Abstract
Allergen-specific immunotherapy is a potential treatment for allergic diseases. We constructed an allergen–cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)-encoding DNA vaccine, administered it directly to antigen-presenting cells (APCs), and investigated its ability and mechanisms to ameliorate allergic airway inflammation in an asthmatic mouse model. An allergen-CTLA-4 DNA plasmid (OVA-CTLA-4-pcDNA3.1) encoding an ovalbumin (OVA) and the mouse CTLA-4 extracellular domain was constructed and transfected into COS-7 cells to obtain the fusion protein OVA-CTLA-4, which was able to bind the B7 ligand on dendritic cells (DCs), and induced CD25+ Foxp3+ regulatory T (Treg) cells by the coculture of naive CD4+ T cells with DCs in vitro. In an animal study, BALB/c mice were sensitized and challenged with OVA to establish the asthmatic model. Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 significantly decreased interleukin-4 (IL-4) and IL-5 levels and eosinophil counts and prevented OVA-induced reduction of the gamma interferon level in the bronchoalveolar lavage fluid. In addition, these mice suffered less severe airway inflammation and had lower levels of OVA-specific IgE and IgG1 titers in serum. Also, high-dose OVA-CTLA-4-pcDNA3.1 vaccination inhibited the development of airway hyperreactivity and prevented OVA-induced reduction of the percentages of Foxp3+ Treg cells in the spleen. Our results indicate that a high dose of allergen-CTLA-4-encoding DNA vaccine was more effective in preventing an allergen-induced Th2-skewed immune response through the induction of Treg cells and may be a new alternative therapy for asthma.
INTRODUCTION
Allergic asthma is a disease characterized by airway inflammation, reversible airway obstruction, and airway hyper-responsiveness to various bronchoconstrictive stimuli. Allergen-specific immunotherapy (SIT) is a conventional treatment for allergic asthma (9, 19), which involves the repetitive application of an allergen by subcutaneous injection or sublingual application. To date, SIT is the treatment most likely to modify the natural course of asthma and reduce the severity of asthma and bronchial responses in patients exposed to inhaled allergens. Allergen DNA vaccines are considered to be one of the most promising immunotherapeutic strategies for the treatment of allergic diseases. However, experimental studies show that allergen DNA vaccines have the major problem of low immunogenicity. This may be because the amount of antigen expressed by transfected cells during the course of vaccination is too low to be effectively captured by antigen-presenting cells (APCs) (6). Therefore, improving the specificity of DNA vaccines administered directly to APCs will increase their efficacy.
Many specific molecules are expressed on the APC surface, including B7 and complement receptors, which offer a wide selection for the design of allergen DNA vaccines to be administered directly to APCs (18). Cytotoxic T lymphocyte associated antigen 4 (CTLA-4), which is mainly expressed on activated T cells, binds B7 ligand on the APC. In the present study, we constructed a DNA vaccine to be administered directly to APCs, comprising an allergen (ovalbumin [OVA]) fused to the CTLA-4 extracellular domain. We investigated whether this allergen-CTLA-4-encoding DNA vaccine could reverse the established Th2 responses in allergic mouse models.
Recently, the induction of regulatory T (Treg) cells has attracted much attention in terms of SIT (3, 11, 20). Treg cells constitutively express the interleukin-2 (IL-2) receptor chain (CD25) at high levels, and the forkhead transcription factor, forkhead box protein 3 (Foxp3), is considered essential for the development and function of Treg cells (1). Indeed, Treg cells are best identified by their expression of Foxp3. In vivo clearance of Treg cells in mice results in allergic airway inflammation, while exogenous infusion of antigen-specific Treg cells can be used to effectively treat airway inflammation (22). The critical ability of Treg cells to control asthma has sparked much interest concerning whether such cells develop or expand in the process of SIT and, if so, which of these promotes the induction of Treg cells. In animal studies, It has been demonstrated that the engagement of B7 ligand on dendritic cells (DCs) by a soluble form of CTLA-4 (CTLA-4-Ig) modifies DCs to express indoleamine 2,3-dioxygenase (IDO), a tryptophan-catabolizing enzyme that induces and activates Treg cells (16). Since CTLA-4-Ig has the potency to induce Treg cells, we investigated whether the OVA-CTLA-4 DNA vaccine encoding fusion protein OVA-CTLA-4 in the present study binds to the B7 ligand on DCs and induces the generation of Treg cells. To establish whether Treg cells play a role in allergen-CTLA-4-encoding DNA vaccination, we also measured the percentages of Treg cells in the spleens of vaccinated mice.
MATERIALS AND METHODS
Animals.
Male BALB/c mice (6 to 10 weeks old) and weighing 18 to 22 g were purchased from the Animal Centre at Jinling Hospital. Animal care and experimental procedures were performed in accordance with the animal ethics regulations of the Institutional Animal Care and Use Committee.
Construction of the OVA-CTLA-4-pcDNA3.1 immunization vector.
OVA and a DNA fragment encoding the extracellular domain of mouse CTLA-4 were amplified by PCR. The two fragments were inserted into a pcDNA3.1(+) vector to generate the recombinant plasmids OVA-pcDNA3.1 and OVA-CTLA-4-pcDNA3.1. The resulting plasmids were then sent to ShengXing Corp. (Nanjing, China) for DNA sequencing. Sequencing confirmed that the two plasmids had been constructed successfully. The large-scale purification of plasmids was conducted by using a Maxiprep GFII Endo-Free kit (Qbiogene, Inc., Montreal, Quebec, Canada) according to the manufacturer's instructions.
