Abstract
The influence of AS03A, a tocopherol oil-in-water emulsion-based adjuvant system, on humoral and cell-mediated responses to A/California/7/2009 H1N1 pandemic vaccine was investigated. In two observer-blind studies, a total of 261 healthy adults aged 18 to 60 years were randomized to receive either AS03A-adjuvanted H1N1 vaccine containing 3.75 μg hemagglutinin (HA) or nonadjuvanted H1N1 vaccine containing 15 or 3.75 μg HA on days 0 and 21. Hemagglutination inhibition (HI) antibody and T-cell responses were analyzed up to day 42. A first dose of AS03A-adjuvanted vaccine (3.75 μg HA) or nonadjuvanted vaccine (15 μg HA) induced HI responses of similar magnitudes that exceeded licensure criteria (e.g., 94 to 100% with titers of ≥40). A lower response following 3.75 μg HA without adjuvant was observed (73% with titers of ≥40). Following a second dose, geometric mean HI titers at day 42 were higher for AS03A-adjuvanted vaccine (636 and 637) relative to nonadjuvanted vaccine (341 for 15 μg HA and 150 for 3.75 μg HA). Over the 42-day period, the increase in frequency of A/H1N1/2009-specific CD4+ T cells was significantly higher in the adjuvanted group than in the nonadjuvanted group. There was no evidence of correlation between baseline CD4+ T-cell frequencies and day 21 HI antibody titers, while there was some correlation (R = 0.35) between day 21 CD4+ T-cell frequencies and day 42 HI titers. AS03A adjuvant enhanced the humoral and CD4+ T-cell-mediated responses to A/H1N1/2009 vaccine. Baseline A/H1N1/2009-specific CD4+ T-cell frequencies did not predict post-dose 1 antibody responses, but there was some correlation between post-dose 1 CD4+ T-cell frequencies and post-dose 2 antibody responses.
INTRODUCTION
Influenza is a highly contagious infectious disease resulting in acute respiratory illness in all age groups. Influenza vaccination has been employed for many years as a primary tool to prevent influenza virus infection and its complications during the annual seasonal epidemics that occur worldwide. More recently, influenza vaccines have been deployed against the pandemic A/H1N1/2009 influenza virus (7, 16, 29, 30) and have also been developed against the highly pathogenic avian influenza A H5N1 virus, which remains a potential pandemic candidate (1, 3, 22, 24, 36).
Advances in adjuvant technology are being applied to several vaccines under development or already developed in order to enhance immunogenicity (12, 18, 37) and, thereby, potentially improve vaccine efficacy. In the case of influenza vaccines, adjuvant is included to address three challenges. The first is the lower response to influenza vaccines observed in elderly subjects relative to younger adults, which is due to the decline in immune function with increasing age (14, 26, 27, 39). This is particularly an issue for seasonal influenza, which disproportionally affects the elderly (34, 35). The second is the rate-limiting nature of influenza antigen production, which is primarily an issue for pandemic influenza when large supplies of vaccine are required in a short time frame. If immunogenicity can be significantly improved by the use of an adjuvant system, then less antigen is required and more vaccine doses can be produced. The third challenge is antigenic drift leading to suboptimal vaccine protection when the vaccine antigen (strain) differs too much from the influenza virus strain that causes the seasonal or pandemic outbreak (4).
The tocopherol oil-in-water emulsion-based adjuvant system AS03 has been successfully employed to enhance the humoral immunogenicity of H5N1 vaccines (22). In addition to antigen sparing, AS03 also promoted cross-immunity against drifted H5N1 strains (21, 22) and induced protection against heterologous lethal H5N1 challenge in ferrets (2).
In this report, we investigate in more depth the influence of AS03 on the immunogenicity of influenza vaccines by evaluating cell-mediated as well as humoral responses to A/California/7/2009 H1N1 pandemic vaccines.
MATERIALS AND METHODS
Study design and participants.
This report presents data from two separate, randomized (1:1), observer-blind phase III studies (designated A and B), each with two parallel groups vaccinated with AS03A-adjuvanted or nonadjuvanted A/H1N1/2009 vaccine, both conducted in one center in Belgium between September and December 2009. The primary objective of study A was to demonstrate that vaccination with two doses of AS03A-adjuvanted A/H1N1/2009 vaccine results in a hemagglutination inhibition (HI) immune response that meets or exceeds European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) criteria (9). The primary objective of study B was to assess the HI immune response to A/H1N1/2009 vaccine with and without AS03A in terms of CHMP criteria. In both studies, assessment of safety was a secondary objective. Exploratory objectives were assessment of cell-mediated immune (CMI) responses and assessment of HI immune responses stratified by previous seasonal vaccination.
In both studies, eligible participants were clinically healthy adults between 18 and 60 years of age at the time of vaccination who provided written informed consent. Subjects with a clinical history suggestive of an influenza infection within 6 months preceding the study start were excluded. Randomization was performed by the sponsor. At the time of vaccination, the responsible on-site personnel accessed the internet randomization system that used a minimization procedure accounting for age (between 18 and 40 years inclusive, above 41 to 50 years inclusive, and above 51 to 60 years inclusive at a ratio or 2:1:1).
The protocol and other relevant study documentation were approved by the appropriate Ethics Committee, and the study was conducted in accordance with good clinical practice and all applicable regulatory requirements, including the Declaration of Helsinki.
Study vaccines.
The monovalent influenza virus A/H1N1/2009 inactivated, split-virion vaccine was manufactured by GlaxoSmithKline (GSK) Biologicals from a vaccine seed virus prepared from the NYMC X-179A strain as recommended by the World Health Organization (WHO) (40). The reassortant virus NYMC X-179A was prepared by New York Medical College, New York, by a classical reassortant methodology from the A/California/7/2009 H1N1 virus and the NYMC X-157A virus (H3N2, hy A/NY/55/2004 × A/PR/8/34 [6:2]). The hemagglutinin (HA), NA, and PB1 genes were donated by A/California/7/2009 H1N1, and the other internal genes (coding for PB2, PA, NP, M, and NS) were donated by NYMC X-157A.
The seed virus was propagated on embryonated hen eggs, and the vaccine was produced by using the licensed manufacturing process for Pandemrix (a trademark of the GlaxoSmithKline group of companies). AS03A, a tocopherol oil-in-water emulsion-based adjuvant system, was manufactured by GSK Biologicals. Each vaccine was administered at a 0.5-ml injection volume. The AS03A-adjuvanted vaccine contained 3.75 μg hemagglutinin (HA) and 11.86 mg tocopherol, while the nonadjuvanted vaccine was formulated to contain either 15 μg HA (study A) or 3.75 μg HA (study B).
Procedures.
