Abstract
Native outer membrane vesicles (NOMV) (not detergent treated), which are prepared from recombinant strains with attenuated endotoxin activity and overexpressed factor H binding protein (fHbp), elicited broad serum bactericidal antibody responses in mice. The amount of overexpressed fHbp required for optimal immunogenicity is not known. In this study we prepared NOMV vaccines from LpxL1 knockout (ΔLpxL1) mutants with penta-acylated lipooligosaccharide and attenuated endotoxin activity. The recombinant strains had wild-type (1×) fHbp expression or were engineered for 3-fold- or 10-fold-increased fHbp expression (3× or 10× fHbp). Control vaccines included NOMV from ΔLpxL1/ΔfHbp mutants or recombinant fHbp. In mice, only the 10× fHbp NOMV vaccine elicited significantly higher serum IgG anti-fHbp antibody titers than the corresponding 1× fHbp NOMV or recombinant fHbp vaccine. The 10× fHbp NOMV vaccine also elicited higher bactericidal responses (P < 0.05) against five group B strains with heterologous PorA than the recombinant fHbp or 1× fHbp NOMV vaccine. The 3× fHbp NOMV vaccine gave higher bactericidal titers against only one strain. Serum bactericidal titers in mice immunized with the control ΔfHbp NOMV vaccines were <1:10, and bactericidal titers in mice immunized with the 10× fHbp NOMV vaccine were <1:10 after adsorption of anti-fHbp antibodies. Mixing antiserum to NOMV vaccines from fHbp knockout mutants with antiserum to recombinant fHbp did not increase anti-fHbp bactericidal titers. Thus, a critical threshold of increased fHbp expression is required for NOMV vaccines to elicit broad serum bactericidal responses, and the antibodies conferring protection are directed primarily at fHbp.
INTRODUCTION
Neisseria meningitidis colonizes the nasopharynges of 10 to 20% of healthy adults. Relatively rarely, the bacterium invades the bloodstream and causes meningitis and/or septicemia (40). While polysaccharide-based vaccines against strains with capsular groups A, C, W-135, and Y are available (27), there is currently no broadly protective vaccine against capsular group B strains. The group B polysaccharide has structural homology with human tissues (12) and is poorly immunogenic, even when conjugated with a carrier protein (20). To date, only detergent-treated outer membrane vesicle (dOMV) vaccines are proven to be effective for prevention of group B disease, and these vaccines were used to control meningococcal group B epidemics in Cuba (35), Norway (5, 6), and New Zealand (15, 23, 31, 39).
The antibody responses to dOMV vaccines are directed mainly at a major porin protein, PorA (38), which is antigenically variable (34). This property limits the utility of dOMV vaccines to prevent endemic meningococcal disease, which is caused by genetically diverse strains (17, 18). The detergent treatment of bacterial cells used to prepare dOMV vaccines extracts lipooligosaccharide (LOS) (8, 14), which decreases endotoxin activity and improves vaccine tolerability (30). The extraction also removes desirable antigens that may elicit bactericidal antibodies (25). In an effort to improve immunogenicity, we and others have prepared native OMV (NOMV) vaccines, which were not treated with detergents (7, 13, 21, 22, 24, 25, 42, 46, 48). In order to attenuate endotoxin activity, the vaccine strains had deleted lpxL1 or lpxL2 genes, which encode late-functioning acyltransferases in the LOS biosynthesis pathway. The resulting mutant LOS molecules are penta- or tetra-acylated instead of hexa-acylated and have substantially decreased endotoxin activity (36, 41). The mutants also were engineered to have increased expression of desirable antigens, such as factor H binding protein (fHbp). In mice, NOMV vaccines with overexpressed fHbp elicited broader bactericidal antibody responses against genetically diverse N. meningitidis isolates than NOMVs prepared from the respective wild-type strains or recombinant protein vaccines containing fHbp (24, 25). The amount of overexpression of fHbp required for broad bactericidal activity and the contribution of antibodies elicited by non-fHbp antigens in NOMV vaccines to the serum bactericidal activity are the topics of investigation in the present study.
MATERIALS AND METHODS
Neisseria meningitidis strains.
The NOMV vaccines were prepared from mutants derived from group B strain H44/76 (Table 1), which naturally expresses relatively high levels of fHbp sequence variant ID 1 as classified in the Neisseria fHbp public database (http://pubmlst.org/neisseria/fHbp/). This protein is assigned to the variant 1 group as described by Masignani et al. (26). Endotoxin activity of the LOS expressed by the H44/76 strain was attenuated by deletion of the lpxL1 gene as described previously (25), which generated the recombinant strain H44/76 ΔLpxL1 with wild-type fHbp expression (1× fHbp) (Table 1). Four additional recombinant strains were derived from strain H44/76 ΔLpxL1. The first fHbp-overexpressing strain was prepared by transforming the recombinant ΔLpxL1 strain with the suicide plasmid pComPindFnrD148A (32), which generated the recombinant strain ΔLpxL1-FNRc. This strain contained the mutant activator of fHbp expression, FNR-D148A, under the control of the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible Ptac promoter, which was integrated into the chromosome. As described in Results, this mutant had 3-fold-increased fHbp expression (3× fHbp). Isogenic fHbp knockout strains of H44/76 ΔLpxL1 and ΔLpxL1-FNRc were obtained by transformation with plasmid pBSΔgna1870-ERM (26). The endogenous fHbp-encoding gene was replaced with an erythromycin cassette, which generated recombinant strains ΔLpxL1ΔfHbp and ΔLpxL1ΔfHbp-FNRc, respectively. A second fHbp-overexpressing strain was generated by transforming ΔLpxL1ΔfHbp with the multicopy plasmid pFP12-gna1870, (19) which in this study was renamed pFP12-fHbp. The plasmid contained the gene encoding fHbp ID 1 and resulted in 10-fold-increased fHbp expression (10× fHbp) (see Results).
