Abstract
The biggest challenge in the serological diagnosis of visceral leishmaniasis (VL) is to find a biomarker with a high specificity. This study was undertaken to identify novel Leishmania donovani antigens to solve the existing problem. The soluble L. donovani promastigote antigen was separated by SDS-PAGE, and a Western blot was probed with pooled sera of five subjects with confirmed VL before (n = 9 pools) and after (n = 9 pools) treatment and at the 6-month follow-up visit (n = 9 pools), healthy controls not from an area of endemicity (n = 9 pools), and healthy controls from an area of endemicity. The antibody response to the identified partially purified antigen was ascertained by an enzyme-linked immunosorbent assay (ELISA) with 70 sera from patients with parasitologically confirmed VL, 48 sera from healthy controls from an area where the disease is not endemic, 60 sera from healthy controls from an area of endemicity, and 42 sera from patients in different disease groups. The eluted protein was subjected to two-dimensional (2D) gel electrophoresis, Western blotted, and probed with sera from patients with confirmed VL and from healthy controls not from an area of endemicity. The antigenic protein was further characterized by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry. The identified protein (BHUP2) corresponds to a cytochrome c-like synthesis protein of 37 kDa. ELISA results were 94% sensitive, whereas specificities with sera from healthy controls from an area of endemicity, healthy controls not from an area of endemicity, and disease controls were 98%, 100%, and 97%, respectively. The antigen identified via a proteomics-based approach has a strong potential for further development as a diagnostic tool for VL.
INTRODUCTION
Visceral Leishmaniasis (VL), commonly known as kala-azar, is the life-threatening clinical form of leishmaniasis characterized by fever, loss of weight, splenomegaly, hepatomegaly, and anemia. It is a major public health problem in several countries, particularly India, Bangladesh, Nepal, Sudan, and Brazil (2). A timely and accurate diagnosis is necessary for cure and also because the antileishmanial drugs for treatment are expensive and associated with adverse events, which can occasionally be serious. The “gold standard” for the diagnosis of VL is through the direct microscopic detection of amastigotes in Giemsa-stained splenic or bone marrow smears, with reported sensitivities ranging from 93.1% to 98.7% (4, 8, 17) for splenic smears and 52% to 85% (1, 17) for bone marrow aspirates. These methods are invasive, painful, and risky. High levels of parasite-specific antibodies are observed prior to the detection of antigen-specific T cell responses (3). One approach adopted to improve diagnostic methods to detect infection with Leishmania donovani is to identify dominant antigens that elicit specific antibodies detectable by serological tests. In the Indian subcontinent, the rK39 antigen, available in the enzyme-linked immunosorbent assay (ELISA) and immunochromatographic (ICT) formats, is widely used for the diagnosis of VL, with excellent sensitivity (10, 12, 14, 15) The specificity in terms of healthy controls not from an area of endemicity is excellent in the Indian subcontinent, whereas it shows 20 to 32% positivity for healthy subjects living in areas where the disease is endemic (13, 15), and this has kept alive the search for a better test. Another antigen, rK28, is in its evaluation phase in different countries; it claims to replace the rK39 antigen, having excellent sensitivity (96.8%) and specificity (96.2%) when tested on a Sudanese population (7). The objective of this study was to identify an L. donovani-specific antigen with high sensitivity and specificity that has the potential to overcome the problems associated with existing serological biomarkers in the Indian subcontinent.
MATERIALS AND METHODS
Study population.
The study was conducted at the Infectious Disease Research Laboratory (IDRL) of Banaras Hindu University (BHU), Varanasi, Uttar Pradesh, and at its field site at the Kala-Azar Medical Research Center (KAMRC), Muzaffarpur, which is in an area where VL is highly endemic. The study was approved by the Ethical Committee of the Institute of Medical Sciences, BHU, Varanasi, and written informed consent was obtained.
Patients.
Sera from 70 patients with parasitologically confirmed VL and 48 healthy individuals living in regions where the disease is endemic (with no history of kala-azar) were collected. Healthy individuals (n = 60) from areas where the disease is not endemic constituted the control cohort, and 42 samples were collected from those who were suffering from diseases like amoebic liver abscess, tuberculosis, and malaria, etc., from the Sir Sunderlal Hospital, BHU. The samples were stored at −20°C.
CSA preparation.