As the appearances of the AS03A-adjuvanted and nonadjuvanted vaccines were different, both studies were observer blind, with vaccinations performed by specific study personnel not involved in the assessment of safety or immunogenicity. Vaccine was administered on days 0 and 21. Blood samples (one for the assessment of antibody responses and one for the assessment of CMI responses) were collected prior to vaccination (day 0) and following dose 1 (day 21) and dose 2 (day 42). Peripheral blood mononuclear cells (PBMCs) for the assessment of CMI responses were isolated by Ficoll-Hypaque (Lymphoprep; Life Technologies, Carlsbad, CA) density centrifugation following standard procedures. The cells were washed twice in Hanks buffered salt solution and cryopreserved in liquid nitrogen until use.
Safety and reactogenicity were evaluated as described previously (30).
Assessment of the humoral immune response.
Serum samples were tested in a validated HI microtiter assay using chicken erythrocytes, as previously described (19), with the A/California/7/2009 vaccine strain used as an antigen.
Assessment of T-cell response.
An adaptation of the method described by Maecker et al. (25) was used in which T cells are restimulated ex vivo by incubation with antigen in the presence of costimulatory antibodies to CD28 and CD49d (38) and brefeldin A to inhibit cytokine secretion and allow intracellular accumulation. The cells were then stained with fluorochrome-conjugated antibodies. CD40L (also known as CD154), a well-documented (5, 11) and potentially more specific activation marker (11, 20), was used instead of CD69 (21).
Ex vivo stimuli.
We used two ex vivo stimuli: (i) A/California/7/2009 H1N1 split antigen containing essentially the two major viral surface glycoproteins H1 and N1 and (ii) an A/California/7/2009 H1 hemagglutinin peptide pool (consisting of 20-mer peptides covering the whole H1 hemagglutinin protein sequence with 15-amino-acid overlaps to ensure all T-cell epitopes are represented) to specifically monitor the T-cell response to H1 antigen. The peptides were synthesized by Eurogentech, Belgium, and shown to have >85% purity by high-performance liquid chromatography (HPLC). Lyophilized peptides were reconstituted in phosphate-buffered saline (PBS)-dimethyl sulfoxide (DMSO) (<0.1% final concentration).
Antibodies.
Antibodies used for cell costimulation were unconjugated and azide-free anti-CD28 and anti-CD49d. Conjugated antibodies used for staining were anti-CD4–peridinin chlorophyll a protein (PerCP), anti-CD8–allophycocyanin–H7 (APC-H7), anti-gamma interferon (IFN-γ)–fluorescein isothiocyanate (FITC), anti-interleukin-2 (IL-2)–allophycocyanin (APC), anti-tumor necrosis factor alpha (TNF-α)–phycoerythrin (PE)–cyanin 7 (PE-Cy7), and anti-CD40L–phycoerythrin (PE) (all BD Pharmingen, San Diego, CA).
Cell stimulation and staining.
Purified PBMCs were thawed, washed twice in culture medium (RPMI 1640; Cambrex, East Rutherford, NJ) supplemented with 10% heat-inactivated fetal calf serum (FCS) (PAA Laboratories GmbH, Austria), 10,000 IU/ml penicillin, 100 μg streptomycin sulfate, 200 mM l-glutamine, 100× minimal essential medium (MEM) with nonessential amino acids, 100 mM sodium pyruvate, and 50 mM 2-mercaptoethanol (all from Life Technologies, Belgium); examined for viability (propidium iodide exclusion using flow cytometry) and counted (Sysmex); washed again; and resuspended to 2 × 107 cells/ml in culture medium. PBMCs (106 cells per well) were incubated in 96-well microtiter plates with costimulatory anti-human CD28 and CD49d antibodies (1/250 dilution each) and stimulated for 20 h at 37°C with either H1N1 split antigen from the A/California/7/2009 strain (final concentration, 1 μg/ml HA) or the H1 peptide pool (final concentration, 1.25 μg/ml of each peptide). Brefeldin A (final concentration, 1 μg/ml; BD Pharmingen) was added for the last 18 h of culture. An unstimulated control (no antigen) and positive control (Staphylococcus enterotoxin B, 1 μg/ml; Sigma-Aldrich, St. Louis, MO) were included in each assay. Following incubation, the cells were washed (PBS containing 1% FCS) and stained with anti-CD4-PerCP and anti-CD8-APC-H7. The cells were then washed again, fixed, and permeabilized with the Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer's instructions and stained with anti-IFN-γ–FITC, anti-IL-2–APC, anti-TNF-α–PE-Cy7, and anti-CD40L–PE. Following washing (Perm/Wash buffer; BD Pharmingen), the cells were analyzed by flow cytometry. Antigen-specific CD4 or CD8 T cells were identified as CD4+ or CD8+ T cells expressing two or more immune markers after stimulation.
Flow cytometry and data analysis.
Cells were analyzed on a FACSCanto flow cytometer (Becton Dickinson, San Jose, CA) using 6-color panels. Data were analyzed with FACSDiva software. Results were expressed as frequencies of CD4+ or CD8+ T cells responding to the antigen and expressing two or more immune markers (among CD40L, IFN-γ, IL-2, and TNF-α) per million total CD4+ or CD8+ T cells. Background (unstimulated control) was subtracted from all values. The remaining positive events were regarded as significant. Samples were only included for analysis if viability was 80% or more.
Statistical analysis.
As planned in the protocol, a final descriptive analysis has been performed for both studies on the humoral immune response and reactogenicity/safety data generated up to day 42. For study A, an additional statistical analysis has been performed on the CMI responses. Humoral and CMI responses are presented for the per-protocol immunogenicity cohort and safety data for the intent-to-treat (total vaccinated) cohort.
The humoral immune response was analyzed by the standard HI test endpoints with 95% confidence intervals (CIs) used by the EMA CHMP for evaluation of influenza vaccines (9). The sample size was calculated on the basis of fulfillment of CHMP criteria for the HI endpoints (9) following two doses of AS03A-adjuvanted vaccine. A sample size of 64 subjects per group was estimated to give a power of at least 97%, assuming 88% for seroconversion and seroprotection rates and an increase of 5.6 to 163 for the geometric mean titer (GMT).
The cellular immune responses were expressed in terms of H1N1 split antigen- or H1 HA (peptide pool)-specific CD4+ T-cell frequency (geometric mean) per million total CD4+ T cells and CD8+ T-cell frequency (geometric mean) per million total CD8+ T cells.
Two post hoc analyses were performed on both humoral and CMI response data. The first analysis was done to assess the benefit of the adjuvant on the CMI response. An analysis of variance model for repeated measures, including age as covariates, and gender, time, treatment, and time by treatment as fixed effects was fitted on the log-transformed frequencies of H1N1- or H1-specific CD4+ T cells identified as expressing two or more immune markers upon in vitro stimulation. The correlation structure of the residuals was selected unstructured, and the interactions including age and gender were assessed but not kept since not significant. Based on this model, geometric means (GMs) with their 95% CIs were reported by time and treatment. The between-treatment ratios of the postvaccination over prevaccination GM ratios were also computed with their 95% CIs. All computations were performed with SAS 9.1.3.
