70 days to resolve the infection. Immunized animals were subsequently challenged with sterile PBS (mock treatment) or wild-type C. neoformans strain H99, and the expression of various cell surface markers associated with an activated or memory phenotype (CD25, CD69, CD44, CD62L, CD45RB, and CCR7) was used to determine the profile of CD4+ and CD8+ T cells in the lungs 72 h after a secondary infection by flow cytometry. Naïve mice served as a control for the flow cytometry experiments. We observed a significant increase in the percentage of CD4+ T cells in the lungs of mice immunized with C. neoformans strain H99γ on day 3 after a secondary challenge compared to naïve mice (Fig. 2A). Furthermore, the percentage of CD69+ CD4+ T cells was significantly increased in immunized mock-infected mice and immunized mice on day 3 after a secondary challenge with wild-type C. neoformans compared to that in naïve mice (Fig. 2B). Although we did not observe a significant difference in the percentage of CD8+ T cells in the lungs of immunized mice prior to or after challenge with wild-type cryptococci from that in naïve mice (Fig. 2A), we did observe a significant increase in the percentage of CD69+ CD8+ T cells within the lungs of immunized mice on day 3 after a secondary infection (Fig. 2C). No differences in the percentage of CD25+ CD4+ (Fig. 2B) or CD25+ CD8+ (Fig. 2C) T cells were detected in any of the groups tested during our studies. Generally, CD4+ T cells within the lungs of C. neoformans strain H99γ-immunized mice on day 3 after a secondary challenge were predominantly CD44+ (Fig. 3A), CCR7− (Fig. 3E), and CD62L− (Fig. 3E) and expressed significantly less CD45RB (Fig. 3A) than did CD4+ T cells from naïve mice. We also observed a significant decrease in the percentage of CD45RB− CD4+ T cells in the lungs of immunized mock-infected mice compared to that of CD4+ T cells from naïve mice (Fig. 3A). No differences in the memory cell phenotype was observed between the CD8+ T cell populations derived from the lungs of naïve, immunized mock-infected, and immunized rechallenged mice (Fig. 3). Taken together, the results suggest that the profile of CD4+ T cells within the lungs of C. neoformans strain H99γ-immunized mice on day 3 after a secondary challenge is indicative of an activated effector memory cell phenotype (1, 31). A high percentage of CD69+ CD4+ T cells appeared to be maintained within the lungs of C. neoformans strain H99γ immunized mice and slightly increased following a second cryptococcal challenge. These results are similar to observations by Lindell et al. showing high CD69 expression on CD4+, but not CD8+, T cells at 12 weeks after C. neoformans infection (23). We additionally show that rechallenge of H99γ-immunized mice with fully virulent cryptococci results in a significant increase in the percentage of CD69+ CD8+ T cells. Importantly, mice immunized with the transgenic IFN-γ-producing strain went on to resolve the primary infection and were not transiently infected at the time of rechallenge. Engagement of CD69 has been shown to augment T cell proliferation, proinflammatory cytokine production, and increased cytotoxic activity of lymphoid cells (27, 32). Also, effector cells migrating to the lungs are characterized by stable CD44 expression, low CD45RB expression, loss of CD62L, and downregulation of CCR7 (1, 29), the phenotype observed in lung CD4+ T cells derived from H99γ-immunized mice following a secondary challenge. The loss of CD62L and CD45RB on CD4+ T cells within the lungs of H99γ-immunized mice appears to be greater following the second cryptococcal challenge (Fig. 3A and E), suggesting that additional cells are recruited to the lungs, a theory supported by the significant increase in CD4+ T cells observed on day 3 after a secondary challenge in C. neoformans strain H99γ-immunized mice (Fig. 2A).
Fig. 2.
