Abstract
In 2005, European Commission directive 2005/744/EC allowed controlled vaccination against avian influenza (AI) virus of valuable avian species housed in zoos. In 2006, 15 Spanish zoos and wildlife centers began a vaccination program with a commercial inactivated H5N9 vaccine. Between November 2007 and May 2008, birds from 10 of these centers were vaccinated again with a commercial inactivated H5N3 vaccine. During these campaigns, pre- and postvaccination samples from different bird orders were taken to study the response against AI virus H5 vaccines. Sera prior to vaccinations with both vaccines were examined for the presence of total antibodies against influenza A nucleoprotein (NP) by a commercial competitive enzyme-linked immunosorbent assay (cELISA). Humoral responses to vaccination were evaluated using a hemagglutination inhibition (HI) assay. In some taxonomic orders, both vaccines elicited comparatively high titers of HI antibodies against H5. Interestingly, some orders, such as Psittaciformes, which did not develop HI antibodies to either vaccine formulation when used alone, triggered notable HI antibody production, albeit in low HI titers, when primed with H5N9 and during subsequent boosting with the H5N3 vaccine. Vaccination with successive heterologous vaccines may represent the best alternative to widely protect valuable and/or endangered bird species against highly pathogenic AI virus infection.
INTRODUCTION
Avian influenza (AI) is an infectious disease caused by type A influenza viruses of the Orthomyxoviridae family. AI virus subtypes are classified according to their surface glycoproteins: hemagglutinin (H1 to H16) and neuraminidase (N1 to N9) (9). To date, highly pathogenic avian influenza (HPAI) viruses are restricted mainly to infections with H5 and H7 subtype viruses, which have caused unprecedented morbidity and mortality in birds within the last few years (2). Aquatic wild birds, including Anatidae (ducks, geese, and swans) and Charadriidae (shorebirds), are widely considered to be the natural reservoir of AI virus (13). Although wild birds were not known to be implicated in the initial HPAI outbreaks, in 2002, an outbreak of H5N1 HPAI virus in Hong Kong caused mortality in a wide range of avian species, including migratory birds and resident waterfowls (6). Since then, the H5N1 subtype of HPAI virus has spread throughout Asia and into Europe and Africa, affecting a large number of species. In 2005, an outbreak killed over 6,000 water birds (mainly bar-headed geese [Anser indicus], great cormorants [Phalacrocorax carbo], Pallas's gulls [Larus ichthyaetus], brown-headed gulls [Larus brunnicephalus], and ruddy shelducks [Tadorna ferruginea]) at the Qinghai Lake National Nature Reserve in northwest China (3). Furthermore, several reports indicate direct bird-to-human transmission in some Asian countries (11, 18). These zoonotic consequences and the ecologic value of protecting avian species have emphasized the need for effective control measures.
Due to unprecedented morbidity and mortality caused by H5N1 HPAI virus and given the value of birds kept in zoos, in 2005 the European Commission directive 2005/744/EC allowed vaccination against AI virus in such birds in zoos, under strict surveillance (7). In the following years, different European countries established preventive vaccination campaigns in zoological institutions. In 2006, 15 Spanish zoos and wildlife centers underwent a vaccination program with a commercial inactivated H5N9 vaccine. Between November 2007 and May 2008, birds from 10 of these centers were vaccinated again with a commercial inactivated H5N3 vaccine, as decided by the Spanish government. The decision of changing the vaccine used in the first AI vaccination program (VP1) was based on experimental results showing that the H5N3 vaccine, a reverse genetics monovalent vaccine, was shown to elicit a strong immune response and protected chickens (10) and ducks (12) from experimental H5N1 infection, with no detection of viral shedding.
The goal of the present study was to compare the seroprotection elicited by inactivated H5N9 and H5N3 vaccines and evaluate the boost effect of H5N3 vaccine in inducing immune responses after priming a wide selection of avian species with H5N9 in Spanish zoos.
MATERIALS AND METHODS
Vaccination.
An inactivated, commercial, water-in-oil adjuvanted H5N9 (A/CK/Italy/22A/H5N9/1998) vaccine (Poulvac i-AI H5N9-, Fort Dodge Animal Health, Weesp, Netherlands), containing at least 128 hemagglutination units (HAU) according to potency test, was used in zoos during the first AI vaccination program (VP1) in Spain. Vaccination against AI virus in some of the zoos began in March 2006, with the remaining zoos vaccinating up to September 2006. More than 2,600 birds were vaccinated in the 15 zoos participating in this study. The birds were vaccinated twice within a 3-week interval via the subcutaneous route. Eighteen months later, between November 2007 and May 2008, a second vaccination program (VP2) was carried out. At that time, an inactivated, commercial, water-in-oil adjuvanted H5N3 (strain rg-A/ck/VN/C58/04) vaccine (Poulvac i-AI H5N3-, Fort Dodge Animal Health, Weesp, Netherlands), containing at least 256 HAU, was used. Ten out of the 15 zoos took part in the second vaccination program. More than 450 birds were vaccinated either once (if they had been previously vaccinated with the H5N9 vaccine) or twice (those being vaccinated for the first time). Most of the animals receiving the vaccine for the first time were born after VP1.
Both vaccines are effective against the virus type in circulation and support the DIVA (differentiating infected from vaccinated animals) principle, as the N antigen differs from N1, which makes it possible to distinguish vaccinated birds from H5N1-infected birds while maintaining acceptable efficacy. Further details may be obtained from the manufacturer. In the two campaigns, the vaccine dose administrated was adapted to body weight. Thus, birds with a body weight of <2 kg were given 0.2 ml, those 2 to 10 kg were given 0.5 ml, and those >10 kg were given 1 ml. Published mean body weights of the different species were used instead of using individual weights (4).
Sampling.
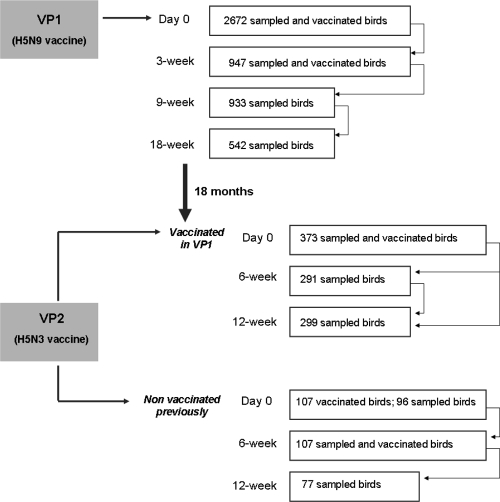
Blood was collected from the right jugular, brachial, or ulnar vein (left or right). In VP1, samples were obtained on the days of both first (n = 2,672 samples from 17 taxonomic orders) and second (n = 947 samples from 17 taxonomic orders) vaccinations, as well as 9 (n = 933 samples from 17 taxonomic orders) and 18 (n = 542 samples from 16 taxonomic orders) weeks following the first vaccination dose. In VP2, blood was collected on the day of vaccination (n = 469 samples from 16 taxonomic orders) and 6 (n = 398 samples from 14 taxonomic orders) and 12 (n = 376 samples from 15 taxonomic orders) weeks after the first vaccination. In VP2, birds receiving an AI vaccine for the first time (107 out of 469) were revaccinated after 6 weeks (Fig. 1).
