Abstract
BK virus (BKV) nephropathy and hemorrhagic cystitis are increasingly recognized causes of disease in renal and hematopoietic stem cell transplant recipients, respectively. Functional characterization of the immune response to BKV is important for clinical diagnosis, prognosis, and vaccine design. A peptide mix (PepMix) and overlapping (OPP) or random (RPP) peptide pools derived from BKV large T antigen (LTA) were used to restimulate 14-day-expanded peripheral blood mononuclear cells (PBMC) from 27 healthy control subjects in gamma interferon (IFN-γ)-specific enzyme-linked immunospot (ELISPOT) assays. A T-cell response to LTA PepMix was detected in 15/27 subjects. A response was frequently observed with peptides derived from the helicase domain (9/15 subjects), while the DNA binding and host range domains were immunologically inert (0/15 subjects). For all nine subjects who responded to LTA peptide pools, the immune response could be explained largely by a 15-mer peptide designated P313. P313-specific CD4+ T-cell clones demonstrated (i) stringent LTA peptide specificity; (ii) promiscuous recognition in the context of HLA-DR alleles; (iii) cross recognition of homologous peptides from the polyomavirus simian virus 40 (SV40); (iv) an effector memory phenotype, CD107a expression, and intracellular production of IFN-γ and tumor necrosis factor alpha (TNF-α); (v) cytotoxic activity in a chromium release assay; and (vi) the ability to directly present cognate antigen to autologous T cells. In conclusion, T-cell-mediated immunity to BKV in healthy subjects is associated with a polyfunctional population of CD4+ T cells with dual T-helper and T-cytotoxic properties. HLA class II promiscuity in antigen presentation makes the targeted LTA peptide sequence a suitable candidate for inclusion in immunotherapy protocols.
INTRODUCTION
BK polyomavirus (BKV) is a small double-stranded DNA virus that is latent in up to 90% of healthy adults. Following kidney transplantation, BKV reactivation has been described for 20 to 60% of subjects. BKV nephropathy, a disease characterized by progressive destruction of the renal parenchyma, develops in up to 10% of transplant patients (12, 15, 17, 35). Interrogation of national databases indicates that this complication has been recognized with increasing frequency in recent years. The cumulative incidence of nephropathy increases progressively as renal transplant recipients are followed up long-term (9). For patients treated with reduced immunosuppression for BK viremia or nephropathy, the 3-year graft survival rate falls from 90% to 79% (adjusted hazard ratio for graft loss = 1.9). Acute rejection episodes in the allograft are two to five times more frequent prior to and following significant BKV infection, presumably because viral infection causes upregulation of immunity directed against allogeneic antigens. Monitoring for BKV infection has become an important safety end point in studies investigating immunosuppressive protocols for renal transplant recipients (40). BKV is also commonly excreted in the urine of bone marrow transplant recipients, and it is associated with mild forms of hemorrhagic cystitis in up to 60% of these individuals and with severe hematuria in 5 to 10% of them (12, 38). BKV-associated hemorrhagic cystitis can also occur in 5% of oncology patients treated with high-dose cyclophosphamide. Considering the fact that approximately 100,000 kidney and bone marrow transplants are performed annually worldwide, there is a significant need to develop immunodiagnostic and immunotherapeutic tools for this growing patient population.
In recent years, significant progress has been made in defining the targets of T-cell-mediated immunity to BKV. Previously, two HLA-A02-directed epitopes, p108 and p44, were localized on the BKV viral capsid 1 (VP1) protein (7, 41). Subsequent work has focused on the large T-antigen (LTA) protein, since it is produced early in the viral life cycle and therefore immune surveillance against LTA epitopes is expected to help maintain viral latency. The first LTA epitope mapping studies were performed on HLA-A02-positive subjects (33, 36), since this allele accounts for 45.6% of the U.S. Caucasian population (http://www.allelefrequencies.net/default.asp). Subsequently, attention was turned to the HLA-A01, -A03, -A24, -B07, and -B08 alleles, which have frequencies of 27.4, 23.8, 12.1, 18.1, and 18.1%, respectively, among Caucasians in the United States (25, 34). Two peptides putatively restricted by HLA-DRB1-03, as well as HLA-DRB1-09 alleles, have also been identified (25).
The cellular phenotypes and functional characteristics of the immune response to BKV have not been evaluated systematically, nor has strict major histocompatibility complex (MHC) restriction been documented rigorously in each case. Reportedly, VP1 and LTA responses are more likely to involve CD4+ than CD8+ T cells (7, 14, 21). However, the results of such studies can be affected by the phase of infection as well as by the length of the peptide used for cell stimulation. There is evidence of cross-reactivity between BKV, JC virus (JCV), and simian virus 40 (SV40) epitopes (21), which could mean that infection by one virus can protect against infection by a second species. However, rigorous studies with pretransplant serology for all three viruses prior to transplantation have not been performed. Moreover, T-cell clones have not been studied to determine if the same or different populations of T cells react to these closely related viral epitopes. Finally, it is becoming increasingly apparent that a study of the magnitude and breadth of the immune response, as measured by commonly used assays, is insufficient to accurately predict virologic outcomes for individual subjects (8, 14, 27). Therefore, a comprehensive understanding of the T-cell-mediated immune response to BKV will require a detailed study of multiple functional immune parameters. The present study was designed to dissect the fine T-cell characteristics of the immune response to BKV at the level of individual T-cell clones.
MATERIALS AND METHODS
Study subjects.
All HLA-typed subjects (n = 27) studied were healthy blood donors or volunteers who agreed to provide samples for this study (Table 1). Peripheral blood mononuclear cells (PBMC) were either purchased from Cellular Technology Ltd. (Shaker Heights, OH) (n = 15) or isolated using Ficoll-Paque Plus (GE Healthcare) from fresh blood samples (n = 6) obtained as a part of the Thomas E. Starzl Transplantation Institute Healthy Control Validation Study (IRB0608014) or from buffy coats (n = 6) made available by the Central Blood Bank of Pittsburgh. Among the 27 subjects, 8 were BKV and JCV seropositive, 9 were BKV seropositive and JCV seronegative, 4 were BKV seronegative and JCV seropositive, and 3 were BKV as well as JCV seronegative. One subject was BKV seropositive but was not tested for JCV, while 2 subjects could not be evaluated for serologic status (Table 1). The serologic testing was performed using a virus-like particle-based enzyme-linked immunosorbent assay (ELISA) as previously described (37).
Table 1.
