Abstract
Tuberculous pericarditis is a rare disease in developed countries. The diagnosis is difficult to set since there are no robust rapid tests, and culture of pericardial fluid for Mycobacterium tuberculosis is often negative. T-SPOT.TB, an enzyme-linked immunospot (ELISPOT) test, measures the gamma interferon response of lymphocytes against tuberculosis antigens and can be performed on blood and body fluids. We describe a patient with tuberculous pericarditis for which the diagnosis was rapidly set by positive T-SPOT.TB results, which were confirmed by isolation of Mycobacterium tuberculosis in pericardial fluid culture. We performed a literature search to assess the diagnostic potential of ELISPOT testing in tuberculous pericarditis. The limited data on this subject indicate that T-SPOT.TB aids in diagnosing active tuberculosis (TB) infection and results in a more rapid decision to start antituberculosis treatment. Enumerating TB-specific lymphocytes and testing blood/compartmental fluid simultaneously can provide useful information on active tuberculous pericarditis.
INTRODUCTION
Pericarditis is a common disease in patients suffering from chest pain: about 5% of patients presenting with chest pain but without myocardial infarction are diagnosed with pericarditis (7). The typical presentation of acute pericarditis is retrosternal chest pain, which is sudden in onset and exacerbates during inspiration. Upon auscultation, a pericardial friction rub is audible. This clinical picture is quite similar to those of pulmonary embolisms and myocardial infarction (13). In the Western world, the cause is infectious in 6 to 8%, neoplasm in 7%, autoimmune in 3 to 5%, and idiopathic in 85 to 90%. The infectious causes comprise viral, bacterial, and tuberculous origins. The incidence of a tuberculous origin of pericarditis is estimated to be at 4% in the Western world and up to 60% in the Republic of South Africa (17, 20, 22).
The diagnosis of tuberculous pericarditis is difficult to set, not only for its nonspecific clinical presentation but also for the lack of useful diagnostic tests. Diagnostic tests used for tuberculous pericarditis are tuberculin skin testing, culture, PCR assays, and acid-fast staining of pericardial fluid. Tuberculin skin testing is a rapid test with a sensitivity ranging from 67 to 72%, cannot differentiate between active and past infections, and can also be positive due to Mycobacterium bovis bacille Calmette-Guérin vaccination (5). Culturing and species determination take 6 to 8 weeks. Furthermore, the sensitivities of both smear and culturing are limited (21). Tuberculous pericarditis has a high mortality and requires the start of adequate treatment at an early phase of disease (16). Therefore, there is a need for rapid and accurate diagnostic tests.
Tuberculosis (TB) results in the activation and proliferation of TB-specific T lymphocytes. These lymphocytes produce proinflammatory cytokines, and one of which is gamma interferon (IFN-γ). T-SPOT.TB is an enzyme-linked immunospot (ELISPOT) assay, an in vitro test which measures the gamma interferon release of activated T cells isolated from the patient's blood. With this test, the response is evoked by the TB-specific antigens early secretory antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10). T-SPOT.TB does not differentiate between past and active TB (1). However, it has successfully been adapted to make the diagnosis of active tuberculosis more likely. A large fraction of activated T cells in response to ESAT-6 have been associated with active infection (6). Furthermore, the diagnosis of active infection can be established by calculating the proportions of activated lymphocytes in blood samples and in specimens obtained from the compartment of infection. There is much experience with this interpretation of testing results for pulmonary TB and extrapulmonary forms such as pleurisy, ascites, and infection of the central nervous system, but very little has been described for ELISPOT testing for tuberculous pericarditis (9, 12, 15).
In this report, we describe a patient with tuberculous pericarditis for which the diagnosis was rapidly set by positive T-SPOT.TB results after 1 day. After 23 days, these results were confirmed by isolation of Mycobacterium tuberculosis in pericardial fluid culture. Furthermore, we researched the literature to assess the diagnostic potential of the ELISPOT assay in tuberculous pericarditis.