Table 1.
Recombinant strains derived from H44/76 that were used to prepare the NOMV vaccines
| Strain | Relevant characteristics | NOMV vaccine (LpxL1 knockout) |
|---|---|---|
| ΔLpxL1 | Derivative of H44/76 with penta-acylated LOS through deletion of lpxL1; with wild-type fHbp expression | 1× fHbp |
| ΔLpxL1-FNRc | Derivative of ΔLpxL1 with constitutively active FNR mutant integrated into the chromosome driving a 3-fold increase in endogenous fHbp-encoding gene expression | 3× fHbp |
| ΔLpxL1ΔfHbp-FNRc | Derivative of ΔLpxL1-FNRc with endogenous fHbp-encoding gene deleted | ΔfHbp, FNRc |
| ΔLpxL1ΔfHbp | Derivative of ΔLpxL1 with endogenous fHbp-encoding gene deleted | ΔfHbp |
| ΔLpxL1ΔfHbp pFP12-fHbp | Derivative of ΔLpxL1ΔfHbp carrying a multicopy plasmid with fHbp-encoding gene, which resulted in a 10-fold increase in fHbp expression compared with wild-type expression | 10× fHbp |
The N. meningitidis test strains used to measure serum bactericidal activity are described in Table 2. All of the recombinant vaccine strains were derived from H44/76; thus, the H44/76 test strain expressed the same PorA included in the NOMV vaccines and expressed an fHbp protein with an amino acid sequence identical to that of the fHbp expressed by the vaccine strains or the recombinant fHbp (ID 1). The other five test strains expressed PorA proteins heterologous to that of the vaccine strains. The test strains expressed subvariants of fHbp in the variant 1 group and are identified by the different fHbp ID numbers. The panel of test strains was selected to represent the most prevalent fHbp sequence variants in the variant 1 group expressed by disease-causing capsular group B strains in the United States, the United Kingdom, and France (28, 33).
Table 2.
Characteristics of Neisseria meningitidis capsular group B strains used to measure serum bactericidal activity
| Straina | fHbp |
Serotype/serosubtype | Clonal complex | LOS immunotyped | fHbp ID prevalence (%) ine: |
|||
|---|---|---|---|---|---|---|---|---|
| IDb | Modular groupc | USA | UK | France | ||||
| H44/76 | 1 | I | B:15:P1.7,16 | 32 | L3,7,9 | 30 | 4 | 20 |
| NM008 | 4 | I | B:4:P1.4 | 41/44 | L3,7,9 | 14 | 24 | 7 |
| GB200 | 13 | I | B:NT:P1.22,9 | 269 | L1 | 5 | 10 | 2 |
| NZ98/254 | 14 | I | B:4:P1.7-2,4 | 41/44 | L3,7,9 | 5 | 7 | 23 |
| NM117 | 15 | IV | B:21:P1.9 | 269 | L1 | 0.3 | 22 | 6 |
| GB101 | 15 | IV | B:NT:P1.19,15 | 269 | L1,8 | |||
H44/76 and its derivatives were used to prepare the NOMV vaccines. It also expressed an fHbp with the same amino acid sequence as that of the recombinant fHbp vaccine.
fHbp identification number assigned in the fHbp database (http://pubmlst.org/neisseria/fHbp/).
Determined by whole-cell ELISA and anti-LOS monoclonal antibodies 2-1-L8, 9-2-L379, 17-1-L1 and 14-1-L-10.
Preparation of NOMVs.
The NOMVs consisted of native outer membrane blebs that were spontaneously released into the culture supernatant during growth of the bacteria. Five recombinant H44/76 strains were used for the preparation of NOMVs (Table 1). In brief, 7 ml modified Catlin 6 medium (48) was inoculated with single colonies and grown to mid-log phase (optical density [OD], 0.6 to 0.7). The starter medium used to grow the recombinant strain ΔLpxL1ΔfHbp-pFP12-fHbp was additionally supplemented with 5 μg/ml chloramphenicol for maintenance of the plasmid. The starter cultures were transferred into flasks containing 100 ml modified Catlin 6 without antibiotics. In order to maintain consistent conditions for each mutant, the medium was supplemented with 1 mM IPTG, which was necessary to induce expression of the mutant protein FNR-D148A. The bacteria were grown at 37°C for 6 h to early stationary phase (OD 1.0 to 1.2). The bacteria were killed by treatment with 0.5% (wt/vol) phenol for 1.5 h at 37°C, and the culture was left overnight at 4°C. After centrifugation, the culture supernatant was filtered (pore size, 0.45 μm) to remove residual cells and subsequently concentrated by filtration through a membrane with a 100-kDa cutoff. NOMVs from the concentrated culture supernatant were collected by ultracentrifugation. The pellet was washed twice with 3% sucrose, resuspended in 3% sucrose, and stored at −20°C. The protein concentration was measured with the bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Rockford, IL). The major proteins in the NOMVs were assessed by SDS-PAGE with Coomassie blue staining (25). Selected bands were cut from the gels and identified by mass spectrometry (11).
Quantification of fHbp in NOMVs.