The objective of the second post hoc analysis was to assess the relationship between HI postvaccination response and preexisting cellular immunity. The correlations (i) between individual HI antibody levels on day 21 and H1N1- or H1-specific CD4+ T-cell frequencies observed at baseline and (ii) between HI antibody levels on day 42 and H1N1- or H1-specific CD4+ T-cell frequencies at day 21 were estimated by computing Spearman partial correlation coefficients adjusted by age, sex, history of vaccination, and prevaccination status.
The safety endpoints (percentage of subjects and 95% CIs) were solicited adverse events and spontaneously reported adverse events. All serious adverse events occurring up to day 42 were described.
Both trials have been registered with ClinicalTrials.gov under no. NCT00968539 and NCT00989287.
RESULTS
Study populations.
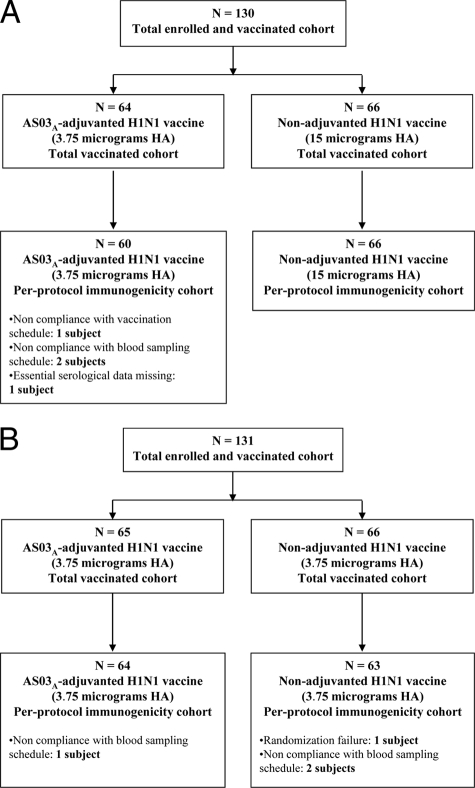
In study A, 130 subjects were enrolled and vaccinated with either 3.75 μg HA of AS03A-adjuvanted A/H1N1/2009 vaccine (n = 64) or 15 μg HA of nonadjuvanted A/H1N1/2009 vaccine (n = 66), and 129 subjects completed the study. In study B, 131 subjects were enrolled and vaccinated with either 3.75 μg HA of AS03A-adjuvanted A/H1N1/2009 vaccine (n = 65) or 3.75 μg HA of nonadjuvanted A/H1N1/2009 vaccine (n = 66), and all completed the study. Figures 1 A and B show the exclusions from immunogenicity assessment. Demographic profiles for each study are shown in Table 1. The percentages of subjects who received seasonal influenza vaccination at least once during the preceding 2 years were 57.8% in the AS03A-adjuvanted group and 40.9% in the nonadjuvanted group in study A and 32.3% in the AS03A-adjuvanted group and 30.3% in the nonadjuvanted group in study B.
Fig. 1.
(A) Participant flowchart for study A; (B) participant flowchart for study B.
Table 1.
Summary of demographic characteristics per protocol cohorts for immunogenicity
| Parameter | Result for: |
|||
|---|---|---|---|---|
| Study A |
Study B |
|||
| AS03A-adjuvanted H1N1 vaccine (3.75 μg HA) | Nonadjuvanted H1N1 vaccine (15 μg HA) | AS03A-adjuvanted H1N1 vaccine (3.75 μg HA) | Nonadjuvanted H1N1 vaccine (3.75 μg HA) | |
| Age at vaccination (yr) | ||||
| Mean (SD) | 40.1 (13.40) | 38.2 (14.10) | 37.2 (14.27) | 38.7 (13.70) |
| Median (range) | 44.5 (19–60) | 40.5 (18–60) | 41.0 (18–60) | 41.0 (20–60) |
| Gender (%) | ||||
| Female | 65.0 | 59.1 | 65.6 | 54.0 |
| Male | 35.0 | 40.9 | 34.4 | 46.0 |
| Geographic ancestry (%) | ||||
| Asian-East Asian | 0.0 | 1.5 | 0.0 | 1.6 |
| White-Caucasian/European | 100 | 98.5 | 100 | 98.4 |
Humoral immune response.
HI data are presented in Table 2. Although GMT values were low prior to vaccination (day 0), antibodies against the vaccine strain were detected (HI titers of ≥1:10; data not shown) in 38.3% and 42.4% of subjects in the two study A groups and 45.3% and 31.7% of subjects in the two study B groups. Overall, 15.1% of subjects in study A and 10.2% of subjects in study B had putatively protective HI titers of ≥1:40 prior to vaccination. When stratified by age, the percentages of subjects with HI titers of ≥1:40 on day 0 in studies A and B, respectively, were 16.4% and 19.0% for 18 to 40 years, 18.8% and 3.1% for 41 to 50 years, and 9.1% and 0% for 51 to 60 years.
Table 2.
Immune response by hemagglutination inhibition antibodies against A/California/7/2009 H1N1 per protocol cohorts for immunogenicity
| Parametera | Result forb: |
|||
|---|---|---|---|---|
| Study A |
Study B |
|||
| AS03A-adjuvanted H1N1 vaccine (3.75 μg HA) (n = 59 or 60)c | Nonadjuvanted H1N1 vaccine (15 μg HA) (n = 66) | AS03A-adjuvanted H1N1 vaccine (3.75 μg HA) (n = 64) | Nonadjuvanted H1N1 vaccine (3.75 μg HA) (n = 63) | |
| Prevaccination, day 0 | ||||
| GMT (95% CI) | 8.8 (7.0–11.1) | 10.8 (8.1–14.4) | 9.6 (7.6–12.0) | 8.4 (6.7–10.6) |
| Seroprotection rate (titer, ≥1:40); % (95% CI) | 11.7 (4.8–22.6) | 18.2 (9.8–29.6) | 10.9 (4.5–21.2) | 9.5 (3.6–19.6) |
| Post-dose 1, day 21 | ||||
| GMT (95% CI) | 335.2 (250.1–449.2) | 310.2 (218.8–439.7) | 424.0 (312.4–575.5) | 96.4 (64.0–145.3) |
| Geometric mean fold rise (95% CI) | 38.1 (28.6–50.7) | 28.7 (20.0–41.2) | 44.4 (33.6–58.7) | 11.4 (8.1–16.3) |
| Seroprotection rate (titer, ≥1:40); % (95% CI) | 100 (94.0–100) | 93.9 (85.2–98.3) | 93.8 (84.8–98.3) | 73.0 (60.3–83.4) |
| Seroconversion rate; % (95% CI) | 98.3 (91.1–100) | 84.8 (73.9–92.5) | 93.8 (84.8–98.3) | 69.8 (57.0–80.8) |
| Post-dose 2, day 42 | ||||
| GMT (95% CI) | 636.3 (520.9–777.3) | 341.0 (259.9–447.3) | 686.7 (567.0–831.7) | 149.7 (108.0–207.7) |
| Geometric mean fold rise (95% CI) | 72.9 (55.4–95.9) | 31.5 (23.1–43.2) | 71.9 (57.0–90.7) | 17.8 (13.3–23.8) |
| Seroprotection rate (titer, ≥1:40); % (95% CI) | 100 (93.9–100) | 100 (94.6–100) | 100 (94.4–100) | 88.9 (78.4–95.4) |
| Seroconversion rate; % (95% CI) | 98.3 (90.9–100) | 92.4 (83.2–97.5) | 100 (94.4–100) | 85.7 (74.6–93.3) |
GMT calculations were performed by taking the antilog of the mean of the log titer transformations. Geometric mean fold rise (seroconversion factor) is defined as geometric mean of the within-subject ratios of the postvaccination reciprocal hemagglutination inhibition titer to the day 0 reciprocal agglutination-inhibition titer. The seroconversion rate for the hemagglutination inhibition antibody response is defined as the percentage of vaccines who have a prevaccination titer of <1:10 and a postvaccination titer of ≥1:40 or a significant increase in antibody titer defined as the percentage of vaccines who have a prevaccination titer of ≥1:10 and at least a 4-fold increase in postvaccination titer.
