Abstract
The Bacillus anthracis exosporium protein BclA contains an O-linked antigenic tetrasaccharide whose terminal sugar is known as anthrose (J. M. Daubenspeck et al., J. Biol. Chem. 279:30945–30953, 2004). We hypothesized that serologic responses to anthrose may have diagnostic value in confirming exposure to aerosolized B. anthracis. We evaluated the serologic responses to a synthetic anthrose-containing trisaccharide (ATS) in a group of five rhesus macaques that survived inhalation anthrax following exposure to B. anthracis Ames spores. Two of five animals (RM2 and RM3) were treated with ciprofloxacin starting at 48 hours postexposure and two (RM4 and RM5) at 72 h postexposure; one animal (RM1) was untreated. Infection was confirmed by blood culture and detection of anthrax toxin lethal factor (LF) in plasma. Anti-ATS IgG responses were determined at 14, 21, 28, and 35 days postexposure, with preexposure serum as a control. All animals, irrespective of ciprofloxacin treatment, mounted a specific, measurable anti-ATS IgG response. The earliest detectable responses were on days 14 (RM1, RM2, and RM5), 21 (RM4), and 28 (RM3). Specificity of the anti-ATS responses was demonstrated by competitive-inhibition enzyme immunoassay (CIEIA), in which a 2-fold (wt/wt) excess of carbohydrate in a bovine serum albumin (BSA) conjugate of the oligosaccharide (ATS-BSA) effected >94% inhibition, whereas a structural analog lacking the 3-hydroxy-3-methyl-butyryl moiety at the C-4" of the anthrosyl residue had no inhibition activity. These data suggest that anti-ATS antibody responses may be used to identify aerosol exposure to B. anthracis spores. The anti-ATS antibody responses were detectable during administration of ciprofloxacin.
INTRODUCTION
Inhalation anthrax has a rapid onset and is usually fatal if untreated. In this form of the disease, spores of Bacillus anthracis are initially presented to the host immune system at the lung surface. Viable infectious spores subsequently outgrow into vegetative cells and proliferate (10, 11, 13, 28). The vegetative bacilli produce the major known virulence factors, i.e., the anthrax toxin proteins protective antigen (PA), lethal factor (LF), and edema factor (EF) together with the poly-γ-d-glutamic acid capsule (γPGA) (25, 31, 32, 36). In the anthrax attacks of 2001, the fatality rate was 45% despite use of antibiotics and supportive treatment (17, 18). Without treatment, fulminant anthrax invariably results in systemic bacteremia, sepsis-induced shock, respiratory distress, extensive hemorrhage, and death (22).
In the event of a bioterrorism-related B. anthracis spore release, a first-line response will be administration of antibiotics and vaccine to individuals at risk of infection as defined by the exposure zone. Defining a B. anthracis exposure zone, however, remains difficult due to the potential for widespread dissemination of B. anthracis spores and the detection limitations of environmental sampling techniques (4, 29). Diagnostic tests with high sensitivity and specificity targeting spore antigens and the host responses to such antigens will be important in addressing this deficit. Currently, the most widely used diagnostics for anthrax rely on culture isolation of the organism, immunohistochemistry for vegetative cell antigens, and PCR for B. anthracis-specific gene targets (15, 30, 35). As a measure of the host systemic response, seroconversion to B. anthracis-specific antigens also has an important role in confirmatory diagnosis, as it may be elicited by low and transient levels of infection (8, 26). A limitation of serologic responses to B. anthracis vegetative cell or toxin antigens, however, is that stimulation of an immune response to these antigens requires some level of spore outgrowth and toxin production, which, although clinically relevant, may be constrained or prevented by antibiotic intervention (9). Thus, a serologic marker that is independent of vegetative cell outgrowth and toxin production and that is not affected by antibiotic treatment might provide a valuable tool for identifying individuals at risk following a release of B. anthracis and an aid in defining human and animal exposure to spores.
We have shown previously that the anthrose oligosaccharide located in the exosporium contains specific antigenic determinants. The structure of this oligosaccharide is a β-Ant-(1→3)-α-l-Rha-(1→3)-α-l-Rha-(1→2)-α-l-Rha tetrasaccharide, in which the anthrosyl residue (Ant) is a 2-O-methyl-4-(3-hydroxy-3-methylbutamido)-4,6-dideoxy-d-glucopyranose (3, 6, 20, 21, 33, 37). Rabbits vaccinated with live or irradiated killed B. anthracis Sterne spores mounted an immune response against a synthetic anthrosyl-containing trisaccharide (ATS) (Fig. 1 A). The critical part of the antigenic region was localized to a 3-methyl butyryl substituent on the anthrosyl residue of the ATS (21). We hypothesized that this B. anthracis spore surface carbohydrate antigen would also elicit a specific, measurable antibody response following aerosol exposure to B. anthracis Ames spores and asymptomatic infection or nonfatal clinical disease. In the present study, we tested this hypothesis using sera from five rhesus macaques that had survived inhalation infection with B. anthracis Ames with or without antibiotic intervention. The data presented provide proof of concept for the utility of the spore surface carbohydrate as a marker of B. anthracis spore exposure and infection during concurrent treatment with ciprofloxacin.