We used an fHbp capture enzyme-linked immunosorbent assay (ELISA) adapted from the assay described by Donnelly et al. (10) to measure the relative amounts of fHbp in NOMVs prepared from the recombinant N. meningitidis strains. In brief, wells of a microtiter plate (Costar) were precoated with rabbit anti-fHbp IgG (1 mg/ml). NOMVs were solubilized by treatment with 5% Empigen BB in 10× phosphate-buffered saline (PBS), 0.25% proclin, and 0.01% methylene blue (final dilution, 1:11) (10). Serial 5-fold dilutions of the solubilized NOMVs (starting from 10 μg/ml of protein) were added to the wells of the microtiter plate and incubated for 16 to 18 h at 4°C. After washing, bound fHbp was detected with a murine anti-fHbp monoclonal antibody (MAb), JAR 5 (2 μg/ml; incubated for 2 h at room temperature). The JAR 5 hybridoma was from a mouse immunized with fHbp ID 1 (44), and the MAb showed broad reactivity with fHbp in the variant 1 group (4). The bound antibody-antigen complex was detected with alkaline phosphatase-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA), which contained 1% normal rabbit serum to eliminate cross-reactive binding to the rabbit IgG used in the capture step. Absorbance at 405 nm was plotted as a measure of the concentration of NOMVs added. The relative amount of fHbp present in the NOMV preparations was determined by comparison of the respective x intercepts in the linear portions of the resultant plots.
LOS immunotype.
The LOS immunotype was determined by whole-cell ELISA. N. meningitidis strains were grown in Mueller-Hinton broth to mid-log phase and heat inactivated at 56°C for 1 h. Cells were washed with PBS and adjusted to an OD of 0.4. The cells were added to wells of a microtiter plate (96-well MaxiSorp plates; Nunc) and dried. To determine the LOS immunotype, serial 5-fold dilutions of anti-LOS monoclonal antibodies (see the footnotes to Table 2) were added and incubated for 3 h at room temperature. Bound antibody was detected with alkaline phosphatase-conjugated rabbit anti-mouse IgG+A+M (Invitrogen).
Mouse immunization.
Female CD1 mice (Charles River Breeding Laboratories) (n = 15 per group for NOMV and recombinant fHbp vaccines and n = 10 for the negative-control group that received aluminum hydroxide only) were immunized intraperitoneally with either NOMV vaccines (2.5 μg of total protein per dose) or recombinant fHbp (20 μg per dose). All vaccines were adsorbed with aluminum hydroxide (600 μg aluminum per dose) in the presence of 10 mM histidine and 9 mg/ml NaCl. Three injections of vaccine were given at intervals of 3 weeks, and blood samples were collected at 3 weeks after the third injection. The sera were separated by centrifugation and stored in aliquots at −20°C.
Serological analysis.
All sera were heat inactivated at 56°C for 30 min to inactivate endogenous complement. Assays were performed on serum pools (five sera per pool; n = 3 pools per vaccine group and 2 pools for the negative-control mice). Total IgG antibody responses to fHbp and LOS were measured by ELISA, which was performed as described elsewhere (25). The antigens on the plate consisted of 1 μg/ml of purified His-tagged recombinant fHbp ID 1 or purified LOS prepared from strain H44/76 ΔLpxL1, which was extracted by the hot phenol-water method (45). Secondary antibody was alkaline phosphatase-conjugated goat anti-mouse IgG (Invitrogen). Complement-mediated serum bactericidal antibody responses were measured as described previously (1) using mid-log-phase bacteria that had been grown in Mueller-Hinton broth supplemented with 0.25% glucose and 0.02 mM cytidine-5′-monophospho–N-acetylneuraminic acid (CMP-NANA) (Sigma-Aldrich). Human complement serum was obtained from a healthy adult with no detectable intrinsic bactericidal activity against N. meningitidis. On the day of the assay, the serum was depleted of IgG by adsorption on a protein G-Sepharose column (Hi Trap protein G HP; GE Healthcare, Piscataway, NJ) (1). The bactericidal titer was defined as the reciprocal serum dilution resulting in 50% killing of bacteria after incubation for 60 min at 37°C.
Adsorption of anti-fHbp antibodies.
Serum pools were adsorbed using a solid-phase matrix (cyanogen bromide-activated agarose; Sigma-Aldrich) with covalently bound purified recombinant His-fHbp ID 1 or recombinant human albumin as a negative control. The matrix was washed to remove the unbound ligand, reactive sites were blocked with 1 M diethanolamine, and additional nonspecific binding sites were blocked with normal mouse serum. The matrix was incubated with the test serum sample for 1 h. After elution with Dulbecco's PBS, the eluate was concentrated to the original volume using Microcon centrifugal filter devices with a 10-kDa cutoff (Millipore, Billerica, MA). Adequacy of adsorption of serum anti-fHbp antibodies was determined by ELISA.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism version 5.01 (GraphPad Software, La Jolla, CA). ELISA and serum bactericidal responses of the groups were expressed as geometric mean titers (GMT) ± 2 standard errors (SE) calculated on log10-transformed titers. The statistical significance of differences in the titers between vaccine groups was performed by one-way analysis of variance (ANOVA). If the overall difference was significant (P < 0.05, two-tailed test), the significance of pairwise differences between individual groups was determined by Tukey's multiple-comparison test.
RESULTS
NOMVs from a recombinant strain with a multicopy plasmid encoding fHbp contain more fHbp than NOMVs from the strain with a mutant activator of fHbp expression.