The European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) criteria for hemagglutination inhibition antibody response in 18 to 60 year olds are a seroprotection rate of >70%, seroconversion rate of >40%, and geometric mean fold rise of >2.5.
n = 60 for prevaccination, day 0, and post-dose 1, day 21, and n = 59 for post-dose 2, day 42.
Stratification by previous seasonal influenza vaccination (Table 3) revealed baseline seroprotection rates and GMTs that were interpreted as comparable for subjects with and without a history of seasonal vaccination.
Table 3.
Immune response stratified by previous seasonal influenza vaccination as determined by hemagglutination inhibition antibodies against A/California/7/2009 H1N1
| Vaccine group | Sampling time point | Previous seasonal influenza vaccination |
No seasonal influenza vaccination |
||||
|---|---|---|---|---|---|---|---|
| n | Seroprotection rate (titer, ≥1:40); % (95% CI) | GMT (95% CI) | n | Seroprotection rate (titer, ≥1:40); % (95% CI) | GMT (95% CI) | ||
| Study A | |||||||
| AS03A-adjuvanted H1N1 | Day 0 | 35 | 14.3 (4.8–30.3) | 9.5 (6.8–13.3) | 25 | 8.0 (1.0–26.0) | 7.9 (5.7–10.9) |
| vaccine (3.75 μg HA) | Day 21 | 35 | 100 (90.0–100) | 270.5 (180.9–404.5) | 25 | 100 (86.3–100) | 452.6 (296.4–691.0) |
| Day 42 | 34 | 100 (89.7–100) | 516.7 (389.6–685.4) | 25 | 100 (86.3–100) | 844.6 (654.8–1,089.4) | |
| Nonadjuvanted H1N1 | Day 0 | 27 | 22.2 (8.6–42.3) | 11.6 (7.8–17.5) | 39 | 15.4 (5.9–30.5) | 10.3 (6.9–15.4) |
| vaccine (15 μg HA) | Day 21 | 27 | 92.6 (75.7–99.1) | 209.6 (122.6–358.5) | 39 | 94.9 (82.7–99.4) | 406.8 (257.1–643.8) |
| Day 42 | 27 | 100 (87.2–100) | 226.3 (145.5–352.0) | 39 | 100 (91.0–100) | 452.8 (325.9–629.1) | |
| Study B | |||||||
| AS03A-adjuvanted H1N1 | Day 0 | 21 | 9.5 (1.2–30.4) | 10.0 (6.4–15.5) | 43 | 11.6 (3.9–25.1) | 9.4 (7.1–12.4) |
| vaccine (3.75 μg HA) | Day 21 | 21 | 85.7 (63.7–97.0) | 289.7 (157.8–531.9) | 43 | 97.7 (87.7–99.9) | 510.7 (360.3–724.1) |
| Day 42 | 21 | 100 (83.9–100) | 499.7 (347.3–718.9) | 43 | 100 (91.8–100) | 802.1 (644.8–997.6) | |
| Nonadjuvanted H1N1 | Day 0 | 17 | 5.9 (0.1–28.7) | 9.4 (6.4–13.7) | 46 | 10.9 (3.6–23.6) | 8.1 (6.0–10.8) |
| vaccine (3.75 μg HA) | Day 21 | 17 | 64.7 (38.3–85.8) | 62.6 (29.6–132.2) | 46 | 76.1 (61.2–87.4) | 113.2 (68.9–185.9) |
| Day 42 | 17 | 76.5 (50.1–93.2) | 83.2 (46.5–148.8) | 46 | 93.5 (82.1–98.6) | 186.0 (126.4–273.7) | |
A substantial increase in GMT following the first dose of either 3.75 μg HA of AS03A-adjuvanted vaccine or 15 μg HA of nonadjuvanted A/H1N1/2009 vaccine in study A was observed with amplitudes considered similar in both groups (335.2 and 310.2). The immune responses in both groups following the first dose met CHMP criteria for influenza vaccines with seroconversion and seroprotection rates (HI titers of ≥1:40) of 98.3% and 100% in the AS03A-adjuvanted group and 84.8% and 93.9% in the nonadjuvanted group.
In study B, a first dose of 3.75 μg HA of AS03A-adjuvanted vaccine induced an observed GMT 4-fold higher than the one estimated after a first dose of 3.75 μg HA of nonadjuvanted A/H1N1/2009 vaccine (424 and 96.4, respectively). The seroconversion and seroprotection rates were 93.8% and 93.8% in the AS03A-adjuvanted group and 69.8% and 73.0% in the nonadjuvanted group. The immune responses in both groups met CHMP criteria.
A second dose of 3.75 μg HA of AS03A-adjuvanted vaccine induced an observed increase of at least 1.6-fold in HI response from day 21 to day 42 (335 to 636 and 424 to 687 in studies A and B, respectively). Lower GMTs were observed at day 42 for both nonadjuvanted vaccine formulations (341 for 15 μg HA and 150 for 3.75 μg HA), although a second dose of the 3.75-μg-HA formulation (but not the 15-μg-HA formulation) did induce an increase of 1.5-fold from day 21 to day 42.
Stratification by previous seasonal influenza vaccination (Table 3) indicated a trend toward lower GMTs at days 21 and 42 in subjects who had previously received seasonal vaccine. This trend was evident in both studies and in both AS03A-adjuvanted and nonadjuvanted vaccine groups.
Cell-mediated immune response (analyzed in study A only).