Fig. 1.
Vaccination and sampling schedule. In VP1, animals were vaccinated twice with an inactivated H5N9 vaccine, at day 0 and 3 weeks after the first dose. Eighteen months later, birds were vaccinated with an inactivated H5N3 vaccine (VP2). In VP2, two groups were differentiated, those being vaccinated for the first time and those that were previously vaccinated with H5N9. Serum samples were collected at all the time points indicated in the figure and tested by cELISA and HI. The numbers of animals tested are also indicated in the rectangles next to each time point.
The official sampling protocol also included collecting cloacal swabs to detect the presence of AI virus by reverse transcription-PCR (RT-PCR), as described previously (13).
Serology.
Sera prior to vaccinations with H5N9 (A/CK/Italy/22A/H5N9/1998) and H5N3 (rg-A/ck/VN/C58/04) were examined for the presence of total antibodies against influenza A nucleoprotein (NP) by a commercial competitive enzyme-linked immunosorbent assay (cELISA) kit (ID VET, Montpellier, France). The cELISA is based on recombinant AI virus NP as the antigen and a conjugated antibody directed against the NP of AI virus. The assay was performed according to manufacturer instructions.
To evaluate the humoral immune response induced after both vaccinations, homologous H5-specific antibody titers were determined by an HI test by following standard procedures (14). Briefly, chicken erythrocytes and 4 HAU of an H5 antigen (GD-Animal Health Service Deventer, Netherlands) were used for the test. Sera from some bird species may cause agglutination of the chicken erythrocytes used in the HI test, which may mask low levels of HI activity. For that reason, before doing the test, sera from all animals were pretreated with a 50% suspension of chicken erythrocytes for 1 h. Fifty microliters of pretreated serum was diluted by 2-fold serial dilution (1:2 to 1:4,096) in phosphate-buffered saline (PBS) solution in U-bottomed microwell plastic plates (Nunc, Copenhagen, Denmark), and 4 HAU of virus was added to each well. Following incubation at room temperature for 30 min, 50 μl of 0.6 to 0.75% chicken red blood cells (RBC) was added to each well, and the plates were incubated at room temperature for 30 to 45 min to allow RBC to settle. The HI titer was determined as the value of the highest dilution of serum causing complete inhibition of the 4 HAU. Vaccine-induced titers of ≥32 were considered to be a measure of vaccine efficacy, and titers <16 were considered negative according to 92/40/EEC guidelines (8). In poultry, HI titers of >16 were shown to indicate protection against infection when animals were challenged with HPAI H7N7 virus after vaccination with inactivated H7 AI vaccines (17). Since performing challenge experiments in valuable zoo species is not possible and in accordance with the European Food Safety Authority (EFSA), we chose an HI titer of 32 as a threshold of protective vaccine efficacy, as vaccine manufacturers do (5).
To evaluate the specific immune response against an HPAI H5N1 virus strain and to test the breadth of antibody response, postvaccination serum was tested against A/Mallard/It/3401/05 (H5N1) and A/Tky/Eng/647/77 (H7N7).
No adverse reactions to vaccination were reported in any of the participating centers.
Statistical analysis.
For each species and for each order, the geometric mean titer (GMT) and the percentage of animals with titers higher than 32 were calculated. Differences of GMT values between orders were tested with the Mann-Whitney test. Statistical analyses were performed using SPSS for Windows, version 17.0.
RESULTS
Humoral response against H5N9 vaccination (VP1).
Detailed data concerning humoral immune response against an inactivated H5N9 vaccine from each order and species studied is provided in Table 1. Before receiving the vaccine, only 33 birds out of 2,672 (1.2%) showed antibodies against AI virus NP when tested by cELISA. Similarly, less than 1% of the animals were seropositive for H5 AI virus by an HI test using the homologous antigen. These 25 birds, presenting HI titers of 32 or higher, belonged to four orders (Phoenicopteriformes [n = 19 birds], Anseriformes [n = 3 birds], Ciconiiformes [n = 2 birds], and Pelecaniformes [n = 1 bird]).
Table 1.
Humoral immune response of avian species in zoos, vaccinated twice (within a 3-week interval) with an inactivated H5N9 vaccine (VP1)a
| Order | Species |
No. of birds | GMT | % of birds with HI titers of ≥32 | |
|---|---|---|---|---|---|
| Common name | Scientific name | ||||
| Anseriformes | Total | 179 | 61 | 67.2 | |
| Mandarin duck | Aix galericulata | 1 | 4 | 0 | |
| Egyptian goose | Alopochen aegyptiacus | 6 | 13 | 33.3 | |
| Northern pintail | Anas acuta | 1 | 256 | 100 | |
| Northern shoveler | Anas clypeata | 1 | 256 | 100 | |
| Baikal teal | Anas formosa | 6 | 228 | 100 | |
| Eurasian wigeon | Anas penelope | 2 | 181 | 100 | |