We prepared NOMV vaccines from the recombinant strains described in Table 1. Proteins in the NOMVs from the three H44/76 recombinant strains that expressed fHbp were separated by SDS-PAGE. The NOMV preparations contained similar levels of the major outer membrane proteins PorA and PorB. As identified by mass spectrometry, fHbp comigrated in the gel with a second protein, OpcA (Fig. 1 A). To quantify the amount of fHbp in the NOMV vaccines, we used an anti-fHbp capture ELISA described in Materials and Methods. The relative quantities of fHbp were correlated with the total protein content of the NOMV preparations (Fig. 1B). Approximately 0.6 μg/ml of NOMV protein from the recombinant ΔLpxL1 strain expressing wild-type levels of fHbp resulted in the same absorption at 405 nm as 0.15 μg/ml of NOMV protein from the recombinant strain ΔLpxL1-FNRc or 0.05 μg/ml from the strain ΔLpxL1-pFP12-fHbp. Thus, the NOMVs from mutant strain ΔLpxL1-FNRc had ∼3-fold-larger amounts of fHbp than NOMVs from strain ΔLpxL1 with wild-type fHbp expression, while the NOMVs from strain ΔLpxL1-pFP12-fHbp had ∼10-fold larger amounts. For convenience, we refer to the NOMV vaccines from these two recombinant strains as 3× and 10× fHbp, respectively, and to NOMVs from the recombinant strain ΔLpxL1 with wild-type fHbp expression as 1× fHbp.
Fig. 1.
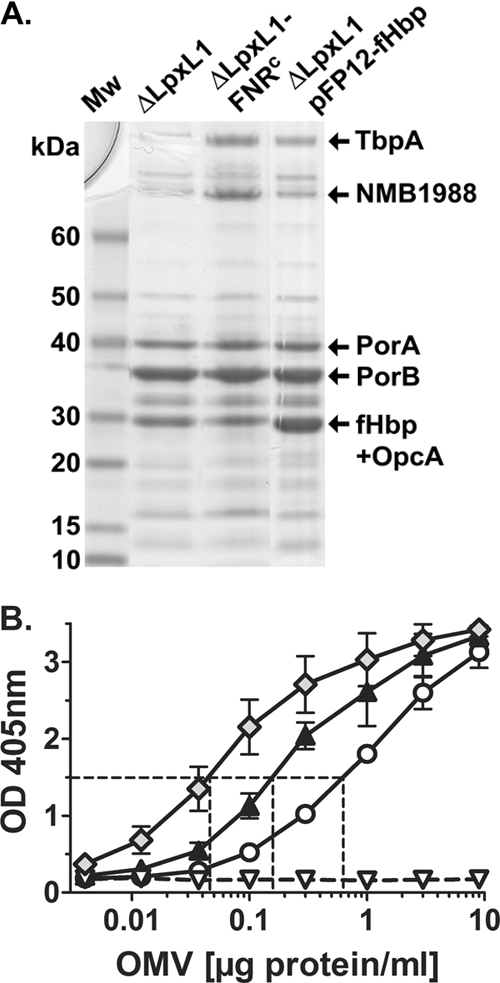
Characterization of NOMV vaccines. (A) SDS-PAGE and Coomassie blue staining of NOMVs prepared from recombinant H44/76 strains (Table 1). The NOMVs from the recombinant ΔLpxL1-FNRc strain had larger amounts of two high-molecular-mass proteins, which in parallel experiments were identified by mass spectrometry as transferrin binding protein A and NMB1988 (9, 37). The NOMVs from the recombinant ΔLpxL1-pFP12-fHbp strain showed larger amounts of a band resolving at ∼30 kDa. In parallel experiments the band resolving in this portion of the gel for NOMV preparations from both the ΔLpxL1-FNRc and ΔLpxL1-pFP12-fHbp recombinant strains contained fHbp and OpcA. (B) Relative amounts of fHbp in NOMV vaccines prepared from recombinant H44/76 strains as measured by a capture ELISA. Circles, ΔLpxL1; black triangles, ΔLpxL1-FNRc; Diamonds, ΔLpxL1-pFP12-fHbp; white triangles, ΔLpxL1ΔfHbp. The NOMV vaccines from the recombinant strains ΔLpxL1-FNRc and ΔLpxL1-pFP12-fHbp contained ∼3- and 10-fold more fHbp, respectively, than the NOMVs from the ΔLpxL1 recombinant strain with wild-type fHbp expression.
The 10× fHbp NOMV vaccine elicits higher IgG serum anti-fHbp antibody responses than the 3× fHbp NOMV vaccine.
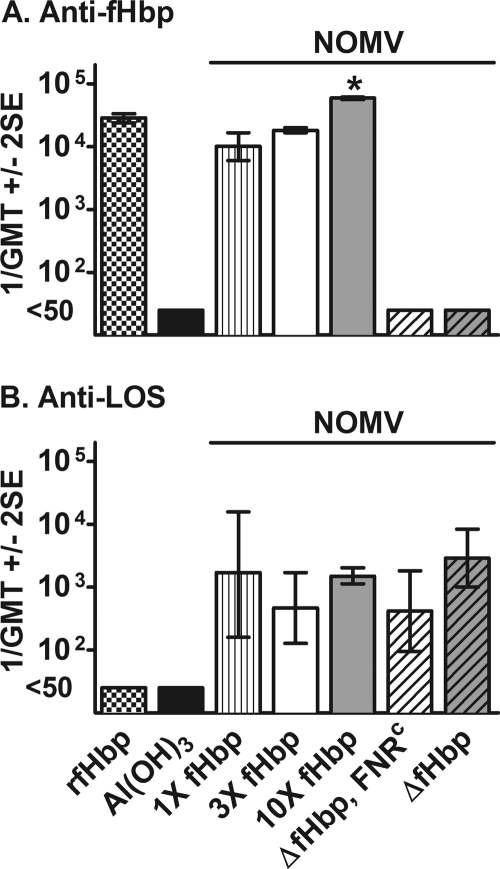
There was no detectable IgG anti-fHbp antibody in sera of mice immunized with the NOMV vaccines prepared from the two ΔfHbp recombinant strains or aluminum hydroxide alone (reciprocal titers of <50) (Fig. 2 A). The reciprocal geometric mean titers (GMT) in mice immunized with the 10× fHbp, 3× fHbp, and 1× fHbp NOMV vaccines were 60,000, 18,300, and 10,000, respectively (P < 0.05 by one-way analysis of variance). The reciprocal GMT in control mice immunized with the recombinant fHbp vaccine (28,500) was not statistically different from that in mice immunized with the 3× fHbp NOMV vaccine and was lower than that in the 10× fHbp NOMV group (P < 0.05).
