Abstract
The differential antibody response measured by the commonly used hemagglutination inhibition (HI) and microneutralization (MN) assays in patients with natural infection and vaccination has not been fully assessed. HI and conventional MN (CMN) assays were performed on sera from 651 patients with natural infection by pandemic H1N1 2009 influenza virus and on sera from 567 recipients of the corresponding vaccine. Surprisingly, the overall seroprotection rates determined by CMN and HI assays in vaccine recipients were only 44.8 and 35.1%, respectively. Antibody titers measured by the CMN assay was significantly higher than that obtained by HI assay in vaccine recipients aged ≥50 years, but these titers were not significantly different among younger vaccine recipients. In contrast, the HI titer was greater than the CMN titer for the age group from 16 to 29 years but was not significantly different in other age groups for natural infection. Lower antibody levels were found in both naturally infected patients and immunized recipients in the older than in the younger age groups, but naturally infected patients exhibited higher HI and CMN titers than did the corresponding vaccine recipients. In addition, we developed a rapid fluorescent focus microneutralization (FFMN) assay to test sera from naturally infected patients. The FFMN assay has a better correlation with CMN than with HI (ρ = 0.810 versus 0.684), which is expected of neutralizing antibody mainly targeted toward the inhibition of viral entry into cells. The higher antibody level elicited by natural infection than by vaccination may be related to differences between antigen presentation by the intramuscular route of vaccination and mucosal viral replication in mucosal cells of the respiratory tract.
INTRODUCTION
The human adaptive immune system reacts to influenza virus infection or vaccination either via humoral response by antibody production or cell-mediated response by T and B lymphocytes. The level of antibody response to influenza virus is measured by either hemagglutination inhibition (HI) or viral neutralization assays in most laboratories (9). HI assay has been considered to be the gold standard for evaluation of immunogenicity in vaccine studies, with an HI titer of ≥40 considered as a surrogate marker for protection (11, 42). This cutoff titer is based on classical studies in the 1970s showing a correlation between HI titer and protection from infection in volunteers inoculated with a circulating strain with or without vaccination (17, 29). However, the HI titer can be affected by the type of red blood cells (RBC) used in the assay, as a result of the differential expression of sialic acid receptors on the surfaces of various RBC, which may affect the binding affinity (37, 38). The HI titer may also be affected in the serum inactivation steps used in removing nonspecific inhibitors (40). Furthermore, HI assays cannot identify neutralizing antibodies that do not inhibit hemagglutination (41). In recent years, viral microneutralization (MN) assay has become a routine test to measure antibody levels in acute infection, cross-reactivity, and vaccine responses (15, 16, 32). This functional assay directly measures the ability of serum antibody to protect cells from cytopathic infection in vitro without involving RBC as a signal and can detect neutralizing antibodies that do not inhibit hemagglutination. Consequently, MN assays are considered more sensitive than the HI assay (2, 12, 32). However, the HI assay is still commonly used in most serological surveys since it is easy to perform. The correlation between HI and MN titer is not well characterized, especially in the setting of the pandemic H1N1 2009 influenza. Discrepancies have been found in different reports. In a previous study involving infected patients, it was found that the MN and HI geometric mean titer (GMT) were similar (7), whereas another report has shown that the MN GMT was higher than the HI GMT for preexisting cross-reactive antibody (16).
We therefore performed a concurrent evaluation of the HI and MN assays in patients with natural infection and in vaccine recipients. For conventional MN (CMN) assays, cytopathic effect is used as the endpoint, but this approach is time-consuming. We modified this assay using monoclonal antibody (MAb) to detect nucleoprotein, which indicates viral entry and antigen expression and does not rely on the observation of a cytopathic effect. To this end, we have developed a rapid fluorescent focus microneutralization (FFMN) assay with a multiplicity of infection (MOI) of 1 to examine viral nucleoprotein expression at 6 h after viral inoculation using indirect immunofluorescent staining of infected cells, and we evaluated this test in patients with natural infection.
MATERIALS AND METHODS
Participants.