| Mallard | Anas platyrhynchos | 8 | 19 | 37.5 | |
| Chiloe wigeon | Anas sibilatrix | 9 | 4 | 0 | |
| Greylag goose | Anser anser | 14 | 24 | 57.1 | |
| Swan goose | Anser cygnoides | 4 | 19 | 50 | |
| Bar-headed goose | Anser indicus | 17 | 234 | 94.1 | |
| Magpie goose | Anseranas semipalmata | 1 | 32 | 100 | |
| Canada goose | Branta canadensis | 1 | 2,048 | 100 | |
| Barnacle goose | Branta leucopsis | 2 | 256 | 100 | |
| Red-breasted goose | Branta ruficollis | 9 | 299 | 100 | |
| Hawaiian goose | Branta sandvicensis | 1 | 512 | 100 | |
| Muscovy duck | Cairina moschata | 16 | 18 | 50 | |
| Ringed teal | Callonetta leucophrys | 3 | 81 | 100 | |
| Cape Barren goose | Cereopsis novaehollandiae | 7 | 32 | 57.1 | |
| Southern screamer | Chauna torquata | 5 | 111 | 80 | |
| Australian wood duck | Chenonetta jubata | 3 | 8 | 33.4 | |
| Andean goose | Chloephaga picta | 3 | 323 | 100 | |
| Black swan | Cygnus atratus | 16 | 146 | 87.5 | |
| Black-necked swan | Cygnus melanocorypha | 1 | 256 | 100 | |
| Mute swan | Cygnus olor | 11 | 53 | 54.5 | |
| Fulvous whistling-duck | Dendrocygna bicolor | 1 | 1,024 | 100 | |
| Marbled duck | Marmaronetta angustirostris | 1 | 32 | 100 | |
| Rosybill | Netta peposaca | 9 | 299 | 88.9 | |
| Red-crested pochard | Netta rufina | 8 | 91 | 62.5 | |
| Knob-billed duck | Sarkidiornis melanotos | 3 | 51 | 66.7 | |
| Ruddy shelduck | Tadorna ferruginea | 6 | 7 | 16.7 | |
| Raja shelduck | Tadorna radjah | 1 | 1,024 | 100 | |
| Common shelduck | Tadorna tadorna | 2 | 362 | 100 | |
| Charadriiformes | Total | 17 | 20 | 47.1 | |
| Eurasian oystercatcher | Haematopus ostralegus | 4 | 23 | 50 | |
| Audouin's gull | Larus audouinii | 1 | 4 | 0 | |
| Caspian gull | Larus cachinnans | 5 | 7 | 20 | |
| Pied Avocet | Recurvirostra avosetta | 5 | 42 | 60 | |
| Masked lapwing | Vanellus miles | 2 | 64 | 100 | |
| Ciconiiformes | Total | 82 | 14 | 33.7 | |
| Abdim's stork | Ciconia abdimii | 1 | 256 | 100 | |
| White stork | Ciconia ciconia | 20 | 13 | 30 | |
| Ibis stork | Ciconia ibis | 3 | 51 | 100 | |
| Scarlet ibis | Eudocimus ruber | 18 | 5 | 5.6 | |
| Northern bald ibis | Geronticus eremita | 4 | 64 | 100 | |
| Marabou stork | Leptoptilos crumeniferus | 9 | 13 | 22.2 | |
| Yellow-billed stork | Mycteria ibis | 1 | 4 | 0 | |
| Roseate spoonbill | Platalea ajaja | 3 | 32 | 66.6 | |
| African spoonbill | Platalea alba | 3 | 128 | 66.7 | |
| African sacred ibis | Threskiornis aethiopicus | 19 | 15 | 37 | |
| Straw-necked ibis | Threskiornis spinicollis | 1 | 8 | 0 | |
| Columbiformes | Total | 79 | 6 | 12.5 | |
| Nicobar pigeon | Caloenas nicobarica | 6 | 20 | 66.7 | |
| Speckled pigeon | Columba guinea | 7 | 4 | 0 | |
| Rock pigeon | Columba livia | 56 | 5 | 7.1 | |
| Common wood pigeon | Columba palumbus | 1 | 4 | 0 | |
| Victoria crowned pigeon | Goura victoria | 2 | 4 | 0 | |
| Barbary dove | Streptopelia risoria | 7 | 10 | 28.6 | |
| Coraciiformes | Total | 27 | 5 | 7.4 | |
| Knobbed hornbill | Aceros cassidix | 2 | 4 | 0 | |
| Mindanao wrinkled hornbill | Aceros leucocephalus | 2 | 4 | 0 | |
| Black hornbill | Anthracoceros malayanus | 2 | 4 | 0 | |
| White-crowned hornbill | Berenicornis comatus | 2 | 4 | 0 | |
| Great hornbill | Buceros bicornis | 1 | 4 | 0 | |
| Rhinoceros hornbill | Buceros rhinoceros | 1 | 4 | 0 | |
| Abyssinian ground hornbill | Bucorvus abyssinicus | 1 | 4 | 0 | |
| Southern ground hornbill | Bucorvus leadbeateri | 8 | 6 | 12.5 | |
| Silvery-cheeked hornbill | Bycanistes brevis | 1 | 4 | 0 | |
| Trumpeter hornbill | Bycanistes bucinator | 1 | 4 | 0 | |
| Gray-cheeked hornbill | Bycanistes subcylindricus | 2 | 4 | 0 | |
| Laughing kookaburra | Dacelo novaeguineae | 4 | 7 | 25 | |
| Falconiformes | Total | 75 | 42 | 64 | |
| Cinereous vulture | Aegypius monachus | 3 | 8 | 33.3 | |
| Steppe eagle | Aquila nipalensis | 3 | 51 | 100 | |
| Verreaux's eagle | Aquila verreauxii | 1 | 128 | 100 | |
| Red-tailed hawk | Buteo jamaicensis | 1 | 16 | 0 | |
| Variable hawk | Buteo poecilochrous | 1 | 128 | 100 | |
| Royal hawk | Buteo regalis | 4 | 32 | 50 | |
| Turkey vulture | Cathartes aura | 4 | 11 | 25 | |
| Short-toed eagle | Circaetus gallicus | 3 | 20 | 66.7 | |
| Black vulture | Coragyps atratus | 1 | 4 | 0 | |
| Lanner falcon | Falco biarmicus | 1 | 512 | 100 | |
| Lesser kestrel | Falco naumanni | 3 | 203 | 100 | |
| Black-chested buzzard eagle | Geranoaetus melanoleucus | 2 | 4 | 0 | |
| Palm-nut vulture | Gypohierax angolensis | 1 | 8 | 0 | |
| White-backed vulture | Gyps africanus | 1 | 4 | 0 | |
| Griffon vulture | Gyps fulvus | 3 | 102 | 100 | |
| Himalayan vulture | Gyps himalayensis | 1 | 256 | 100 | |
| White-tailed eagle | Haliaeetus albicilla | 2 | 32 | 100 | |
| Bald eagle | Haliaeetus leucocephalus | 4 | 54 | 50 | |
| African fish eagle | Haliaeetus vocifer | 4 | 38 | 50 | |
| Black kite | Milvus migrans | 3 | 64 | 66.7 | |
| Red kite | Milvus milvus | 5 | 194 | 100 | |