A total of 1 × 108 parasites were harvested from stationary-phase promastigote cultures in cold 1× phosphate-buffered saline (PBS) at pH 7.2 for crude soluble antigen (CSA) preparations. After washing and centrifugation, the pellet was resuspended in 1× PBS, and an equal volume of complete protease inhibitor cocktail (Sigma) was added. Lysis of parasite cells was done by 6 alternate cycles of freezing (at −70°C) and thawing (at room temperature), followed by sonication. The supernatant was collected by centrifugation at 4,000 rpm for 10 min, and the protein was quantified by use of a BCA (bicinchoninic acid) kit (Thermo Scientific) (9).
SDS-PAGE.
The CSA extract was electrophoresed on a 12% polyacrylamide gel according to the method of Laemmli (6).
Western blotting.
CSA (45 μg/well) of L. donovani was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). CSA was then immunoblotted (16), by Western blotting (Mini-Protean II, multiscreen; Bio-Rad), onto a polyvinylidene difluoride (PVDF) membrane (0.45-μm pore size; Millipore) at 20 V for 30 min. The membrane was further treated with sera (1:100 in PBS) from different study groups for 1 h at room temperature. Alkaline phosphatase conjugated with goat anti-human IgG (1:1,000) was used as a secondary antibody. At the end, color was developed by using BCIP-NBT (5-bromo-4-chloro-3-indolylphosphate plus Nitro Blue thiazole) as a substrate (Promega). The obtained bands were analyzed with an Alpha Imager (Alpha Innotech).
Partial purification of protein from SDS-PAGE gels.
The 37-kDa protein band corresponding to the protein marker was excised directly from the SDS-PAGE gel with a sterile scalpel, crushed, and incubated overnight in an elution buffer (50 mM Tris-HCl, 150 mM NaCl, and 0.1 mM EDTA [pH 7.5]) at 37°C. The solution was centrifuged at 10,000 rpm at 10°C for 20 min, and the obtained supernatant was quantified for protein by the BCA method.
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was done as described elsewhere previously, with some modifications (5). Microtiter plates (Nunc) were coated with the eluted 37-kDa BHUP2 protein (100 ng/well) as a target antigen in carbonate buffer (pH 9.6) overnight at 4°C, and the plate was then blocked with 1% bovine serum albumin (BSA) in 1× PBS for 2 h at room temperature to prevent nonspecific binding. Sera (1:100 dilution) of different sets were added and incubated at 25°C for 1 h. Serum antibody titers were measured with horseradish peroxidase (HRP)-conjugated goat anti-human IgG (1:16,000) secondary antibody, using trimethylene benzidine (TMB; Promega) as a substrate. The reaction was stopped by the addition of 1 N H2SO4 to the mixture, and the optical density (OD) was measured at 450 nm by an ELISA plate reader (Spectromax 190; Molecular Devices). The cutoff value was determined as the means ± 2 standard deviations (SDs) above the mean absorbance of sera from healthy controls not from an area of endemicity. The diagnostic accuracy of the BHUP2 protein was evaluated by calculating the ROC (receiver operating characteristic) value, which was 0.98.
Two-dimensional (2D) gel electrophoresis.
Isoelectric focusing (IEF) was done with immobilized pH gradient gel strips (IPG strips; Bio-Rad) with a pH range of 3 to 10. Five micrograms of eluted protein was applied in 125 μl of rehydration buffer per IPG strip. The sample-containing rehydration buffer was loaded overnight at room temperature by in-gel reswelling under mineral oil to prevent the oxidation of protein and drying of the gel strip. The loaded IPG strip was connected with the electrode of a Protean IEF cell (Bio-Rad, India), followed by electric parameters of 20 min at 100 V and 50 μA, 30 min at 250 V and 50 μA, 2 h at 4,000 V, and 3 h at 10,000 V. The IPG strip was then equilibrated in equilibrium buffer and then run for the second dimension on a resolving SDS-PAGE gel. The gel was stained with a highly sensitive silver staining kit (Pierce silver stain kit; Thermo Scientific) according to the manufacturer's instructions.
Mass spectrometry.
The protein spot after silver staining was excised and subjected to protein sequencing analysis by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) analysis at the Molecular Biophysics Unit (MBU) of the Indian Institute of Science, Bangalore, India.
Statistical analysis.
Data analysis was done with SPSS 16.0 software. A comparative evaluation was done by a nonparametric t test. The peak lists of the mass spectra were used for peptide mass fingerprint analysis with Mascot software together with the NCBI sequence database. The protein was identified by using the following parameters: database, eukaryote (eukaryotes); enzyme, trypsin; variable modification, oxidation (M); fixed modification, carbamidomethyl (C); mass value, monoisotopic; protein mass, unrestricted; peptide mass tolerance, ±100 ppm, peptide charge state, +1; maximum number of missed cleavages, 1. Analyses of the post-source decay data sets were done by peptide mass fingerprinting with Mascot software.