Patients with natural infection were randomly selected from those who suffered from pandemic H1N1 2009 influenza virus infection confirmed by either reverse transcriptase PCR or viral culture and donated their convalescent plasma for the treatment of patients with severe pandemic influenza (18, 19, 49). The vaccine recipients received a monovalent, split-virus, inactivated, nonadjuvanted vaccine containing 15 μg of hemagglutinin of influenza A/California/07/2009 (H1N1) virus (Panenza; Sanofi Pasteur, France). All blood samples were taken within 243 days after vaccination or symptom onset in natural infection. The present study was approved by the Institutional Review Board of the Hospital Authority of Hong Kong.
HI assay.
The HI assay was carried out in 96-well microtiter plates after removal of nonspecific inhibitors in the serum with receptor-destroying enzyme (RDE; 1:3 [Denka Seiken Co., Ltd., Tokyo, Japan]), incubated overnight at 37°C, and heat inactivated at 56°C for 30 min. Serial 2-fold dilutions of RDE-treated serum from 1:10 were mixed with four hemagglutinin units of the pandemic H1N1 A/HK/415742/2009 virus, followed by incubation at room temperature for 1 h. Next, 0.5% turkey erythrocytes were added to the serum-virus mixture, followed by further incubation at room temperature for 30 min.
CMN assay.
The CMN assay for the pandemic H1N1 A/HK/415742/2009 was carried out in microtiter plates with neutralization of the virus cytopathic effect as the endpoint in Madin-Darby canine kidney (MDCK) cells according to a previously described method (19). Briefly, serial serum dilutions in duplicate started from 1:10 were mixed with 100 50% tissue culture infective doses for 2 h at 37°C and added to MDCK cells. At 1 h after infection, the serum-virus mixtures were removed, and serum-free minimal essential medium with 2 μg of TPCK (l-1-tosylamide-2-phenylethyl chloromethyl ketone)-treated trypsin (TPCK-Trypsin; Sigma Immunochemical)/ml was added to each well. The plates were incubated for either 3 or 4 days at 37°C, and the cytopathic effect was observed in order to determine the highest serum dilution that protected ≥50% of the cells from cytopathology in these wells. Positive and negative control sera and virus back titration to confirm the viral inoculum were included in each assay.
Production of MAbs against the conserved nucleoprotein of influenza A virus.
The preparation of MAb against nucleoprotein of influenza virus A H1N1 WSN strain was performed as previously described, with some modifications (30, 50). Briefly, BALB/c mice were first immunized intraperitoneally with 10 μg of recombinant nucleoprotein of influenza A virus, and were then given five boosters of the same doses of nucleoprotein of influenza A virus at 10-day intervals. Three days after a final booster with 100 μg of nucleoprotein, the splenocytes were fused with NS-1 myeloma cells (ATCC TIB-18). The hybridoma cell lines were screened for the production of antibodies against recombinant protein by enzyme-linked immunosorbent assay. Positive hybridoma cells were cloned by limiting dilution. The isotype of each MAb was determined by the use of a commercially available mouse MAb isotyping kit (Zymed Laboratories, Carlsbad, CA). The preparation of ascitic fluid and purification of MAb AN5 was described previously (50).
FFMN assay.
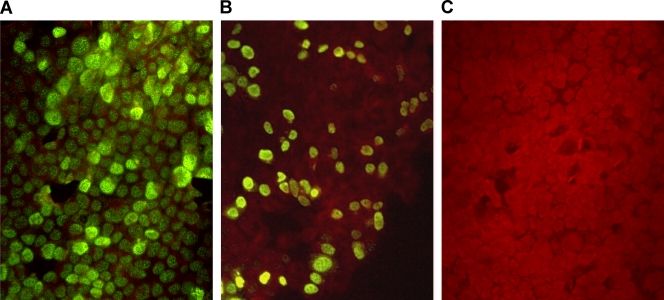
The infection procedure was similar to the CMN assay as described above except that the infection dose and the infection time were 1 MOI and 6 h, respectively. The seeded cells were then fixed in chilled acetone and methanol (1:1) at −20°C for 10 min and stained with MAb (AN5) against influenza A virus nucleoprotein at 37°C for 45 min. This was followed by the addition of goat anti-mouse fluorescein-labeled conjugate (Millipore, California) and further incubation at 37°C for 45 min. The percentage of positive cells was examined, and the FFMN titer was taken as the highest serum dilution at which the percentage of fluorescent foci gave a 50% reduction compared to virus only control (Fig. 1) (47).