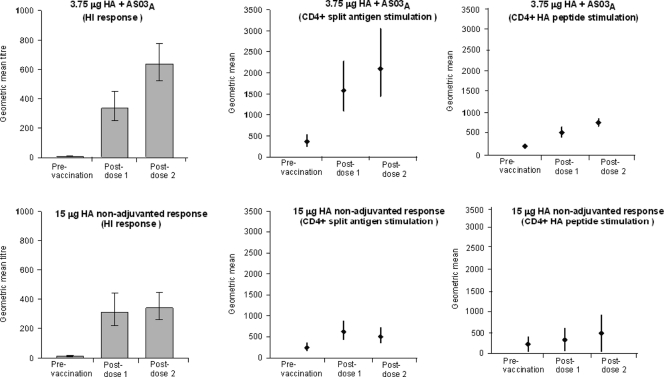
Geometric means of the frequencies of CD4+ T cells and CD8+ T cells specific for A/California/7/2009 H1N1 split antigen or the A/California/7/2009 H1 peptide pool on day 0 (prevaccination), day 21, and day 42 are presented in Table 4. Plots of the adjusted geometric means of the frequencies of CD4+ T cells are presented in Fig. 2. This analysis demonstrates that the CD4+ T-cell response increased in both vaccine groups over the whole 42-day period and that the increase was significantly higher in the adjuvanted group than that in the nonadjuvanted group (GM ratio of 2.82 and P = 0.0080 with H1N1 split antigen and GM ratio of 1.72 and P = 0.0084 with the H1 peptide pool). A significantly higher CD4+ T-cell response was already observed over the period from day 0 to day 21 in the AS03A-adjuvanted group compared with the nonadjuvanted group following stimulation with the H1 peptide pool (GM ratio, 1.76; P = 0.0104), and although not significant (P = 0.1079), the same trend was observed following stimulation with H1N1 split antigen (GM ratio, 1.73). Another observation is that the postvaccination GM values recorded following stimulation with split antigen in the AS03A-adjuvanted group were higher than those recorded following stimulation with the H1 peptide pool as well as those recorded following either stimulus in the nonadjuvanted group.
Table 4.
Geometric means of the frequencies of immunological marker-positive CD4+ T cells or immune marker-positive CD8+ T cells specific for A/California/7/2009 H1N1 split antigen or H1 peptide poola
| Stimulating antigen | Vaccine group | Geometric mean/million CD4+ or CD8+ T cells (CV) |
|||||
|---|---|---|---|---|---|---|---|
| CD4+ |
CD8+ |
||||||
| Day 0 | Day 21 | Day 42 | Day 0 | Day 21 | Day 42 | ||
| H1N1 split antigen | Nonadjuvanted H1N1 vaccine (15 μg HA) | 249.76 (370.82) | 627.20 (209.64) | 509.49 (310.28) | 2.80 (472.00) | 2.56 (338.13) | 2.71 (517.90) |
| AS03A-adjuvanted H1N1 vaccine (3.75 μg HA) | 356.75 (141.84) | 1,552.96 (323.60) | 2,044.63 (200.45) | 3.57 (519.80) | 4.61 (794.93) | 1.97 (311.29) | |
| H1 peptide pool | Nonadjuvanted H1N1 vaccine (15 μg HA) | 215.73 (115.14) | 319.26 (134.23) | 471.80 (40.92) | 3.03 (457.01) | 1.98 (277.97) | 5.97 (954.90) |
| AS03A-adjuvanted H1N1 vaccine (3.75 μg HA) | 197.90 (126.65) | 516.65 (72.97) | 741.75 (47.89) | 2.61 (470.14) | 4.92 (723.98) | 5.26 (873.57) | |
Marker positive is defined as positive for at least two immunological markers among CD40L, IFN-γ, IL-2, and TNF-α.
Fig. 2.
Adjusted geometric means of the frequencies of CD4+ T cells specific for A/California/7/2009 H1N1 split antigen or A/California/7/2009 H1 peptide pool (presented with hemagglutination inhibition antibody geometric mean titers at the same time points).
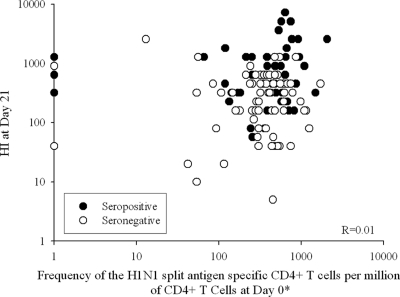
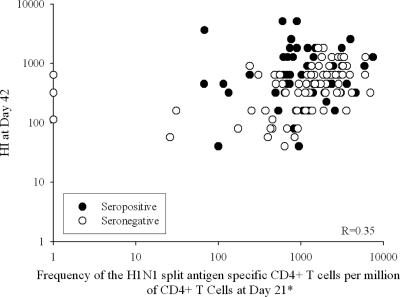
There was no evidence of a correlation between HI antibody levels on day 21 after the first vaccination and the frequency of CD4+ T cells specific for A/California/7/2009 H1N1 split antigen observed at baseline (R = 0.01) (Fig. 3). There was, however, some correlation between HI antibody levels on day 42 (after the second vaccination) and the frequency of CD4+ T cells specific for A/California/7/2009 H1N1 split antigen observed at day 21 (R = 0.35) (Fig. 4). Similar results were obtained between HI antibody levels on days 21 and 42 and the peptide-pool-specific CD4+ T cells (R = 0.11 and 0.35, respectively).
Fig. 3.
Scatter plot of hemagglutination inhibition antibodies at day 21 versus frequency of CD4+ T cells specific for A/California/7/2009 H1N1 split antigen at day 0 (prevaccination) (per protocol cohort for immunogenicity). R, Spearman's partial correlation coefficient adjusted by age, sex, history of vaccination, and prevaccination status (seropositive or seronegative). *, positive for at least two immunological markers among CD40L, IFN-γ, IL-2, and TNF-α.
Fig. 4.
Scatter plot of hemagglutination inhibition antibodies at day 42 versus frequency of CD4+ T cells specific for A/California/7/2009 H1N1 split antigen at day 21 (per protocol cohort for immunogenicity). R, Spearman's partial correlation coefficient adjusted by age, sex, history of vaccination, and prevaccination status (seropositive or seronegative). *, positive for at least two immunological markers among CD40L, IFN-γ, IL-2, and TNF-α.
Vaccination did not have any detectable impact on the frequencies of antigen-specific CD8+ T cells (Table 4) as measured on day 21 or 42.
Reactogenicity and safety.
In each study, the observed frequencies of injection site symptoms were higher in the AS03A-adjuvanted groups than those in the nonadjuvanted groups. Following both vaccine doses, pain at the injection site was the only local solicited symptom reported for the nonadjuvanted groups (35.4% and 30.3% following the first and second doses in the 15-μg-HA nonadjuvanted group in study A and 24.2% and 17.2% in the 3.75-μg-HA nonadjuvanted group in study B). In the AS03A-adjuvanted groups, injection site pain was reported in over 89% of subjects following both vaccine doses but was mainly mild to moderate, with only one to two subjects with grade 3 pain after each dose. Reports of injection site redness and swelling were much less frequent, and no cases were over 100 mm. The median duration of injection site pain after each dose was 3 days in the AS03A-adjuvanted groups in both studies (1 to 2 days for grade 3 pain) and 1 to 2 days in the nonadjuvanted group in both studies (data not shown).