HLA typing and viral serology of healthy subjects tested in this studya
| Subject | HLA-A type |
HLA-B type |
HLA-DR type |
Serology |
LTA PepMix response | ||||
|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A1 | A2 | A1 | A2 | BKV | JCV | ||
| 1 | 11 | 24 | 51 | 60 | 04 | 08 | − | + | + |
| 2 | 01 | 01 | 08 | 08 | 0101 | 0701 | + | NT | + |
| 3 | 03 | 29 | 44 | 65 | 1301 | 0701 | + | + | + |
| 4 | 03 | 25 | 35 | 62 | 07 | 16 | + | + | − |
| 5 | 01 | 02 | 44 | 63 | 11 | 13 | − | − | − |
| 6 | 01 | 02 | 44 | 63 | 12 | 14 | + | − | + |
| 7 | 24 | 30 | 13 | 51 | 07 | 13 | − | + | − |
| 8 | 0101 | 2901 | 0801 | 1501 | 0301 | 0301 | + | + | − |
| 9 | 02 | 26 | 70 | 70 | 1101 | 1301 | NT | NT | − |
| 10 | 0101 | 2402 | 5201 | 2701 | 1501 | 1501 | + | + | + |
| 11 | 0101 | 6801 | 5701 | 5703 | 0701 | 0901 | + | − | + |
| 12 | 0101 | 0201 | 0801 | 60-4002 | 0301 | 1601 | + | − | + |
| 13 | 0101 | 0201 | 1402 | 4102 | 1302 | 1303 | + | + | + |
| 14 | 0101 | 0101 | 1402 | 4102 | 1302 | 1303 | + | + | + |
| 15 | 0201 | 0301 | 4402 | 4402 | 0401 | 0901 | − | + | − |
| 16 | 0201 | 0301 | 1801 | 1801 | 0401 | 1407 | + | − | + |
| 17 | 0201 | 0301 | 0702 | 0801 | 0102 | 0301 | + | + | − |
| 18 | 0201 | 0301 | 60-4002 | 4402 | 0404 | 1201 | + | − | + |
| 19 | 0201 | 0301 | 0702 | 2705 | 0801 | 1501 | + | − | − |
| 20 | 0201 | 0301 | 1501 | 2705 | 1101 | 1101 | + | − | − |
| 21 | 0201 | 0301 | 0702 | 4402 | 1101 | 1301 | + | + | − |
| 22 | 01 | 30 | 38 | 57 | 04 | 11 | + | − | + |
| 23 | 03 | 24 | 07 | 51 | 0801 | 1501 | NT | NT | + |
| 24 | 0101 | 3301 | 0801 | 1402 | 0102 | 1501 | − | − | + |
| 25 | 0205 | 2402 | 0801 | 5001 | 0701 | 1501 | − | − | − |
| 26 | 2402 | 3201 | 0801 | 5501 | 0301 | 1501 | − | + | − |
| 27 | 0207 | 2402 | 3901 | 4601 | 0803 | 0901 | + | − | + |
Data shown in bold are for subjects who were positive for a response to BKV LTA PepMix. NT, not tested. There were 18 BKV-seropositive subjects, 7 BKV-seronegative subjects, 2 subjects not tested for BKV, 12 JCV-seropositive subjects, 12 JCV-seronegative subjects, 3 subjects not tested for JCV, 8 BKV- and JCV-seropositive subjects, 3 BKV- and JCV-seronegative subjects, 9 BKV-seropositive and JCV-seronegative subjects, and 4 BKV-seronegative and JCV-seropositive subjects.
PepMix and peptides used.
The following peptide mixtures (PepMix) and peptides were used for this study. (i) CEF Peptide Pool Plus (Cellular Technology Ltd.), which contains cytomegalovirus (CMV), Epstein-Barr virus (EBV), and influenza virus peptides, was used as a positive control. An HIV Gag protein-derived 15-mer PepMix and peptide P37 (KCKEFHPDKGGDEDK) served as negative controls. (ii) A BKV LTA-derived 15-mer PepMix was used to enable a global assessment of T-cell-mediated immunity directed against this viral protein. (iii) A BKV VP1-derived 15-mer PepMix was used to evaluate cross-reactivity between the VP1 and LTA proteins. (iv) One hundred seventy overlapping BKV LTA-derived individually synthesized peptides, each of which was a 15-mer and shifted 4 amino acids with respect to the preceding peptide, were used in an attempt to elicit both CD4+ and CD8+ T-cell responses (24). These peptides spanned the entire 695-amino-acid sequence of BKV LTA (strain Dunlop; Swiss-Prot accession no. P03071). (v) BKV Dunlop strain LTA peptide P313 (PYHFKYHEKHFANAI; amino acids 313 to 327) was also used. (vi) The JCV LTA (strain MAD1; Swiss-Prot accession no. P03072) homologous peptide P312 (PNHFNHHEKHYYNAQ; amino acids 312 to 326) was included as a JCV peptide. (vii) The SV40 LTA (strain 776; Swiss-Prot accession no. Q9WND0) homologous peptide P312 (PSHYKYHEKHYANAA; amino acids 312 to 326) was included as an SV40 peptide.
All 15-mer peptides and the PepMix for BKV LTA and VP1 and for HIV Gag protein were purchased from JPT Peptide Technologies (Berlin, Germany). Peptides i to iv were preparative-grade peptides used for screening purposes, while peptides v to vii, used for functional analyses, were >95% pure as shown by high-pressure liquid chromatography (HPLC).
Design of BKV LTA peptide pools.
Peptides spanning the full-length BKV LTA were designated P1 to P170, where P1 refers to a peptide consisting of the first 15 amino acids (LTA positions 1 to 15), P2 refers to the next peptide, overlapping P1 by 11 amino acids (LTA positions 5 to 19), and so on. A two-dimensional analytic grid (1, 18) was used to distribute all 170 peptides into 13 overlapping peptide pools (OPP1 to -12) and 13 random peptide pools (RPP1 to -13). Each pool contained 13 peptides, except for OPP13 and RPP13, which contained 14 peptides each (Fig. 1). Stock solutions (10 mg/ml) of each peptide were made, and 20 μl of each of the 13 peptides was mixed to obtain a peptide pool stock solution of 4 μg/ml/peptide, which was subsequently diluted 5-fold to get a 1-μg/ml final concentration of each peptide.
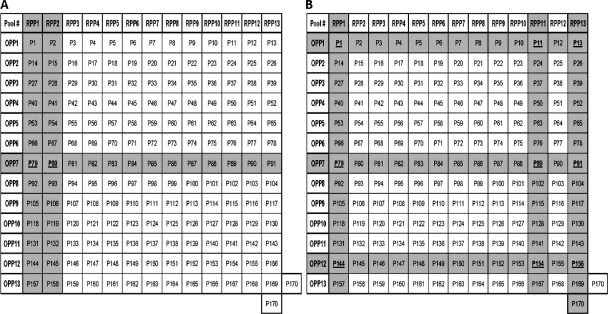
Fig. 1.
Use of two-dimensional peptide matrix to identify candidate peptides in subject 1 (A) and subject 5 (B). The shaded rows and columns indicate the peptide pools that tested positive in the ELISPOT assay. All 170 peptides (P1 to P170) tested were arranged in a two-dimensional grid, wherein columns and rows designate the peptides that constitute the corresponding random and overlapping peptide pools, respectively. This two-dimensional design of the peptide matrix ensures that each and every peptide is present in only two individual pools. Therefore, when a single peptide of the pool is recognized in an assay, either two separate pools will be positive or, along with those two, a third pool, which has the adjacent overlapping peptide, will also test positive, and the candidate peptide can be identified readily. In this manner, testing of 170 individual peptides can be replaced by testing of 26 peptide pools without any loss of information on peptide immunogenicity.
Antigen-specific expansion of PBMC with IL-2.
PBMC (5 × 106/ml) were cultured at 37°C in a 5% CO2 incubator in 24-well tissue culture plates in RPMI complete medium (RPMI 1640 supplemented with 2 mM l-glutamine, 10 mM HEPES, 100 IU/ml penicillin-streptomycin, 1 mM sodium pyruvate, and 12% fetal calf serum [FCS], all purchased from Sigma, St. Louis, MO), with a 1-μg/ml final concentration of each peptide added individually or as part of a PepMix or peptide pool. After 5 days, recombinant human interleukin-2 (IL-2) (Proleukin; Chiron, Emeryville, CA) was added to a final concentration of 30 IU/ml, followed by expansion for an additional 9 days. Every 2 days thereafter, 50% of the culture medium was replaced with fresh medium containing IL-2. Finally, the cells were collected on day 14 for gamma interferon-specific enzyme-linked immunospot (IFN-γ ELISPOT) assays.