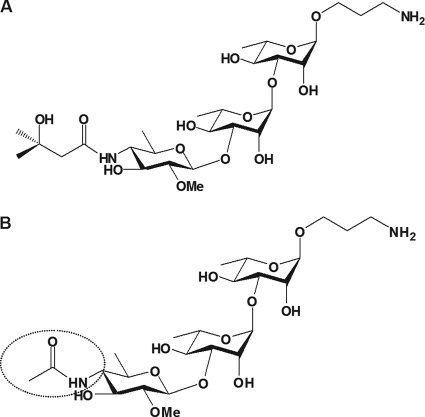
Fig. 1.
(A) Structure of the synthetic anthrose trisaccharide (ATS) composed of a terminal anthrosyl residue attached to a rhamnan disaccharide. Also shown is the amino propyl spacer used in the ATS-protein conjugates. This synthetic structure is based on the oligosaccharide moiety of the glycoprotein BclA from the exosporium of B. anthracis (3). (B) Structure of a synthetic analog (HNAc) of the anthrosyl-containing trisaccharide. The compound is N acetylated at the C-4" and therefore lacks most of the 3-hydroxy-3-methyl-butyryl moiety of the native anthrosyl-containing oligosaccharide (circled).
MATERIALS AND METHODS
Aerosol challenge of rhesus macaques.
All animal procedures were implemented at Battelle Biomedical Research Center (BBRC), Columbus, OH, under protocols approved by CDC and Battelle Institutional Animal Care and Use Committees (IACUC). All animals were monitored for clinical signs and symptoms throughout the course of the study, and the stringent requirements for care and use set down by CDC IACUC guidelines, which adhere to the National Institutes of Health guidelines for the care and use of laboratory animals, were followed (23). All aspects of the study were designed to minimize stress in the animals. Animals judged to be moribund after spore challenge were humanely euthanized. Aerosol challenge and blood collection protocols have been described elsewhere (2). The five animals (RM1 to RM5) reported on in this study were survivors from an infection study involving a larger cohort of 24 rhesus macaques (Macaca mulatta) that were exposed to a median dose of 246 ± 40 50% lethal dose (LD50) equivalents of B. anthracis Ames spores, with an exposure/fatality ratio of 94.7% in naïve animals (A. E. Boyer et al., unpublished data). RM1 survived infection without intervention. RM2 to RM5 received ciprofloxacin (twice a day [BID], 16 mg/kg, 14 days) starting at either 48 h postexposure (RM2 and RM3) or 72 h postexposure (RM4 and RM5).
Bacteremia.
For confirmation of infection, whole blood samples were assessed for bacteremia at 42 days prior to exposure (negative control), at 12, 24, 30, 36, 48, 72, 96, 120, 144, and 168 h postexposure, and at euthanasia. Approximately 10 to 40 μl of whole blood was inoculated by loop onto a blood agar plate and incubated at 37 ± 2°C for a minimum of 48 h. Positive B. anthracis bacteremia was indicated by the presence of white colonies, 4 to 10 mm in diameter, with a rough appearance and irregular edges. Specific grading of bacteremia is reported elsewhere (2).
LF quantification.