Activation marker expression on CD4+ and CD8+ T cells prior to and following a secondary challenge. BALB/c mice received an intranasal inoculation with either sterile PBS (white bars) or C. neoformans strain H99γ and were allowed 70 days to resolve the infection. C. neoformans strain H99γ-immunized mice were subsequently given a second intranasal challenge with sterile PBS (light gray bars) or C. neoformans strain H99 (dark gray bars), and the cell surface expression of CD3, CD4, CD8, CD25, and CD69 was determined by flow cytometry analysis of whole lung dispersions. Shown are cumulative data from four experiments using 5 mice per group. Results are expressed as means ± the standard errors of the means. Asterisks indicate where significant differences (P < 0.05) from naïve mice were observed.
Fig. 3.
Memory marker expression on CD4+ and CD8+ T cells prior to and following a secondary challenge. BALB/c mice received an intranasal inoculation with either sterile PBS (white bars) or C. neoformans strain H99γ and were allowed 70 days to resolve the infection. C. neoformans strain H99γ-immunized mice were subsequently given a second intranasal challenge with sterile PBS (light gray bars) or C. neoformans strain H99 (dark gray bars), and the expression of CD3, CD4, CD8, CD44, CD45B, CD62, and CCR7 was determined by flow cytometry analysis of whole lung digests. Shown are cumulative data from four experiments using 5 mice per group. Results are expressed as means ± the standard errors of the means. Asterisks indicate where significant differences (P < 0.05) from naïve mice were observed.
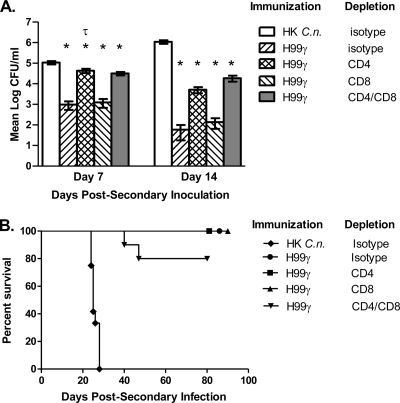
Nonetheless, for any cryptococcal vaccine strategy to be effective, it must elicit protection in populations most at risk for developing life-threatening cryptococcosis (i.e., T cell-deficient individuals). Consequently, our goal was to evaluate protection against pulmonary cryptococcosis in mice rendered experimentally T cell deficient following immunization with C. neoformans strain H99γ compared to immunocompetent mice immunized with HK C. neoformans cells. BALB/c mice were immunized with C. neoformans strain H99γ or HK C. neoformans and allowed 70 days to resolve the infection. Mice immunized with C. neoformans strain H99γ were then treated with an isotype control antibody or depleted of CD4+ and/or CD8+ T cells using complement-fixing antibodies beginning on day 70 postimmunization. Mice immunized with HK C. neoformans received sterile PBS. Subsequently, all mice received a second challenge with wild-type C. neoformans 48 h following the initial antibody treatments that were continued throughout the observation period. Figure 4A shows that the pulmonary fungal burden of all mice immunized with C. neoformans strain H99γ, irrespective of immunodeficiency, was significantly lower on days 7 and 14 after a secondary inoculation than that of HK C. neoformans-immunized mice on the respective day. A significant increase in the pulmonary fungal burden was also observed in C. neoformans strain H99γ-immunized mice that were depleted of CD4+ T cells compared to that of immunocompetent C. neoformans strain H99γ-immunized mice on day 7 after a secondary inoculation. Importantly, the additional depletion of CD8+ T cells in CD4-depleted mice did not result in a further increase in the pulmonary fungal burden (Fig. 4A), suggesting a dominant role for CD4+ T cells in mediating protection during the efferent phase of C. neoformans infection. However, the fungal burden in C. neoformans strain H99γ-immunized mice was not significantly different from that in C. neoformans strain H99γ-immunized mice depleted of CD4+ and/or CD8+ T cells on day 14 after a secondary inoculation. The pulmonary fungal burden analysis suggested that protective anamnestic responses to pulmonary cryptococcosis can be generated in vaccinated T cell-deficient hosts, a suggestion supported by subsequent survival studies. These data demonstrate that it is possible for previously vaccinated individuals to mount a protective immune response to Cryptococcus, even after becoming immunocompromised due to HIV or as a result of the use of immunosuppressive therapies in order to prevent rejection following organ transplantation.