Fig. 1.
FFMN assay. (A) Influenza virus-infected MDCK cells without neutralizing antibody show fluorescent foci in the nuclei of >80% of the cells (MOI = 1). (B) Image showing 50% inhibition of fluorescent foci. (C) Image showing 100% inhibition of fluorescent foci.
Statistical analysis.
Statistical analysis was performed using SPSS software (version 17.0) for Windows. The gender ratios and seroprotection rates between naturally infected patients and vaccine recipients were compared by using the χ2 test. Antibody titers were expressed as the GMT. HI or MN titers below the limit of detection were arbitrarily assigned a value of 5. All statistical calculation involving the GMT was performed with log-transformed titers. MN and HI titers from the same patient group were compared by using the Wilcoxon signed-rank test, while antibody titers between naturally infected patients and vaccine recipients were compared by using the Mann-Whitney U test. Correlation between age and antibody titer, and of the antibody titer between different assays, were assessed by using Spearman's rank correlation coefficient test. A P value of <0.05 was considered statistically significant.
RESULTS
The demographics of the 651 patients with natural infection and 567 vaccine recipients are shown in Table 1. There was no significant difference in gender ratio in age sectors between 16 and 59 years. The antibody level was not available for the naturally infected individuals older than 60 years since none of them came back for convalescent plasma donation. Therefore, in subsequent analyses of the antibody titers comparing between natural infection and vaccination, only patients in the same age group were compared.
Table 1.
Demographics of naturally infected patients and vaccine recipients
| Parameter | Naturally infected patients | Vaccine recipients |
|---|---|---|
| No. (%) of 16- to 59-yr-old femalesa | 331 (50.8) | 116 (52.0) |
| No. of individuals per age group (yr) | ||
| 16–29 | 382 | 15 |
| 30–39 | 133 | 21 |
| 40–49 | 100 | 74 |
| 50–59 | 36 | 113 |
| 60–69 | 0 | 112 |
| 70–79 | 0 | 181 |
| 80–89 | 0 | 51 |
| Total | 651 | 567 |
P = 0.762.
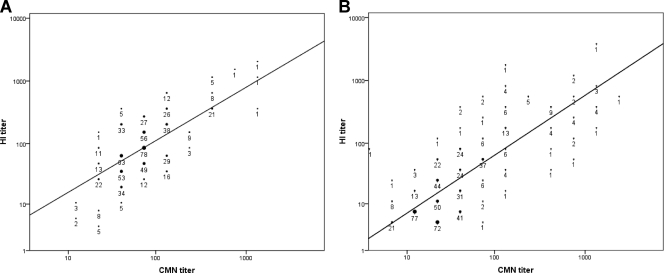
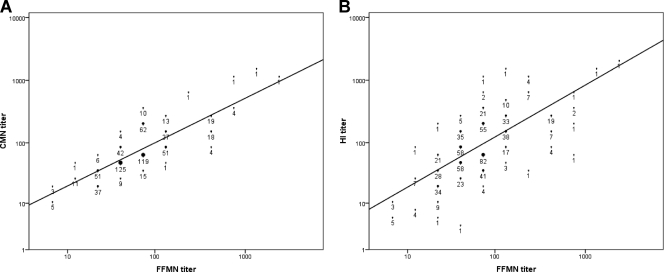
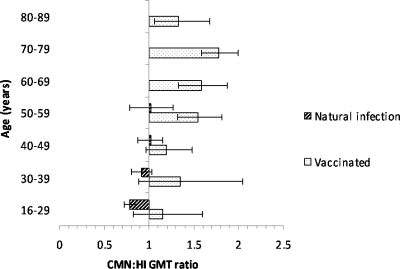
Antibody levels measured by CMN and HI were well correlated for both patients with natural infection (ρ = 0.596, P < 0.001) (Fig. 2A) and vaccine recipients (ρ = 0.726, P < 0.001) (Fig. 2B). For the natural infection group, we also determined the neutralizing antibody titer by FFMN assay, and there was better correlation between FFMN and CMN (ρ = 0.810, P < 0.001) (Fig. 3A) than between FFMN and HI (ρ = 0.684, P < 0.001) (Fig. 3B). For the natural infection cohort, the HI GMT was significantly higher than the CMN GMT for the 16- to 29-year-old group, but no significant differences were found in the other age groups (Fig. 4). Conversely, in the vaccine recipients, the CMN GMT was higher than the HI GMT for all age groups and statistically significant for those 50 years old or older. A lower antibody titer was associated with older age for both infected patients and vaccine recipients (Table 2).