| Hooded vulture | Necrosyrtes monachus | 6 | 81 | 83.3 | |
| Egyptian vulture | Neophron percnopterus | 2 | 362 | 100 | |
| Osprey | Pandion haliaetus | 3 | 40 | 66.7 | |
| Harris's hawk | Parabuteo unicinctus | 2 | 256 | 100 | |
| Honey buzzard | Pernis apivorus | 1 | 4 | 0 | |
| Southern caracara | Polyborus plancus | 4 | 54 | 75 | |
| King vulture | Sarcoramphus papa | 2 | 64 | 100 | |
| White-headed vulture | Trigonoceps occipitalis | 1 | 4 | 0 | |
| Andean condor | Vultur gryphus | 3 | 4 | 0 | |
| Galliformes | Total | 69 | 30 | 59.4 | |
| Vulturine guineafowl | Acryllium vulturinum | 3 | 25 | 66.7 | |
| Lady Amherst's pheasant | Chrysolophus amherstiae | 3 | 8 | 33.3 | |
| Golden pheasant | Chrysolophus pictus | 1 | 4 | 0 | |
| Great curassow | Crax rubra | 1 | 32 | 100 | |
| Red junglefowl | Gallus gallus | 26 | 55 | 69.2 | |
| Silver pheasant | Lophura nycthemera | 2 | 4 | 0 | |
| Indian peafowl | Pavo cristatus | 31 | 29 | 61.3 | |
| Common pheasant | Phasianus colchicus | 2 | 4 | 0 | |
| Gruiformes | Total | 31 | 10 | 25.8 | |
| Blue crane | Anthropoides paradisea | 3 | 4 | 0 | |
| Demoiselle crane | Anthropoides virgo | 10 | 8 | 20 | |
| Black crowned crane | Balearica pavonina | 1 | 4 | 0 | |
| Gray crowned crane | Balearica regulorum | 11 | 18 | 45.5 | |
| Seriema | Cariama cristata | 3 | 6 | 0 | |
| Common crane | Grus grus | 3 | 13 | 33.3 | |
| Passeriformes | Total | 9 | 8 | 11.1 | |
| Pied crow | Corvus albus | 3 | 4 | 0 | |
| Carrion crow | Corvus corone | 1 | 4 | 0 | |
| Corn bunting | Emberiza calandra | 1 | 16 | 0 | |
| Rosy starling | Pastor roseus | 1 | 128 | 100 | |
| Red-billed chough | Pyrrhocorax pyrrhocorax | 1 | 16 | 0 | |
| Common blackbird | Turdus merula | 2 | 4 | 0 | |
| Pelecaniformes | Total | 31 | 15 | 38.7 | |
| Great white pelican | Pelecanus onocrotalus | 20 | 32 | 60 | |
| Pink-backed pelican | Pelecanus rufescens | 8 | 4 | 0 | |
| Great cormorant | Phalacrocorax carbo | 3 | 4 | 0 | |
| Phoenicopteriformes | Total | 93 | 122 | 86 | |
| Lesser flamingo | Phoeniconaias minor | 19 | 143 | 89 | |
| Chilean flamingo | Phoenicopterus chilensis | 5 | 256 | 80 | |
| American flamingo | Phoenicopterus ruber | 69 | 111 | 85.5 | |
| Piciformes | Total | 3 | 13 | 33.3 | |
| Toco toucan | Ramphastos toco | 1 | 4 | 0 | |
| Keel-billed toucan | Ramphastos sulfuratus | 1 | 128 | 100 | |
| Black-mandibled toucan | Ramphastos ambiguus | 1 | 4 | 0 | |
| Psittaciformes | Total | 177 | 15 | 42.9 | |
| Blue-fronted amazon | Amazona aestiva | 3 | 8 | 33.3 | |
| Orange-winged amazon | Amazona amazonica | 2 | 16 | 50 | |
| Yellow-shouldered amazon | Amazona barbadensis | 9 | 30 | 56 | |
| Festive amazon | Amazona festiva | 5 | 21 | 40 | |
| Yellow-crowned amazon | Amazona ochrocephala | 3 | 32 | 66.7 | |
| Red-spectacled amazon | Amazona pretrei | 3 | 10 | 0 | |
| Vinaceous amazon | Amazona vinacea | 3 | 10 | 0 | |
| Hyacinth macaw | Anodorhynchus hyacinthinus | 1 | 128 | 100 | |
| Great green macaw | Ara ambigua | 3 | 51 | 100 | |
| Blue-and-yellow macaw | Ara ararauna | 27 | 16 | 66.7 | |
| Red-and-green macaw | Ara chloroptera | 17 | 9 | 23.5 | |
| Scarlet macaw | Ara macao | 15 | 37 | 86.7 | |
| Military macaw | Ara militaris | 13 | 19 | 38.5 | |
| Red-fronted macaw | Ara rubrogenys | 13 | 16 | 53.8 | |
| Chestnut-fronted macaw | Ara severa | 4 | 8 | 25 | |
| Blue-crowned parakeet | Aratinga acuticaudata | 1 | 4 | 0 | |
| Finsch's parakeet | Aratinga finschi | 1 | 4 | 0 | |
| White cockatoo | Cacatua alba | 8 | 9 | 25 | |
| Sulfur-crested cockatoo | Cacatua galerita | 5 | 11 | 40 | |
| Goffins cockatoo | Cacatua goffini | 1 | 8 | 0 | |
| Salmon-crested cockatoo | Cacatua moluccensis | 1 | 32 | 100 | |
| Western corella | Cacatua pastinator | 8 | 4 | 0 | |
| Yellow-crested cockatoo | Cacatua sulphurea | 1 | 64 | 100 | |
| Eclectus parrot | Eclectus roratus | 7 | 16 | 57.1 | |
| Golden parakeet | Guarouba guarouba | 6 | 11 | 16.7 | |
| Scaly-headed parrot | Pionus maximilianii | 1 | 4 | 0 | |
| Pesquet's parrot | Psittrichas fulgidus | 1 | 4 | 0 | |
| African gray parrot | Psittacus erithacus | 15 | 13 | 40 | |
| Sphenisciformes | Total | 16 | 9 | 18.8 | |
| Humboldt penguin | Spheniscus humboldti | 5 | 21 | 60 | |
| African penguin | Spheniscus demersus | 11 | 6 | 0 | |
| Strigiformes | Total | 12 | 7 | 16.7 | |
| Little owl | Athene noctua | 2 | 11 | 50 | |
| Eurasian eagle owl | Bubo bubo | 7 | 7 | 14.3 | |
| Snowy owl | Bubo scandiacus | 2 | 4 | 0 | |
| Barn owl | Tyto alba | 1 | 4 | 0 | |
| Struthioniformes | Total | 33 | 11 | 30.3 | |
| Emu | Dromaius novaehollandiae | 9 | 7 | 22.2 | |
| Greater rhea | Rhea americana | 19 | 9 | 21.1 | |
| Ostrich | Struthio camelus | 5 | 37 | 80 | |
| All | 933 | 103 | 48.2 | ||
The geometric mean titers (GMT) and the percentages of birds with a postvaccination serum hemagglutination inhibition (HI) titer of ≥32 shown were measured 6 weeks after the second vaccination.