A 24-year-old male born in Kenya and who recently immigrated to the Netherlands was admitted to the emergency department of Diakonessenhuis Utrecht, Netherlands, with symptoms including dry cough, fever, night sweats, and chest tightness for 3 weeks. His past medical history was unremarkable, and he did not use any medication. Physical examination revealed normal vital signs, a body temperature of 37.2°C, a pericardial friction rub upon auscultation, and inguinal lymphadenopathy. Blood tests showed increased C-reactive protein levels of 64 mg/liter, an erythrocyte sedimentation rate of 63 mm/1 h, normal leukocyte counts (5.1 × 10e9 leukocytes/liter), and normocytic anemia (hemoglobin level, 7.0 mmol/liter). An electrocardiogram showed a sinus rhythm without any abnormalities at admission but changed from normal to negative T waves in II, III, AVF, V4, V5, and V6 during the first day of hospitalization. Chest radiograph showed an increased heart figure but, otherwise, no abnormalities. A computed tomography scan showed pericardial fluid (2 cm in size), mediastinal and hilar lymphadenopathy, and a small multinodular consolidation in the right upper lobe. Transesophageal echocardiography showed pericardial fluid at 1.5 to 2.5 cm in size, a slight cardiac inflow restriction, an increased diameter of the inferior caval vein, decreased left ventricle function, and a swinging heart. The differential diagnosis included TB, viral pericarditis, HIV, sarcoidosis, autoimmune vasculitis, and neoplasms. Drainage of the pericardial fluid was performed. Pericardial fluid showed effusion with predominantly lymphocytes (Table 1). Viral detection, conventional bacterial cultures, and autoimmune diagnostic tests were negative. Ziehl-Neelsen staining and an auramine smear on pericardial fluid were negative. Pericardial fluid was cultured on specific media for M. tuberculosis. T-SPOT.TB tests with blood and pericardial fluid were performed: the number of spot-forming units on blood were borderline positive (see Materials and Methods for definition) and on pericardial fluid were highly positive, which aids the diagnosis of active pericarditis tuberculosis (Table 1 and Fig. 1). Based on this finding, the patient was treated with isoniazid at 300 mg, rifampin at 600 mg, pyrazinamide at 1,600 mg, and ethambutol at 2,000 mg (all once daily), combined with a tapering course of prednisolone starting at 30 mg. On this treatment, the patient clinically improved. After 23 days, the diagnosis of tuberculous pericarditis was definitively confirmed by culturing of acid-fast rods from pericardial fluid, which were determined to be M. tuberculosis.
Table 1.
Results of biochemistry tests on PF and TB testing of patients reported to have tuberculous pericarditis by positive ELISPOT assaya
| Characteristic | Value for published case with positive ELISPOT test (study that presented case) |
||||
|---|---|---|---|---|---|
| 1 (11) | 2 (2) | 3 (14) | 4 (14) | 5 (this study)b | |
| PF testing | |||||
| No. of leukocytes/μl | 2,450 | 4,000 | 800 | 6,030 | 5,000 |
| % lymphocytes | 95 | 25 | 66 | 25 | 86 |
| % histiocytes | Not reported | 72 | 8 | 7 | 6 |
| % granulocytes | Not reported | 3 | 26 | 68 | 8 |
| Glucose (mg/dl) | 24 | 22 | Not done | 27 | 47 |
| Total protein (g/liter) | 47 | 60 | Not done | 58 | 53 |
| Lactate deoxygenase (U/liter) | 1,880 | 2,256 | Not done | Not done | 881 |
| TB testing | |||||
| Culture for M. tuberculosis | Positive | Negative | Positive | Negative | Positive |
| M. tuberculosis PCR of PF | Negative | Negative | Not done | Not done | Negative |
| Auramine/ZN staining | Negative | Negative | Negative | Negative | Negative |
| No. of blood ESAT-6 SFU (ISL) | 14 | 62 (310) | 8 | 12 | 4 (17) |
| No. of blood CFP-10 SFU (ISL) | 8 | 150 (750) | 97 | 0 | 10 (42) |
| No. of PF ESAT-6 SFU (ISL) | 120 | 20 (320) | Not done | Not done | >400 (>1,852) |
| No. of PF CFP-10 SFU (ISL) | 56 | 57 (912) | Not done | Not done | >400 (>1,852) |
| PF/blood ratio of no. of ESAT-6/10e6 lymphocytes | 8.57c | 1.03 | Not done | Not done | >109 |
| PF/blood ratio of no. of CFP-10/10e6 lymphocytes | 7.00c | 1.22 | Not done | Not done | >44 |
PF = pericardial fluid, TB = tuberculosis, SFU = spot-forming units, ISL = number of IFN-γ-secreting lymphocytes/10e6 lymphocytes in pericardial fluid, ZN = Ziehl-Neelsen.