Fig. 4.
Role of CD4+ and CD8+ T cells during the efferent response to pulmonary C. neoformans infection. BALB/c mice received an initial immunization with HK C. neoformans (C.n.) or C. neoformans strain H99γ in 50 μl of sterile PBS and were allowed 70 days to resolve the infection. Mice were then treated with isotype control antibodies or depleted of CD4+ T cells and/or CD8+ T cells 48 h prior to a challenge with C. neoformans strain H99 in 50 μl of sterile PBS. Depletion was maintained throughout the observation period. (A) Lungs were excised on days 7 and 14 after a secondary inoculation, and the cryptococcal burden was quantified. Pulmonary fungal burden data are cumulative for three experiments using 5 mice per time point. Separate mice were used for each time point. Results are expressed as the mean log CFU per milliliter ± the standard error of the mean. Asterisks indicate where significant decreases (P < 0.05) from mice immunized with HK C. neoformans were observed. The symbol τ indicates where significant increases (P < 0.05) from immunocompetent mice immunized with C. neoformans strain H99γ were observed. (B) Alternatively, the survival of challenged mice was monitored twice daily and mice that appeared moribund or did not maintain normal habits (grooming) were sacrificed by CO2 inhalation. The survival data shown are from one experiment using 10 mice per experimental group.
C. neoformans strain H99γ-immunized mice treated with isotype control antibodies or depleted of CD4+ or CD8+ T cells exhibited 100% survival of a second pulmonary challenge with the fully virulent wild-type strain. Furthermore, 80% of C. neoformans strain H99γ-immunized mice depleted of both CD4+ and CD8+ T cells survived the secondary challenge, compared to the 100% mortality observed in immunocompetent mice immunized with HK C. neoformans (Fig. 4B). Cultures of lung tissues from surviving mice revealed no detectable viable cryptococci at the termination of the survival experiment (day 80). Similarly, no brain dissemination was observed in H99γ-immunized mice treated with isotype control or anti-CD4 depletion antibodies and extremely low brain carriage was observed in whole brain cultures derived from anti-CD8 antibody-treated mice (7.3 ± 4.3 CFU) or dually anti-CD4- and anti-CD8-treated mice (1 ± 0.78 CFU). Again, we observed >98% depletion of CD4+ and/or CD8+ T cells in pulmonary and spleen leukocytes of protected mice upon the conclusion of the survival study (day 80) compared to the levels in control mice (data not shown).
C. neoformans is typically most aggressive in individuals with impaired T cell function (i.e., individuals with AIDS, those with lymphoid malignancies, and recipients of immunosuppressive therapies) (3, 4, 8–14, 19–22). The studies described herein highlight the potential for inducing protection against cryptococcosis in immunocompromised hosts. Previous vaccination strategies using recombinant forms of heat shock protein 60 from H. capsulatum (7) and Paracoccidioides brasiliensis (6) and live H. capsulatum (39), B. dermatitidis, (39, 40), and Candida albicans (33) yeast cells have supported the feasibility of developing fungal vaccines for use in patients with severe T cell deficiencies. Each study has enlightened us as to the importance of CD4+ and/or CD8+ T cells during the afferent phase of vaccination and the efferent phase of infection. Cumulatively, those studies show, as we do herein, that T cells are essential for the induction of protective immunity following vaccination. We additionally demonstrate protection during the efferent phase of the immune response to a fungal infection of mice depleted of both CD4+ and CD8+ T cells. While our results concur with those of previous studies suggesting a compensatory role for CD8+ T cells to mediate protection against fungal pathogens in the absence of CD4+ T cells (24, 39), our findings also allude to the capacity of an innate population of immune cells and/or B cells to compensate for the lack of T cells during the efferent phase of the immune response to C. neoformans infection. Although previous studies in our lab have shown that B cells are not required for the generation of protective immunity against pulmonary cryptococcosis in our model system (38), they may still play a compensatory and thus protective role during the efferent phase of the immune response in T cell-deficient hosts. Also, recent studies have suggested that natural killer cells, which have clearly been shown to have microbicidal activity against C. neoformans (25), may acquire some form of antigen-specific immunological memory (reviewed in reference 36). Studies to resolve which cellular populations are present during the efferent response in T cell-depleted mice are under way. Future studies utilizing mice depleted of or genetically deficient in certain effector cell populations and/or the adoptive transfer of cellular subsets into these mice are needed to “flesh out” the cellular population mediating efferent-phase protection. Nonetheless, our study provides proof of the concept that vaccines designed to combat C. neoformans infections are capable of inducing potent, long-lasting anticryptococcal immunity in immunocompromised patients.