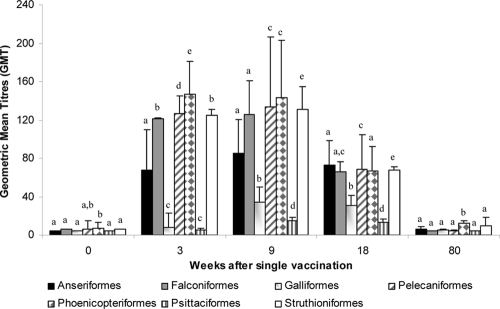
HI antibody titers 3 weeks after the first vaccination (at the time of the second vaccination) (n = 947 birds) and 9 (n = 933 birds) and 18 (n = 542 birds) weeks after the first dose were determined. After the first vaccine dose, the geometric mean titer (GMT) was 81, and 31.8% of birds reached a serum antibody titer of ≥32 against the H5 antigen. On average, after the booster vaccination, the GMT reached 103, and 51.4% had a titer of ≥32 against the H5 antigen. To evaluate longer-lasting immunity, titers 15 weeks after the second vaccination were studied. More than 45% of the birds were considered positive, and the overall GMT was 59. Of the 7 taxonomic orders for which more than 45 individuals were subjected to serological follow-up, 6 reached mean titers greater than 32 (Fig. 2). Falconiformes, Pelecaniformes, Phoenicopteriformes, and Struthioniformes presented HI titers over 120. In contrast, Psittaciformes and Galliformes showed the lowest GMT values. However, only Phoenicopteriformes reached prevalences over 75% of antibody titers at 32 or higher. Over 50% of birds belonging to the orders of Galliformes, Falconiformes, and Anseriformes reached a serum antibody titer of ≥32.
Fig. 2.
Humoral immune response following vaccination with an inactivated H5N9 vaccine (VP1). An inactivated H5N9 vaccine was used and administered twice within a 3-week interval. Bars represent the geometric mean titers (GMT) with standard errors (SE) of different taxonomic orders at different time points. The statistical significance of the difference (Mann-Whitney test) between taxonomic orders for each time point is indicated with a letter (P < 0.05).
Humoral response against H5N3 vaccination (VP2).
Detailed data concerning humoral immune response against an inactivated H5N3 vaccine from each order and species studied are provided in Table 2. Of 469 birds tested prior to VP2, 190 tested positive by the cELISA (40%). Most of the seropositive birds were from the following orders: Phoenicopteriformes (n = 74), Anseriformes (n = 51), Psittaciformes (n = 16), and Ciconiiformes (n = 15). However, only 26 out of 190 animals were not vaccinated in the previous vaccination program (VP1). By HI test, 279 out of 469 (60%) birds were seronegative for H5 AIV.
Table 2.
Humoral immune response of avian species in zoos vaccinated twice (within a 6-week interval) with an inactivated H5N3 vaccine (VP2)a
| Group | Order | Species |
No. of birds | GMT | % of birds with HI titers of ≥32 | |
|---|---|---|---|---|---|---|
| Common name | Scientific name | |||||
| Nonvaccinated in VP1 | Anseriformes | Total | 44 | 10 | 11 | |
| Anseriformes | Egyptian goose | Alopochen aegyptiacus | 4 | 4 | 0 | |
| Anseriformes | Mallard | Anas platyrhynchos | 12 | 10 | 0 | |
| Anseriformes | Greylag goose | Anser anser | 2 | 4 | 0 | |
| Anseriformes | Bar-headed goose | Anser indicus | 2 | 32 | 100 | |
| Anseriformes | Magpie goose | Anseranas semipalmata | 1 | 4 | 0 | |
| Anseriformes | Hawaiian goose | Branta sandvicensis | 5 | 16 | 20 | |
| Anseriformes | Cape Barren goose | Cereopsis novaehollandiae | 1 | 4 | 0 | |
| Anseriformes | Andean goose | Chloephaga picta | 4 | 11 | 0 | |
| Anseriformes | Black swan | Cygnus atratus | 6 | 16 | 0 | |
| Anseriformes | Mute swan | Cygnus olor | 1 | 16 | 0 | |
| Anseriformes | Fulvous whistling-duck | Dendrocygna bicolor | 1 | 64 | 100 | |
| Anseriformes | Rosybill | Netta peposaca | 5 | 7 | 20 | |
| Columbiformes | Total | 5 | 16 | 40 | ||
| Columbiformes | Common wood pigeon | Columba palumbus | 1 | 4 | 0 | |
| Columbiformes | Diamond dove | Geopelia cuneata | 2 | 64 | 100 | |
| Columbiformes | Barbary dove | Streptopelia turtur | 2 | 8 | 0 | |
| Coraciiformes | Total | 2 | 4 | 0 | ||
| Coraciiformes | White-crowned hornbill | Berenicornis comatus | 2 | 4 | 0 | |
| Falconiformes | Total | 4 | 49 | 75 | ||
| Falconiformes | Common buzzard | Buteo buteo | 2 | 108 | 100 | |
| Falconiformes | Griffon vulture | Gyps fulvus | 1 | 4 | 0 | |
| Falconiformes | Black kite | Milvus migrans | 1 | 128 | 100 | |
| Galliformes | Total | 11 | 187 | 100 | ||
| Galliformes | Red junglefowl | Gallus gallus | 5 | 56 | 100 | |
| Galliformes | Indian peafowl | Pavo cristatus | 6 | 512 | 100 | |
| Gruiformes | Total | 1 | 4 | 0 | ||
| Gruiformes | Demoiselle crane | Anthropoides virgo | 1 | 4 | 0 | |
| Pelecaniformes | Total | 4 | 152 | 75 | ||
| Pelecaniformes | Great white pelican | Pelecanus onocrotalus | 3 | 512 | 100 | |
| Pelecaniformes | Great cormorant | Phalacrocorax carbo | 1 | 4 | 0 | |
| Phoenicopteriformes | Total | 4 | 8 | 0 | ||
| Phoenicopteriformes | American flamingo | Phoenicopterus ruber | 4 | 8 | 0 | |
| Strigiformes | Total | 2 | 11 | 50 | ||
| Strigiformes | Barn owl | Tyto alba | 1 | 4 | 0 | |
| Strigiformes | Spectacled owl | Pulsatrix perspicillata | 1 | 32 | 100 | |
| Vaccinated in VP1 | Anseriformes | Total | 91 | 20 | 42 | |
| Anseriformes | White-cheeked pintail | Anas bahamensis | 1 | 4 | 0 | |
| Anseriformes | Chestnut teal | Anas castanea | 2 | 4 | 0 | |
| Anseriformes | Mallard | Anas platyrhynchos | 11 | 4 | 0 | |
| Anseriformes | Greylag goose | Anser anser | 3 | 40 | 33.3 | |
| Anseriformes | Emperor goose | Anser canagicus | 5 | 4 | 0 | |
| Anseriformes | Swan goose | Anser cygnoides | 5 | 9 | 20 | |