IFN-γ ELISPOT assays.
Multiscreen 96-well plates (Millipore, Bedford, MA) were precoated with anti-human IFN-γ monoclonal antibody (MAb) (Mabtech Inc., Mariemont, OH). PBMC expanded with the appropriate peptides for 14 to 15 days were washed, and aliquots (1 × 105 cells/well) were restimulated with PepMix-, peptide pool-, or HLA-A/DR allele-matched individual peptide-loaded B-lymphoblastoid cell lines (B-LCL) as antigen-presenting cells (APC) (PBMC/B-LCL ratio, 5:1). Following 18 to 24 h of incubation with the appropriate antigen, IFN-γ spots were developed and spot counts enumerated using an ELISPOT reader (Cellular Technology Ltd.). Negative-control wells contained PBMC incubated with HIV Gag PepMix or peptide P37. Positive-control wells contained PBMC stimulated with CEF PepMix or phorbol myristate acetate (PMA)-calcium ionomycin. Background spot counts were evaluated by spot counts derived from wells that contained (i) unloaded B-LCL plus unstimulated PBMC (mean ± standard deviation [SD] = 184 ± 136; median = 180), (ii) unloaded B-LCL plus PBMC stimulated with CEF PepMix (mean ± SD = 124 ± 94; median = 93), or (iii) unloaded B-LCL plus PBMC stimulated with BKV LTA PepMix (mean ± SD = 131 ± 88; median = 126). The response to the antigenic peptides of interest was considered positive only when the spot count was at least two times more than that observed in all background wells.
Generation of P313-specific CD4+ T-cell clones.
For three subjects, cloning was initiated from PBMC expanded for 14 days. CD8+ T cells, B cells, and NK cells were depleted using a CD4+ T-cell negative isolation kit II (Miltenyi Biotec, Germany). Purified CD4+ T cells or CD4+ CD154+ T cells were sorted using a BD FACS Aria single-cell sorter (BD Biosciences, San Jose, CA) at a concentration of 1 cell/well. Sorted cells were supported by a feeder mix as previously described (44, 46). Clones that reached a density of 1 × 106 cells/ml/well were screened for an IFN-γ response to P313 in ELISPOT assays, and 40 selected clones from each subject were expanded further for functional characterization. The cloning efficiency was 7% for CD4+ T cells and 17 to 21% for CD4+ CD154+ T cells.
Multicolor flow cytometric analysis of T-cell clone phenotype and function.
Analysis of cell surface marker expression and intracellular cytokines was performed using a BD LSR II flow cytometer (BD Biosciences). Fluorochrome-conjugated MAbs were used to study the expression of various surface receptors, namely, CD4, CD45RA, CD45RO, CD62L, CCR7, HLA-DR, CD56, CD38, CD86, CD28, FAS, and FAS-L. Matched isotype control antibodies were used as negative controls (BD Biosciences). For surface staining, cells were incubated with the indicated MAb for 30 min at 4°C. The cells were then washed with fluorescence-activated cell sorter (FACS) buffer (Dulbecco's phosphate-buffered saline [DPBS] supplemented with 1% fetal bovine serum [FBS] and 0.05% NaN3), fixed with 1% paraformaldehyde, and analyzed. Data were analyzed using BD FACSDiva (BD Biosciences) or FlowJo (v.7.6.1; Tree Star, Inc., Ashland, OR) software.
For intracellular cytokine staining (ICS) analysis, T-cell clones were restimulated overnight at 37°C and 5% CO2 with peptide P313- or P37-pulsed B-LCL in the presence of CD28/CD49d reagent (BD Fast Immune) plus anti-CD107a MAb (eBiosciences), in addition to brefeldin A and monensin (both from Sigma), added 1 h after the MAb. The cells were then stained with anti-CD4 MAb for 30 min at 4°C, washed twice with FACS buffer, and fixed and permeabilized with Cytofix/Cytoperm reagent (BD Biosciences) per the manufacturer's instructions. Various intracellular cytokine antibodies, including those for tumor necrosis factor alpha (TNF-α), IFN-γ, IL-2, IL-4, IL-10, IL-21, and IL-17A (BD Biosciences), were added, incubated for 30 min at 4°C, washed, and analyzed.
51Chromium release cytotoxicity assays.
Target cells consisted of 1 × 106 autologous or HLA-DR-matched or -mismatched B-LCL labeled with 100 μCi 51Cr (Perkin Elmer, Boston, MA) at 37°C in 5% CO2 for 1 h (45). Cells were then washed, loaded with peptide P313 or negative peptide P37 (1 μg/ml) for 1 h at 37°C, washed again to remove the unbound peptides, and used as target cells at 5 × 103 cells/well in a 96-well plate (Costar; Corning, NY) containing effector CD4+ T-cell clones at different effector/target (E/T) ratios (50:1, 25:1, 12.5:1, 6.25:1, 3:1, and 1.5:1). Supernatants were harvested after 4 h of incubation at 37°C in 5% CO2. 51Cr release was measured using an automatic gamma counter (model 28037; ICN Micromedic Systems, Huntsville, AL). Total 51Cr release was determined by lysis of target cells in 1% Triton X-100. Spontaneous 51Cr release was measured in the absence of effector cells. The percent specific lysis was calculated using the following formula: [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100.
T-cell antigen presentation assay.
T-cell clones were pulsed with 1 μg/ml of P313 or control peptide P37 for 1 h at 37°C, gamma irradiated (5,000 rads), washed three times to remove unbound peptides, and used as antigen-presenting cells in an 18- to 24-h IFN-γ ELISPOT assay. Unpulsed autologous T-cell clones (1 × 104/well) were used as effector cells, at an effector/target ratio of 5:1.
Evaluations of T-cell cross-reactivity.
Peptide P313-stimulated PBMC (1 × 105) or CD4+ T-cell clones (1 × 104) were restimulated with autologous or HLA-DR-matched B-LCL not loaded or loaded with BKV LTA peptide P313, homologous JC virus LTA peptide P312, homologous SV40 LTA peptide P312, or negative-control peptide P37 (1 μg/ml) at a 5:1 ratio. After 24 h of incubation, IFN-γ spots were developed and counted using an automated ELISPOT counter. The response was considered positive when the spot count was two times above background. Background IFN-γ counts for PBMC were 193 ± 21 spots for unpulsed B-LCL and 178 ± 64 spots for B-LCL pulsed with negative-control peptide P37. With cloned CD4+ T cells, the background signal was zero (0 ± 0 spots).
Statistical analysis.
IFN-γ ELISPOT counts were tabulated in a Microsoft Excel spreadsheet. Mean counts for different stimulation conditions were compared by the Mann-Whitney rank sum test, using SigmaStat 3.01A for Windows (Systat Software Inc., Richmond, CA). P values of <0.05 were regarded as statistically significant.
RESULTS
Frequency of BKV LTA-sensitized T cells in healthy subjects.