We considered 400 SFU as the maximum discernible number of SFU.
Ratio shown in SFU.
Fig. 1.
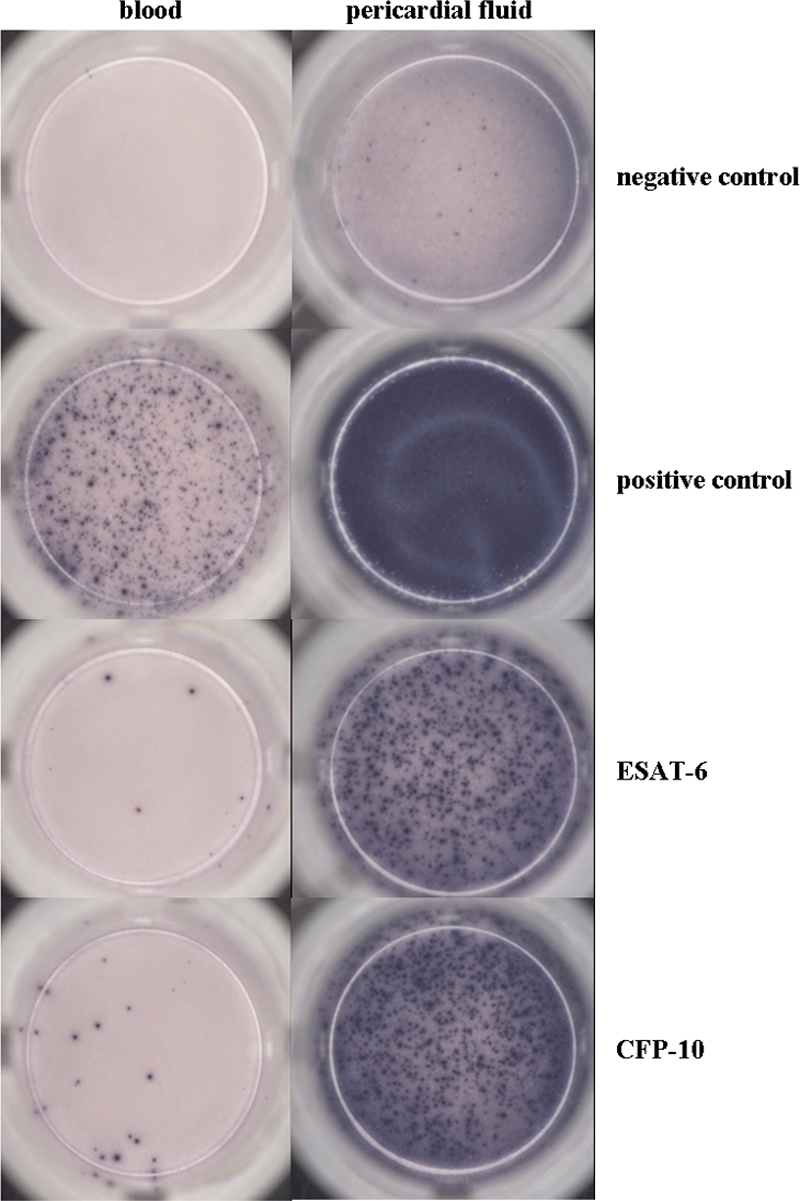
Results of TSPOT.TB tests performed on blood and pericardial fluid. Lymphocytes producing IFN-γ against Mycobacterium tuberculosis are visible as blue spots. Negative controls were stimulated with medium only. The lymphocyte functional capacity (positive controls) was verified with PHA. Table 1 contains the numbers of spot-forming units.