| Anseriformes | Barnacle goose | Branta leucopsis | 1 | 4 | 0 | |
| Anseriformes | Red-breasted goose | Branta ruficollis | 3 | 16 | 33.3 | |
| Anseriformes | Hawaiian goose | Branta sandvicensis | 1 | 16 | 0 | |
| Anseriformes | Cape Barren goose | Cereopsis novaehollandiae | 1 | 11 | 0 | |
| Anseriformes | Andean goose | Chloephaga picta | 6 | 4 | 0 | |
| Anseriformes | Ashy-headed goose | Chloephaga poliocephala | 2 | 4 | 0 | |
| Anseriformes | Ruddy-headed goose | Chloephaga rubidiceps | 7 | 4 | 0 | |
| Anseriformes | Black swan | Cygnus atratus | 6 | 102 | 83.3 | |
| Anseriformes | Black-necked swan | Cygnus melancoryphus | 3 | 6 | 0 | |
| Anseriformes | Rosybill | Netta peposaca | 6 | 323 | 100 | |
| Anseriformes | Red-crested pochard | Netta rufina | 2 | 181 | 100 | |
| Anseriformes | Ruddy shelduck | Tadorna ferruginea | 14 | 61 | 78.6 | |
| Anseriformes | Common shelduck | Tadorna tadorna | 12 | 85 | 83.3 | |
| Charadriiformes | Total | 4 | 13 | 50.0 | ||
| Charadriiformes | Caspian gull | Larus cachinnans | 4 | 13 | 50 | |
| Ciconiiformes | Total | 25 | 16 | 44 | ||
| Ciconiiformes | White stork | Ciconia ciconia | 3 | 4 | 0 | |
| Ciconiiformes | Glossy ibis | Plegadis falcinellus | 17 | 31 | 64.7 | |
| Ciconiiformes | African sacred ibis | Threskiornis aethiopicus | 5 | 4 | 0 | |
| Columbiformes | Total | 9 | 4 | 0 | ||
| Columbiformes | Common wood pigeon | Columba palumbus | 9 | 4 | 0 | |
| Coraciiformes | Total | 6 | 9 | 33.3 | ||
| Coraciiformes | Knobbed hornbill | Aceros cassidix | 2 | 4 | 0 | |
| Coraciiformes | Mindanao wrinkled hornbill | Aceros leucocephalus | 2 | 4 | 0 | |
| Coraciiformes | Black hornbill | Anthracoceros malayanus | 2 | 45 | 100 | |
| Falconiformes | Total | 7 | 9 | 28.6 | ||
| Falconiformes | Turkey vulture | Cathartes aura | 2 | 64 | 100 | |
| Falconiformes | Himalayan vulture | Gyps himalayensis | 1 | 4 | 0 | |
| Falconiformes | Bald eagle | Haliaeetus leucocephalus | 1 | 4 | 0 | |
| Falconiformes | Hooded vulture | Necrosyrtes monachus | 1 | 4 | 0 | |
| Falconiformes | Harris's hawk | Parabuteo unicinctus | 2 | 4 | 0 | |
| Galliformes | Total | 22 | 437 | 95.5 | ||
| Galliformes | Indian peafowl | Pavo cristatus | 22 | 437 | 95.5 | |
| Gruiformes | Total | 6 | 9 | 33.3 | ||
| Gruiformes | Blue crane | Anthropoides paradisea | 3 | 4 | 4 | |
| Gruiformes | Demoiselle crane | Anthropoides virgo | 2 | 45 | 100 | |
| Gruiformes | Gray crowned crane | Balearica regulorum | 1 | 4 | 0 | |
| Passeriformes | Total | 1 | 4 | 0 | ||
| Passeriformes | European greenfinch | Carduelis chloris | 1 | 4 | 0 | |
| Pelecaniformes | Total | 8 | 4 | 0 | ||
| Pelecaniformes | Pink-backed pelican | Pelecanus rufescens | 8 | 4 | 0 | |
| Phoenicopteriformes | Total | 91 | 18 | 29.7 | ||
| Phoenicopteriformes | Lesser flamingo | Phoeniconaias minor | 31 | 4 | 0 | |
| Phoenicopteriformes | Chilean flamingo | Phoenicopterus chilensis | 9 | 276 | 100 | |
| Phoenicopteriformes | American flamingo | Phoenicopterus ruber | 51 | 27 | 35.3 | |
| Psittaciformes | Total | 7 | 58 | 100 | ||
| Psittaciformes | Red-and-green macaw | Ara chloroptera | 1 | 32 | 100 | |
| Psittaciformes | Military macaw | Ara militaris | 3 | 32 | 100 | |
| Psittaciformes | Eclectus parrot | Eclectus roratus | 3 | 128 | 100 | |
| Sphenisciformes | Total | 16 | 10 | 0 | ||
| Sphenisciformes | African penguin | Spheniscus demersus | 4 | 4 | 0 | |
| Sphenisciformes | Humboldt penguin | Spheniscus humboldti | 12 | 14 | 0 | |
| Strigiformes | Total | 3 | 4 | 0 | ||
| Strigiformes | Eurasian eagle owl | Bubo bubo | 2 | 4 | 0 | |
| Strigiformes | Snowy owl | Nyctea scandiaca | 1 | 4 | 0 | |
| Struthioniformes | Total | 3 | 128 | 33.3 | ||
| Struthioniformes | Emu | Dromaius novaehollandiae | 1 | 16 | 0 | |
| Struthioniformes | Greater rhea | Rhea americana | 2 | 362 | 100 | |
| Total nonvaccinated in VP1 | 77 | 19 | 23.4 | |||
| Total vaccinated in VP1 | 299 | 16 | 38.5 | |||
| All | 376 | 18 | 33.2 | |||
The geometric mean titers (GMT) and the percentages of birds with a postvaccination serum hemagglutination inhibition (HI) titer of ≥32 shown were measured 6 weeks after the second vaccination. Animals are grouped into two groups: the nonvaccinated in VP1 and the ones that were vaccinated in VP1.
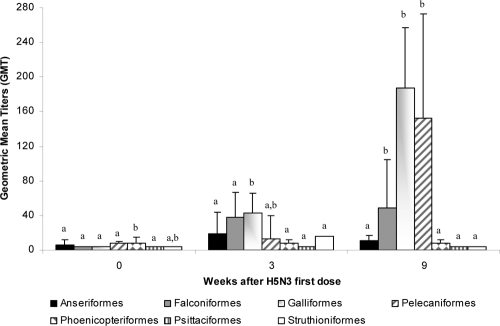
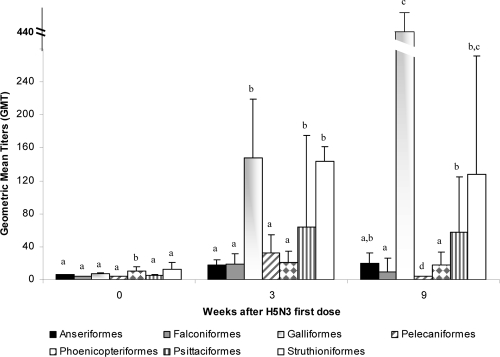
In VP2, antibody titers at 6 (n = 398 samples) and 12 (n = 376 samples) weeks postvaccination were studied. In both cases, the number of seropositive animals was around 40%, and the overall GMTs were different between those animals vaccinated in the previous vaccination program (VP1 with H5N9) and those vaccinated for the first time with H5N3 (Fig. 3 and 4). Six weeks after the second dose of the H5N3 vaccine, Galliformes and Pelecaniformes orders (that were included in the VP2 with only the H5N3 vaccine) manifested a GMT higher than 150 (Fig. 3). The Falconiformes order showed a weaker response, with a GMT of 50. The other birds that had not been vaccinated previously had a GMT of less than 32. Among animals vaccinated in VP1, Galliformes showed a very high response (GMT = 437) 12 weeks after receiving the H5N3 vaccine. The Psittaciformes and Struthioniformes orders reached seropositivity with a GMT of 58 and 128, respectively (Fig. 4).