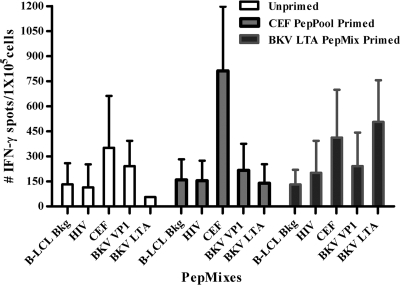
In our preliminary experiments, PBMC stimulated overnight with BKV LTA PepMix did not elicit an IFN-γ+ response in the ELISPOT assay. Thus, we stimulated PBMC with specific antigen for 5 days and expanded them with IL-2 for 9 days before screening for an IFN-γ+ response. Expanded PBMC from 24 of 27 subjects initially stimulated with the CEF peptide pool responded to rechallenge with the CEF peptide pool (791 ± 384 spots/1 × 105 cells) (Fig. 2) but not to rechallenge with BKV LTA PepMix. Expanded PBMC from 15 of 27 subjects initially stimulated with BKV LTA PepMix responded to rechallenge with BKV LTA PepMix (473 ± 263 spots/1 × 105 cells) (Fig. 2). No IFN-γ+ responses were obtained with HIV or BKV VP1 PepMix. Twelve of the 15 subjects who responded to BKV LTA PepMix were BKV seropositive, while 2 were BKV seronegative and 1 subject's serostatus was not known (Table 1).
Fig. 2.
IFN-γ+ responses of expanded PBMC to HIV, CEF, BKV VP1, and BKV LTA peptide mixtures. The color code indicates the stimulating peptide used during the 14-day PBMC expansion step. The x axis depicts the peptides used during the 18 to 24 h of incubation performed during the ELISPOT assay. B-LCL Bkg, background results obtained with lymphoblastoid cell lines without peptide antigens. Data are expressed as mean spot counts and SD for all subjects tested in this study (n = 27). Statistically significant differences were seen between the mean ELISPOT counts of (i) the unprimed/CEF-stimulated group versus the CEF-primed/CEF-stimulated group (n = 27; P < 0.001) and (ii) the unprimed/BKV LTA-stimulated group versus the BKV LTA-primed/BKV LTA-stimulated group (n = 27; P < 0.001).
Response of in vitro-expanded PBMC to BKV LTA-derived OPP and RPP.
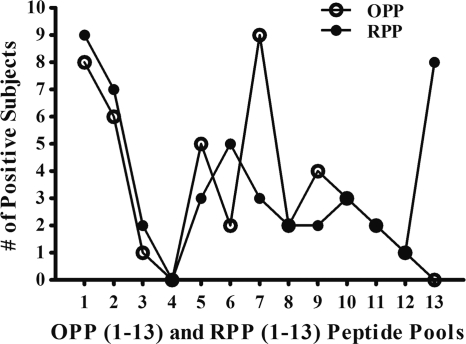
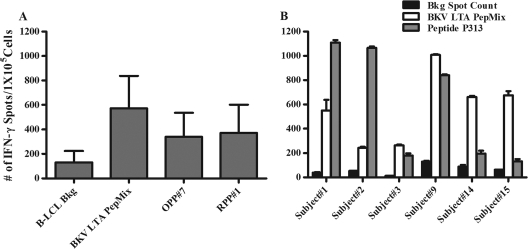
Expanded PBMC from 15 subjects who responded to BKV LTA-derived PepMix were rechallenged with BKV LTA OPP and RPP (Fig. 3). Most commonly, subjects responded to OPP1 and -7 (8/15 and 9/15 subjects, respectively) (Fig. 3). Notably, OPP7 generated an IFN-γ+ response that was more than half of the response elicited by BKV LTA PepMix (Fig. 4A). However, no response was seen with OPP4 or -13. Among the 13 RPP tested, RPP1 and -13 induced an IFN-γ+ response in 9/15 and 8/15 subjects, respectively. Correlating the RPP and OPP response patterns indicated that the IFN-γ+ response in subject 1 was derived from peptides P79 and P80 (Fig. 1A). The IFN-γ+ response patterns in other subjects were more complex. For example, subject 5 reacted to OPP1, -7, and -12 and to RPP1, -11, and -13 (Fig. 1B). Potential peptides that can explain these findings are P1, P79, P144, P11, P89, P154, P13, P91, and P156. Additional testing with individual peptides would be required to identify the immunogenic peptides in this particular subject. All further studies below were performed with P79, which corresponds to BKV LTA amino acid positions 313 to 327 and is referred to henceforth as P313.
Fig. 3.
Recognition frequencies of BKV LTA OPP and RPP by healthy subjects who responded to BKV LTA PepMix (n = 15). PBMC were stimulated with BKV LTA PepMix for 5 days and further expanded with IL-2 for 14 to 15 days. Expanded PBMC were tested for an IFN-γ response to BKV LTA in ELISPOT assays using OPP and RPP as antigens and HLA-A allele-matched B-LCL as APC. The response with peptide pools was considered positive when the IFN-γ spot counts were two times more than the background.
Fig. 4.
IFN-γ response in BKV LTA PepMix-immunoreactive subjects stimulated by peptide pools or peptide P313. (A) PBMC were stimulated with BKV LTA PepMix for 5 days, further expanded with IL-2 for 14 to 15 days, and restimulated with peptide pools for 18 to 24 h, using HLA-A allele-matched B-LCL under the indicated conditions (n = 15). B-LCL Bkg, background results obtained with lymphoblastoid cell lines without peptide antigens. Note that overlapping peptide pool OPP7 (9/15 subjects) and random peptide pool RPP1 (9/15 subjects) generated signals that were approximately half of that elicited by BKV LTA PepMix (15/15 subjects). (B) OPP7- and RPP1-positive subjects (n = 6) were tested individually for an IFN-γ+ response to unloaded (black bars), BKV LTA PepMix-loaded (white bars), or P313-loaded (gray bars) HLA-DR allele-matched B-LCL as APC. The data are expressed as mean spot counts and SD.
In vitro-expanded T-cell responses to P313.
The IFN-γ+ response to P313 was tested in 14-day-expanded PBMC from 6 of 9 subjects who reacted to OPP7 and RPP1, and all 6 subjects tested responded (Fig. 4B). This was further confirmed using T-cell lines derived from each of the 6 P313-positive subjects (data not shown). These T-cell lines responding to peptide P313 were analyzed by FACS to determine if the IFN-γ+ response was derived from CD4+ or CD8+ T cells. The IFN-γ+ response to peptide P313 was detected on CD4+ but not on CD8+ T cells (data not shown). Since the peptide P313-specific IFN-γ+ response was limited to CD4+ T cells, only CD4+ T cells were studied in the subsequent cloning experiments. The IFN-γ+ response to peptide P313 was confirmed by ELISPOT assays with 10 to 26 CD4+ T-cell clones obtained from each of three subjects (1,013 ± 174 spots/1 × 104 cells). Ten clones from one subject were tested extensively for specificity and found to be nonresponsive to 21 different peptides derived from DNA polymerase alpha, DNA binding, and p53 binding domains of BKV LTA protein.
Cross recognition of SV40 and JCV homologues of BKV LTA peptide P313.