ACKNOWLEDGMENTS
We thank Jose Lopez-Ribot and Sarah Hardison for critical reading of the manuscript.
This work was supported by grant RO1 AI071752-04 from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (F.L.W.).
The content of this report is solely our responsibility and does not necessarily represent the official views of NIAID of the NIH.
We declare no conflicts of interest.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Bingaman A. W., et al. 2005. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur. J. Immunol. 35:3173–3186 [DOI] [PubMed] [Google Scholar]
- 2. Bozzette S. A., et al. 1991. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. California Collaborative Treatment Group N. Engl. J. Med. 324:580–584 [DOI] [PubMed] [Google Scholar]
- 3. Chuck S. L., Sande M. A. 1989. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N. Engl. J. Med. 321:794–799 [DOI] [PubMed] [Google Scholar]
- 4. Collins V. P., Gellhorn A., Trimble J. R. 1951. The coincidence of cryptococcosis and disease of the reticulo-endothelial and lymphatic systems. Cancer 4:883–889 [DOI] [PubMed] [Google Scholar]
- 5. Cutler J. E., Deepe G. S., Jr., Klein B. S. 2007. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 5:13–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Bastos Ascenço Soares R., Gomez F. J., de Almeida Soares C. M., Deepe G. S., Jr 2008. Vaccination with heat shock protein 60 induces a protective immune response against experimental Paracoccidioides brasiliensis pulmonary infection. Infect. Immun. 76:4214–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deepe G. S., Jr., Gibbons R. S. 2002. Cellular and molecular regulation of vaccination with heat shock protein 60 from Histoplasma capsulatum. Infect. Immun. 70:3759–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diamond R. D., Bennett J. E. 1974. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann. Intern. Med. 80:176–181 [DOI] [PubMed] [Google Scholar]
- 9. Dismukes W. E. 1988. Cryptococcal meningitis in patients with AIDS. J. Infect. Dis. 157:624–628 [DOI] [PubMed] [Google Scholar]
- 10. Duperval R., Hermans P. E., Brewer N. S., Roberts G. D. 1977. Cryptococcosis, with emphasis on the significance of isolation of Cryptococcus neoformans from the respiratory tract. Chest 72:13–19 [DOI] [PubMed] [Google Scholar]
- 11. Eng R. H., Bishburg E., Smith S. M., Kapila R. 1986. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am. J. Med. 81:19–23 [DOI] [PubMed] [Google Scholar]
- 12. Gal A. A., Koss M. N., Hawkins J., Evans S., Einstein H. 1986. The pathology of pulmonary cryptococcal infections in the acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 110:502–507 [PubMed] [Google Scholar]
- 13. Gendel B. R., Ende M., Norman S. L. 1950. Cryptococcosis; a review with special reference to apparent association with Hodgkin's disease. Am. J. Med. 9:343–355 [DOI] [PubMed] [Google Scholar]
- 14. Goldstein E., Rambo O. N. 1962. Cryptococcal infection following steroid therapy. Ann. Intern. Med. 56:114–120 [DOI] [PubMed] [Google Scholar]
- 15. Herring A. C., Lee J., McDonald R. A., Toews G. B., Huffnagle G. B. 2002. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect. Immun. 70:2959–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huffnagle G. B., Lipscomb M. F. 1998. Cells and cytokines in pulmonary cryptococcosis. Res. Immunol. 149:387–396 (Discussion, 149:512-514.) [DOI] [PubMed] [Google Scholar]
- 17. Huffnagle G. B., Lipscomb M. F., Lovchik J. A., Hoag K. A., Street N. E. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 55:35–42 [DOI] [PubMed] [Google Scholar]
- 18. Huffnagle G. B., et al. 1996. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529–4536 [PubMed] [Google Scholar]