Fig. 2.
IgG antibody responses of immunized mice as measured by ELISA. (A) Anti-fHbp. (B) Anti-LOS. The antigen on the plate was recombinant fHbp ID1 or LOS prepared from recombinant strain H44/76 ΔLpxL1. *, P < 0.05 for 10× fHbp NOMVs compared to NOMVs with 1× or 3× fHbp or recombinant fHbp vaccine (by Tukey's multiple comparison test). The differences between the respective anti-LOS antibody titers were not significant (P > 0.05).
The IgG anti-LOS antibody responses of control mice immunized with the recombinant fHbp vaccine or aluminum hydroxide alone were below detection (1/GMT, <50). There were no significant differences between the respective reciprocal GMTs of the serum anti-LOS antibody responses of mice immunized with the different NOMV vaccines (P > 0.05) (Fig. 2B).
The 10× fHbp NOMV vaccine elicits higher serum bactericidal antibody responses than the 3× fHbp NOMV vaccine against strains with heterologous PorA.
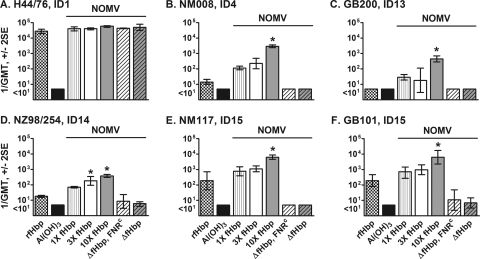
All of the NOMV vaccines as well as the recombinant fHbp vaccine elicited high serum bactericidal antibody responses against the wild-type strain H44/76 (reciprocal GMT, >20,000) (Fig. 3 A), which had a PorA that matched the PorA in the NOMV vaccines prepared from the recombinant H44/76 strains. This test strain also expressed fHbp sequence variant ID 1, which was the variant used in the recombinant fHbp vaccine and in the fHbp NOMV vaccines.
Fig. 3.
Human complement-mediated serum bactericidal antibody responses of mice. The H44/76 test strain (A) was the parent of recombinant H44/76 strains used to prepare the NOMV vaccines. The amino acid sequence of fHbp of this test strain also matched those of the fHbp in the NOMV and recombinant fHbp vaccines. The other five test strains (B to F) each expressed a PorA heterologous to that of the H44/76 vaccine strain and had heterologous fHbp sequence variants in the variant 1 group compared with the fHbp antigen in the vaccines (see text). *, P < 0.05 for the 10× fHbp or 3× fHbp NOMV vaccine group compared to NOMVs from strains with 1× fHbp expression (by Tukey's multiple-comparison test). ID, fHbp sequence variant identification number from the fHbp database (http://pubmlst.org/neisseria/fHbp/).
The remaining five test strains had PorA proteins heterologous to that of the vaccine strain and expressed fHbp sequence variants with amino acid identity ranging from 87% (strains NM117 and GB101, fHbp ID 15) to 96% (strain NM008, fHbp ID 4) to that of the vaccine strain. Against each of these strains, the 10× fHbp NOMV vaccine elicited higher geometric mean titers (P < 0.05) than the 1× fHbp NOMV vaccine or the recombinant fHbp vaccine. In contrast, mice immunized with the 3× fHbp NOMV vaccine had higher titers (P < 0.05) against only one strain (NZ98/254). Against four of the five heterologous strains (Fig. 3B, C, E, and F), the GMT in the 10× fHbp NOMV vaccine group also was significantly higher than that in the 3× fHbp NOMV vaccine group (P < 0.05).
Bactericidal antibody responses elicited by the 10× NOMV vaccine against strains with heterologous PorA are directed predominantly against fHbp.
Despite high serum anti-LOS antibody titers (Fig. 2B), mice immunized with control NOMV vaccines from the two isogenic ΔfHbp recombinant strains developed low or undetectable serum bactericidal antibody responses against all five strains with a heterologous PorA (1/GMT, ≤10) Fig. 3B to F). The lack of serum bactericidal activity was consistent with undetectable serum anti-fHbp antibody responses elicited by the NOMV vaccines from the ΔfHbp recombinant strains (Fig. 2A).