Fig. 2.
Correlation of antibody titers determined by CMN and HI assays in naturally infected patients (A) and vaccine recipients (B).
Fig. 3.
Correlation of antibody titers in naturally infected patients. (A) Correlation between FFMN and CMN assays; (B) correlation between FFMN and HI assays.
Fig. 4.
Differences between the antibody responses measured by the HI and CMN assays.
Table 2.
Spearman correlation between age and antibody titer
| Comparison | Spearman correlationa |
|
|---|---|---|
| Naturally infected patients | Vaccine recipients (up to age 59 yr) | |
| Age vs log HI (ρ) | −0.206* | −0.147† |
| Age vs log CMN (ρ) | −0.098† | −0.140† |
| Age vs log FFMN (ρ) | −0.122* | NA |
*, P < 0.01; †, P < 0.05. NA, not applicable.
The antibody titers in naturally infected patients and vaccine recipients were compared for subjects 16 to 59 years old (Table 3). Significantly higher CMN and HI GMTs were found in naturally infected patients, except for the CMN GMT for the age group from 16 to 29 years. The seroprotection rate was also significantly higher in naturally infected patients than in vaccine recipients (Table 4). In vaccine recipients of all age groups, the overall seroprotection rates were determined to be 44.8 and 35.1% by the CMN and HI assays, respectively, whereas the seroprotection rates for those 16 to 59 years old after natural infection were determined to be 90.0 and 86.0% by the CMN and HI assays, respectively.
Table 3.
Difference in antibody titer between natural infection and vaccinationa
| Age group (yr) | CMN assay |
HI assay |
||||
|---|---|---|---|---|---|---|
| GMT (95% CI) |
P | GMT (95% CI) |
P | |||
| Natural infection | Vaccination | Natural infection | Vaccination | |||
| 16–29 | 78.42 (72.34–85.01) | 43.87 (19.10–100.79) | 0.135 | 99.82 (90.21–110.46) | 38.19 (15.18–96.12) | 0.043 |
| 30–39 | 61.65 (54.15–70.19) | 41.34 (20.64–82.81) | 0.046 | 67.36 (53.33–80.55) | 30.72 (14.42–65.44) | 0.022 |
| 40–49 | 67.27 (58.56–77.28) | 34.11 (25.06–46.43) | <0.001 | 66.35 (55.30–79.60) | 28.55 (19.14–42.58) | <0.001 |
| 50–59 | 61.10 (47.47–78.64) | 29.98 (23.39–38.47) | <0.001 | 59.93 (40.58–88.51) | 19.40 (14.98–25.11) | <0.001 |
CI, confidence interval.
Table 4.
Seroprotection rate after natural infection or vaccination
| Age group (yr) | CMN ≥ 40 |
HI ≥ 40 |
||||
|---|---|---|---|---|---|---|
| Seroprotection (%) |
P | Seroprotection (%) |
P | |||
| Natural infection | Vaccination | Natural infection | Vaccination | |||
| 16–29 | 91.9 | 53.8 | <0.001 | 90.3 | 60.0 | 0.003 |
| 30–39 | 84.2 | 47.6 | 0.001 | 78.2 | 47.6 | 0.003 |
| 40–49 | 91.0 | 44.6 | <0.001 | 86.0 | 45.9 | <0.001 |
| 50–59 | 88.9 | 38.9 | <0.001 | 69.4 | 32.7 | <0.001 |
There were 57 patients with natural infection for which paired sera were available, collected at medians of 33 days (range, 24 to 74 days) and 333 days (range, 275 to 385 days) for the first and second titers, respectively (Table 5). Less than 10% of the patients had antibody levels reduced by 4-fold, as measured by the CMN or HI assay.