- 19. Husain S., Wagener M. M., Singh N. 2001. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg. Infect. Dis. 7:375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keye J. D., Jr., Magee W. E. 1956. Fungal diseases in a general hospital; a study of 88 patients. Am. J. Clin. Pathol. 26:1235–1253 [DOI] [PubMed] [Google Scholar]
- 21. Kovacs J. A., et al. 1985. Cryptococcosis in the acquired immunodeficiency syndrome. Ann. Intern. Med. 103:533–538 [DOI] [PubMed] [Google Scholar]
- 22. Lewis J. L., Rabinovich S. 1972. The wide spectrum of cryptococcal infections. Am. J. Med. 53:315–322 [DOI] [PubMed] [Google Scholar]
- 23. Lindell D. M., Ballinger M. N., McDonald R. A., Toews G. B., Huffnagle G. B. 2006. Immunologic homeostasis during infection: coexistence of strong pulmonary cell-mediated immunity to secondary Cryptococcus neoformans infection while the primary infection still persists at low levels in the lungs. J. Immunol. 177:4652–4661 [DOI] [PubMed] [Google Scholar]
- 24. Lindell D. M., Moore T. A., McDonald R. A., Toews G. B., Huffnagle G. B. 2005. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. J. Immunol. 174:7920–7928 [DOI] [PubMed] [Google Scholar]
- 25. Marr K. J., et al. 2009. Cryptococcus neoformans directly stimulates perforin production and rearms NK cells for enhanced anticryptococcal microbicidal activity. Infect. Immun. 77:2436–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitchell T. G., Perfect J. R. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moretta A., et al. 1991. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J. Exp. Med. 174:1393–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park B. J., et al. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530 [DOI] [PubMed] [Google Scholar]
- 29. Román E., et al. 2002. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 196:957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saag M. S., et al. 2000. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America Clin. Infect. Dis. 30:710–718 [DOI] [PubMed] [Google Scholar]
- 31. Sallusto F., Geginat J., Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745–763 [DOI] [PubMed] [Google Scholar]
- 32. Sancho D., Gomez M., Sanchez-Madrid F. 2005. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 26:136–140 [DOI] [PubMed] [Google Scholar]
- 33. Saville S. P., Lazzell A. L., Chaturvedi A. K., Monteagudo C., Lopez-Ribot J. L. 2008. Use of a genetically engineered strain to evaluate the pathogenic potential of yeast cell and filamentous forms during Candida albicans systemic infection in immunodeficient mice. Infect. Immun. 76:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Horst C. M., et al. 1997. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group N. Engl. J. Med. 337:15–21 [DOI] [PubMed] [Google Scholar]
- 35. Vibhagool A., et al. 2003. Discontinuation of secondary prophylaxis for cryptococcal meningitis in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy: a prospective, multicenter, randomized study. Clin. Infect. Dis. 36:1329–1331 [DOI] [PubMed] [Google Scholar]
- 36. Vivier E., et al. 2011. Innate or adaptive immunity? The example of natural killer cells. Science 331:44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wormley F. L., Jr, Perfect J. R., Steele C., Cox G. M. 2007. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect. Immun. 75:1453–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wozniak K. L., et al. 2009. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS One 4:e6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wüthrich M., Filutowicz H. I., Warner T., Deepe G. S., Jr., Klein B. S. 2003. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J. Exp. Med. 197:1405–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wüthrich M., Filutowicz H. I., Warner T., Klein B. S. 2002. Requisite elements in vaccine immunity to Blastomyces dermatitidis: plasticity uncovers vaccine potential in immune-deficient hosts. J. Immunol. 169:6969–6976 [DOI] [PubMed] [Google Scholar]