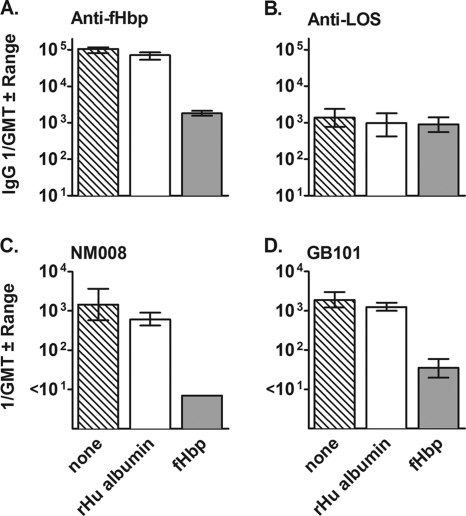
To investigate the antigenic target of bactericidal antibodies elicited by the 10× fHbp NOMV, we adsorbed anti-fHbp antibodies from two of the serum pools from the immunized mice and measured serum bactericidal activity against two strains, NM008 and GB101, with heterologous PorA VR types. By ELISA, approximately 98% of the anti-fHbp antibodies were removed from the sera by adsorption on the fHbp column (Fig. 4 A) while the levels of anti-LOS antibodies did not change (Fig. 4B). Adsorption of the anti-fHbp antibodies resulted in a decrease in the bactericidal titer from ∼1:1,000 to <1:10 against strain NM008 and from ∼1:1,000 to 1:35 against strain GB101 (Fig. 4C and D, respectively). These results were consistent with fHbp being the main target of serum bactericidal antibodies.
Fig. 4.
Effect of absorption of serum anti-fHbp antibodies on bactericidal activity. Two serum pools from mice immunized with 10× fHbp NOMVs were incubated with recombinant fHbp or, as a negative control, recombinant human albumin (rHu albumin), which were covalently linked to cyanogen bromide-activated agarose. Adsorption on the fHbp column depleted 98% of the anti-fHbp antibodies (A) without a significant effect on anti-LOS antibody titer (B). Depletion of anti-fHbp antibodies eliminated or removed the majority of serum bactericidal activity against strain NM008 or GB101 with a heterologous PorA (C and D).
Lack of cooperative serum bactericidal activity between anti-fHbp antibodies elicited by the recombinant fHbp vaccine and antibodies elicited by ΔfHbp NOMV vaccines.
Previous studies indicated that for antibodies directed at “minor” protein antigens, a critical density of immune complexes on the bacterial surface was required to elicit bactericidal activity, and this threshold was reached only when more than one minor antigen was targeted (47). Evidence for cooperative serum bactericidal activity between antibodies to fHbp and Neisseria heparin binding antigen in adults immunized with a multicomponent recombinant vaccine was also recently reported (43). Therefore, it was possible that the high bactericidal titers elicited in mice by the NOMV vaccine with 10× fHbp resulted from cooperative bactericidal activity between antibodies directed at fHbp and antibodies elicited by other antigens in the NOMV vaccine. To investigate this possibility, we mixed a serum pool from mice immunized with the recombinant fHbp vaccine with serum pools from mice immunized with the ΔfHbp NOMV vaccines or, as a negative control, a serum pool from mice immunized with aluminum hydroxide only (Table 3). We measured serum bactericidal activity of the mixed sera against two strains with heterologous PorA. Strain NM008 had an LOS immunotype homologous to that of the vaccine strain (L3,7,9). Strain GB101 had a heterologous LOS immunotype (L1,8). Against both test strains the respective reciprocal anti-fHbp bactericidal titers of the antiserum to the recombinant fHbp vaccine were not significantly augmented by the addition of antiserum from mice immunized with the ΔfHbp NOMV vaccines. Thus, there was no evidence that antibodies elicited by non-fHbp antigens in the NOMV vaccines from the ΔfHbp recombinant strains cooperated and augmented bactericidal activity of the anti-fHbp antibodies elicited by the recombinant fHbp vaccine.
Table 3.
Serum bactericidal titers of mixtures of antisera
| Antiserum Aa | Antiserum Ba | 1/titerb |
||
|---|---|---|---|---|
| Serum bactericidal activityc |
IgG anti-fHbp | |||
| Strain GB101 | Strain NM008 | |||
| ΔfHbp NOMV | Al(OH)3without antigen | <10 | <10 | <50 |
| ΔfHbp, FNRc NOMV | Al(OH)3without antigen | <10 | <10 | <50 |
| Recombinant fHbp | Al(OH)3without antigen | 90 | 10 | 11,000 |
| Recombinant fHbp | ΔfHbp NOMV | 80 | 10 | 11,000 |
| Recombinant fHbp | ΔfHbp, FNRc NOMV | 70 | 12 | 11,000 |
| 10× fHbp NOMV | Al(OH)3 | 4,600 | 1,600 | 20,000 |
Equal volumes of antisera A and B were mixed and assayed for bactericidal activity.
Refers to the dilution of the mixed sera.
Strain GB101 expresses an LOS immunotype (L1,8) heterologous to that of the H44/76 recombinant vaccine strains, while strain NM008 expresses a homologous LOS immunotype (L3,7,9).
DISCUSSION
Detergent-treated meningococcal OMV vaccines are safe (29, 30) and effective (reviewed in reference 16). Their major limitation is a strain-specific serum bactericidal antibody response, which in infants is directed largely against antigenically variable PorA (38). In the present study, we prepared NOMV vaccines from recombinant ΔLpxL1 strains of H44/76 in which either the gene encoding fHbp ID 1 was deleted or fHbp expression was increased 3-fold or 10-fold compared to wild-type fHbp expression, which in strain H44/76 is naturally high (26, 33). Our most important finding was that only the 10× fHbp NOMV vaccine consistently elicited high IgG anti-fHbp titers and high serum bactericidal antibody responses against strains with heterologous PorA. Several lines of evidence indicated that anti-fHbp antibodies were principally responsible for this bactericidal activity. First, depletion of anti-fHbp antibodies from sera of mice immunized with the 10× fHbp NOMV vaccine eliminated or substantially decreased serum bactericidal activity. Second, sera from control mice immunized with NOMV vaccines prepared from ΔfHbp recombinant strains had minimal or no bactericidal activity. Third, there was no evidence of augmentation of bactericidal activity of anti-fHbp antibodies elicited by the recombinant fHbp vaccine when the serum pool was mixed with a serum pool from the mice immunized with NOMV vaccines from the ΔfHbp recombinant strains (Table 3). Collectively, the results indicated that a relatively large increase in fHbp expression in the NOMV vaccine was required for optimal fHbp immunogenicity, that the principal antigenic target of the bactericidal responses was fHbp, and that other antibodies elicited by the NOMV vaccine did not contribute to the observed bactericidal activity.