Expression and purification of ectopic protein.
COS-7 cells were seeded in 100-mm culture flasks (2 × 106 cells/flask) in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (Gibco, Grand Island, NY). The expression plasmids OVA-pcDNA3.1 and OVA-CTLA-4-pcDNA3.1 were transfected into COS-7 cells by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. After 72 h, the supernatants were collected, and ectopic protein OVA and OVA-CTLA-4 were purified from the culture supernatants by using anti-OVA antibodies (Zhongshan Co., Beijing, China) coupled to CNBr-Sepharose 4B affinity chromatography (Amersham Biosciences, Piscataway, NJ). The purified protein OVA and OVA-CTLA-4 were confirmed by Western blotting with anti-OVA antibodies.
Detection of OVA-CTLA-4 binding to B7 ligands on DCs in vitro.
Mouse bone marrow-derived DCs were generated as previously described (2). After 8 days of culture with 10 ng of granulocyte-macrophage colony-stimulating factor/ml and 1 ng of interleukin-4 (IL-4)/ml, DCs were purified by using anti-CD11c-coated microbeads (Miltenyi Biotec, Auburn, CA) and treated with purified OVA (100 ng/ml) or OVA-CTLA-4 (100 ng/ml) for 4 h. To study the interaction of OVA-CTLA-4 and B7 ligands on DCs, coimmunoprecipitation experiments were performed to detect the interactions between them. To stabilize potentially transient or weak interactions, whole cells were subjected to chemical cross-linking by incubation with the cleavable cross-linker dithiobis(succinimidyl propionate) (DSP; Pierce) as described previously (7). Cells were lysed by adding lysis buffer (25 mM Tris-HCl [pH 8.0], 50 mM NaCl, 0.5% sodium deoxycholate, 0.5% Triton X-100) containing a protease inhibitor mixture and incubated by mixing them at 4°C for 30 min. Lysates were cleared by centrifugation (15,000 × g for 15 min). Anti-CD80 or anti-CD86 antibody was added to the diluted samples, and after overnight incubation at 4°C protein A-coupled agarose beads (15 μl) were added, followed by further incubation for at least 8 h at 4°C. After three washes with Tris buffer, the beads were suspended in SDS-PAGE loading buffer (0.125 M Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 0.1 M dithiothreitol, 0.01% bromphenol blue) and boiled for 5 min, a process which also cleaves the cross-linker DSP. After centrifugation at 14,000 × g for 5 min, the supernatants were analyzed by Western blotting with anti-OVA antibody.
Induction of Foxp3+ Treg cells by OVA-CTLA-4 in vitro.
Autologous CD4+ T cells were obtained from the spleen by using antibody-coated paramagnetic MultiSort MicroBeads (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. After detaching, the CD4+ T cells were stained with CD25 MicroBeads (Miltenyi Biotec), and then CD4+ CD25+ T cells were positively selected and CD4+ CD25− T cells were negatively selected. Separation was controlled by flow cytometry (FCM) (CD4+ CD25− T cells > 95%). CD4+ CD25− T cells at 4 × 105 cells/well were incubated with 4 × 104 DCs in the presence of medium, 100 ng of OVA/ml. or 100 ng of OVA-CTLA-4/ml at 37°C in 5% CO2-humidified air. After 72 h of incubation, the cells were harvested to examine the expression of CD25 and Foxp3 using mouse regulatory T cell staining kit (eBioscience, Inc.).
Immunization protocols.
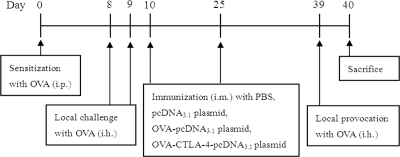
Mice were anesthetized and immunized via injection of 100 μl of inoculum into the tibialis anterior muscle by using a 1-ml syringe. Mice were divided randomly into six groups (n = 6 mice per group) as follows: (i) mice treated and sensitized and challenged with phosphate-buffered saline (PBS) (control); (ii) mice treated with PBS and sensitized and challenged with OVA (model); (iii) mice treated with the pcDNA3.1 plasmid (100 μg/mouse) and sensitized and challenged with OVA (pcDNA3.1); (iv) mice treated with OVA-pcDNA3.1 (100 μg/mouse) and sensitized and challenged with OVA (OVA-pcDNA3.1); (v) mice treated with a low dose of OVA-CTLA-4-pcDNA3.1 (50 μg/mouse) and sensitized and challenged with OVA [OVA-CTLA-4-pcDNA3.1(L)]; and (vi) mice treated with a high dose of OVA-CTLA-4-pcDNA3.1 (100 μg/mouse) and sensitized and challenged with OVA [OVA-CTLA-4-pcDNA3.1(H)]. The establishment of the mouse allergic asthma model and the vaccination protocols have been described previously (14). In brief, mice were sensitized to OVA (grade V; Sigma-Aldrich, St. Louis, MO) by intraperitoneal injection of 100 μl of alum-precipitated antigen comprising 10 μg of OVA absorbed onto 4 mg of aluminum potassium sulfate (Pierce Biotechnology, Rockford, IL) on day 0, followed by inhalation of 1% OVA (grade II; Sigma-Aldrich) diluted in PBS for 30 min on days 8 and 9. The mice were then vaccinated with PBS, pcDNA3.1, OVA-pcDNA3.1, or OVA-CTLA-4-pcDNA3.1 on days 10 and 25. On day 39 the mice were exposed to aerosol challenge with 1% OVA (grade II) diluted in PBS for 30 min (Fig. 1). At 24 h after the final challenge, blood, bronchoalveolar lavage fluid (BALF), lungs, and spleens were harvested for further analysis.