MATERIALS AND METHODS
T-SPOT.TB (Oxford Immunotec, Oxford, United Kingdom) with the peripheral blood mononuclear cell (PBMC) fraction was performed, according to the instructions obtained from the manufacturer. Shortly, venous blood was collected in Vacutainer CPT tubes (catalog no. 362782; BD, Franklin Lakes, NJ), and PBMCs were harvested. Cells were harvested from the pericardial fluid after being spun down. For each sample, 4 wells of a 96-well anti-IFN-γ-coated test plate were inoculated with 2.5 × 105 cells. RPMI medium was added to the first well as an unstimulated control, and phytohemagglutinin (PHA) was added to the second well to verify the viability of the mononuclear cells. Panel A (CFP-10 peptides) and panel B (ESAT-6 peptides) were added to the third and fourth well, respectively. Plates were incubated for 16 to 20 h at 37°C and 5% CO2. After incubation, plates were washed, and IFN-γ bound to the bottom of the wells was detected using a BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate/Nitro Blue Tetrazolium) color reaction. Interpretation of the results was performed according to the criteria defined by the manufacturer (Oxford Immunotec, Oxford, United Kingdom). Spots were counted by two independent observers. A positive test well, either in CFP-10 or ESAT-6, was predefined as containing at least 6 spot-forming cells (SFCs) and more than twice the number of SFCs present in the negative-control well.
Pericardial fluid was inoculated using a MGIT system (Becton Dickinson, Shannon, Ireland). The positive culture was verified using acid-fast staining using auramine and PCR for M. tuberculosis, and the positive culture was subsequently referred to the National Institute for Public Health and the Environment (RIVM) for identification and antimicrobial susceptibility testing.
Review criteria.
We performed a literature search in PubMed to assess the potential role of IFN-γ-based diagnostic tests in tuberculous pericarditis by searching the following keywords in the title or abstract: ((((((((((((((interferon) OR gamma interferon) OR interferon-γ) OR igra) OR elispot) OR Interferon-γ-Release-Assay) OR enzyme-linked-immunospot) OR enzyme-linked-immuno-spot) OR enzyme linked immunospot) OR enzyme linked immunospot) OR Gamma interferon-Release-Assay) OR tspot) OR TSPOT.TB) OR T-cell-based assay) OR quantiferron) AND ((((pericard) OR pericarditis) OR pericardial) OR pericardium). Documents from up to November 2010 were retrieved and screened by their titles and abstracts for their relevancy on the topic. We excluded non-English-language abstracts and articles.
RESULTS
Two large prospective studies carried out in the Republic of South Africa have measured nonspecific gamma interferon levels in pericardial fluids, tested by enzyme-linked immunoassays, and investigated their diagnostic value for tuberculous pericarditis (3, 18). These studies found that nonspecific IFN-γ levels have very good predictive test characteristics for tuberculous pericarditis in both HIV- and non-HIV-infected patients (19). The first study found that applying a cutoff value of ≥200 pg/ml resulted in sensitivity and specificity levels of 100%, and the second study found an optimal cutoff value of ≥50 pg/ml, with a sensitivity of 92% and a specificity of 100%. However, there have been reports of nontuberculous infectious pericarditis with much higher pericardial levels of nonspecific IFN-γ than these reported cutoff values (4). Therefore, it is uncertain whether these excellent prognostic characteristics can also be found in developed countries in which nontuberculous infectious pericarditis (bacterial and viral) is just as prevalent as tuberculous pericarditis (13).
Two case reports of patients with tuberculous pericarditis with positive ELISPOT assays of pericardial fluid have been published (2, 11). The first report described a patient with acute tuberculous pericarditis for which diagnosis had been confirmed by a positive M. tuberculosis culture. The other report described a patient with recurrent pericarditis. The onset of symptoms had started 3 months before ELISPOT testing. Furthermore, we have found two case series describing the diagnostic aid of ELISPOT testing in extrapulmonary forms of TB (10, 14). These studies reported that three patients with tuberculous pericarditis tested positive by an ELISPOT assay of blood. Unfortunately, no ELISPOT testing on pericardial fluid was performed, and little data were described in these cases. We retrieved unpublished additional data of two cases and present these in Table 1 (14).