In a phase 1 trial, Keiser et al. immunized adults with an NOMV vaccine from a mutant with attenuated endotoxin activity (ΔLpxL1), which also was engineered to overexpress fHbp (21, 48). The LOS of the mutant also had a truncated oligosaccharide chain. They found that most of the serum bactericidal antibody response was directed against LOS, with only a minor contribution of anti-fHbp antibodies. Similar results were observed in an earlier study with this vaccine in CD1 mice (48). Our findings provide a plausible explanation for the low anti-fHbp antibody responses to the Keiser vaccine, since by Western blotting the recombinant strain used to prepare the NOMV vaccine had only approximately 2-fold-increased fHbp expression compared to the parent wild-type strain (48). Also, the recombinant strain was described as expressing fHbp levels similar to those of the H44/76 wild-type strain (21), which would correspond to the 1× fHbp NOMV vaccine in our study. In CD1 mice, only the NOMV vaccine (10×) with 10-fold-increased fHbp elicited broad serum anti-fHbp bactericidal antibody responses against strains with heterologous PorA; the 3× and 1× fHbp NOMV vaccines were less effective.
All of the NOMV vaccines containing fHbp elicited higher serum bactericidal titers against three of the five PorA-mismatched strains than the control recombinant fHbp vaccine (Fig. 3B, C, and D). Our hypothesis is that the fHbp NOMV vaccines elicited a different anti-fHbp antibody repertoire than the recombinant fHbp vaccine, which can affect bactericidal activity (3, 4). An alternative hypothesis is that there were different anti-fHbp IgG subclass responses to the different vaccine formulations, but previously published data did not support this possibility (19). A third possibility was that antibodies directed at other antigens in the NOMV vaccine augmented bactericidal activity of the anti-fHbp antibodies. As described above, this hypothesis also was unlikely, since adding antisera from mice immunized with NOMV vaccines from ΔfHbp recombinant strains did not augment the bactericidal activity of antiserum to the recombinant fHbp (Table 3).
In conclusion, NOMV vaccines from ΔLpxL1 recombinant strains with increased fHbp expression have the potential to be safe for use in humans and efficacious. As shown in this study, high levels of overexpressed fHbp (10× over wild-type fHbp expression by strain H44/76) are needed in the NOMV vaccine to elicit high anti-fHbp titers and broad bactericidal activity.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants R01 AI 046464 and AI 082263 (to D.M.G.) from the National Institute of Allergy and Infectious Diseases, NIH. Oliver Koeberling and Isabel Delany are employees of Novartis Vaccines and Diagnostics, Siena, Italy. Dan M. Granoff is principal investigator of laboratory research conducted on behalf of Children's Hospital Oakland Research Institute, which is funded by grants from Novartis Vaccines and Diagnostics, and Sanofi Pasteur. He also holds a consultancy from Novartis.
We are grateful to Laura Amodeo for providing expert technical assistance; Francesca Oriente, who prepared the FNR construct used to prepare the ΔLpxL1-FNRc recombinant strain; and Danilo Donnarumma for mass spectrometry studies.
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Beernink P. T., Caugant D. A., Welsch J. A., Koeberling O., Granoff D. M. 2009. Meningococcal factor H-binding protein variants expressed by epidemic capsular group A, W-135, and X strains from Africa. J. Infect. Dis. 199:1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beernink P. T., Granoff D. M. 2009. The modular architecture of meningococcal factor H-binding protein. Microbiology 155:2873–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beernink P. T., et al. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186:3806–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beernink P. T., et al. 2008. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate, factor H-binding protein. Infect. Immun. 76:4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bjune G., Gronnesby J. K., Hoiby E. A., Closs O., Nokleby H. 1991. Results of an efficacy trial with an outer membrane vesicle vaccine against systemic serogroup B meningococcal disease in Norway. NIPH Ann. 14:125–130 (Discussion, 14:130–132) [PubMed] [Google Scholar]
- 6. Bjune G., et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093–1096 [DOI] [PubMed] [Google Scholar]
- 7. Bonvehi P., et al. 2010. Three doses of an experimental detoxified L3-derived lipooligosaccharide meningococcal vaccine offer good safety but low immunogenicity in healthy young adults. Clin. Vaccine Immunol. 17:1460–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Claassen I., et al. 1996. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine 14:1001–1008 [DOI] [PubMed] [Google Scholar]
- 9. Delany I., Grifantini R., Bartolini E., Rappuoli R., Scarlato V. 2006. Effect of Neisseria meningitidis fur mutations on global control of gene transcription. J. Bacteriol. 188:2483–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donnelly J., et al. 2010. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc. Natl. Acad. Sci. U. S. A. 107:19490–19495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrari G., et al. 2006. Outer membrane vesicles from group B Neisseria meningitidis Delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 6:1856–1866 [DOI] [PubMed] [Google Scholar]
- 12. Finne J., Leinonen M., Makela P. H. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357 [DOI] [PubMed] [Google Scholar]
- 13. Fisseha M., et al. 2005. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect. Immun. 73:4070–4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fredriksen J. H., et al. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67–79 (Discussion, 14:79–80) [PubMed] [Google Scholar]