Toxemic infection was confirmed by quantitative analysis of anthrax toxin lethal factor (LF) levels in plasma taken 42 days prior to exposure (negative control) and at 12, 18, 24, 30, 36, 48, 72, 96, 120, 144, and 168 h and 14, 21, 28, and 35 days postexposure (2). Briefly, LF in a sample was captured by two anti-LF monoclonal antibodies (MAb) covalently linked to magnetic Dynabeads MyOne Tosylactivated beads (Invitrogen Corp., Carlsbad, CA) and then mixed with 30 μl buffer and a synthetic peptide substrate optimized for cleavage by LF. This mixture was incubated at 37°C for 2 h, and then a 3-μl volume was analyzed by isotope dilution matrix-assisted laser desorption ionization mass spectrometry (MS). The remaining LF-substrate mixture was incubated for an additional 16 h and then analyzed by MS again with the appropriate standard curves and quality control materials described below. To achieve the necessary dynamic range and to account for high-sensitivity rapid-response sample analysis, two standard curves were prepared in plasma, one for 20-μl (low volume [LV]) and the other for 200-μl (high volume [HV]) sample volumes. In addition four quality control samples (QCs) were prepared from human plasma pools (Interstate Blood Bank, Memphis, TN) using recombinant LF (List Biological Laboratory, Campbell, CA) and validated. The LV standard curve ranged from 0.025 to 100 ng/ml, with QCs at 6.3, 1.25, and 0.14 ng/ml. LV analysis at 2 h of incubation covered a dynamic range of 0.125 to 50 ng/ml, and that at 18 h covered 0.025 to 10 ng/ml. The HV standards and samples were analyzed at 2 h for high-sensitivity qualitative diagnostics (limit of detection, 0.025 ng/ml) and at 18 h for quantification, which covered 0.005 to 1 ng/ml and included QCs at 0.14 and 0.014 ng/ml. The relative standard deviations (SDs) for QC validation ranged from 8.5 to 14.7% (M. Gallegos-Candela, unpublished data).
Detection of anti-ATS IgG in rhesus macaque sera.
Sera from the five rhesus macaques that survived inhalation anthrax were obtained at 30 days preexposure and 14, 21, 28, and 35 days postexposure and analyzed for an anti-ATS IgG response by enzyme-linked immunosorbent assay (ELISA). Chemical synthesis of the anthrosyl-containing oligosaccharide, its structural analogs, and conjugation to protein carriers have been described elsewhere (21). Briefly, Immulon 2 HB flat-bottom 96-well microtiter plates (Thermo Labsystems, Franklin, MA) were coated with 100 μl/well of the ATS-keyhole limpet hemocyanin (KLH) conjugate at a concentration of 0.03 μg/ml of carbohydrate content (0.5 μg/ml by protein content) or with 0.5 μg/ml KLH (Pierce Biotechnology, Inc., Rockford, IL) in coating buffer (0.01 M phosphate-buffered saline [PBS], pH 7.4). Plates were covered with plastic wrap, stored at +4°C, and used within 7 days. For ELISA, plates were washed three times in wash buffer (0.01 M PBS [pH 7.4], 0.1% Tween 20) using an ELX405 microplate washer (BioTek Instruments Inc., Winooski, VT). Serial dilutions (100 μl/well) of serum in dilution buffer (0.01 M PBS [pH 7.4], 5% skim milk, 0.5% Tween 20) were added and plates incubated for 60 min at 37°C. After plates were washed three times in wash buffer, horseradish peroxidase (HRPO)-conjugated goat anti-rhesus monkey IgG (Research Diagnostics, Inc., Flanders, NJ) was added at a dilution of 1:10,000 (100 μl/well) and the incubation continued for 60 min at 37°C. Plates were washed three times in wash buffer, and bound conjugate was detected colorimetrically by using 100 μl/well 2,2≪-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS)–H2O2 substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Color development was for 15 min and was stopped by addition of 100 μl of peroxidase stop solution (Kirkegaard and Perry Laboratories, Gaithersburg, MD) to each well. Optical density (OD) values were read within 15 min. Samples were tested in duplicate in each of two independent experiments, and the average OD and standard deviation (SD) were calculated. Anti-ATS IgG responses were expressed as a titer based on a reactivity threshold (RT). The RT was determined from the average OD value plus one SD from the sera of 114 naïve rhesus macaques tested against ATS-KLH by ELISA. Each naïve serum was tested twice at a 1:100 dilution in dilution buffer. From these data the RT was calculated as an OD value of 0.317. Serum titers were assigned as the reciprocal value of the first discrete serum dilution which resulted in an OD reading above the RT.
Anti-ATS IgG inhibition ELISA.
To confirm the specificity of the anti-ATS antibody response, the anti-ATS IgG ELISA described above was modified to a competitive-inhibition format using a 2- to 128-fold (wt/wt) carbohydrate excess of a conjugate (ATS-BSA) consisting of ATS and bovine serum albumin (BSA) (Pierce Biotechnology, Rockford, IL). A noncompetitive synthetic trisaccharide analog conjugated to BSA (HNAc-BSA) which lacked the 3-hydroxy-3-methyl-butyryl moiety at the C-4" of the anthrosyl residue was used as the specificity control (Fig. 1B). Unconjugated BSA was used as a noninhibiting reagent control. Dilution buffer served as the 100% binding activity reference (0% inhibition). Serum samples were tested in duplicate in each of three independent experiments and the results reported as the average percent inhibition with standard error (SE).