Following the first dose, the most frequently reported systemic solicited adverse event was fatigue (34.9% in the AS03A-adjuvanted group and 27.7% in the 15-μg-HA nonadjuvanted group in study A and 47.7% in the AS03A-adjuvanted group and 33.3% in the 3.75-μg-HA nonadjuvanted group in study B). Systemic symptoms tended to occur more frequently in the AS03A-adjuvanted groups, in particular headache (27.0% versus 16.9% in study A and 38.5% versus 24.2% in study B) and muscle aches (31.7% versus 9.2% in study A and 26.2% versus 9.1% in study B).
Following the second dose, fatigue remained the most common solicited adverse event (45.2% in the AS03A-adjuvanted group and 19.7% in the 15-μg-HA nonadjuvanted group in study A and 43.8% in the AS03A-adjuvanted group and 23.4% in the 3.75-μg-HA nonadjuvanted group in study B). In both studies, systemic symptoms were more common in the AS03A-adjuvanted groups, and in study A, incidences also tended to be higher in this group than following the first dose. In all groups and after each vaccine dose, the majority of symptoms were mild to moderate in intensity, with a median duration of 1 to 3 days, and most were considered by the investigators to be related to vaccination (data not shown). The incidence of fever ≥38°C was low (zero to three reports following each dose), and the rates were similar in both adjuvanted and nonadjuvanted groups.
In both adjuvanted and nonadjuvanted groups, the percentages of doses followed by at least one spontaneously reported adverse event within the 42-day period were, respectively, 53.1% and 48.5% in study A and 38.0% and 30.8% in study B. The percentages of doses followed by at least one spontaneously reported grade 3 adverse event were still, respectively, 5.5% and 6.1% in study A and 3.9% and 3.1% in study B. The percentages of doses followed by spontaneously reported adverse events considered by the investigators as related to vaccination were 11.0% in the AS03A-adjuvanted group and 5.3% in the nonadjuvanted group in study A and 13.2% in the AS03A-adjuvanted group and 8.5% in the nonadjuvanted group in study B. Two grade 3 spontaneously reported events considered by the investigators as related to vaccination (influenza-like illness and malaise) were reported in the AS03A-adjuvanted group in study A. One subject reported a serious adverse event (migraine) 14 days after the first vaccination dose in the nonadjuvanted group in study A; this event, which resolved, was considered by the investigator as not related to vaccination.
DISCUSSION
Previously, the AS03 adjuvant system has been administered with H5N1 vaccines to a large number of adults (6, 22, 32) without raising any clinically observable safety concerns when monitored for up to 6 months following two-dose primary vaccination and also following a third (booster) dose administered at 6 months (33) or 14 months (23) after primary vaccination. Data from a smaller number of adults from the two studies presented here and from a previous study (30) also indicate no clinically observable safety concerns when AS03 is administered with A/H1N1/2009 vaccine. This report focuses on the immune responses and in particular addresses (i) the impact of the AS03 adjuvant system on humoral and T-cell responses to A/H1N1/2009, (ii) whether preexisting T-cell immunity influences postvaccination humoral responses, and (iii) whether previous seasonal influenza vaccination influences postvaccination humoral responses. Before addressing these three points, we first discuss the immune response to A/H1N1/2009 in the context of experience with H5N1 and preexisting humoral immunity.
In contrast to the experience with H5N1 where two doses of nonadjuvanted vaccine with HA levels above 30 μg are required to fulfill licensure criteria in at least more than half of the vaccinees (3, 22, 36), the results presented here demonstrate that a single dose of A/H1N1/2009 vaccine containing either 15 μg HA without adjuvant or 3.75 μg HA with adjuvant is highly immunogenic in adults and sufficient to fulfill regulatory acceptance criteria for pandemic influenza vaccines. This suggests the existence of some level of immunological priming of the population for the A/H1N1/2009 strain, despite the fact that it is antigenically and genetically distinct from recently circulating influenza virus A H1N1 strains (13, 15). This is supported by the observation that, prior to vaccination, 32% to 45% of our Belgian study participants and 48% to 78% of adults in another study in the United States (29) had baseline detectable antibodies against the A/H1N1/2009 strain, while only 0.7% to 3% of subjects in H5N1 vaccine studies had baseline detectable antibodies against H5N1 strains (3, 22, 33, 36). As subjects with a medical history suggestive of influenza in the 6 months prior to the study were excluded, preexisting antibodies are unlikely to be due to prior infection with A/H1N1/2009, although there may have been some asymptomatic cases.
Receipt of seasonal vaccination within the preceding 2 years did not appear to influence prevaccination seroprotection rates in our studies. This does not rule out cross-reactive priming by previous seasonal vaccination as HI titers at baseline may not be the most appropriate parameter to detect prior immune activation. Also, even if some level of cross-reactive HI response was induced by prior seasonal vaccination, it may not have been strong enough to be still detectable 1 to 2 years later. In another vaccination study, it was reported that baseline A/H1N1/2009 HI antibody levels were higher in adults, but not children, with seasonal vaccination in any of the five preceding years (29). The authors suggested, however, that seasonal influenza vaccination may have been a confounder of another factor, such as exposure (by infection or vaccination) to strains with similar epitopes which were in circulation prior to the 2004-2005 season (29). In children, Cowling et al. (8) found little cross-reactive neutralizing antibody response to A/H1N1/2009 virus following 2008 seasonal influenza vaccination but did find statistically significant cross-reactive responses to A/H1N1/2009 in those who had seasonal A/H1N1 infection, although few individuals had antibody titers of ≥1:40. Hancock et al. (17) also concluded that recent seasonal influenza vaccines induced little or no cross-reactive antibody responses to A/H1N1/2009.
In the case of H5N1 vaccination, the use of AS03 was found to significantly decrease the amount of antigen required to elicit an acceptable response (22). A similar effect of AS03 on A/H1N1/2009 was exemplified in study B, where adjuvanted and nonadjuvanted formulations were compared at the same 3.75-μg-HA dosage and the HI GMTs following both the first and the second vaccinations were higher for the AS03A-adjuvanted formulation. Furthermore, in terms of the kinetics of the HI immune response, the adjuvanted formulation induced a further increase in HI antibody levels after the second vaccination, whereas for the nonadjuvanted formulation, the increase was more modest. This is clearly evident in study A, where comparable GMTs were observed after the first vaccination for the AS03A-adjuvanted 3.75-μg HA and nonadjuvanted 15-μg HA formulations, whereas after the second vaccination, the GMT was higher for the AS03A-adjuvanted formulation. Greenberg et al. (16) also observed that a second dose of nonadjuvanted A/H1N1/2009 vaccine conferred little additional benefit in terms of HI responses in adults. This difference in the kinetics of the HI immune response between adjuvanted and nonadjuvanted formulations was also observed for H5N1 (22).