RESULTS
Western blotting.
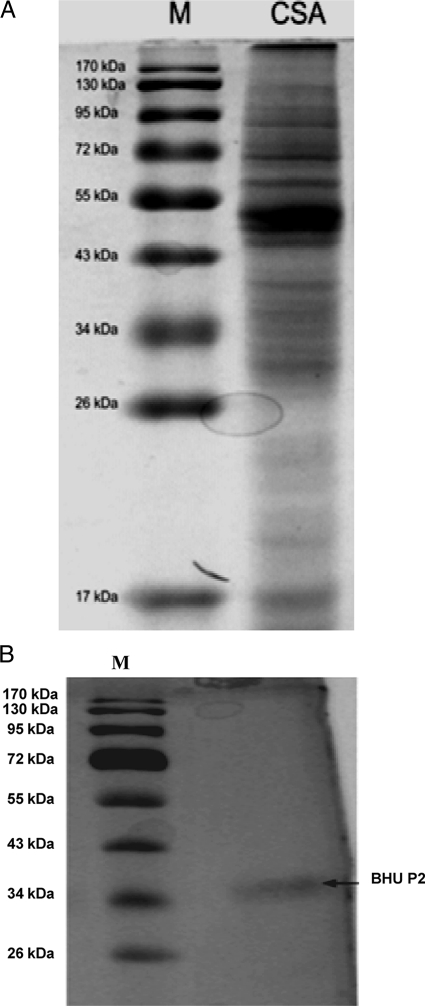
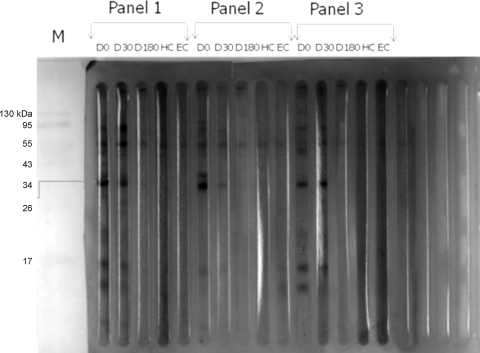
The protein profile of CSAs of L. donovani promastigotes showed a number of protein bands of different molecular weights when separated on 12% SDS-PAGE gels (Fig. 1 A). Soluble antigens trapped in a PVDF membrane were reacted with pooled sera of each group. A number of antigenic bands were recognized by the serum samples of different groups with different frequencies and intensities. The 37-kDa (BHUP2) soluble antigen of L. donovani promastigotes showed a strong reactivity with all serum samples (9/9) from the VL patients (Fig. 2). Out of nine serum samples, only three (33.3%) serum samples from the 6-month follow-up visit hybridized with this protein. No reaction was observed for sera of healthy subjects from an area of endemicity and healthy subjects not from an area of endemicity. The identified protein was eluted from the SDS-PAGE gel through a specific gel elution method, as shown in Fig. 1B.
Fig. 1.
(A) Protein profile of crude soluble antigen (6) of L. donovani promastigotes separated on 12% SDS-PAGE gels. Lane 1, protein marker (M); lane 2, crude soluble antigen. (B) Eluted protein as a single protein band stained with sensitive silver stain. Lane 1, protein marker; lane 2, eluted protein.
Fig. 2.
Western blotting profile of crude soluble antigens of L. donovani promastigotes hybridized with sera from different groups. Shown in each panel are sera from patients with parasitologically confirmed VL before (D 0) and after (D 30) treatment and at the 6-month follow-up visit (D 180), healthy controls not from an area of endemicity (HC), and healthy controls from an area of endemicity (EC). Arrows indicate the BHUP2 protein, which was recognized by the sera of VL patients pre- and posttreatment but not by the sera of our control groups.
ELISA.
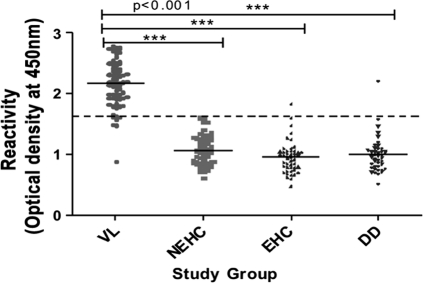
ELISA with the 37-kDa (BHUP2) protein was 94% sensitive (66/70 samples; 95% confidence interval [CI], 86.2 to 97.7%), whereas the specificities with sera from healthy subjects from an area of endemicity, healthy subjects not from an area of endemicity, and disease controls were 98% (47/48 samples; 95% CI, 92.6 to 100%), 100% (60/60; 95% CI, 92.6 to 100%), and 97% (41/42; 95% CI, 92.6 to 100%), respectively (Fig. 3).