Fig. 1.
Immunization protocol.
Histological analysis of lung tissue.
At 24 h after the final allergen challenge, the excised lungs were harvested and fixed with neutral buffered 4% formalin and embedded in paraffin. The left lobes of each lung were sectioned (3 to 5 μm) and stained with hematoxylin and eosin (H&E). Cellular infiltrates were assessed in five randomly selected fields with a microscope at ×100 magnification. Inflammatory changes were graded on a scale of 0 to 5 for perivascular and bronchiolar eosinophilia, epithelial damage, and edema (24).
Bronchoalveolar lavage.
To investigate the differential cell count in the BALF, the trachea was exposed and cannulated, and the lungs were gently instilled twice with 500 μl of cold PBS. The volume, total cell number, and composition of the BALF samples were recorded. Samples were centrifuged (500 × g for 5 min at 4°C), resuspended, and loaded onto slides by using a Cytospin. Differential cell counts were performed in duplicate on coded slides using 200 cells from each sample. BALF was stored at −70°C, and the levels of IL-4, IL-5, and gamma interferon (IFN-γ) were determined by using a specific enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (eBioscience, San Diego, CA; sensitivity > 2 pg/ml).
Measurement of OVA-specific IgE, IgG1, and IgG2a levels in serum.
The measurement of anti-OVA-specific IgE, IgG1, and IgG2a antibodies was performed by using ELISA. Briefly, 96-well plates were coated overnight with rat anti-mouse IgE (10 μg), rat anti-mouse IgG1 (20 μg), or rat anti-mouse IgG2a (20 μg) in 100 μl of PBS and then blocked for 1 h at room temperature with 200 μl of PBS-casein/well. Fifty microliters of each diluted serum (1/5 for IgE, 1/1,000 for IgG1, and 1/10 for IgG2a) was added per well, followed by incubation for 2 h at room temperature. After washing, OVA (1 μg/100 μl) and peroxidase-labeled rabbit anti-OVA IgG (240 ng/100 μl) were sequentially added, followed by incubation for 1 h at room temperature. Finally, 96-well plates were washed with buffer containing tetramethylbenzidine dihydrochloride hydrate (1 μl/100 μl) and H2O2 (1 μl/100 μl). The peroxidase reaction was stopped by adding H2SO4, and the optical density was measured at 450 nm.
Measurement of AHR.
The airway hyper-reactivity (AHR) was analyzed 24 h after the last OVA aerosol challenge. For analysis of the AHR, methacholine-induced airflow obstruction tests of conscious mice placed in a whole-body plethysmograph (model PLY 3211; Buxco Electronics, Troy, NY) were performed, and the dimensionless parameter known as the enhanced pause (Penh) was measured as previously described (10). Briefly, the mice were placed in the plethysmograph chamber and exposed to an aerosol of normal saline solution (baseline readings) and then to cumulative concentrations of β-methacholine ranging from 3 to 48 mg/ml. The aerosol was generated by an ultrasonic nebulizer and drawn through the chamber for 2 min. The inlet was then closed, and Penh readings were taken for 3 min and averaged; the values were then reported as the Penh index.
Detection of CD25+ Foxp3+ Treg cells in spleen CD4+ T cells.
CD4+ T cells were obtained from spleensby using antibody-coated paramagnetic MultiSort MicroBeads (MACS; Miltenyi Biotec) according to the manufacturer's protocol. After detachment, spleen CD4+ T cells were labeled by using a mouse regulatory T cell staining kit (Miltenyi Biotec) to detect the expression of CD25 and Foxp3. Ten thousand spleen CD4+ T cells were collected from each sample, and the proportion of CD25+ Foxp3+ T cells was analyzed by flow cytometry with CellQuest software (Becton Dickinson, Mountain View, CA).
Statistical analysis.
Data were expressed as means ± the standard deviations. Differences between groups were analyzed by using SPSS for Windows (version 8.0) and unpaired two-tailed parametric Student t test or analysis of variance. P values of <0.05 were considered statistically significant.
RESULTS
OVA-CTLA-4 binds to B7 ligands on DCs in vitro.
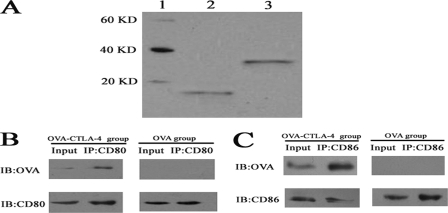
The expression plasmids OVA-pcDNA3.1 and OVA-CTLA-4-pcDNA3.1 were constructed sequentially and validated by sequencing. After the two plasmids were transfected into COS-7 cells, the ectopic expression protein OVA and OVA-CTLA-4 were purified from the culture medium of transfected COS-7 cells by using CNBr-Sepharose 4B affinity chromatography. Western blot analysis of purified OVA identified a 13.1-kDa protein, while purified OVA-CTLA-4 identified a 28.4-kDa protein, a finding in agreement with the combined molecular masses of the extracellular domain of murine CTLA-4 (15.3 kDa) and OVA (Fig. 2 A).
Fig. 2.