In Table 1, we present the results of biochemical tests on pericardial fluid and of the diagnostic tuberculosis tests of these two case reports and of the two cases in the extrapulmonary tuberculosis series, together with our case. In 3 cases, the diagnosis was confirmed by a positive culture; the other 2 probably had tuberculous pericarditis based on the clinical presentation and/or histopathological findings. The case report of recurrent pericarditis by Biglino et al. had some results that were not in support of active tuberculous pericarditis (2). First, the M. tuberculosis culture of pericardial fluid was negative; however, culturing is not very sensitive, and therefore, this result does not rule out the diagnosis of tuberculous pericarditis. Furthermore, the number of ESAT-6 spot-forming units was not very high, and moreover, the pericardial fluid/blood ratio of spot-forming units was not increased. If there is an isolated organ infection by tuberculosis, one would expect an increased infected organ fluid/blood ratio, as has been shown in patients with, for example, tuberculous pleurisy (15). In addition to this, the inflammatory pattern showed a predominant increase in histiocytes, which does not match with those of the 3 cases confirmed by isolation of M. tuberculosis: these cases predominantly had lymphocytes in pericardial fluid (Table 1). Most cases of recurrent pericarditis are probably caused by autoimmune disease, although tuberculous pericarditis cases with a recurrent course have been described (8). Treatment with prednisolone in addition to antituberculosis medication was probably adequate for both tuberculous and autoimmune causes of disease. Therefore, it is uncertain whether the positive ELISPOT results were due to active TB infection or past infection in this case. Alternatively, the first onset of pericarditis could have been due to M. tuberculosis infection, provoking an autoimmune response causing the recurrent course of disease.
The T-SPOT.TB test provides a diagnosis within 2 days, has good testing characteristics, and differentiates between infection by M. tuberculosis and previous vaccination, unlike the tuberculin skin test. The drawbacks of the T-SPOT.TB test are the relatively high labor intensity to perform the test and the inability of the test to differentiate between active and past tuberculosis infections. Several methods have been suggested to improve the predictive value for an active infection. The fraction of TB-specific lymphocytes can be enumerated by compensating for the initial lymphocyte numbers in the sample. This fraction can be expressed as the number of IFN-γ-secreting lymphocytes/10e6 lymphocytes. Larger fractions of IFN-γ-secreting lymphocytes, particularly those triggered by ESAT-6, could be indicative of a progressive infection (6). Furthermore, an elevated M. tuberculosis-specific T-cell response in an isolated compartment of TB infection is suggestive of active TB infection. To test this, peripheral blood and compartmental fluid can be measured in the same T-SPOT.TB run. The compartmental fluid/blood ratio also can be expressed either as a ratio of IFN-γ-secreting lymphocytes or as a ratio of spot-forming units. The first method of expressing the ratio describes a difference in the inflammatory pattern, and the second describes a difference in the inflammatory intensity of the infected compartment compared to that of blood. Calculation of both of these parameters provides complementary arguments for an active TB infection.
DISCUSSION
We conclude that diagnosing tuberculous pericarditis is difficult in developed countries, since the prevalence of tuberculous pericarditis is low, and therefore, diagnostic tests with high sensitivity/specificity are needed to achieve useful predictive testing characteristics. This report illustrates that T-SPOT.TB aids in diagnosing active TB infection, particularly if TB-specific lymphocytes are enumerated and blood/compartmental fluid are tested simultaneously. Compared to traditional TB tests, T-SPOT.TB using pericardial fluid results in a more rapid decision to start TB treatment. In the future, large trials will be needed to assess the testing characteristics of T-SPOT.TB in diagnosing tuberculous pericarditis.