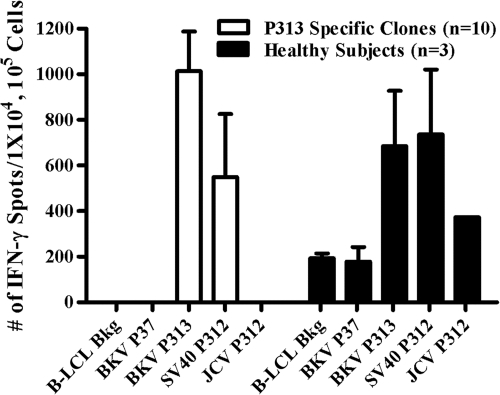
Since BKV shares >75% homology with the closely related human polyomavirus JCV and with SV40, we tested whether P313-stimulated, 14-day-expanded PBMC (n = 3) and P313-specific CD4+ T-cell clones (n = 10) from subject 2 would exhibit a cross-reactive IFN-γ+ response upon rechallenge with either JCV P312 or SV40 P312 peptide in an ELISPOT assay (Fig. 5). As expected, expanded PBMC from all three subjects and all 10 CD4+ T-cell clones showed a specific IFN-γ+ response to P313 rechallenge (684 ± 243 and 1,013 ± 173 spots/1 × 104 cells, respectively). An IFN-γ+ response was also seen in all expanded PBMC and T-cell clones upon rechallenge with SV40 P312 (579 ± 276 and 736 ± 284 spots/1 × 104 cells, respectively). However, expanded PBMC from only 1 of the 3 subjects showed an IFN-γ+ response to JCV P312 (373 spots/1 × 105 cells), while no response was seen in any of the P313-specific CD4+ T-cell clones derived from the second subject.
Fig. 5.
P313-specific T cells and clones recognize homologous SV40 and JCV peptides. To verify cross recognition between BKV LTA P313 and the homologous peptides JCV LTA P312 and SV40 LTA P312, IFN-γ+ responses were assessed in an ELISPOT assay using PBMC from three subjects (black bars) and 10 P313-specific CD4+ T-cell clones from one subject (white bars). Responses to BKV P313 and SV40 P312 were seen in all 3 subjects and 10/10 clones. In contrast, only subject 3 (1/3 subjects) recognized JCV P312. Data from positive stimulations are expressed as mean spot counts and SD. A negative-control peptide, P37, elicited no response in any of the clones evaluated (spot count = zero).
Cognate antigen presentation by P313-specific CD4+ T-cell clones.
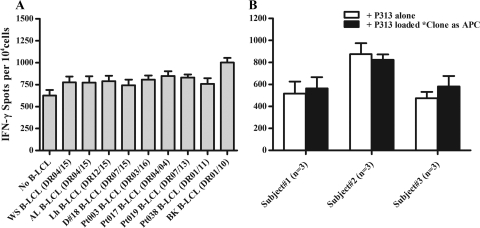
P313-specific clones responded by IFN-γ production to free peptide in the absence of APC (Fig. 6A). IFN-γ+ responses were not significantly affected when a panel of B-LCL was added to facilitate peptide antigen presentation. This observation suggested that at a high cell density, the cloned T cells themselves were capable of efficiently presenting peptide antigen to each other. The antigen presentation ability of T-cell clones was also demonstrated directly by replacing B-LCL with peptide-pulsed gamma-irradiated autologous T-cell clones (Fig. 6B). At a lower cell density, cognate antigen presentation of clones to each other became impaired and could be augmented by adding HLA-DR-matched B-LCL to the system (Fig. 7).
Fig. 6.
Cognate antigen presentation by P313-specific CD4+ T-cell clones. (A) T-cell clones (n = 5) from a healthy subject were assessed for an IFN-γ+ response to peptide P313 in an ELISPOT assay, and data are presented as means and SD. Even in the absence of B-LCL (first bar), P313 elicited an IFN-γ+ response (625 ± 144 spots/1 × 104 cells). This immune response, which was felt to reflect the ability of the CD4+ T-cell clones to present peptide antigens to each other, was increased only modestly when any one of nine different B-LCL with diverse HLA alleles were used as APC. No IFN-γ+ response was observed if P313 was omitted or replaced by the irrelevant peptide P37 (data not shown). (B) Cloned T cells from three different subjects responded to P313-pulsed gamma-irradiated (5,000 rads) (*) autologous T-cell clones as antigen-presenting cells. The signals obtained in this manner (black bars) are comparable to those obtained by stimulation directly with 1 μg/ml of free P313 peptide (white bars). The irrelevant peptide P37 did not elicit any response in this system (data not shown).
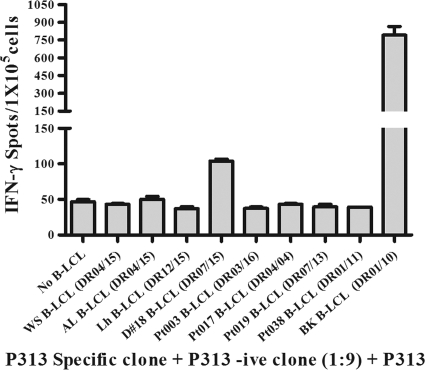
Fig. 7.
Evaluation of HLA-DR restriction in recognition of P313. P313-specific CD4+ T-cell clones from an HLA-DR01/07 subject were diluted 1:9 with irrelevant clones and tested for an IFN-γ+ response to P313, using a panel of B-LCL with diverse HLA-DR types as APC, and the data are expressed as mean spot counts and SD. Two B-LCL, with HLA-DR07/15 and -01/10, generated signals that were clearly above the background. Matching the HLA-DR allele was a necessary but not sufficient condition for antigen presentation, since no significant IFN-γ+ response was observed with one HLA-DR01/11- and one HLA-DR07/15-positive B-LCL.
HLA-DR-restricted, promiscuous recognition of BKV P313.
To determine if P313-specific CD4+ T-cell clones recognize P313 in an HLA-DR-restricted manner, we tested five different clones from a DR01/07-positive subject for the IFN-γ+ response to P313 by using B-LCL with matched or mismatched DR alleles as APC in an ELISPOT assay. In order to limit T-cell to T-cell antigen presentation by the clones, the cells were diluted with irrelevant CD4+ T-cell clones (1:9). As shown in Fig. 7, DR01/07 CD4+ T-cell clones showed an IFN-γ+ response to P313 above the background only with B-LCL expressing shared DR alleles with either DR01 (794 ± 73 spots/1 × 105 cells) or DR07 (104 ± 3 spots/1 × 105 cells), not with B-LCL expressing mismatched DR alleles. While these findings confirm that P313 is recognized in an HLA-DR-restricted manner, recognition of P313 was nevertheless promiscuous, since T-cell lines from 6 different subjects with various HLA-DR alleles (subjects 1, 2, 3, 16, 24, and 27) (Table 1) showed an IFN-γ+ response to P313.
Phenotypic and functional characterization of P313-specific CD4+ T-cell clones.
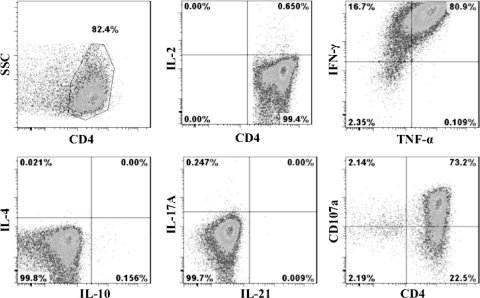
Three P313-specific CD4+ T-cell clones generated from different subjects were examined for surface expression of various markers by multicolor flow cytometry. All clones expressed CD45RO but not CD45RA, CD62L, CCR7, CD28, or CD127, consistent with an effector memory phenotype (Fig. 8). The majority of clones expressed the activation markers HLA-DR (96 to 99%), CD38 (96 to 98%), and CD86 (99 to 100%), while a smaller proportion (2 to 10%) expressed CD56. Staining for intracellular cytokines indicated that most cells produced IFN-γ (80 to 85%) and TNF-α (65 to 70%), while a predominance of CD107a surface staining (66 to 70%) (Fig. 9) suggested cytolytic granule translocation and exocytosis. A small proportion of cells produced IL-2 (0.6 to 1.6%), but no intracellular staining was observed for IL-4, IL-10, IL-17A, or IL-21 (Fig. 9).