Statistical analyses.
Differences in the anti-ATS IgG titers were analyzed using the Kruskal-Wallis analysis of variance on ranks test procedure in SigmaPlot 11 (Systat Software, Inc., San Jose, CA).
RESULTS
Confirmation of infection.
Infection was confirmed in the five animals (RM1 to RM5) that survived aerosol exposure to B. anthracis. Of these animals, only RM1 did not receive ciprofloxacin. All five animals exhibited transient bacteremia at between 30 h and 72 h postexposure, and all were nonbacteremic by 96 h postexposure (Table 1). In addition, LF quantification (Table 2) demonstrated that toxemia was detectable as early as 18 h postexposure (in RM2, RM3, and RM5). All animals were toxemic by 36 h postexposure, and all animals remained toxemic until at least day 7 postexposure. The untreated animal RM1 had low LF levels on all sampling dates for which LF was quantified. At no time during the study did RM1 LF levels rise above a maximum of 7.68 ng/ml (at 72 h postexposure) (Table 2). These parameters of bacteremia and toxemia are consistent with a high exposure/fatality ratio in untreated rhesus macaques under similar study conditions (2; Boyer et al., unpublished data).
Table 1.
Determination of infection in rhesus macaques by measurement of bacteremia after aerosol exposure to B. anthracis Ames spores
| Animal | Gender | Challenge wt (kg) | LD50 equivalentc |
Bacteremia on day: |
First ciprofloxacin administration (h) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (12 h) | 1 |
2 (48 h) | 3 (72 h) | 4 (96 h) | 5 (120 h) | 6 | 7 | |||||||
| 24 h | 30 h | 36 h | ||||||||||||
| RM1 | Male | 4.0 | 230 | − | − | − | − | + | − | − | − | − | − | NAa |
| RM2 | Male | 4.2 | 266 | − | − | + | + | − | − | − | − | − | − | 48 |
| RM3 | Female | 5.4 | 265 | − | − | + | + | +/−b | − | − | − | − | − | 48 |
| RM4 | Male | 5.6 | 207 | − | − | − | − | + | + | − | − | − | − | 72 |
| RM5 | Female | 2.8 | 243 | − | − | − | + | + | − | − | − | − | − | 72 |
NA, not applicable.
+/−, low positive result with only one to five colonies in the primary streak.
For Bacillus anthracis Ames, LD50 equivalent = inhaled dose (CFU/animal)/55,000 (where 55,000 is the reported LD50 of anthrax spores in rhesus monkeys [2]).
Table 2.
Determination of infection in rhesus macaques by measurement of lethal factor after aerosol exposure to B. anthracis Ames spores
| Animal | Gender | Challenge wt (kg) | LD50 equivalentb | Lethal factor (ng/ml) on day: |
First ciprofloxacin administration (h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 |
1 |
2 (48 h) | 3 (72 h) | 4 (96 h) | 5 (120 h) | 6 | 7 | 14 | ||||||||
| 12 h | 18 h | 24 h | 30 h | 36 h | ||||||||||||
| RM1 | Male | 4.0 | 230 | 0.03 | 1.60 | 7.68 | 1.96 | 1.87 | 1.54 | 1.65 | 0.165 | NAa | ||||
| RM2 | Male | 4.2 | 266 | 0.031 | 0.18 | 4.12 | 50.28 | 41.35 | 6.14 | 1.53 | 0.35 | 0.10 | 0.21 | 48 | ||
| RM3 | Female | 5.4 | 265 | 0.013 | 0.24 | 5.13 | 30.21 | 55.18 | 5.70 | 3.83 | 1.06 | 0.41 | 0.31 | 48 | ||
| RM4 | Male | 5.6 | 207 | 0.21 | 0.06 | 4.87 | 54.99 | 6.14 | 1.67 | 0.95 | 0.44 | 72 | ||||
| RM5 | Female | 2.8 | 243 | 0.015 | 0.05 | 0.39 | 5.48 | 24.66 | 18.45 | 5.04 | 1.50 | 0.58 | 0.19 | 72 | ||
NA, not applicable.
For Bacillus anthracis Ames, LD50 equivalent = inhaled dose (CFU/animal)/55,000 (where 55,000 is the reported LD50 of anthrax spores in rhesus monkeys [2]).
Anti-ATS IgG responses in rhesus macaques.