ACKNOWLEDGMENT
We thank Po-Ren Hsueh of the National Taiwan University College of Medicine.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Arend S. M., et al. 2007. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am. J. Respir. Crit. Care Med. 175:618–627 [DOI] [PubMed] [Google Scholar]
- 2. Biglino A., Crivelli P., Concialdi E., Bolla C., Montrucchio G. 2008. Clinical usefulness of ELISPOT assay on pericardial fluid in a case of suspected tuberculous pericarditis. Infection 36:601–604 [DOI] [PubMed] [Google Scholar]
- 3. Burgess L. J., Reuter H., Carstens M. E., Taljaard J. J., Doubell A. F. 2002. The use of adenosine deaminase and interferon-gamma as diagnostic tools for tuberculous pericarditis. Chest 122:900–905 [DOI] [PubMed] [Google Scholar]
- 4. de Souza A. L., et al. 2006. Cytokine activation in purulent pericarditis caused by Neisseria meningitidis serogroup C. Int. J. Cardiol. 113:419–421 [DOI] [PubMed] [Google Scholar]
- 5. Diel R., Loddenkemper R., Nienhaus A. 2010. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest 137:952–968 [DOI] [PubMed] [Google Scholar]
- 6. Doherty M., Wallis R. S., Zumla A. 2009. Biomarkers for tuberculosis disease status and diagnosis. Curr. Opin. Pulm. Med. 15:181–187 [DOI] [PubMed] [Google Scholar]
- 7. Fruergaard P., et al. 1996. The diagnoses of patients admitted with acute chest pain but without myocardial infarction. Eur. Heart J. 17:1028–1034 [DOI] [PubMed] [Google Scholar]
- 8. Imazio M., Trinchero R., Shabetai R. 2007. Pathogenesis, management, and prevention of recurrent pericarditis. J. Cardiovasc. Med. (Hagerstown) 8:404–410 [DOI] [PubMed] [Google Scholar]
- 9. Jafari C., et al. 2009. Bronchoalveolar lavage enzyme-linked immunospot for a rapid diagnosis of tuberculosis: a Tuberculosis Network European Trialsgroup study. Am. J. Respir. Crit. Care Med. 180:666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim S. H., et al. 2007. Diagnostic usefulness of a T-cell based assay for extrapulmonary tuberculosis. Arch. Intern. Med. 167:2255–2259 [DOI] [PubMed] [Google Scholar]
- 11. Kobashi Y., et al. 2010. Rapid diagnosis of tuberculous pericarditis by ELISPOT assay. Scand. J. Infect. Dis. 42:712–715 [DOI] [PubMed] [Google Scholar]
- 12. Kosters K., et al. 2008. Rapid diagnosis of CNS tuberculosis by a T-cell interferon-gamma release assay on cerebrospinal fluid mononuclear cells. Infection 36:597–600 [DOI] [PubMed] [Google Scholar]
- 13. Lange R. A., Hillis L. D. 2004. Clinical practice. Acute pericarditis. N. Engl. J. Med. 351:2195–2202 [DOI] [PubMed] [Google Scholar]
- 14. Liao C. H., et al. 2009. Diagnostic performance of an enzyme-linked immunospot assay for interferon-gamma in extrapulmonary tuberculosis varies between different sites of disease. J. Infect. 59:402–408 [DOI] [PubMed] [Google Scholar]
- 15. Losi M., et al. 2007. Use of a T-cell interferon-gamma release assay for the diagnosis of tuberculous pleurisy. Eur. Respir. J. 30:1173–1179 [DOI] [PubMed] [Google Scholar]
- 16. Mayosi B. M., et al. 2008. Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. S. Afr. Med. J. 98:36–40 [PubMed] [Google Scholar]
- 17. Permanyer-Miralda G., Sagrista-Sauleda J., Soler-Soler J. 1985. Primary acute pericardial disease: a prospective series of 231 consecutive patients. Am. J. Cardiol. 56:623–630 [DOI] [PubMed] [Google Scholar]
- 18. Reuter H., Burgess L., van Vuuren W., Doubell A. 2006. Diagnosing tuberculous pericarditis. QJM 99:827–839 [DOI] [PubMed] [Google Scholar]
- 19. Reuter H., Burgess L. J., Carstens M. E., Doubell A. F. 2006. Characterization of the immunological features of tuberculous pericardial effusions in HIV positive and HIV negative patients in contrast with non-tuberculous effusions. Tuberculosis (Edinb.) 86:125–133 [DOI] [PubMed] [Google Scholar]
- 20. Reuter H., Burgess L. J., Doubell A. F. 2005. Epidemiology of pericardial effusions at a large academic hospital in South Africa. Epidemiol. Infect. 133:393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strang G., et al. 1991. Bedside culture to confirm tuberculous pericarditis. Lancet 338:1600–16011684009 [Google Scholar]
- 22. Zayas R., et al. 1995. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am. J. Cardiol. 75:378–382 [DOI] [PubMed] [Google Scholar]