Fig. 8.
Phenotypic characterization of peptide P313-specific CD4+ T-cell clones by flow cytometry. The figure shows representative data from one of three independent experiments collected from the CD4+ T-cell population in the forward/side scatter (FSC/SSC) lymphocyte gate. The analyzed cells expressed CD45RO but not CD45RA, CCR7, CD62L, CD28, or FAS/FAS-L. Several surface molecules typically seen in activated lymphocytes and antigen-presenting cells were upregulated, including HLA-DR (96 to 99%), CD38 (96 to 99%), and CD86 (99 to 100%). A small proportion of cells expressed the NK cell marker CD56 (9 to 21%).
Fig. 9.
Cytokine profile of P313-specific CD4+ T-cell clones characterized by multicolor flow cytometry. CD4/CD107a surface staining and intracellular staining for various cytokines were performed as described in Materials and Methods. The figure shows representative data from one of three independent experiments for the CD4+ T-cell population in the forward/side scatter (FSC/SSC) lymphocyte gate. The cloned T cells produced Th1 cytokines TNF-α (65 to 80%), IFN-γ (80 to 97%), and IL-2 (very little [0.6 to 1.6%]) upon 18 to 24 h of rechallenge with peptide P313. No intracytoplasmic staining for IL-4, IL-10, IL-17A, or IL-21 was observed. The degranulation marker CD107a (66 to 75%) was highly upregulated upon rechallenge with peptide P313.
The cytotoxic function of these T-cell clones was confirmed in a 51Cr release assay using P313-loaded and unloaded autologous or HLA-DR allele-matched B-LCL targets (Fig. 10). The latter experiments demonstrated dose-dependent and specific killing in up to 55% of target cells at an effector-to-target ratio of 25:1. The absence of surface staining for FAS and FAS-L suggests that this cytotoxic function is dependent on granule exocytosis and is not mediated by the FAS-FAS-L pathway. Taken together, our characterization studies show that P313-specific CD4+ T-cell clones are polyfunctional, with T-helper as well as T-cytotoxic function and the ability to secrete two different effector cytokines, IFN-γ and TNF-α.
Fig. 10.
51Cr release cytotoxicity assay. Three clones each from subjects 1, 2, and 3 were assayed for the ability to kill autologous or HLA-DR allele-matched B-LCL pulsed with peptide P313. A standard 4-h 51Cr release assay was performed with E/T ratios varying from 1.5:1 to 50:1. A dose-dependent cytolytic effect was demonstrated. Results are expressed as mean percent specific lysis (means and SD; n = 3). No killing was observed when HLA-DR allele-mismatched B-LCL pulsed with P313 were used as target cells (data not shown).
Peptide P313 sequence conservancy analysis.
A consensus amino acid sequence derived from 160 published BKV whole-genome sequences was used for peptide P313 conservancy analysis. Among 160 sequences, 140 differed from P313 only at the C-terminal position (isoleucine replaced by threonine) (Table 2), and 1/160 sequences had a phenylalanine replaced by cysteine at position 323. Thus, incorporation of P313 in a therapeutic vaccine peptide can be expected to provide broad coverage against most BKV strains circulating in nature. A comparison of the BKV P313 peptide sequence with the homologous JCV peptide P312 sequence (consensus of 450 JCV amino acid sequences derived from the corresponding whole-genome sequences) and the homologous SV40 peptide P312 sequence (consensus of 30 SV40 complete amino acid sequences) showed more frequent amino acid substitutions. P313 maps to the zinc finger of the DNA polymerase alpha region within the helicase domain of BKV LTA. Peptides derived from the DNA binding and host range domains of LTA (OPP4 and -13, respectively) were not immunogenic. This may reflect the availability of appropriate endogenously processed sequences for proteasomal digestion or selective survival of exogenous LTA proteins released from dead infected cells.
Table 2.
Peptide P313 sequence homology among known naturally occurring strains of polyomaviruses BKV, JCV, and SV40
| Peptide | Residue at BKV peptide position (JCV and SV40 peptide position)a: |
No. of strains covered | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 313 (312) | 314 (313) | 315 (314) | 316 (315) | 317 (316) | 318 (317) | 319 (318) | 320 (319) | 321 (320) | 322 (321) | 323 (322) | 324 (323) | 325 (324) | 326 (325) | 327 (326) | ||
| BKV peptides | 160 | |||||||||||||||
| BKV Dunlop P313 | P | Y | H | F | K | Y | H | E | K | H | F | A | N | A | I | 19 |
| BKV P313 variant 1 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | T | 140 |
| BKV P313 variant 2 | * | * | * | * | * | * | * | * | * | * | C | * | * | * | T | 1 |
| JCV peptides | 450 | |||||||||||||||
| JCV MAD1 P312 | * | N | * | * | N | H | * | * | * | * | Y | Y | * | * | Q | 444 |
| JCV P312 variant 1 | * | * | * | * | * | * | * | * | * | * | H | * | * | * | * | 2 |
| JCV P312 variant 2 | * | * | * | * | * | * | * | * | * | * | F | * | * | * | * | 1 |
| JCV P312 variant 3 | * | * | * | * | * | * | * | * | * | * | * | S | * | * | * | 1 |
| JCV P312 variant 4 | * | * | * | * | * | * | * | * | * | * | * | C | * | * | * | 1 |
| JCV P312 variant 5 | * | * | * | * | * | * | * | * | * | * | * | N | * | * | * | 1 |
| SV40 peptides | 30 | |||||||||||||||
| SV40 776 P312 | * | S | * | Y | * | * | * | * | * | * | Y | * | * | * | A | 29 |
| SV40 P312 variant 1 | * | * | * | * | * | H | * | * | * | * | * | * | * | * | * | 1 |
*, amino acid homology of BKV peptide P313 with the homologous peptides JCV P312 and SV40 P312.
DISCUSSION
Cell-mediated immunity to BKV is considered essential for maintaining successful viral latency in healthy subjects. Transition from the latent to the lytic phase of the viral life cycle is heralded by expression of LTA, which has been recognized to elicit T-cell responses in mice (29, 30). In humans, LTA-derived peptides bind multiple MHC class I and II alleles and are recognized by sensitized CD8+ as well as CD4+ T cells (25). However, peptides described to date elicit relatively weak immune responses limited to a small proportion of tested subjects. This underscores the need to identify immunodominant peptides that could be potential components of a vaccine against BKV. Hence, we set out to discover additional BKV LTA-derived peptides that can elicit strong T-cell responses, using PBMC from healthy subjects.
To overcome the recognized limitations of bioinformatics-based methods, we utilized the overlapping peptide pool approach to identify immunodominant peptides that would be recognized across multiple HLA alleles (20). We identified several highly reactive peptide pools, from which an immunodominant epitope, P313, was used to test and characterize T-cell responses. As reported by others, we were unable to detect T-cell responses to BKV LTA without initial expansion of PBMC in vitro. This problem likely reflects low frequencies of BKV-sensitized cells in peripheral blood (6, 41, 49) and could be overcome by culturing PBMC with BKV LTA PepMix in the presence of IL-2 prior to ELISPOT testing. Notably, several other studies also found it necessary to expand peripheral blood mononuclear cells for comparable durations prior to being able to detect virus-sensitized cells. The responses detected in this fashion appear to be functionally important, since high frequencies of cytotoxic T cells correlate with resolution of BKV infection in kidney transplant patients (6, 14, 41, 49) and of the related polyomavirus JCV infection in patients with progressive multifocal encephalopathy (10). The resulting increase in frequency of detectable BKV-reactive T cells could be due to expansion of preexisting memory cells, since the majority of the subjects tested (12/15 subjects) were BKV seropositive. For BKV-seronegative subjects, this could represent de novo activation of naïve T cells in vitro. However, the latter observation is also clinically relevant, since it demonstrates that lymphocytes that have not encountered viral antigens before can be sensitized to BKV for the purposes of adoptive immunotherapy.