The baseline and postchallenge anti-ATS antibody responses were determined for the five rhesus macaques that survived aerosol challenge with B. anthracis Ames. There were no detectable, above-threshold anti-ATS IgG responses in the baseline serum of any test animal (−30 days). All five animals mounted an anti-ATS response that was highly specific for the terminal anthrosyl group. The earliest responses were detected on day 14 in RM1, RM2, and RM5, with average titers of 100, 100, and 350, respectively (Table 3; Fig. 2). Rhesus macaque RM4 responded by day 21 (titer = 150) and RM3 by day 28 (titer = 250). From day 14 to day 35 the anti-anthrose IgG titer increased 56-fold (rising from 100 to 5,600) for RM1, 9-fold (rising from 100 to 900) for RM2, greater than 6-fold (rising from <RT to 600) for RM3, and 4.57-fold (rising from 350 to 1,600) for RM5. The response mounted by animal RM4 rose to 175 relative to prechallenge responses but increased only slightly between day 21 and day 28 postexposure. There was no RM4 sample available to test the day 35 response. While the untreated animal RM1 did not respond earlier than the four animals that received ciprofloxacin treatment, one-way analysis of variance performed on the anti-anthrose IgG titers showed that on days 21, 28, and 35 the titers in RM1 were significantly higher than the titers in the other animals (P < 0.001). This indicates that antibiotic intervention during infection may have an impact on the magnitude of the detectable anti-ATS antibody response.
Table 3.
Average anti-ATS IgG titers in rhesus macaque sera 30 days before and 14, 21, 28, and 35 days after exposure to B. anthracis Ames aerosolized spores
| Animal | Avg titera on day: |
||||
|---|---|---|---|---|---|
| −30 | 14 | 21 | 28 | 35 | |
| RM1 | <RT2 | 100 | 1,600b | 3,200 | 5,600 |
| RM2 | <RT | 100 | 600 | 800b | 900 |
| RM3 | <RT | <RT | <RT | 250 | 600b |
| RM4 | <RT | <RT | 150 | 175b | NS |
| RM5 | <RT | 350 | NS | NS | 1,600b |
Titer averages are based on four separate OD measurements in two independent experiments. RT, reactivity threshold; NS, no sample available for testing.
Serum used in the inhibition assay.
Fig. 2.
Anti-ATS IgG responses in rhesus macaques (RM) that survived inhalation anthrax. Animals were exposed to 246 ± 40 LD50 equivalents of B. anthracis Ames spores. Animal RM1 was an untreated control. Two of the animals (RM2 and RM3) were treated with ciprofloxacin starting at 48 h. Animals RM4 and RM5 received ciprofloxacin starting at 72 h postexposure. Anti-ATS IgG was determined by ELISA. Error bars indicate the standard deviations of four titer determinations based on OD measurements from two separate experiments.
Specificity of the anti-ATS IgG response.
The specificity of the interaction of the rhesus macaque infection sera with the ATS-KLH conjugate was assessed by competitive inhibition with increasing amounts (2- to 128-fold excess) of the ATS-BSA conjugate or the BSA conjugate of a structural analog lacking the 3-hydroxy-3-methyl-butyryl moiety at the C-4" of the anthrosyl residue (Fig. 1B) (21). Serum dilutions for specificity analyses were selected on the basis of available quantities for testing and a minimum anti-ATS reactivity titer of 175 (Table 3). A 2-fold excess (wt/wt) of the ATS-BSA conjugate relative to the carbohydrate coating antigen effected greater than 94% inhibition. Under the same experimental conditions, the BSA reagent control and structural analog conjugate control resulted in less than 20% inhibition (Fig. 3). These data indicate that the anti-ATS antibody response elicited due to inhalation anthrax in these animals was specific for the trisaccharide component and was directed primarily at the terminal anthrosyl group.
Fig. 3.
Percent inhibition of anti-ATS IgG binding to ATS-KLH-coated ELISA plates. Specificity of the anti-ATS response was demonstrated by competitive inhibition immunoassay, adding the ATS-BSA conjugate or the HNAc-BSA conjugate at a 2-fold excess (wt/wt) of carbohydrate together with the serum to the microtiter wells. Addition of buffer only served as the no-inhibitor control and was the 100% binding activity reference point for calculating percent inhibition in the wells to which BSA, ATS-BSA, or HNAc-BSA was added. Selected test serum dilutions were 1:400 (RM1, day 21), 1:400 (RM2, day 28), 1:200 (RM3, day 35), 1:100 (RM4, day 28), and 1:800 (RM5, day 35). Bars represent the means from three separate experiments. Error bars indicate the standard errors.