OVA-CTLA-4 binds to B7 ligands on DCs in vitro. (A) Purified OVA (lane 2) and OVA-CTLA-4 (lane 3) were determined by Western blot analysis. Lane 1, protein markers. (B) DCs were treated with OVA or OVA-CTLA-4 and lysed for immunoprecipitation with the anti-CD80 antibody. Precipitated proteins were determined by Western blot analysis with anti-OVA antibody. OVA was observed in the OVA-CTLA-4 treated group, whereas no protein was observed in the OVA-treated group. (C) DCs were treated with OVA or OVA-CTLA-4 and lysed for immunoprecipitation with the anti-CD86 antibody. Precipitated proteins were determined by Western blot analysis with anti-OVA antibody. OVA was observed in the OVA-CTLA-4-treated group, while no protein was observed in the OVA-treated group.
To investigate whether OVA-CTLA-4 binds to B7 ligands on DCs in vitro, the DCs were treated with OVA or OVA-CTLA-4 and lysed for immunoprecipitation with anti-CD80 or anti-CD 86 antibody. Precipitated proteins were separated on an SDS-PAGE gel and visualized by Western analysis with anti-OVA antibody (Fig. 2B and C). A single protein was observed by using an OVA-specific antibody in the OVA-CTLA-4-treated group. In contrast, no protein was observed in the OVA-treated group. These results establish that OVA-CTLA-4 can form a complex with B7 ligands on DCs in vitro.
Induction of Treg cells by OVA-CTLA-4 in vitro.
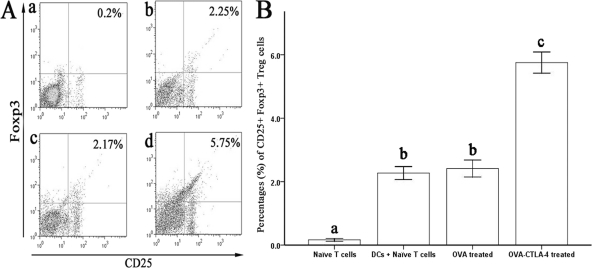
To determine whether OVA-CTLA-4 could induce Treg cells from CD4+ CD25− T cells or not, naive CD4+ CD25− T cells were isolated from the spleen and cocultured with DCs in the presence of OVA or OVA-CTLA-4. After 3 days of culture, The percentages of Foxp3+ Treg cells were significantly increased in the OVA-CTLA-4-treated group compare to that in the OVA-treated group (P < 0.05). This result suggested that OVA-CTLA-4 has the potential to induce Treg cells in vitro. (Fig. 3).
Fig. 3.
Induction of Treg cells by OVA-CTLA-4 in vitro. (A) Representative results of the percentages of CD25+ Foxp3+ Treg cells from naive CD4+ CD25− T cells only (a) or cocultured with DCs in the presence of medium (b), OVA (c), or OVA-CTLA-4 (d). (B) The percentages of CD25+ Foxp3+ Treg cells were significantly increased in mice from the OVA-CTLA-4-treated group. The values are represent means ± the standard deviations (n = 5). Values with different letters showed significant differences (P < 0.05).
Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 reduces airway inflammation.
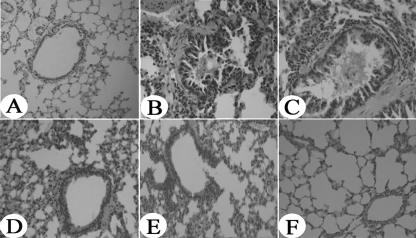
Mice were sacrificed 24 h after the final allergen challenge. Histological analysis was performed to investigate the efficacy of the DNA vaccines in protecting against airway inflammation. The severity of the cellular infiltration, edema, and epithelial damage are shown in Table 1. OVA induced a significant increase in eosinophil infiltration in OVA-sensitized and -challenged mice treated with PBS or pcDNA3.1 compared to mice sensitized, challenged, and treated with PBS (P < 0.01). The degree of cellular infiltration around the central bronchi, alveoli, and blood vessels was significantly decreased in OVA-sensitized and challenged mice treated with a high dose of OVA-CTLA-4-pcDNA3.1 compared to OVA-sensitized and -challenged mice treated with PBS (P < 0.01), pcDNA3.1 (P < 0.01), OVA-pcDNA3.1 (P < 0.05), or a low dose of OVA-CTLA-4-pcDNA3.1 (P < 0.05). Extensive infiltration of inflammatory cells, goblet cell hyperplasia, and the production of mucin was increased in OVA-sensitized and -challenged mice treated with PBS (Fig. 4 B) or with pcDNA3.1 (Fig. 4C). Treatment with a high dose of OVA-CTLA-4-pcDNA3.1 (Fig. 4F) significantly reduced OVA-induced infiltration of inflammatory cells into the airways and had a greater therapeutic effect than did OVA-pcDNA3.1 (Fig. 4D) or a low dose of OVA-CTLA-4-pcDNA3.1 (Fig. 4E).
Table 1.
Severity of inflammatory cell infiltration into lung tissues after immunization
| Treatment group | Mean infiltration ± SDa |
||
|---|---|---|---|
| Perivascular and peribronchiolar eosinophilia | Edema | Epithelial damage | |
| Control | 0† | 0† | 0† |
| Model | 8.58 ± 0.58* | 8.25 ± 0.67* | 8.16 ± 0.54* |
| pcDNA3.1 | 7.37 ± 0.62* | 7.86 ± 0.59* | 7.63 ± 0.47* |
| OVA-pcDNA3.1 | 3.68 ± 0.37*‡ | 3.96 ± 0.37*‡ | 3.64 ± 0.28*‡ |
| OVA-CTLA-4-pcDNA3.1(L) | 3.49 ± 0.48*‡ | 3.73 ± 0.52*‡ | 3.51 ± 0.34*‡ |
| OVA-CTLA-4-pcDNA3.1(H) | 1.18 ± 0.16† | 1.16 ± 0.19† | 0.97 ± 0.15† |
*, P < 0.05 compared to the control and OVA-CTLA-4-pcDNA3.1(H) groups; †, P < 0.01 compared to the model and pcDNA3.1 groups; ‡, P < 0.05 compared to the model and pcDNA3.1 groups.