Fig. 3.
Humoral immune response following vaccination with an inactivated H5N9 vaccine (VP1). An inactivated H5N9 vaccine was used and administered twice within a 3-week interval. Bars represent the geometric mean titers (GMT) with standard errors (SE) of different taxonomic orders. The statistical significance of the difference (Mann-Whitney test) between taxonomic orders for each time point is indicated with a letter (P < 0.05).
Fig. 4.
Humoral immune response in birds vaccinated with an inactivated H5N3 vaccine (VP2) and vaccinated previously with an inactivated H5N9 vaccine in VP1. An inactivated H5N3 vaccine was used and administered once. Bars represent the geometric mean titers (GMT) with standard errors (SE) of different taxonomic orders. The statistical significance of the difference (Mann-Whitney test) between taxonomic orders for each time point is indicated with a letter (P < 0.05).
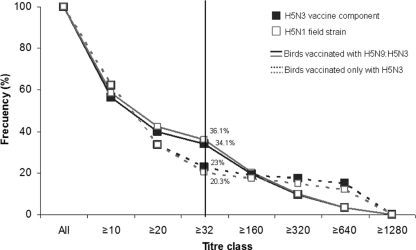
After H5N3 vaccination, 338 birds were evaluated for the presence of serum antibody titers against an HPAI H5N1 strain circulating in Europe (A/Mallard/It/3401/05) and for the presence of A/Tky/Eng/647/77 (H7N7)-specific antibodies. The response obtained against H5N1 was compared to those elicited against the H5N3 vaccine component. Moreover, two groups were differentiated between those being H5N9 and H5N3 vaccinated and those receiving only the H5N3 vaccine. The frequencies of birds reaching a seroprotective titer (≥32) are similar when testing antibody titers against H5N1 as well as for the vaccine compound in both the studied groups (Fig. 5). No immune response against the H7N7 strain was detected in any of the studied animals.
Fig. 5.
Comparison of serum hemagglutination inhibition (HI) antibody titers against the H5N3 vaccine and H5N1 field virus following vaccination with either a single vaccine (H5N3) or two successive heterologous vaccines (H5N9 and H5N3). HI titers against the vaccine component (A/ck/VN/C58/04; H5N3) and the field strain (A/Mallard/It/3401/05; H5N1) were determined in 338 birds 12 weeks after starting VP2.
Virus detection.
No AIV antigen was detected in collected cloacal swabs in VP1. Prior to VP2, two animals that were RT-PCR positive were probably exposed to AI virus during this time interval. Both animals were from the Phoenicopteriformes order.
DISCUSSION
In the present work, we demonstrate that carrying out two vaccination programs with successive heterologous vaccines in wild animals from Spanish zoos can be the key to widely protect species from taxonomic orders which did not develop HI antibody to a unique vaccine. In 2005, when the European Commission directive 2005/744/EC allowed vaccination against avian influenza (AI) in zoos (7), other European countries also embarked on the mass vaccination program in zoo birds. Lately, results from some of the zoos, judging the efficacy of different vaccine formulations used, have been reported (1, 15, 16). Comparison of different vaccine formulations in eliciting a strong humoral response could be instrumental to decide future vaccination programs against AI virus.
In 2006, both Spain (data from present study, VP1) and Denmark (1) used inactivated H5N9 vaccines from different manufacturers in their vaccination programs in zoo birds. We observed that 51.4% of the H5N9-vaccinated birds in Spanish zoos had an HI titer of ≥32 after booster vaccination, with an overall GMT of 103. The present data were comparatively lower than those previously reported by Bertelsen et al. (1), also using the H5N9 vaccine, where 76% of the zoo birds developed a titer of 32 with a GMT of 137. The differences in seroprotection efficacy between our results and those reported by Bertelsen et al. (1) may be due to different amounts of antigen or adjuvants used in the vaccine preparation, since the inactivated H5N9 vaccine studied by the Danish group was derived from a different manufacturer. Moreover, it should be noted that the present work is comprised of a large number of exotic birds (n = 933 after booster vaccination) from various orders, which may influence the amount of the overall GMT. This fact may also explain the heterogeneity in the antibody responses that we observed in serological analysis in vaccinated birds, which varied greatly, not only between taxonomic orders but also between species of a single order and even within species. Similar observations with an inactivated H7N1 vaccine were published by Philippa et al. (15), who described a high seroprotection rate of 81.5% and an overall GMT of 190, with variations in HI titers among different bird orders examined. In general, based on the serological analysis from a huge number of H5N9-vaccinated Spanish zoo birds, we observed that more than 75% of birds from Phoenicopteriformes manifested a GMT of ≥32, and from the other 15 orders studied after booster vaccination, 12 had a protection rate less than 50%.
For the second vaccination program (VP2), the Spanish Ministry replaced the H5N9 vaccine with an H5N3 recombinant vaccine. The decision was based on the results given by the manufacturer, showing that H5N3 (a reverse genetics vaccine), besides protecting chickens (10) and ducks (12) from experimental AI infection, also prevented viral shedding. Masking disease signs while the bird continues to shed viruses may be a serious problem both for valuable exotic birds and humans. Thus, limiting virus shedding and further transmission is of extreme importance.