DISCUSSION
The infectious disease continuum that culminates in fulminant inhalation anthrax includes exposure to spores and subsequent infection. Currently, candidacy for postexposure disease prevention (PEP) interventions is based solely on an individual's suspected exposure to B. anthracis. There are no available clinical tests or assays to distinguish B. anthracis-exposed individuals at risk of disease. It is probable, therefore, that many individuals may receive unnecessary PEP due to minimal (if any) risk for developing anthrax.
We reported previously that the ATS component of the B. anthracis exosporium glycoprotein BclA elicits a specific IgG immune response to the terminal anthrosyl group in rabbits vaccinated intramuscularly with live or irradiated killed B. anthracis Sterne spores (21). We hypothesized, therefore, that the anthrosyl group was an important epitope on the B. anthracis spore surface and that aerosol exposure of rhesus macaques to virulent B. anthracis Ames spores would consequently induce a specific antibody response against the ATS and the anthrosyl moiety in particular. This study tested this hypothesis using a synthetic ATS-KLH conjugate ELISA to detect anti-ATS IgG antibody responses in the sera of five rhesus macaques that were exposed to aerosolized B. anthracis Ames spores and had survived inhalation anthrax. All five animals demonstrated bacteremia and toxemia consistent with infection. We evaluated the potential for using host anti-ATS IgG as a serologic marker of B. anthracis spore exposure and nonfatal anthrax when ciprofloxacin was administered as an antibiotic medical countermeasure. Ciprofloxacin was administered to four of the five test subjects in a clinically relevant time frame postexposure (48 to 72 h) for an emergency response to an anthrax outbreak. In addition, there was one untreated survivor of inhalation anthrax. The data clearly demonstrate that all five animals mounted a specific IgG antibody response against the anthrosyl component of the ATS. Importantly, the specific anti-ATS response was detectable in animals treated with ciprofloxacin.
These data confirm (i) the utility of a spore carbohydrate antigen as a biomarker for infection and (ii) that the anti-ATS antibody response is detectable during concomitant postexposure administration of antimicrobials (R. W. Carlson, G.-J. Boons, T. Buskas, B. Choudhury, E. Kannenberg, C. Leoff, A. Mehta, E. Saile, J. Rauvolfova,C. Quinn, P. Wilkins, M. Vasan, and M. A. Wolfert, U.S. patent applications 2009/0246200 A1 and 2010/0233174 A9). In this context, even though it may require 14 days or more for a detectable response, an anti-ATS antibody reaction may be a valuable tool for ascertaining spore exposure in asymptomatic individuals, thus aiding targeted intervention with anthrax medical countermeasures. While it is feasible that the onset of the response was earlier than 14 days, there is invariably an associated lag period between antigenic challenge and the onset of the primary IgG response (26). Evaluation of anti-ATS IgM responses is under way in this laboratory. Ideally, an early host response to ATS could be used to provide immediate point-of-care (POC) information that facilitates rapid decision making during an emergency response. The time dependency for a serologic response is therefore a potential limitation of this approach. In practice, however, this may not be an impediment to the value of an anti-ATS assay because of the lag between incidence occurrence and collection of acute- and convalescent-phase sera.
Although much has been learned since the anthrax emergency response of the fall of 2001, those events remain the only practicable example of the time frame for collection and evaluation of serum samples following a bioterrorism event and indicate that in an emergency response serum sample acquisition is likely (advantageously) to coincide directly with the acute and convalescent phases of a specific antibody response (26). The value of quantitative determination of specific antibody responses in such a scenario was demonstrated during the anthrax letter attacks in 2001. These data indicated that the initial mail attack occurred more than a week earlier than was first estimated and placed the location of that incident in New York, NY, ∼1,200 miles north of the original estimate of Palm Beach County, FL (26). These antitoxin serology data were limited, however, in that the anti-PA antibody responses were detectable only in confirmed clinical cases and unable to determine exposure of asymptomatic persons (1, 8, 14, 26, 27). These analyses did not affect the immediate and likely life-saving responses of intervention with antibiotics (16, 17). Of additional importance and value is that the duration of a specific antibody response may be such that spore exposure can be determined retrospectively in persons who have late presentation of disease or who are only later realized to have been exposed (26).
It is feasible that the magnitude of an anti-ATS antibody response may be related to the level of spore exposure and the degree of vegetative cell development (infection). The contribution of vegetative cell development to the anti-ATS IgG response was demonstrated previously by Mehta et al. by comparing live spore and irradiated killed spore preparations in rabbits (21). In the present study this effect may be indicated in the response of the single untreated animal, in which the anti-ATS titers were significantly higher than those in the four animals receiving ciprofloxacin. In such a scenario one might speculate that antibiotic treatment accelerated clearance of replicating vegetative cells, resulting in a lower anti-ATS response. It is also possible that the level of spore exposure will influence significantly the magnitude and duration of an anti-ATS IgG response. Ongoing and future studies will evaluate these aspects of the anti-ATS response.