Fig. 4.
Reduced lung inflammation in high-dose OVA-CTLA-4-pcDNA3.1-vaccinated mice. Histological findings of lung tissues (original magnification, ×200) in mice from the control group (A), the model group (B), the pcDNA3.1 group (C), the OVA-pcDNA3.1 group (D), the OVA-CTLA-4-pcDNA3.1(L) group (E), and the OVA-CTLA-4-pcDNA3.1(H) group (F) were analyzed by using H&E staining.
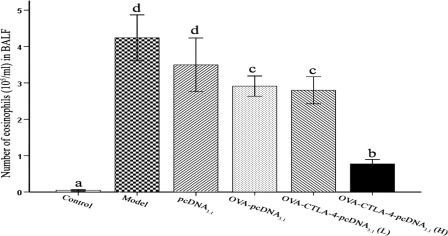
Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 inhibits infiltration of eosinophils into the BALF.
To further study DNA vaccine-generated prophylactic effects on airway inflammation, we assessed the allergen-induced infiltration of eosinophils in BALF. The number of eosinophils in the BALF was significantly higher in OVA-sensitized and -challenged mice treated with PBS or with pcDNA3.1 compared to those sensitized, challenged, and treated with PBS (P < 0.01, Fig. 5). The amount of eosinophilia in high-dose OVA-CTLA-4-pcDNA3.1-treated mice was significantly lower than in OVA-sensitized and -challenged mice treated with PBS (P < 0.01), pcDNA3.1 (P < 0.01), OVA-pcDNA3.1 (P < 0.05), or a low dose of OVA-CTLA-4-pcDNA3.1 (P < 0.05).
Fig. 5.
Decreased infiltration of eosinophils into BALF fluid in high-dose OVA-CTLA-4-pcDNA3.1-vaccinated mice. The number of eosinophils in BALF harvested in mice from the control group, the model group, the pcDNA3.1 group, the OVA-pcDNA3.1 group, the OVA-CTLA-4-pcDNA3.1(L) group, and the OVA-CTLA-4-pcDNA3.1(H) group were analyzed by counting. The values represent means ± the standard deviations (n = 5). Values with different letters showed significant differences (P < 0.05).
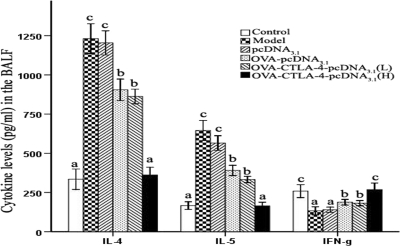
Effects of OVA-CTLA-4-pcDNA3.1 vaccination on cytokine concentrations in the BALF.
To investigate whether DNA vaccines can reverse the disequilibrium of Th1/Th2 cytokine production, the levels of IL-4, IL-5, and IFN-γ in the BALF were measured by ELISA. As shown in Fig. 6, the levels of IL-4 and IL-5 were markedly increased in OVA-sensitized and -challenged mice treated with PBS or pcDNA3.1 compared to those sensitized, challenged, and treated with PBS (P < 0.01), while high-dose OVA-CTLA-4-pcDNA3.1-treated mice showed a significant reduction in the levels of IL-4 and IL-5 compared to OVA-sensitized and -challenged mice treated with PBS (P < 0.01), pcDNA3.1 (P < 0.01), OVA-pcDNA3.1 (P < 0.05), or a low dose of OVA-CTLA-4-pcDNA3.1 (P < 0.05). Treatment with a high dose of OVA-CTLA-4-pcDNA3.1 significantly prevented the OVA-induced reduction in IFN-γ level compared to OVA-sensitized and -challenged mice treated with PBS (P < 0.01), pcDNA3.1 (P < 0.01), OVA-pcDNA3.1 (P < 0.05), or low dose of OVA-CTLA-4-pcDNA3.1 (P < 0.05).
Fig. 6.
Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 decreased IL-4 and IL-5 levels and prevented the OVA-induced reduction of the IFN-γ level in the BALF. Cytokine production of IL-4, IL-5, and IFN-γ in the BALF from mice in the control group, the model group, the pcDNA3.1 group, the OVA-pcDNA3.1 group, the OVA-CTLA-4-pcDNA3.1(L) group, and the OVA-CTLA-4-pcDNA3.1(H) group was assessed by ELISA. The values represented means ± the standard deviations (n = 5). Values with different letters showed significant differences (P < 0.05).
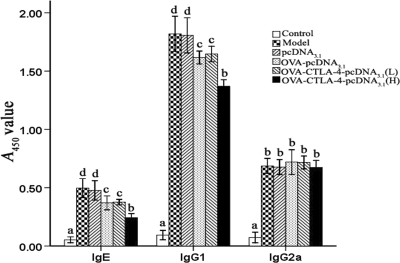
Inhibition of OVA-specific IgE and IgG1 by vaccination with a high dose of OVA-CTLA-4-pcDNA3.1.