As we lack precise knowledge on what a well-validated protective antibody level is, in particular for this new A/California strain, the clinical implications of a higher GMT for AS03A-adjuvanted vaccine are not clear. Apart from promoting better antibody persistence, it may, for example, signal a difference in the quality of the priming of the B-cell or CD4+ T-cell memory response. In this respect, it is of note that in study A, although the antibody GMTs were comparable on day 21 after one dose, a post hoc inferential analysis indicated that the frequency of H1-specific CD4+ T cells was higher in the AS03A-adjuvanted group than that in the nonadjuvanted group. Furthermore, after a second dose, this difference between the groups was sustained, resulting in significantly higher H1N1- and H1-specific CD4+ T-cell response amplitudes over the 42-day period, which mirrors the difference in HI antibody response kinetics between the groups over the same period. Studies on A/H1N1/2009 booster vaccination (following both one- and two-dose primary schedules) should provide further information on the quality of priming with and without AS03A in terms of the induction of immune memory as monitored by both antibody and CD4+ T-cell responses. We also performed a post hoc analysis to examine whether preexisting CD4+ T-cell immunity could (partially) explain the variability of the postvaccination HI antibody responses. Our observations indicated that prevaccination CD4+ T-cell immunity did not predict antibody responses as no evidence of a relationship was found between the frequency of A/H1N1/2009-specific CD4+ T cells at baseline (day 0) and the post-dose 1 HI antibody titer at day 21. A moderate level of correlation was, however, observed between the frequency of A/H1N1/2009-specific CD4+ T cells at day 21 and the post-dose 2 HI antibody titer at day 42. It may be that we observed such a correlation between A/H1N1/2009-specific CD4+ T cells and HI antibody levels only after the first vaccine dose because the post-dose 1 CD4 memory pool is more homogeneous and specific for the A/H1N1/2009 vaccine, whereas the CD4 memory population before vaccination is more heterogeneous and is likely to be the consequence of a more complex cross-reactive immunity arising from previous influenza vaccinations or infections. In that setting, the vaccine amplifies a rare A/H1N1/2009-specific memory population—the frequencies of these rare cells may not be reflected in the total prevaccination frequency. Further studies, specifically designed to assess immune markers as predictors of responses to vaccination, are required to confirm this correlation.
Although we could not discern a clear effect of seasonal vaccination within the preceding 2 years on prevaccination status, there was a consistent trend, in both studies and for both AS03A-adjuvanted and nonadjuvanted A/H1N1/2009 vaccines, of a diminished HI antibody response in subjects who had previously received seasonal vaccine. Plennevaux et al. (29) also reported that previous seasonal influenza vaccination was associated with a lower HI GMT after a first dose of nonadjuvanted A/H1N1/2009 vaccine. Similarly prior vaccination with either seasonal influenza vaccine (10, 28) or nonadjuvanted H5N1 vaccine (23) was found to have a negative impact on immune responses to subsequent H5N1 vaccination. The immunological mechanisms underlying this phenomenon and its clinical implications are not clear. Cowling et al. (8) reported that children aged 6 to 15 years who received seasonal trivalent influenza vaccine (TIV) in 2008 were found to have nonstatistically significant higher rates of subsequent serologically confirmed infection with A/H1N1/2009 in 2009, although no clinical differences were noted in terms of influenza-like illness (ILI) attack rates compared to subjects who did not receive TIV. Wrammert et al. (41) have demonstrated that influenza TIV vaccination expands rare memory B cells that have very high specificity for the vaccine, showing that the vaccine was very efficient at capturing preexisting memory. Should seasonal TIV vaccination actually have the ability to change this existing memory B-cell population, it is likely that subsequent vaccination will be affected by this change. Whether that is positive or negative likely depends on the level of cross-reactivity conferred by the initial seasonal vaccination. Therefore, it is possible that previous nonadjuvanted seasonal influenza vaccination leads to a “skewing” of the T-cell and/or B-cell repertoires toward epitopes specific for those vaccines, thus limiting the selection and expansion of those T- and B-cell clones that display the strongest specificity for A/H1N1/2009 (28, 31). Clearly, the influence of previous seasonal vaccination on responses to A/H1N1/2009 vaccination and the potential impact of adjuvant in this process warrant further in depth investigation.
In conclusion, the AS03A adjuvant system enhances the humoral and CD4+ T-cell-mediated responses to A/H1N1/2009 vaccine. Baseline A/H1N1/2009-specific CD4+ T-cell frequencies do not seem to predict post-dose 1 antibody responses, although a correlation suggests a relationship between post-dose 1 CD4+ T-cell frequencies and post-dose 2 antibody responses.
ACKNOWLEDGMENTS
The sponsor was involved in all stages of the study conduct and analysis. The sponsor was responsible for all costs associated with the development and the publishing of the present article. G. Leroux-Roels was an investigator for this and other studies of the sponsor, and W. Dewé, F. Roman, K. Walravens, and E. Hanon are employed by the GSK group of companies.
F. De Boever, C. Maes, J. Willekens, and F. Clément have no conflicts of interest to declare.
All authors participated in the design or implementation of the study or analysis and interpretation of the data, the writing of the manuscript, and the decision to submit it for publication. Geert Leroux-Roels was the principal investigator who conducted the trial, together with the team of the Center for Vaccinology (CEVAC). Fien De Boever was responsible for the coordination of the clinical team at CEVAC. Frédéric Clément was responsible for the sample preparation for all analyses and for the measurement of the cellular immune responses. Cathy Maes and Julie Willekens were coinvestigators responsible for the recruitment and selection of eligible subjects, data collection and safety follow-up. François Roman managed the study at GlaxoSmithKline (GSK) Belgium. Walthère Dewé was responsible for the statistical input. Karl Walravens coordinated the serological laboratory teams and, together with Emmanuel Hanon and François Roman, was involved in data analysis and interpretation. All authors had full access to the data and had final responsibility to submit the manuscript for publication.
We are grateful to the New York Medical College, New York, for providing the vaccine virus strain. The authors are indebted to the study volunteers, participating clinicians, study doctors and trial nurses, and laboratory technicians at the study site, as well as to the sponsor's project staff, for support and contributions throughout the study. We are grateful to all teams of GSK Biologicals for their contribution to this study, in particular Roger Bernhard and Urban Lundberg and the clinical and serological laboratory teams; Philippe Moris and the Human Cellular Immunity Team, who characterized the cell-mediated immune responses; David Moreels and Sarah Van de Voorde for preparation of the study protocols and related study documentation; Koen Ceulemans and Els Praet for global study management; Dorothy Slavin, clinical safety representative, Carine Maggetto and Philippe Marius, clinical data coordinators, Mohamed Oujaa, and Isabelle Carletti for input on statistical analysis; and Edith Lépine for project management. We would like to address special thanks to Paul Gillard for leading the clinical team and for involvement in the studies and Robbert van der Most, Philippe Moris, and Paul Gillard for critical reading of the manuscript and very helpful suggestions. Finally, we thank Miriam Hynes (freelancer, United Kingdom), who provided medical writing services on behalf of GSK Biologicals, and Isabelle Gautherot (GSK Biologicals, Belgium) for editorial assistance and manuscript coordination.