Another facet of the anti-ATS response that is currently under investigation in this and other laboratories is its diagnostic specificity (5, 34). For example, Dong et al. (6) reported that synthesis of anthrose, of which ATS is a component, is not restricted solely to B. anthracis but may occur in other closely related Bacillus species, although at lower levels, and may correlate with virulence phenotypes within the B. cereus lineage (6, 12). It will be of value to determine if infections with other Bacillus species are also capable of eliciting an anti-ATS response. Having already determined a crucial motif of the anthrose epitope (21), we are in a position to structurally optimize our capture antigens to enhance specificity of detection (24).
We conclude that rhesus macaques that survive exposure to and infection from aerosols of B. anthracis mount an anti-ATS IgG response. These data provide proof of concept for the antigenicity of the exosporium anthrose trisaccharide as a marker of B. anthracis spore exposure and infection. Future studies will evaluate enhanced analytic sensitivity of the anti-ATS antibody detection assay and determine the diagnostic sensitivity and specificity of the anti-ATS antibodies and their utility as a diagnostic tool (7, 19). Collectively these data will contribute to our ability to quickly detect exposure to B. anthracis spores.
ACKNOWLEDGMENTS
This work was supported by NIAID grant R21 AI059577 (to R.W.C.), by NIH grant GM065248 (to G.-J.B.), and in part by DOE grant DE-FG02-93ER20097 (to the CCRC). E.S. was funded in part through the Atlanta Research and Education Foundation (AREF), Atlanta, GA. Part of the work was performed by Battelle Biomedical Research Center, Columbus, OH, under contract SPO700-00-D-3180. We also gratefully acknowledge funding support from the Biomedical Advance Research and Development Authority (BARDA).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 9 March 2011.
REFERENCES
- 1. Baggett H. C., et al. 2005. No evidence of a mild form of inhalational Bacillus anthracis infection during a bioterrorism-related inhalational anthrax outbreak in Washington, D.C., in 2001. Clin. Infect. Dis. 41:991–997 [DOI] [PubMed] [Google Scholar]
- 2. Boyer A. E., et al. 2009. Kinetics of lethal factor and poly-d-glutamic acid antigenemia during inhalation anthrax in rhesus macaques. Infect. Immun. 77:3432–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daubenspeck J. M., et al. 2004. Novel oligosaccharide side chains of the collagen-like region of BclA, the major glycoprotein of the Bacillus anthracis exosporium. J. Biol. Chem. 279:30945–30953 [DOI] [PubMed] [Google Scholar]
- 4. Dewan P. K., et al. 2002. Inhalational anthrax outbreak among postal workers, Washington, D.C., 2001. Emerg. Infect. Dis. 8:1066–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhénin S. G., Moreau V., Nevers M. C., Créminon C., Djedaïni-Pilard F. 2009. Sensitive and specific enzyme immunoassays for antigenic trisaccharide from Bacillus anthracis spores. Org. Biomol. Chem. 7:5184–5199 [DOI] [PubMed] [Google Scholar]
- 6. Dong S., et al. 2008. Anthrose biosynthetic operon of Bacillus anthracis. J. Bacteriol. 190:2350–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong S., et al. 2010. Characterization of the enzymes encoded by the anthrose biosynthetic operon of Bacillus anthracis. J. Bacteriol. 192:5053–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doolan D. L., et al. 2007. The US Capitol bioterrorism anthrax exposures: clinical epidemiological and immunological characteristics. J. Infect. Dis. 195:174–184 [DOI] [PubMed] [Google Scholar]
- 9. Friedlander A. M., et al. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239–1243 [DOI] [PubMed] [Google Scholar]
- 10. Glomski I. J., Piris-Gimenez A., Huerre M., Mock M., Goossens P. L. 2007. Primary involvement of pharynx and Peyer's patch in inhalational and intestinal anthrax. PLoS Pathog. 3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guidi-Rontani C., Weber-Levy M., Labruyère E., Mock M. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9–17 [DOI] [PubMed] [Google Scholar]
- 12. Han C. S., et al. 2006. Pathogenomic sequence analysis of Bacillus cereus and Bacillus thuringiensis isolates closely related to Bacillus anthracis. J. Bacteriol. 188:3382–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanna P. C., Ireland J. A. 1999. Understanding Bacillus anthracis pathogenesis. Trends Microbiol. 7:180–182 [DOI] [PubMed] [Google Scholar]