As expected from DNA sequence homology, BKV P313-specific T-cell clones showed cross-reactivity with the homologous SV40 peptide P312. However, there was only limited cross-reactivity to JCV P312. Such variability in responses to peptides with comparable homologies could be due to differences in MHC binding affinity or in the preexisting T-cell repertoire or to gene polymorphisms that affect immune responses (32). Similar to our findings, cross recognition of SV40 and JCV LTA peptides binding HLA-A02 and HLA-B07/B08 by BKV-reactive CD8+ T cells was noted in another study (25). Others have reported similar cross recognition of BKV, SV40, and JCV VP1-derived peptides (7, 21). Phenotypic and functional characterization of P313-specific T-cell clones revealed that all clones were CD4+ CD28− and had the effector memory phenotype (CD45RO+ CD45RA− CCR7− CD62L−). Although this phenotype reflects expansion under in vitro conditions, it has relevance for the design of future T-cell therapy protocols dependent upon ex vivo generation of BKV-sensitized cells. While CD4+ T cells can present antigens directly only to cells that display MHC class II antigens (an unlikely scenario at sites of BKV latency in healthy subjects), these cells can certainly provide help to CD8+ T cells as well as B cells and can facilitate differentiation of antigen-specific naïve cells into memory cells capable of secondary responses (4, 47). In this regard, the expression of CD154 (CD40L) by these cells is notable and suggests an ability to promote affinity maturation and immunoglobulin class switching in B cells, which would lead to the production of virus-neutralizing antibodies.
The pattern of intracellular cytokine production (IFN-γ+ TNF-α+ IL-2± IL-4−) of the T-cell clones studied corresponds to that of highly differentiated Th1 cells, which are believed to confer antiviral protection. Similar to our findings, BKV-sensitized T cells were noted to produce both IFN-γ and TNF-α in a prior study of healthy subjects (49). P313-specific CD4+ T-cell clones also expressed CD107a, a marker that correlates with release of perforin and granzyme B (19). The absence of CD95L (FASL) expression indicates that their cytolytic function, as demonstrated in chromium release assays, is likely dependent upon cytotoxic granule exocytosis rather than the CD95L-CD95-mediated killing of target cells (2, 28, 48). Such polyfunctional CD4+ T cells that exhibit both helper and cytotoxic functions have been reported to arise in the context of other viral infections (43, 49).
A notable observation in this study was that subjects with diverse HLA-DR types were reactive to P313. Promiscuous binding of T-helper epitopes to both class I and class II MHC molecules is now well recognized (3, 13, 24, 31). Therefore, strict HLA-DR restriction is not a prerequisite for the development of a successful cell-mediated immune response to BKV. Indeed, a lack of stringent HLA-DR restriction would facilitate the development of peptide-based viral vaccines that have broad applicability across subjects with diverse HLA-DR alleles. The possibility that P313 is presented by other classical and nonclassical MHC molecules, such as HLA-DQ, HLA-DP, HLA-E, and CD1, was not examined in our study (5, 11, 39). P313-specific CD4+ T-cell clones expressed HLA-DR and presented the cognate peptide P313 to autologous clones in the absence of APC. Similar to these findings, activated human T cells have been reported to present influenza virus hemagglutinin and HIV gp120 to cognate T cells, suggesting that although T cells lack an antigen-processing function, they are capable of presenting cognate peptides when in an activated state (16, 22, 23, 26, 42). The clinical relevance of our observation is uncertain, since BKV cannot infect T cells directly. However, it is possible that activated T cells can process exogenous LTA for MHC loading in vivo.
In conclusion, we have identified an immunodominant peptide, P313, derived from BKV LTA, which shows sequence conservation across major BKV genotypes and the related species SV40 and JCV. P313-specific CD4+ T-cell responses were detected in PBMC of the majority of subjects following stimulation and expansion with BKV LTA. These T-cell responses were found to be HLA-DR restricted yet promiscuous, with cross-reactivity to homologous SV40 and JCV peptides. Antigen-specific CD4+ T-cell clones exhibited T-helper and T-cytotoxic functions, suggesting that they would be suitable for adoptive immunotherapy in immunosuppressed patients with BKV reactivation. Our data also identify P313 as a potential candidate for inclusion in a peptide-based vaccine that would be effective in subjects with diverse HLA alleles.
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Anthony D. D., Lehmann P. V. 2003. T-cell epitope mapping using the ELISPOT approach. Methods 29:260–269 [DOI] [PubMed] [Google Scholar]
- 2. Appay V., et al. 2002. Characterization of CD4(+) CTLs ex vivo. J. Immunol. 168:5954–5958 [DOI] [PubMed] [Google Scholar]
- 3. Axelsson-Robertson R., et al. 2010. Extensive major histocompatibility complex class I binding promiscuity for Mycobacterium tuberculosis TB10.4 peptides and immune dominance of human leucocyte antigen (HLA)-B*0702 and HLA-B*0801 alleles in TB10.4 CD8 T-cell responses. Immunology 129:496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casazza J. P., et al. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 203:2865–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castelli F. A., et al. 2002. HLA-DP4, the most frequent HLA II molecule, defines a new supertype of peptide-binding specificity. J. Immunol. 169:6928–6934 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y., et al. 2008. BKV and JCV large T antigen-specific CD8+ T cell response in HLA A*0201+ kidney transplant recipients with polyomavirus nephropathy and patients with progressive multifocal leukoencephalopathy. J. Clin. Virol. 42:198–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y. P., et al. 2006. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J. Virol. 80:3495–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daucher M., et al. 2008. Virological outcome after structured interruption of antiretroviral therapy for human immunodeficiency virus infection is associated with the functional profile of virus-specific CD8+ T cells. J. Virol. 82:4102–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dharnidharka V. R., Cherikh W. S., Abbott K. C. 2009. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation 87:1019–1026 [DOI] [PubMed] [Google Scholar]
- 10. Du Pasquier R. A., et al. 2003. Low frequency of cytotoxic T lymphocytes against the novel HLA-A*0201-restricted JC virus epitope VP1(p36) in patients with proven or possible progressive multifocal leukoencephalopathy. J. Virol. 77:11918–11926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falk C. S., Nossner E., Frankenberger B., Schendel D. J. 2000. Non-MHC-restricted CD4+ T lymphocytes are regulated by HLA-Cw7-mediated inhibition. Hum. Immunol. 61:1219–1232 [DOI] [PubMed] [Google Scholar]