To assess the potential utility of DNA vaccine in modulating the immune response, we measured OVA-specific IgE, IgG1, and IgG2a levels in serum 24 h after the final allergen challenge by ELISA. As shown in Fig. 7, OVA-allergic PBS-treated mice had significantly increased serum titers of OVA-specific IgE, IgG1, and IgG2a compared to those sensitized, challenged, and treated with PBS (P < 0.01), indicating that the OVA sensitizations were successful. Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 yielded significantly lower levels of OVA-specific IgE and IgG1 titers compared to OVA-sensitized and challenged mice treated with PBS, pcDNA3.1, OVA-pcDNA3.1, or a low dose of OVA-CTLA-4-pcDNA3.1 (P < 0.01). OVA-specific IgG2a was not significantly affected by any of the treatments. The data indicated that vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 was more effective in suppressing OVA-specific IgE and IgG1 responses.
Fig. 7.
Decreased OVA-specific IgE and IgG1 titers in serum in high doses of OVA-CTLA-4-pcDNA3.1-vaccinated mice. The serum levels of OVA-specific IgE, IgG1, and IgG2a in mice from the control group, the model group, the pcDNA3.1 group, the OVA-pcDNA3.1 group, the OVA-CTLA-4-pcDNA3.1(L) group, and the OVA-CTLA-4-pcDNA3.1(H) group were assessed by ELISA. The values represent as means ± the standard deviations (n = 5). Values with different letters showed significant differences (P < 0.05).
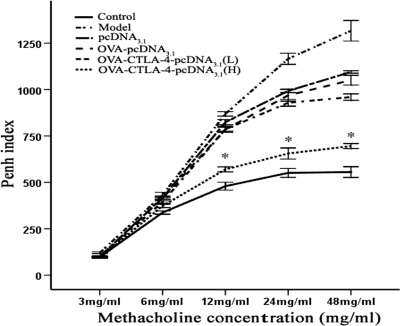
Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 inhibits the development of AHR.
To investigate whether OVA-CTLA-4-pcDNA3.1 vaccination is relevant to OVA-induced changes in lung function, we compared the development of AHR in each group of mice after OVA aerosol challenge. As shown in Fig. 8, OVA-sensitized and -challenged mice treated with PBS or pcDNA3.1 developed significant AHR compared to mice sensitized, challenged, and treated with PBS (P < 0.01). After the methacholine concentration reached 12 mg/ml, the Penh index in the high-dose OVA-CTLA-4-pcDNA3.1 group was significantly lower than in OVA-sensitized and challenged mice treated with PBS, pcDNA3.1, OVA-pcDNA3.1, or a low dose of OVA-CTLA-4-pcDNA3.1 (P < 0.01). These results indicated that a high dose of OVA-CTLA-4-pcDNA3.1 vaccination can significantly inhibit the development of AHR in allergic asthma mice.
Fig. 8.
Reduced AHR to cholinergic stimuli in high-doses OVA-CTLA-4-pcDNA3.1-vaccinated mice. Reactivity to methacholine was measured by body plethysmograph, and the data represent the percent increases in Penh values versus baseline values from normal mice at corresponding concentrations of methacholine. Decreased reactivity was seen at all concentrations of methacholine in mice from the OVA-CTLA-4-pcDNA3.1(H) group compared to those from the model group, the pcDNA3.1 group, the OVA-pcDNA3.1 group, and the OVA-CTLA-4-pcDNA3.1(L). *, P < 0.01.
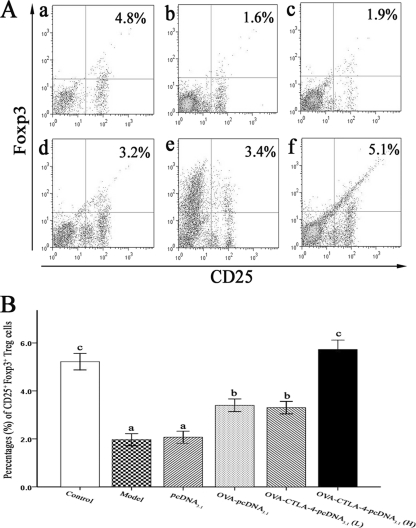
Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 prevents the reduction of percentages of Treg cells in vivo.
The induction of Treg cells plays a key role in conventional SIT. To determine whether vaccination with OVA-CTLA-4-pcDNA3.1 could induce Treg cells, we examined the percentage of CD25+ Foxp3+ Treg cells in vivo. The results showed that OVA challenge induced a significant reduction in the percentages of CD25+ Foxp3+ Treg cells within the spleen CD4+ T cell population in OVA-sensitized and -challenged mice treated with PBS or pcDNA3.1 compared to animals sensitized, challenged, and treated with PBS (P < 0.01, Fig. 9). Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 significantly prevented the OVA-induced reduction of percentages of CD25+ Foxp3+ Treg cells compared to OVA-sensitized and -challenged mice treated with PBS, pcDNA3.1, OVA-pcDNA3.1 or a low dose of OVA-CTLA-4-pcDNA3.1 (P < 0.01).
Fig. 9.
Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 prevented the OVA-induced reduction of percentages of CD25+ Foxp3+ Treg cells in the spleen. (A) Representative results of percentages of CD25+ Foxp3+ Treg cells in spleens from mice in the control group (a), the model group (b), the pcDNA3.1 group (c), the OVA-pcDNA3.1 group (d), the OVA-CTLA-4-pcDNA3.1(L) group (e), and the OVA-CTLA-4-pcDNA3.1(H) group (f). (B) The percentages of CD25+ Foxp3+ Treg cells were significantly increased in mice from the OVA-CTLA-4-pcDNA3.1(H) group. The values represented means ± the standard deviations (n = 5). Values with different letters showed significant differences (P < 0.05).