- 15. Galloway Y., Stehr-Green P., McNicholas A., O'Hallahan J. 2009. Use of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 years. Int. J. Epidemiol. 38:413–418 [DOI] [PubMed] [Google Scholar]
- 16. Granoff D. M. 2010. Review of meningococcal group B vaccines. Clin. Infect. Dis. 50(Suppl. 2):S54–S65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holst J. 2007. Strategies for development of universal vaccines against meningococcal serogroup B disease: the most promising options and the challenges evaluating them. Hum. Vaccin. 3:290–294 [DOI] [PubMed] [Google Scholar]
- 18. Holst J., et al. 2005. The concept of “tailor-made,” protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 23:2202–2205 [DOI] [PubMed] [Google Scholar]
- 19. Hou V. C., Koeberling O., Welsch J. A., Granoff D. M. 2005. Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. J. Infect. Dis. 192:580–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jennings H. J., Lugowski C. 1981. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J. Immunol. 127:1011–1018 [PubMed] [Google Scholar]
- 21. Keiser P. B., et al. 2011. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 29:1413–1420 [DOI] [PubMed] [Google Scholar]
- 22. Keiser P. B., et al. 2010. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine 28:6970–6976 [DOI] [PubMed] [Google Scholar]
- 23. Kelly C., Arnold R., Galloway Y., O'Hallahan J. 2007. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Am. J. Epidemiol. 166:817–823 [DOI] [PubMed] [Google Scholar]
- 24. Koeberling O., Giuntini S., Seubert A., Granoff D. M. 2009. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin. Vaccine Immunol. 16:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koeberling O., Seubert A., Granoff D. M. 2008. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J. Infect. Dis. 198:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masignani V., et al. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maslanka S. E., et al. 1998. Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from Montana immunized with a licensed polysaccharide vaccine. Infect. Immun. 66:2453–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy E., et al. 2009. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis.. J. Infect. Dis. 200:379–389 [DOI] [PubMed] [Google Scholar]
- 29. Nokleby H. 2007. Neurological adverse events of immunization: experience with an aluminum adjuvanted meningococcal B outer membrane vesicle vaccine. Expert Rev. Vaccines 6:863–869 [DOI] [PubMed] [Google Scholar]
- 30. Nokleby H., et al. 2007. Safety review: two outer membrane vesicle (OMV) vaccines against systemic Neisseria meningitidis serogroup B disease. Vaccine 25:3080–3084 [DOI] [PubMed] [Google Scholar]
- 31. O'Hallahan J., Lennon D., Oster P. 2004. The strategy to control New Zealand's epidemic of group B meningococcal disease. Pediatr. Infect. Dis. J. 23:S293–S298 [PubMed] [Google Scholar]
- 32. Oriente F., Scarlato V., Delany I. 2010. Expression of factor H binding protein of meningococcus responds to oxygen limitation through a dedicated FNR-regulated promoter. J. Bacteriol. 192:691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pajon R., Beernink P. T., Harrison L. H., Granoff D. M. 2010. Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine 28:2122–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sacchi C. T., et al. 2000. Diversity and prevalence of PorA types in Neisseria meningitidis serogroup B in the United States, 1992–1998. J. Infect. Dis. 182:1169–1176 [DOI] [PubMed] [Google Scholar]
- 35. Sierra G. V., et al. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195–207(Discussion, 14:208–210) [PubMed] [Google Scholar]
- 36. Steeghs L., Tommassen J., Leusen J. H., van de Winkel J. G., van der Ley P. 2004. Teasing apart structural determinants of ‘toxicity’ and ‘adjuvanticity’: implications for meningococcal vaccine development. J. Endotoxin Res. 10:113–119 [DOI] [PubMed] [Google Scholar]
- 37. Sun Y. H., Bakshi S., Chalmers R., Tang C. M. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269–1273 [DOI] [PubMed] [Google Scholar]
- 38. Tappero J. W., et al. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520–1527 [DOI] [PubMed] [Google Scholar]
- 39. Thornton V., et al. 2006. Safety and immunogenicity of New Zealand strain meningococcal serogroup B OMV vaccine in healthy adults: beginning of epidemic control. Vaccine 24:1395–1400 [DOI] [PubMed] [Google Scholar]
- 40. Trotter C. L., Gay N. J., Edmunds W. J. 2006. The natural history of meningococcal carriage and disease. Epidemiol. Infect. 134:556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van der Ley P., et al. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 69:5981–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van de Waterbeemd B., et al. 2010. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine 28:4810–4816 [DOI] [PubMed] [Google Scholar]
- 43. Vu D. M., Wong T. T., Granoff D. M. 2011. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and neisserial heparin binding antigen. Vaccine 29:1968–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Welsch J. A., Rossi R., Comanducci M., Granoff D. M. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J. Immunol. 172:5606–5615 [DOI] [PubMed] [Google Scholar]
- 45. Westphal O., Jann K., Himmelspach K. 1983. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog. Allergy 33:9–39 [PubMed] [Google Scholar]
- 46. Weynants V., et al. 2009. Genetically modified L3,7 and L2 lipooligosaccharides from Neisseria meningitidis serogroup B confer a broad cross-bactericidal response. Infect. Immun. 77:2084–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weynants V. E., et al. 2007. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. Immun. 75:5434–5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zollinger W. D., et al. 2010. Design and evaluation in mice of a broadly protective meningococcal group B native outer membrane vesicle vaccine. Vaccine 28:5057–5067 [DOI] [PubMed] [Google Scholar]