- 12. Fioriti D., et al. 2005. BKV infection and hemorrhagic cystitis after allogeneic bone marrow transplant. Int. J. Immunopathol. Pharmacol. 18:309–316 [DOI] [PubMed] [Google Scholar]
- 13. Frahm N., et al. 2007. Extensive HLA class I allele promiscuity among viral CTL epitopes. Eur. J. Immunol. 37:2419–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ginevri F., et al. 2007. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am. J. Transplant. 7:2727–2735 [DOI] [PubMed] [Google Scholar]
- 15. Hariharan S. 2006. BK virus nephritis after renal transplantation. Kidney Int. 69:655–662 [DOI] [PubMed] [Google Scholar]
- 16. Hewitt C. R., Feldmann M. 1989. Human T cell clones present antigen. J. Immunol. 143:762–769 [PubMed] [Google Scholar]
- 17. Hirsch H. H., Randhawa P. 2009. BK virus in solid organ transplant recipients. Am. J. Transplant. 9(Suppl. 4):S136–S146 [DOI] [PubMed] [Google Scholar]
- 18. Hoffmeister B., et al. 2003. Mapping T cell epitopes by flow cytometry. Methods 29:270–281 [DOI] [PubMed] [Google Scholar]
- 19. Jongert E., et al. 2010. Functional characterization of in vivo effector CD4(+) and CD8(+) T cell responses in acute toxoplasmosis: an interplay of IFN-gamma and cytolytic T cells. Vaccine 28:2556–2564 [DOI] [PubMed] [Google Scholar]
- 20. Koralnik I. J., et al. 2002. Association of prolonged survival in HLA-A2(+) progressive multifocal leukoencephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J. Immunol. 168:499–504 [DOI] [PubMed] [Google Scholar]
- 21. Krymskaya L., et al. 2005. Cross reactivity of T lymphocytes recognizing a human cytotoxic T-lymphocyte epitope within BK and JC virus VP1 polypeptides. J. Virol. 79:11170–11178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanzavecchia A., Roosnek E., Gregory T., Berman P., Abrignani S. 1988. T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature 334:530–532 [DOI] [PubMed] [Google Scholar]
- 23. LaSalle J. M., Ota K., Hafler D. A. 1991. Presentation of autoantigen by human T cells. J. Immunol. 147:774–780 [PubMed] [Google Scholar]
- 24. Leen A. M., et al. 2008. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J. Virol. 82:546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J., et al. 2006. T-cell responses to peptide fragments of the BK virus T antigen: implications for cross-reactivity of immune response to JC virus. J. Gen. Virol. 87:2951–2960 [DOI] [PubMed] [Google Scholar]
- 26. Mannie M. D. 2001. T cell-mediated antigen presentation: a potential mechanism of infectious tolerance. Immunol. Res. 23:1–21 [DOI] [PubMed] [Google Scholar]
- 27. Mattes F. M., et al. 2008. Functional impairment of cytomegalovirus specific CD8 T cells predicts high-level replication after renal transplantation. Am. J. Transplant. 8:990–999 [DOI] [PubMed] [Google Scholar]
- 28. Mitra-Kaushik S., Cruz J., Stern L. J., Ennis F. A., Terajima M. 2007. Human cytotoxic CD4+ T cells recognize HLA-DR1-restricted epitopes on vaccinia virus proteins A24R and D1R conserved among poxviruses. J. Immunol. 179:1303–1312 [DOI] [PubMed] [Google Scholar]
- 29. Mylin L. M., Bonneau R. H., Lippolis J. D., Tevethia S. S. 1995. Hierarchy among multiple H-2b-restricted cytotoxic T-lymphocyte epitopes within simian virus 40 T antigen. J. Virol. 69:6665–6677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mylin L. M., et al. 1995. Cytotoxic T lymphocyte escape variants, induced mutations, and synthetic peptides define a dominant H-2Kb-restricted determinant in simian virus 40 tumor antigen. Virology 208:159–172 [DOI] [PubMed] [Google Scholar]
- 31. Nakagawa M., Kim K. H., Gillam T. M., Moscicki A. B. 2007. HLA class I binding promiscuity of the CD8 T-cell epitopes of human papillomavirus type 16 E6 protein. J. Virol. 81:1412–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nie S., Lin S. J., Kim S. K., Welsh R. M., Selin L. K. 2010. Pathological features of heterologous immunity are regulated by the private specificities of the immune repertoire. Am. J. Pathol. 176:2107–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Provenzano M., et al. 2006. Characterization of highly frequent epitope-specific CD45RA+/CCR7+/− T lymphocyte responses against p53-binding domains of the human polyomavirus BK large tumor antigen in HLA-A*0201+ BKV-seropositive donors. J. Transl. Med. 4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramaswami B., et al. 2009. HLA-A01-, -A03-, and -A024-binding nanomeric epitopes in polyomavirus BK large T antigen. Hum. Immunol. 70:722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramos E., Hirsch H. H. 2006. Polyomavirus-associated nephropathy: updates on a persisting challenge. Transpl. Infect. Dis. 8:59–61 [DOI] [PubMed] [Google Scholar]
- 36. Randhawa P., et al. 2006. Detection of CD8+ T-cells sensitized to BK virus large T-antigen in healthy volunteers and kidney transplant recipients. Hum. Immunol. 67:298–302 [DOI] [PubMed] [Google Scholar]
- 37. Randhawa P. S., Gupta G., Vats A., Shapiro R., Viscidi R. P. 2006. Immunoglobulin G, A, and M responses to BK virus in renal transplantation. Clin. Vaccine Immunol. 13:1057–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rao K. V., et al. 2009. Intravesicular cidofovir for the management of BK virus-associated cystitis. Biol. Blood Marrow Transplant. 15:391–392 [DOI] [PubMed] [Google Scholar]
- 39. Salerno-Goncalves R., Fernandez-Vina M., Lewinsohn D. M., Sztein M. B. 2004. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J. Immunol. 173:5852–5862 [DOI] [PubMed] [Google Scholar]
- 40. Schold J. D., et al. 2009. Treatment for BK virus: incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl. Int. 22:626–634 [DOI] [PubMed] [Google Scholar]
- 41. Sharma M. C., et al. 2006. Cross-reactive CTL recognizing two HLA-A*02-restricted epitopes within the BK virus and JC virus VP1 polypeptides are frequent in immunocompetent individuals. Virology 350:128–136 [DOI] [PubMed] [Google Scholar]
- 42. Siliciano R. F., et al. 1988. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell 54:561–575 [DOI] [PubMed] [Google Scholar]
- 43. van de Berg P. J., van Leeuwen E. M., ten Berge I. J., van Lier R. 2008. Cytotoxic human CD4(+) T cells. Curr. Opin. Immunol. 20:339–343 [DOI] [PubMed] [Google Scholar]
- 44. Wankowicz-Kalinska A., et al. 2003. Immunopolarization of CD4+ and CD8+ T cells to type-1-like is associated with melanocyte loss in human vitiligo. Lab. Invest. 83:683–695 [DOI] [PubMed] [Google Scholar]
- 45. Whiteside T. L. 2001. Measurement of cytotoxic activity of NK/LAK cells. Curr. Protoc. Immunol. 2001:Unit 7.18 [DOI] [PubMed] [Google Scholar]
- 46. Yssel H., Spits H. 2002. Generation and maintenance of cloned human T cell lines. Curr. Protoc. Immunol. 2002:Unit 7.19 [DOI] [PubMed] [Google Scholar]
- 47. Zaunders J. J., et al. 2004. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood 103:2238–2247 [DOI] [PubMed] [Google Scholar]
- 48. Zheng C. F., et al. 2007. Cytotoxic CD4+ T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood 109:2049–2057 [DOI] [PubMed] [Google Scholar]
- 49. Zhou W., et al. 2007. Functional characterization of BK virus-specific CD4+ T cells with cytotoxic potential in seropositive adults. Viral Immunol. 20:379–388 [DOI] [PubMed] [Google Scholar]