DISCUSSION
Asthma has become one of the most serious health problems worldwide. SIT is the most widely used therapy for asthma. However, due to the long-lasting nature of the treatment, side effects are frequent. Therefore, it is often difficult to achieve the desired therapeutic effect due to poor patient compliance. Allergen DNA vaccination has sparked considerable interest because it has fewer side effects and is easier to use (25). Recently, many studies have demonstrated that plasmid DNAs encoding for certain allergen genes result in the induction of Th1 immune responses while concomitantly inhibiting Th2 responses. DNA-based immunization is effective in modulating allergen-specific IgE responses and may provide a novel therapeutic approach to allergic diseases and asthma (8, 13). However, although DNA vaccines show great promise for preventing and treating allergic asthma in mouse models, their potency is limited, especially in large animals and humans. Modification of DNA vaccines to enhance efficiency has been investigated extensively. DNA vaccines are commonly administered by intramuscular injection, with the majority of DNA being taken up by muscle cells. Hence, immune responses appear to be generated by a combination of the incidental transfection of APCs, and APC sampling of antigens from transfected muscle cells, which is the predominant source of expressed antigen. Cross-presentation of antigens from transfected cells by APCs is thought to be the major mechanism facilitating Th1-skewed immune responses (12). Therefore, targeting of antigens to professional APCs raises the possibility of improving the efficacy of DNA vaccines (5, 21). CTLA-4 represents an attractive ligand for targeting vaccine antigens to APCs. The extracellular domain of CTLA4 is involved in binding to the B7 ligand (CD80 and CD86) on APCs (5), which are potent initiators of immune responses. Several animal experiments have reported that a CTLA-4 fusion vaccine could induce potent immune responses and antitumor or anti-microorganism activities in vivo (23, 26).
In the present study, we constructed an allergen-CTLA-4-encoding DNA vaccine, OVA-CTLA-4-pcDNA3.1, and obtained the fusion protein OVA-CTLA-4 by affinity chromatography. The immunoprecipitation analyses demonstrated that protein OVA-CTLA-4 was capable of binding to the B7 ligand on DCs and supported the consideration that CTLA-4 fused allergens are more easily captured by APCs than a mono-allergen. In our study, we also showed that protein OVA-CTLA-4 induced the generation of Treg cells in vitro. Since Treg cells negatively regulate immune responses, it was an interesting problem to define whether protein OVA-CTLA-4 has a paradoxical role in controlling Th2-skewing responses by Treg cells, but the beneficial Th1 immune responses were also damaged. For our animal study, a well-established mouse model of allergic asthma was used to identify the allergen DNA vaccine with the most potent anti-allergic effects. From the results, it is evident that vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 was most effective in reducing the allergic response.
One dominant characteristic of asthmatic mouse is the presence of eosinophils (17). After vaccination with a high dose of OVA-CTLA-4-pcDNA3.1, the bronchial influx of eosinophils in the lung tissues or BALF was reduced compared to those associated with the other vaccines. Moreover, the reduction of Th2 cytokines in BALF and decreased serum levels of OVA-specific IgE and IgG1 were also observed. It is known that Th2-produced IL-4 is essential for the switch to the IgG1 and IgE isotype. The results of the present study verified a correlation between reduced IL-4 in the BALF and reduced OVA-specific IgE and IgG1. OVA-IgG2a, a Th1-related immunoglobulin titer, was not affected after treatment with any of the vaccines. Another hallmark of allergic asthma in animal model is AHR. Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 most successfully reduced the increased airway response to methacholin.
In particular, Treg cell responses in vivo were investigated in the present study. The results demonstrated that the percentage of Treg cells in OVA-sensitized and challenged mice treated with PBS was decreased, an observation similar to previous findings (15), suggesting the deficiency of Treg cells to inhibit the overactivation of immune response. Vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 effectively induced higher percentages of Treg cells in the spleen than did the mono-allergen vaccine (OVA-pcDNA3.1) or low dose OVA-CTLA-4-pcDNA3.1 therapy. These data may be helpful in showing that the development of Treg cells by allergen-CTLA-4-encoding DNA vaccine occurs in a dose-dependent manner and that the mechanisms underlying allergen-CTLA-4-encoding DNA vaccination for inhibiting the Th2 responses and airway inflammation are mediated by the induction of Treg cells. Th1 responses are more susceptible to regulation by Treg cells than Th2 responses (4). However, in our study, vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 failed to downregulate the Th1 type cytokine, IFN-γ, in the BALF compared to other vaccinated mice. This result may be due to the cross-presentation of the expression product of DNA vaccines by APCs, which induced Th1 responses.
In conclusion, vaccination with a high dose of OVA-CTLA-4-pcDNA3.1 decreased the eosinophil infiltration, the production of Th2 cytokines, and the OVA-IgE and OVA-IgG1 levels in serum and also reduced AHR. These results were partially related to the induction of Treg cells. Thus, the administration of allergen-CTLA-4-encoding vaccine directly to APCs may offer a new alternative therapy for patients with allergic asthma.
ACKNOWLEDGMENTS
This study was supported by a grant from the China Postdoctoral Science Foundation (grant 20070411050) and by Jiangsu Planned Projects for Postdoctoral Research Funds (grant 0701023B).
Footnotes
Published ahead of print on 23 February 2011.