Footnotes
Published ahead of print on 30 March 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Banzhoff A., et al. 2009. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 4:e4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baras B., et al. 2008. Crossprotection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS One 3:e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bresson J. L., et al. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367:1657–1664 [DOI] [PubMed] [Google Scholar]
- 4. Carrat F., Flahault A. 2007. Influenza vaccine: the challenge of antigenic drift. Vaccine 25:6852–6862 [DOI] [PubMed] [Google Scholar]
- 5. Chattopadhyay P. K., Yu J., Roederer M. 2005. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat. Med. 11:1113–1117 [DOI] [PubMed] [Google Scholar]
- 6. Chu D. W., et al. 2009. Immunogenicity and tolerability of an AS03-adjuvanted prepandemic influenza vaccine. A phase III study in a large population of Asian adults. Vaccine 27:7428–7435 [DOI] [PubMed] [Google Scholar]
- 7. Clark T. W., et al. 2009. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N. Engl. J. Med. 361:2424–2435 [DOI] [PubMed] [Google Scholar]
- 8. Cowling B. J., et al. 2010. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin. Infect. Dis. 51:1370–1379 [DOI] [PubMed] [Google Scholar]
- 9. European Committee for Proprietary Medicinal Products 24 January 2007, posting date. Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside the core dossier context (EMEA/CHMP/VWP/263499/2006). European Agency for the Evaluation of Medicinal Products, London, United Kingdom [Google Scholar]
- 10. FDA 2007. Vaccines and related biological products advisory committee presentation on Sanofi Pasteur's H5N1 vaccine, p. 20 U.S. Food and Drug Administration; http://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4282S1_6.ppt Accessed 13 April 2010 [Google Scholar]
- 11. Frentsch M., et al. 2005. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med. 11:1118–1124 [DOI] [PubMed] [Google Scholar]
- 12. Garçon N., Chomez P., Van Mechelen M. 2007. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev. Vaccines 6:723–739 [DOI] [PubMed] [Google Scholar]
- 13. Garten R. J., et al. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodwin K., Viboud C., Simonsen L. 2006. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24:1159–1169 [DOI] [PubMed] [Google Scholar]
- 15. Greenbaum J. A., et al. 2009. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. U. S. A. 106:20365–20370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenberg M. E., et al. 2009. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 2009. 361:2405–2413 [DOI] [PubMed] [Google Scholar]
- 17. Hancock K., et al. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361:1945–1952 [DOI] [PubMed] [Google Scholar]
- 18. Harandi A. M., Davies G., Olesen O. F. 2009. Vaccine adjuvants: scientific challenges and strategic initiatives. Expert Rev. Vaccines 8:293–298 [DOI] [PubMed] [Google Scholar]
- 19. Hehme N. W., et al. 2002. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin. Drug Invest. 22:751–769 [Google Scholar]
- 20. Kirchhoff D., et al. 2007. Identification and isolation of murine antigen-reactive T cells according to CD154 expression. Eur. J. Immunol. 37:2370–2377 [DOI] [PubMed] [Google Scholar]
- 21. Leroux-Roels I., et al. 2008. Broad clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One 3:e1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leroux-Roels I., et al. 2007. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine. Lancet 370:580–589 [DOI] [PubMed] [Google Scholar]
- 23. Leroux-Roels I., et al. 2010. Priming with AS03A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomized primary study. Vaccine 28:849–857 [DOI] [PubMed] [Google Scholar]
- 24. Levie K., et al. 2008. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J. Infect. Dis. 198:642–649 [DOI] [PubMed] [Google Scholar]
- 25. Maecker H. T., Maino V. C., Picker L. J. 2000. Immunofluorescence analysis of T-cell responses in health and disease. J. Clin. Immunol. 20:391–399 [DOI] [PubMed] [Google Scholar]
- 26. McElhaney J. E. 2009. Prevention of infectious diseases in older adults through immunization: the challenge of the senescent immune response. Expert Rev. Vaccines 8:593–606 [DOI] [PubMed] [Google Scholar]
- 27. McElhaney J. E., Dutz J. P. 2008. Better influenza vaccines for older people: what will it take?. J. Infect. Dis. 198:632–634 [DOI] [PubMed] [Google Scholar]
- 28. Nolan T., et al. 2008. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine 26:6383–6391 [DOI] [PubMed] [Google Scholar]
- 29. Plennevaux E., Sheldon E., Blatter M., Reeves-Hoché M. K., Denis M. 2010. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 375:41–48 [DOI] [PubMed] [Google Scholar]
- 30. Roman F., et al. 2010. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine 28:1740–1745 [DOI] [PubMed] [Google Scholar]
- 31. Roman F., Vaman T., Kafeja F., Hanon E., Van Damme P. 2010. AS03A-adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin. Infect. Dis. 51:668–677 [DOI] [PubMed] [Google Scholar]
- 32. Rümke H. C., et al. 2008. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine 26:2378–2388 [DOI] [PubMed] [Google Scholar]
- 33. Schwarz T. F., et al. 2009. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine 27:6284–6290 [DOI] [PubMed] [Google Scholar]
- 34. Thompson W. W., et al. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333–1340 [DOI] [PubMed] [Google Scholar]
- 35. Thompson W. W., et al. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186 [DOI] [PubMed] [Google Scholar]
- 36. Treanor J. J., Campbell J. D., Zangwill K. M., Rowe T., Wolff M. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 354:1345–1351 [DOI] [PubMed] [Google Scholar]
- 37. Vogel F. R., Caillet C., Kusters I. C., Haensler J. 2009. Emulsion based adjuvants for influenza vaccines. Expert Rev. Vaccines 8:483–492 [DOI] [PubMed] [Google Scholar]
- 38. Waldrop S. L., Davis K. A., Maino V. C., Picker L. J. 1998. Normal human CD4+ memory T-cells display broad heterogeneity in their activation threshold for cytokine synthesis. J. Immunol. 161:5284–5295 [PubMed] [Google Scholar]
- 39. Weinberger B., Herndler-Brandstetter D., Schwanninger A., Weiskopf D., Grubeck-Loebenstein B. 2008. Biology of immune responses to vaccines in elderly persons. Clin. Infect. Dis. 46:1078–1084 [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization 27 May 2009, posting date. Availability of a candidate reassortant vaccine virus for the novel influenza A (H1N1) vaccine development X-179A. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/swineflu/candidates_X-179a/en/ Accessed 13 April 2010 [Google Scholar]
- 41. Wrammert J., et al. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]