Table 5.
Difference in antibody level in 57 patients with natural infectiona
| Change | Antibody level (%) |
|
|---|---|---|
| CMN assay | HI assay | |
| Reduction by 4-fold | 2 (3.5) | 4 (7.0) |
| Reduction by 2-fold | 13 (22.8) | 9 (15.8) |
| No difference | 18 (31.6) | 23 (40.4) |
| Increase by 2-fold | 15 (26.3) | 14 (24.6) |
| Increase by 4-fold | 3 (5.3) | 6 (10.5) |
| Increase by 8-fold | 5 (8.8) | 0 (0) |
| Increase by 16-fold | 1 (1.8) | 1 (1.8) |
The first and second titers were collected at medians of 33 days (range, 24 to 74 days) and 333 days (range, 275 to 385 days) after symptom onset.
DISCUSSION
This study characterized antibody response to influenza virus in individuals after natural infection or vaccination by using HI and MN assays. The 2009 pandemic influenza provided a unique opportunity for assessing the immune response to influenza virus as most individuals, especially the younger population, do not have preexisting cross-reactive antibodies (16, 25).
For vaccine recipients, the GMT of CMN was higher than that of HI for all age groups but was statistically significant only for the older age groups (≥50 years). This is consistent with a seasonal H1N1 vaccine study conducted in which neutralizing, but nonhemagglutinating antibody was more often found in the elderly population (31). Conversely, for patients with natural infection, the GMT of HI was higher than that of CMN in the 16- to 29-year-old age group, but there were no significant differences in the other age groups. There are several explanations for the differences between the CMN and HI titers. Antibodies that neutralize virus may not inhibit hemagglutination and hence are not detected by the HI assay. These include neutralizing antibodies targeting the stem region of the hemagglutinin (3, 10, 41, 45), the neuraminidase (26, 27, 39), or the M2 ectodomain (46). On the other hand, some antibodies that inhibit hemagglutination may not have viral neutralizing activity (4) and therefore are not detected by the CMN assay. Furthermore, for the HI assay, virus strains and the host source of the RBC can affect the binding avidity. Finally, there are nonspecific inhibitors of hemagglutination in the human sera, designated α, β, and δ (33). Removal of these inhibitors are required during the HI assay but not during the MN assay (1). Since the β and δ inhibitors may also have neutralizing activity, some of the neutralizing activity may be related to these factors.
The antibody titer was higher in naturally infected patients than in vaccine recipients, independent of the method of measurement. During a natural infection, live virus first infects and replicates at the mucosal surface, triggering both local and systemic immune response. Local humoral immunity is characterized by increased mucosal and secretory IgA, which is not present in individuals vaccinated via the intramuscular route. In addition to local infection, live virus can also disseminate systemically and can be found in the gut or in the blood (43, 44). In a recent study, similar peak levels of MN titer were found in patients with pandemic 2009 H1N1 infection or vaccine recipients (23). However, the sample size was small, and only military personnel aged between 18 and 28 were recruited in the study. For the similar age group in the present study, no significant difference in antibody level to the pandemic H1N1 virus between infected patients and vaccine recipients was detected by the CMN assay.
For both infected patients and vaccine recipients, we have observed a trend toward lower antibody titer in older age groups, which is similar to the findings of another study performed in Hong Kong (25). A meta-analysis by Goodwin et al. showed that the elderly have a lower rate of seroconversion compared to younger populations (13), and a recent analysis of breakthrough pandemic H1N1 infection in vaccinated individuals found that age was the only significant risk factor in multivariate analysis (48). It is known that the antibody response in the elderly is suboptimal, including poorer IgG response against both protein and polysaccharide antigens (34).
In the present study, the seroprotection rate after vaccination was lower than those reported in the initial clinical trials with a single dose or two doses of the pandemic H1N1 monovalent vaccine (15, 28, 51). Poor immunogenicity has also been reported in studies involving young healthcare workers and in the elderly (8, 20). In Hong Kong, the pandemic influenza vaccine was given as a single dose, and this may explain the relatively low seroprotection rate in the population in the present study. Several studies have shown the benefit of a second booster dose (22), but this was disputed in another study (8). Irrespective of its efficacy compared to previous seasonal vaccines, even a lower HI or MN titer could be associated with decreased disease severity and mortality if such vaccine recipients get infected.