REFERENCES
- 1. Abe M., et al. 2008. Foxp3 expression on normal and leukemic CD4+ CD25+ T cells implicated in human T-cell leukemia virus type-1 is inconsistent with Treg cells. Eur. J. Haematol. 81:209–217 [DOI] [PubMed] [Google Scholar]
- 2. Ahrens B., et al. 2009. BCG priming of dendritic cells enhances T regulatory and Th1 function and suppresses allergen-induced Th2 function in vitro and in vivo. Int. Arch. Allergy Immunol. 150:210–220 [DOI] [PubMed] [Google Scholar]
- 3. Akdis M., Akdis C. A. 2007. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 119:780–791 [DOI] [PubMed] [Google Scholar]
- 4. Cosmi L., et al. 2004. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood 103:3117–3121 [DOI] [PubMed] [Google Scholar]
- 5. Cusi M. G., et al. 2004. Efficient delivery of DNA to dendritic cells mediated by influenza virosomes. Vaccine 22:735–739 [DOI] [PubMed] [Google Scholar]
- 6. Deliyannis G., Boyle J. S., Brady J. L., Brown L. E., Lew A. M. 2000. A fusion DNA vaccine that targets antigen-presenting cells increases protection from viral challenge. Proc. Natl. Acad. Sci. U. S. A. 97:6676–6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta N., et al. 2006. Identification and characterization of p63 (CKAP4/ERGIC-63/CLIMP-63), a surfactant protein A binding protein, on type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 291:L436–L446 [DOI] [PubMed] [Google Scholar]
- 8. HuangFu T., Lim L. H., Chua K. Y. 2006. Efficacy evaluation of Derp1 DNA vaccine for allergic asthma in an experimental mouse model. Vaccine 24:4576–4581 [DOI] [PubMed] [Google Scholar]
- 9. Jacobsen L. 2001. Preventive aspects of immunotherapy: prevention for children at risk of developing asthma. Ann. Allergy Asthma Immunol. 87:43–46 [DOI] [PubMed] [Google Scholar]
- 10. Jang A. S., et al. 2004. Changes in the expression of NO synthase isoforms after ozone: the effects of allergen exposure. Respir. Res. 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jutel M., Akdis C. A. 2008. T-cell regulatory mechanisms in specific immunotherapy. Chem. Immunol. Allergy 94:158–177 [DOI] [PubMed] [Google Scholar]
- 12. Knutson K. L., Disis M. L. 2005. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 54:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lombardi V., Singh A. K., Akbari O. 2010. The role of costimulatory molecules in allergic disease and asthma. Int. Arch. Allergy Immunol. 151:179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maecker H. T., et al. 2001. Vaccination with allergen-IL-18 fusion DNA protects against, and reverses established, airway hyperreactivity in a murine asthma model. J. Immunol. 166:959–965 [DOI] [PubMed] [Google Scholar]
- 15. Matsumoto K., et al. 2009. Frequency of Foxp3+ CD4+ CD25+ T cells is associated with the phenotypes of allergic asthma. Respirology 14:187–194 [DOI] [PubMed] [Google Scholar]
- 16. Miwa N., et al. 2005. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-gamma increase in normal pregnancy but decrease in spontaneous abortion. Mol. Hum. Reprod. 11:865–870 [DOI] [PubMed] [Google Scholar]
- 17. Ohta K., Yamashita N. 1999. Apoptosis of eosinophils and lymphocytes in allergic inflammation. J. Allergy Clin. Immunol. 104:14–21 [DOI] [PubMed] [Google Scholar]
- 18. Rohrbach F., et al. 2005. Targeted delivery of the ErbB2/HER2 tumor antigen to professional APCs results in effective antitumor immunity. J. Immunol. 174:5481–5489 [DOI] [PubMed] [Google Scholar]
- 19. Rolland J. M., Douglass J., O'Hehir R. E. 2000. Allergen immunotherapy: current and new therapeutic strategies. Expert Opin. Invest. Drugs 9:515–527 [DOI] [PubMed] [Google Scholar]
- 20. Rolland J. M., Gardner L. M., O'Hehir R. E. 2009. Allergen-related approaches to immunotherapy. Pharmacol. Ther. 121:273–284 [DOI] [PubMed] [Google Scholar]
- 21. Ruffini P. A., et al. 2004. Genetic fusions with viral chemokines target delivery of nonimmunogenic antigen to trigger antitumor immunity independent of chemotaxis. J. Leukoc. Biol. 76:77–85 [DOI] [PubMed] [Google Scholar]
- 22. Ryanna K., Stratigou V., Safinia N., Hawrylowicz C. 2009. Regulatory T cells in bronchial asthma. Allergy 64:335–347 [DOI] [PubMed] [Google Scholar]
- 23. Sloots A., et al. 2008. DNA vaccines targeting tumor antigens to B7 molecules on antigen-presenting cells induce protective antitumor immunity and delay onset of HER-2/Neu-driven mammary carcinoma. Clin. Cancer Res. 14:6933–6943 [DOI] [PubMed] [Google Scholar]
- 24. Underwood S., Foster M., Raeburn D., Bottoms S., Karlsson J. A. 1995. Time course of antigen-induced airway inflammation in the guinea-pig and its relationship to airway hyperresponsiveness. Eur. Respir. J. 8:2104–2113 [DOI] [PubMed] [Google Scholar]
- 25. Verhagen J., Taylor A., Akdis C. A., Akdis M. 2005. Advances in allergen-specific immunotherapy. Expert Opin. Biol. Ther. 5:537–544 [DOI] [PubMed] [Google Scholar]
- 26. Zhang F., et al. 2007. Enhanced efficacy of CTLA-4 fusion anti-caries DNA vaccines in gnotobiotic hamsters. Acta Pharmacol. Sin. 28:1236–1242 [DOI] [PubMed] [Google Scholar]