Fig. 3.
ELISA reactivity of sera with the 37-kDa (BHUP2) protein. The study groups were composed of patients with visceral leishmaniasis (VL) (n = 70), healthy controls not from an area of endemicity (NEHC) (n = 60), healthy controls from an area of endemicity (EHC) (n = 48), and patients with different diseases (DD) (n = 42). MALDI-TOF results showed that the identified protein is a cytochrome c-like synthesis protein. The full lines indicate the average ELISA index (EI) for each assayed group. The dashed line represents the cutoff value. Each dot represents an individual subject.
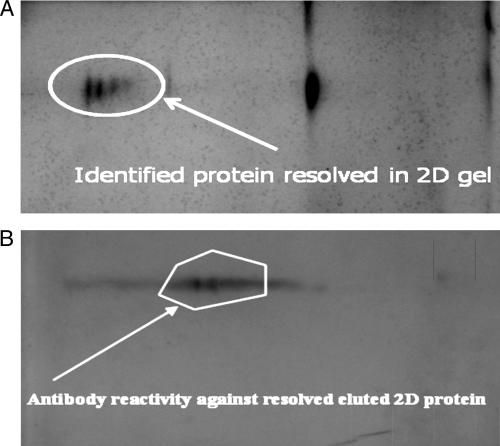
2D gel electrophoresis and MALDI-TOF characterization.
The 2D gel electrophoresis profile of the 37-kDa fraction revealed a mixture of 3 to 4 proteins (Fig. 4 A). Upon Western blotting of the resolved 2D gel and probing with the sera from patients with confirmed VL and from healthy controls not from an area of endemicity separately, only two protein spots reacted with sera from the VL patients (Fig. 4B), whereas none reacted with sera from healthy controls not from an area of endemicity. We excised the first of the two recognized protein spots from the 2D gel for MALDI-TOF characterization. The peak lists of the MALDI-TOF analysis were used for peptide mass fingerprint analysis with Mascot software (Matrix Science) together with the NCBI sequence database. A search of the protein sequence obtained from the mass spectrometry analysis recognized it as a cytochrome c-like synthesis protein of 37 kDa. Attempts to characterize the second protein failed due to the low yield of the protein.
Fig. 4.
(A) 2D gel electrophoresis of the eluted BHUP2 protein. The circle around the spot is the eluted protein. (B) Western blotting of the eluted protein separated on a 2D gel, hybridized with sera from patients with parasitologically confirmed VL. 2D gel electrophoresis of the eluted protein revealed 3 to 4 protein spots, and out of these protein spots, only two of them showed antibody reactivity against the eluted protein.
DISCUSSION
Through a series of experiments we identified a novel 37-kDa L. donovani antigen (BHUP2) with excellent sensitivity and specificity, as confirmed by ELISA. This protein was recognized as a cytochrome c-like synthesis protein on blasting peptide mass fingerprints (PMFs) with the NCBI protein sequence database. The recognized 37-kDa (BHUP2) protein revealed great potential as a serological marker for the diagnosis of Indian visceral leishmaniasis. ELISA results with the BHUP2 protein were 94% sensitive (66/70 samples) and 98.6% specific (148/150). Thus, fewer than 2% of healthy individuals from an area of endemicity were positive with the BHUP2 antigen, as opposed to the up to 32% positivity reported previously for the rK39 antigen (12, 13). There are several other diseases, like malaria, typhoid fever, and tuberculosis, etc., whose signs and symptoms overlap with those of VL, that also show positivity with rK39 (11). Thus, there is an urgent need for a diagnostic assay that could specifically diagnose active VL. This study was done to discover a specific immunoreactive marker for the early diagnosis of visceral leishmaniasis, so in our experiments, we chose this 37-kDa immunoreactive fraction for evaluation for this disease, which was compared with markers from sera from healthy controls from an area of endemicity, healthy controls not from an area of endemicity, and patients with different diseases to give comparable specificity and sensitivity data.