In the naturally infected patients for whom paired sera were available, only 3.5 and 7% of patients, respectively, had a decline of antibody titer of ≤4-fold, as measured by CMN and HI assays. This is consistent with studies reporting long-lasting antibody levels in patients after influenza virus infection (35). The long persistence of antibody may be related to the prolonged circulation of antibody-secreting cells (21).
We have found good correlation between the two neutralization assays. The main advantage of FFMN over CMN is the much shorter time for neutralization result, because FFMN is based on viral entry and nucleoprotein expression at MOI of 1 but not on viral cytopathology associated with many complete cycles of viral replication. A previous study on avian influenza virus examined nucleoprotein expression after an 18-h incubation period (32), but these researchers used a much lower viral inoculum that therefore requires viral replication to achieve infection of all cells. In our study, we used a much larger inoculum which required no viral replication but depended on viral entry only and therefore could shorten the incubation time to 6 h. It is known that a viral neutralization assay may have poor interlaboratory reproducibility (36). Whether FFMN reduces this variability may merit further investigation.
There are several limitations in the present study. We do not have data for antibody response in naturally infected patients ≥60 or those aged ≤15 years old. The elderly are important since they are the most vulnerable to severe influenza virus infection (24). Children and adolescents are disproportionately affected by pandemic influenza because they lack cross-reactive antibodies (16). The antibody response in these age groups will provide insights into the level of protection after natural infection. Another limitation is that there were relatively few vaccine recipients in the younger age groups because they were not included as a targeted at-risk group in the pandemic H1N1 vaccine program of Hong Kong. Third, we do not have paired archive sera in vaccine recipients, and this prevented analysis of the change in antibody titers in this population. Finally, we do not have the information of the prior vaccination history of the naturally infected patients or vaccine recipients. A study in Taiwan has shown that children who received seasonal influenza vaccine prior to the pandemic influenza vaccine have a lower seroconversion rate (6).
The concept of a protective level of HI antibody originated from studies in the 1970s (29). Although commonly used as a surrogate marker of protection in vaccine studies, there have not been any formal studies on the level of MN titer and protection. Recently, lower MN titer after vaccination have been reported to be associated with failure of protection from 2009 influenza A virus (H1N1) (23). We demonstrated here significant differences in antibody titer measured by HI and MN assay, which is especially apparent in vaccine recipients. The difference in the subtype of the antibody can be important. For pandemic influenza, a lower IgG2 level correlates with more severe infection (5, 14). Further studies are required to understand the functional differences in antibodies with discrepant neutralizing and hemagglutination-inhibiting properties.
ACKNOWLEDGMENTS
We are grateful for the support of the National Science and Technology Major Project of China (grant 2009ZX10004-306), the Ted Sun Foundation, the Clinical Infectious Diseases Research Endowment Fund from Teresa Wong On Yik, the Research Fund for the Control of Infectious Diseases of the Food and Health Bureau, and the Research Grants Council of the Hong Kong Special Administrative Region, China.