Vaccination with inactivated recombinant H5N3 vaccine was equally effective as VP1 in eliciting high titers of HI antibodies against H5 among most of the bird orders studied, except for birds belonging to Psittaciformes, which did not develop HI antibodies to either vaccination protocol. Interestingly, however, priming with H5N9 and subsequently boosting with the H5N3 vaccine induced a significant antibody response in Psittaciformes birds, albeit at lower titers than the others. Similarly, Galliformes and Struthioniformes birds responded to the H5N3 vaccine with much higher HI titers after booster vaccination. This strategy (prime-boost) could be used in some of the orders or species which do not respond to a unique vaccine. However, we also have to carefully pay attention to the antibody titer length. As shown in Fig. 2, GMT after 18 months decreased drastically. Thus, some of the orders receiving H5N3 vaccine only once, because they were previously vaccinated with H5N9 (Fig. 4), did not show high titers. Philippa et al. (16), based on previous reports, have pointed to the need of a revaccination between 6 to 10 months after vaccination to maintain seroprotective titers among different wild species in zoos. This was similar to the results we obtained in VP1 18 months after the single vaccination, where seroprotection titers started to decrease. The effect of a booster vaccination is seen clearly in VP2, in those animals nonvaccinated previously in VP1 (Fig. 3), especially for the orders of Galliformes and Pelecaniformes, where GMT increased four times. These results are similar to those obtained by Philippa et al. (16), after booster vaccination increased the GMT by 30% (from 50.5% after single vaccination to 80.5% after booster vaccination) (16).
To design future vaccination strategies in exotic wild birds, it is important to evaluate both the response against the vaccine and the durability of HI antibodies. Sera 80 weeks after a single H5N9 dose were analyzed. On average, the birds had titers less than 20, meaning that 1.5 years after vaccination, we cannot detect HI titers in serum samples.
Antibody titers against HPAI H5N1 showed a similar trend as those against the homologous strain, with 34.1% of birds developing a titer of ≥32 (animals vaccinated with successive vaccines, H5N9 and H5N3) and 20.3% of the animals receiving only the H5N3 vaccine showing seroprotective titers. However, both groups showed lower titers than the results reported by Philippa et al. (16), where 61.2% of the birds had a titer of ≥40 against the HPAI strain tested, and more than 80% had a seroprotective titer against the homologous strain.
Taking into account that inactivated H5N3 vaccine induces strong immune responses and, more importantly, limits viral shedding (sterile immunity), a prime (H5N9)-boost (H5N3) vaccine strategy in future vaccination programs within exotic valuable zoo birds and in particular in the Psittaciformes, Galliformes, and Struthioniformes orders would be more adequate and advisable. Together with increased biosecurity measures and monitoring, vaccination may represent the best alternative to protect valuable and/or endangered bird species against HPAI virus infection. However, variations in elicited antibody responses among different bird orders and species must be carefully scrutinized in designing future vaccination programs. This will not only protect vaccinated birds from infection but also restrict further dissemination of otherwise devastating HPAI virus.
ACKNOWLEDGMENTS
This work was partially supported by the AGL2007-60434/GAN project funded by the Spanish Government and by the EUROFLU project (SP5B-CT-2007-044098) funded by the European Union.
We are grateful to staff at participating zoos for their collaboration and kind help in data compilation, including Rocío Canales Merino (Safari Park Vergel), Loles Carbonell (Jardín Zoológico de Valencia), Sergio Fernández Hernández (Selwo Marina and Selwo Aventura), Daniel García Párraga (L'Oceanogràfic), Candelaria González Villavicencio (Águilas Jungle Park), Ayose Melián Melián (Palmitos Park), Tania Monreal Pawlowsky (Marineland Mallorca), Miguel Angel Quevedo Muñoz (Zoo Botánico Jerez), José María Rodríguez Linde (Oasys Parque del Desierto de Tabernas), and Fernanda Valdés García (Senda del Retiro), as well as staff at Faunia, Zoo Aquarium de Madrid, Zoo de Fuengirola, and Parc Zoològic de Barcelona.
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Bertelsen M. F., Klausen J., Holm E., Grøndahl C., Jørgensen P. H. 2007. Serological response to vaccination against avian influenza in zoo-birds using an inactivated H5N9 vaccine. Vaccine 25:4345–4349 [DOI] [PubMed] [Google Scholar]
- 2. Capua I., Alexander D. J. 2006. The challenge of avian influenza to the veterinary community. Avian Pathol. 35:189–205 [DOI] [PubMed] [Google Scholar]
- 3. Chen H., et al. 2006. Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China. J. Virol. 80:5976–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Hoyo J., Elliot A., Sargata J. 2005. Handbook of the birds of the world. Lynx Editions, Barcelona, Spain [Google Scholar]
- 5. EFSA 2007. Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the commission related with the vaccination against avian influenza of H5 and H7 subtypes as a preventive measure carried out in member states in birds kept in zoos under community approved programmes. EFSA-Q.-2006-156 European Food Safety Authority, Parma, Italy [Google Scholar]
- 6. Ellis T. M., et al. 2004. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 33:492–505 [DOI] [PubMed] [Google Scholar]
- 7. European Commission 2005. Requirements for the prevention of highly pathogenic avian influenza caused by influenza A virus of subtype H5N1 in susceptible birds kept in zoos in the member states. Commission decision 2005/744/EC. Off. J. Eur. Comm. L279:75–78 [Google Scholar]
- 8. European Commission 1992. Community measures for the control of avian influenza. Council directive 92/40/EEC. Off. J. Eur. Comm. L167:1–15 [Google Scholar]
- 9. Fouchier R. A., et al. 2005. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 79:2814–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar M., Chu H. J., Rodenberg J., Krauss S., Webster R. G. 2007. Association of serologic and protective responses of avian influenza vaccines in chickens. Avian Dis. 51(Suppl. 1):481–483 [DOI] [PubMed] [Google Scholar]
- 11. Lin Y. P., et al. 2000. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc. Natl. Acad. Sci. U. S. A. 97:9654–9658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Middleton D., et al. 2007. Efficacy of inactivated vaccines against H5N1 avian influenza infection in ducks. Virology 359:66–71 [DOI] [PubMed] [Google Scholar]
- 13. Munster V. J., et al. 2006. Towards improved influenza A virus surveillance in migrating birds. Vaccine 24:6729–6733 [DOI] [PubMed] [Google Scholar]
- 14. Palmer D. F., Dowdle W. R., Coleman M. T., Schild G. C. 1975. Haemagglutination inhibition test. Advanced laboratory techniques for influenza diagnosis: procedural guide, p. 25–62 U.S. Department of Health, Education and Welfare, Atlanta, GA [Google Scholar]
- 15. Philippa J. D., et al. 2005. Highly pathogenic avian influenza (H7N7): vaccination of zoo birds and transmission to non-poultry species. Vaccine 23:5743–5750 [DOI] [PubMed] [Google Scholar]
- 16. Philippa J., et al. 2007. Vaccination against highly pathogenic avian influenza H5N1 virus in zoos using an adjuvanted inactivated H5N2 vaccine. Vaccine 25:3800–3808 [DOI] [PubMed] [Google Scholar]
- 17. van der Goot J. A., Koch G., de Jong M. C. M., van Boven M. 2005. Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proc. Natl. Acad. Sci. U. S. A. 102:18141–18146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu X., Subbarao, Cox N. J., Guo Y. 1999. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261:15–19 [DOI] [PubMed] [Google Scholar]