- 14. Hsu V. P., et al. 2002. Opening a Bacillus anthracis-containing envelope, Capitol Hill, Washington, D.C.: the public health response. Emerg. Infect. Dis. 8:1039–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. International Office of Epizootics, World Health Organization, and Food and Agriculture Organization of the United Nations 2008. Anthrax in humans and animals, 4th ed. World Health Organization, Geneva, Switzerland [Google Scholar]
- 16. Jefferds M. D., et al. 2002. Adherence to antimicrobial inhalational anthrax prophylaxis among postal workers, Washington, D.C., 2001. Emerg. Infect. Dis. 8:1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jernigan D. B., et al. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jernigan J. A., et al. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kubler-Kielb J., et al. 2008. Saccharides cross-reactive with Bacillus anthracis spore glycoprotein as an anthrax vaccine component. Proc. Natl. Acad. Sci. U. S. A. 105:8709–8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuehn A., et al. 2009. Development of antibodies against anthrose tetrasaccharide for specific detection of Bacillus anthracis spores. Clin. Vaccine Immunol. 16:1728–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta A. S., et al. 2006. Synthesis and antigenic analysis of the BclA glycoprotein oligosaccharide from the Bacillus anthracis exosporium. Chemistry 12:9136–9149 [DOI] [PubMed] [Google Scholar]
- 22. Mock M., Mignot T. 2003. Anthrax toxins and the host: a story of intimacy. Cell. Microbiol. 5:15–23 [DOI] [PubMed] [Google Scholar]
- 23. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 24. Oberli M. A., et al. 2010. Molecular analysis of carbohydrate-antibody interactions: case study using a Bacillus anthracis tetrasaccharide. J. Am. Chem. Soc. 132:10239–10241 [DOI] [PubMed] [Google Scholar]
- 25. Preisz H. 1909. Experimentelle Studien über Virulenz, Empfänglichkeit und Immunität beim Milzbrand. Z. Immunitätsforschung. 5:341–452 [Google Scholar]
- 26. Quinn C. P., et al. 2004. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J. Infect. Dis. 190:1228–1236 [DOI] [PubMed] [Google Scholar]
- 27. Quinn C. P., et al. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 8:1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ross J. M. 1957. The pathogenesis of anthrax following the administration of spores by the respiratory route. J. Pathol. Bacteriol. LXXIII:485–494 [Google Scholar]
- 29. Sanderson W. T., et al. 2004. Bacillus anthracis contamination and inhalational anthrax in a mail processing and distribution center. J. Appl. Microbiol. 96:1048–1056 [DOI] [PubMed] [Google Scholar]
- 30. Shieh W. J., et al. 2003. The critical role of pathology in the investigation of bioterrorism-related cutaneous anthrax. Am. J. Pathol. 163:1901–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith H., Keppie J., Stanley J. L. 1955. The chemical basis of the virulence of Bacillus anthracis. V. The specific toxin produced by B. anthracis in vivo. Br. J. Exp. Pathol. 36:460–472 [PMC free article] [PubMed] [Google Scholar]
- 32. Stanley J. L., Smith H. 1961. Purification of factor I and recognition of a third factor of the anthrax toxin. J. Gen. Microbiol. 26:49–63 [DOI] [PubMed] [Google Scholar]
- 33. Steichen C., Chen P., Kearney J. F., Turnbough C. L., Jr 2003. Identification of the immunodominant protein and other proteins of the Bacillus anthracis exosporium. J. Bacteriol. 185:1903–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamborrini M., et al. 2009. Immuno-detection of anthrose containing tetrasaccharide in the exosporium of Bacillus anthracis and Bacillus cereus strains. J. Appl. Microbiol. 106:1618–1628 [DOI] [PubMed] [Google Scholar]
- 35. Turnbull P. C. B. 1998. Guidelines for the surveillance and control of anthrax in humans and animals, 3rd ed. World Health Organization, Geneva, Switzerland [Google Scholar]
- 36. Welkos S. L. 1991. Plasmid-associated virulence factors of non-toxigenic (pX01−) Bacillus anthracis. Microb. Pathog. 10:183–198 [DOI] [PubMed] [Google Scholar]
- 37. Werz D. B., Seeberger P. H. 2005. Total synthesis of antigen Bacillus anthracis tetrasaccharide—creation of an anthrax vaccine candidate. Angew Chem. Int. Ed. Engl. 44:6315–6318 [DOI] [PubMed] [Google Scholar]