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Ananthanarayan R., Paniker C. K. 1960. Non-specific inhibitors of influenza viruses in normal sera. Bull. World Health Organ. 22:409–419 [PMC free article] [PubMed] [Google Scholar]
- 2. Benne C. A., et al. 1998. Comparison of neutralizing and hemagglutination-inhibiting antibody responses to influenza A virus vaccination of human immunodeficiency virus-infected individuals. Clin. Diagn. Lab. Immunol. 5:114–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bommakanti G., et al. 2010. Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc. Natl. Acad. Sci. U. S. A. 107:13701–13706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cascino I., et al. 1986. A nonneutralizing human IgM monoclonal antibody inhibiting hemagglutination of H3N2 influenza A strains. Hybridoma 5:307–318 [DOI] [PubMed] [Google Scholar]
- 5. Chan J. F., et al. 2011. Lower serum immunoglobulin G2 level is associated with cytokine dysregulation in severe cases of pandemic H1N1 2009 influenza. Clin. Vaccine Immunol. 18:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chao D. Y., et al. 2011. Serological response and persistence in schoolchildren with high baseline seropositive rate after receiving 2009 pandemic influenza A (H1N1) vaccine. Vaccine 29:617–623 [DOI] [PubMed] [Google Scholar]
- 7. Chen M. I., et al. 2010. Serological response in RT-PCR confirmed H1N1 2009 influenza a by hemagglutination inhibition and virus neutralization assays: an observational study. PLoS One 5:e12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheong H. J., et al. 2011. Immunogenicity and safety of influenza A (H1N1) 2009 monovalent inactivated split vaccine in Korea. Vaccine 29:523–527 [DOI] [PubMed] [Google Scholar]
- 9. Eichelberger M., et al. 2008. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development in Bethesda, Maryland, December 10–11, 2007. Vaccine 26:4299–4303 [DOI] [PubMed] [Google Scholar]
- 10. Ekiert D. C., et al. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Food and Drug Administration 2007. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. Food and Drug Administration, Washington, DC [Google Scholar]
- 12. Frank A. L., Puck J., Hughes B. J., Cate T. R. 1980. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J. Clin. Microbiol. 12:426–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodwin K., Viboud C., Simonsen L. 2006. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24:1159–1169 [DOI] [PubMed] [Google Scholar]
- 14. Gordon C. L., et al. 2010. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G2 subclass deficiency. Clin. Infect. Dis. 50:672–678 [DOI] [PubMed] [Google Scholar]
- 15. Greenberg M. E., et al. 2009. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 361:2405–2413 [DOI] [PubMed] [Google Scholar]
- 16. Hancock K., et al. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361:1945–1952 [DOI] [PubMed] [Google Scholar]
- 17. Hobson D., Curry R. L., Beare A. S., Ward-Gardner A. 1972. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (Lond.) 70:767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung I. F., et al. 2011. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 52:447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hung I. F., et al. 2010. Effect of clinical and virological parameters on the level of neutralizing antibody against pandemic influenza A virus H1N1 2009. Clin. Infect. Dis. 51:274–279 [DOI] [PubMed] [Google Scholar]
- 20. Igari H., et al. 2010. Immunogenicity of a monovalent pandemic influenza A H1N1 vaccine in healthcare workers of a university hospital in Japan. Microbiol. Immunol. 54:618–624 [DOI] [PubMed] [Google Scholar]
- 21. Jones P. D., Ada G. L. 1987. Persistence of influenza virus-specific antibody-secreting cells and B-cell memory after primary murine influenza virus infection. Cell. Immunol. 109:53–64 [DOI] [PubMed] [Google Scholar]
- 22. Liang X. F., et al. 2010. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 375:56–66 [DOI] [PubMed] [Google Scholar]
- 23. Liu W., et al. 2010. Clinical and immunological characteristics of patients with 2009 pandemic influenza A (H1N1) virus infection after vaccination. Clin. Infect. Dis. 51:1028–1032 [DOI] [PubMed] [Google Scholar]
- 24. Louie J. K., et al. 2009. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA 302:1896–1902 [DOI] [PubMed] [Google Scholar]
- 25. Mak G. C., et al. 2010. Sero-immunity and serologic response to pandemic influenza A (H1N1) 2009 virus in Hong Kong. J. Med. Virol. 82:1809–1815 [DOI] [PubMed] [Google Scholar]
- 26. Matrosovich M. N., Matrosovich T. Y., Gray T., Roberts N. A., Klenk H. D. 2004. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 78:12665–12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohuchi M., Asaoka N., Sakai T., Ohuchi R. 2006. Roles of neuraminidase in the initial stage of influenza virus infection. Microbes Infect. 8:1287–1293 [DOI] [PubMed] [Google Scholar]