Our results suggest that the BHUP2 protein can be used as a diagnostic marker for VL. However, a limitation of the BHUP2 antigen is that its sensitivity is slightly lower than the benchmark of 95%. However, these experiments were done with antigens eluted from gel; a recombinant antigen devoid of impurities might provide better results. Since the antibody response to the antigen persists in a significant proportion of patients, it does not have a prognostic value and cannot be used to detect relapses; however, high specificity is its highlight.
In summary, the result of this study showed that the crude antigen of the 37-kDa fraction gave encouraging results with an ELISA in terms of antibody reactivity. Our protein shows high specificity, which is one of the highest of all the antigens used for the detection of leishmaniasis. If results with a recombinant form are similar or better, this will be another candidate to be used for the detection of VL.
ACKNOWLEDGMENTS
We thank all the staff of the Kala Azar Medical Research Centre (KAMRC), a unit of the Sitaram Memorial Trust, for their assistance in the collection of samples used in the experiments.
The study was partially funded by the National Institute of Allergy and Infectious Disease (NIAID) and the DMID funding mechanism of the Tropical Medicine Research Center (grant number P50AI074321). S.K. and D.K. thank the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing fellowships.
We declare no potential conflicts of interest.
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Bryceson A. 1996. Leishmaniasis, p. 1213–1243 In Cook G. C. (ed.), Manson's tropical diseases. W. B. Saunders, London, United Kingdom [Google Scholar]
- 2. Desjeux P. 1996. Leishmaniasis. Public health aspects and control. Clin. Dermatol. 14:417–423 [DOI] [PubMed] [Google Scholar]
- 3. Ghose A. C., Haldar J. P., Pal S. C., Mishra B. P., Mishra K. K. 1980. Serological investigations on Indian kala-azar. Clin. Exp. Immunol. 40:318–326 [PMC free article] [PubMed] [Google Scholar]
- 4. Ho E. A., Soong T. H., Li Y. 1948. Comparative merits of sternum, spleen and liver punctures in the study of human visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 41:629–636 [DOI] [PubMed] [Google Scholar]
- 5. Hommel M., Peters W., Ranque J., Quilici M., Lanotte G. 1978. The micro-ELISA technique in the serodiagnosis of visceral leishmaniasis. Ann. Trop. Med. Parasitol. 72:213–218 [DOI] [PubMed] [Google Scholar]
- 6. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:682–685 [DOI] [PubMed] [Google Scholar]
- 7. Pattabhi S., et al. Design, development and evaluation of rK28-based point-of-care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Negl. Trop. Dis. 4:e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siddig M., Ghalib H., Shillington D. C., Petersen E. A. 1988. Visceral leishmaniasis in the Sudan: comparative parasitological methods of diagnosis. Trans. R. Soc. Trop. Med. Hyg. 82:66–68 [PubMed] [Google Scholar]
- 9. Smith P. K., et al. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85 [DOI] [PubMed] [Google Scholar]
- 10. Sundar S., Pai K., Sahu M., Kumar V., Murray H. W. 2002. Immunochromatographic strip-test detection of anti-K39 antibody in Indian visceral leishmaniasis. Ann. Trop. Med. Parasitol. 96:19–23 [DOI] [PubMed] [Google Scholar]
- 11. Sundar S., Rai M. 2002. Laboratory diagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 9:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sundar S., Reed S. G., Singh V. P., Kumar P. C., Murray H. W. 1998. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet 351:563–565 [DOI] [PubMed] [Google Scholar]
- 13. Sundar S., et al. 2002. Noninvasive management of Indian visceral leishmaniasis: clinical application of diagnosis by K39 antigen strip testing at a kala-azar referral unit. Clin. Infect. Dis. 35:581–586 [DOI] [PubMed] [Google Scholar]
- 14. Sundar S., et al. 2007. Comparative evaluation of parasitology and serological tests in the diagnosis of visceral leishmaniasis in India: a phase III diagnostic accuracy study. Trop. Med. Int. Health 12:284–289 [DOI] [PubMed] [Google Scholar]
- 15. Sundar S., et al. 2006. Serological diagnosis of Indian visceral leishmaniasis: direct agglutination test versus rK39 strip test. Trans. R. Soc. Trop. Med. Hyg. 100:533–537 [DOI] [PubMed] [Google Scholar]
- 16. Towbin H., Staehelin T., Gordon J. 1979. Electrophoretic transfer of polyacrylamide gels to nitrocellulose sheet: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zijlstra E. E., et al. 1992. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans. R. Soc. Trop. Med. Hyg. 86:505–507 [DOI] [PubMed] [Google Scholar]