- 28. Plennevaux E., Sheldon E., Blatter M., Reeves-Hoche M. K., Denis M. 2010. Immune response after a single vaccination against 2009 influenza A H1N1 in U. S. A.: a preliminary report of two randomised controlled phase 2 trials. Lancet 375:41–48 [DOI] [PubMed] [Google Scholar]
- 29. Potter C. W., Oxford J. S. 1979. Determinants of immunity to influenza infection in man. Br. Med. Bull. 35:69–75 [DOI] [PubMed] [Google Scholar]
- 30. Qiu L. W., et al. 2009. Development of an antigen capture immunoassay based on monoclonal antibodies specific for dengue virus serotype 2 nonstructural protein 1 for early and rapid identification of dengue virus serotype 2 infections. Clin. Vaccine Immunol. 16:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Remarque E. J., de Bruijn I. A., Boersma W. J., Masurel N., Ligthart G. J. 1998. Altered antibody response to influenza H1N1 vaccine in healthy elderly people as determined by HI, ELISA, and neutralization assay. J. Med. Virol. 55:82–87 [DOI] [PubMed] [Google Scholar]
- 32. Rowe T., et al. 1999. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 37:937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryan-Poirier K. A., Kawaoka Y. 1991. Distinct glycoprotein inhibitors of influenza A virus in different animal sera. J. Virol. 65:389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siegrist C. A., Aspinall R. 2009. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9:185–194 [DOI] [PubMed] [Google Scholar]
- 35. Smith A. J., Davies J. R. 1976. Natural infection with influenza A (H3N2): the development, persistence, and effect of antibodies to the surface antigens. J. Hyg. (Lond.) 77:271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stephenson I., Das R. G., Wood J. M., Katz J. M. 2007. Comparison of neutralizing antibody assays for detection of antibody to influenza A/H3N2 viruses: an international collaborative study. Vaccine 25:4056–4063 [DOI] [PubMed] [Google Scholar]
- 37. Stephenson I., Wood J. M., Nicholson K. G., Charlett A., Zambon M. C. 2004. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 103:91–95 [DOI] [PubMed] [Google Scholar]
- 38. Stephenson I., Wood J. M., Nicholson K. G., Zambon M. C. 2003. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza hemagglutinin. J. Med. Virol. 70:391–398 [DOI] [PubMed] [Google Scholar]
- 39. Su B., et al. 2009. Enhancement of the influenza A hemagglutinin (HA)-mediated cell-cell fusion and virus entry by the viral neuraminidase (NA). PLoS One 4:e8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Subbarao E. K., Kawaoka Y., Ryan-Poirier K., Clements M. L., Murphy B. R. 1992. Comparison of different approaches to measuring influenza A virus-specific hemagglutination inhibition antibodies in the presence of serum inhibitors. J. Clin. Microbiol. 30:996–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sui J., et al. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. The European Agency for the Evaluation of Medicinal Products (EMEA) Committee for Proprietary Medicinal Products (CPMP) 1997. Note for guidance on the harmonization of requirements for influenza vaccines. EMEA, London, United Kingdom [Google Scholar]
- 43. To K. K., et al. 2010. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J. Med. Virol. 82:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. To K. K., et al. 2010. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis. 50:850–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang T. T., et al. 2010. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc. Natl. Acad. Sci. U. S. A. 107:18979–18984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y., et al. 2009. Monoclonal antibody recognizing SLLTEVET epitope of M2 protein potently inhibited the replication of influenza A viruses in MDCK cells. Biochem. Biophys. Res. Commun. 385:118–122 [DOI] [PubMed] [Google Scholar]
- 47. Wang Z., Mo C., Kemble G., Duke G. 2004. Development of an efficient fluorescence-based microneutralization assay using recombinant human cytomegalovirus strains expressing green fluorescent protein. J. Virol. Methods 120:207–215 [DOI] [PubMed] [Google Scholar]
- 48. Wichmann O., et al. 2010. Pandemic influenza A (H1N1) 2009 breakthrough infections and estimates of vaccine effectiveness in Germany 2009-2010. Eur. Surveill. 15:19561. [PubMed] [Google Scholar]
- 49. Wong H. K., et al. 2010. Practical limitations of convalescent plasma collection: a case scenario in pandemic preparation for influenza A (H1N1) infection. Transfusion 50:1967–1971 [DOI] [PubMed] [Google Scholar]
- 50. Xu H., et al. 2006. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: implications for early diagnosis and serotyping of dengue virus infections. J. Clin. Microbiol. 44:2872–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu F. C., et al. 2009. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 361:2414–2423 [DOI] [PubMed] [Google Scholar]