Abstract
The mediators of protective immunity against cholera are currently unknown, but memory B-cell responses may play a central role in facilitating long-term and anamnestic responses against Vibrio cholerae, the cause of cholera. We compared memory B-cell responses in adults with natural cholera in Bangladesh (n = 70) to responses in Bangladeshi adults after one-dose (n = 30) or two-dose (n = 30) administration of an oral killed cholera vaccine, WC-rBS (Dukoral; Crucell), assessing the responses at the acute stage of disease or prevaccination and then on days 3, 30, 90, 180, 270, and 360. Individuals with natural cholera developed prominent vibriocidal and plasma anti-cholera toxin B subunit (CtxB) and lipopolysaccharide (LPS) IgG and IgA responses, but these responses returned to baseline by 1 year of follow-up. Vaccinees developed plasma anti-CtxB and anti-LPS IgG and IgA responses that were generally comparable to those in individuals recovering from natural disease, but vibriocidal responses were lower in vaccinees than in infected patients. Individuals recovering from natural disease developed memory B-cell IgG and IgA anti-CtxB and anti-LPS responses by day 30, and these responses were detectable through at least days 180 to 360. In contrast, we detected no IgA or IgG memory B-cell responses to LPS in vaccinees; anti-CtxB IgA responses were only detectable on day 30, and anti-CtxB IgG responses were detectable until days 90 to 180, compared to days 270 to 360 in patients. These findings may explain in part the relatively short-term protection afforded by oral cholera vaccination compared to natural disease.
INTRODUCTION
Cholera is endemic in more than 50 countries and continues to be a major cause of severe diarrheal disease in resource-limited settings (41). Cholera is caused by Vibrio cholerae O1 and O139, and it is estimated that 5 million cases of cholera occur each year, resulting in 100,000 deaths (40, 42). Cholera can occur in explosive outbreaks, as evidenced by the outbreak beginning in 2008 in Zimbabwe that affected over 100,000 individuals and resulted in more than 4,000 deaths (15), as well as outbreaks in 2010 in Pakistan (39) and Haiti (10).
V. cholerae has an environmental reservoir, existing in freshwater and brackish water in many regions of the world, and V. cholerae shed by an infected human is hyperinfectious to other humans (22). Both features contribute to ongoing human disease. Partly in recognition of the fact that safe water and improved hygiene will not be immediate realities to those most affected by cholera, the World Health Organization recently issued an updated position statement on the role that cholera vaccines should play in limiting the cholera disease burden (41). Currently, two oral cholera vaccines are licensed and available: a killed V. cholerae O1 vaccine supplemented with recombinant nontoxic cholera toxin B subunit (CtxB; WC-rBS; Dukoral; Crucell) and a bivalent killed V. cholerae O1/O139 vaccine not containing supplemental CtxB (O1/O139 WC; Shanchol-India, ORC-VAX-Viet Nam) (15, 21, 33). Both types of vaccines are safe and immunogenic and are usually administered in two doses separated by 1 to 6 weeks (26, 27). O1/O139 WC provided ca. 70% protection in a recent field study in Kolkata (32) and is currently being evaluated in a larger field trial in Bangladesh. WC-rBS provides 85 to 90% protective efficacy against cholera in the few months following a two-dose regimen (19), but this efficacy falls toward baseline within 24 to 36 months of vaccination, especially in children who may not have had previous exposure like the adults (2). In comparison, natural cholera induces protection that lasts for years or decades after infection (17).
We have recently shown that anti-V. cholerae memory B-cell responses occur following natural cholera (8, 12) and that these responses may be important mediators of the duration of protection against subsequent cholera. Therefore, we were interested in whether oral cholera vaccination also induced anti-V. cholerae memory B-cell responses, whether these responses differed following one or two doses of vaccine, and whether the responses differed from those seen following natural disease.
MATERIALS AND METHODS
Study design and subject enrollment.
The study was conducted in an urban area in Mirpur, Dhaka, Bangladesh, between October 2008 and June 2010. We enrolled 60 healthy adult males (n = 29) and nonpregnant females (n = 31) aged 18 to 45 years (median age, 28.5 years). We administered a single dose of oral cholera vaccine WC-rBS (Dukoral) to 30 subjects and two doses separated by 2 weeks to another 30 subjects. We obtained blood samples (10 ml) before vaccination (preimmune, day 0) and 3 days after ingestion of each vaccine dose, and again 30, 90, 180, 270, and 360 days after initial vaccination. At each time point, we assayed plasma for vibriocidal antibodies and anti-CtxB and V. cholerae O1 Ogawa lipopolysaccharide (LPS) responses. We assessed the antigen-specific IgG and IgA memory B cells by enzyme-linked immunospot (ELISPOT) assay by measuring circulating antibody-secreting cells (ASC), as described below, on study days 0, 30, 90, 180, 270, and 360.
We compared immune responses following WC-rBS vaccination to those occurring following natural cholera in 70 adult cholera patients (male, n = 38; female, n = 32) (8, 16, 37). Adult patients presenting with severe, acute watery diarrhea who had a positive stool culture for V. cholerae O1 and were without significant comorbid conditions were eligible for inclusion in the study. We analyzed immune responses at the acute stage (day 2) and days 30, 90, 180, 270, and 360 after the onset of disease. The present study and all immunologic analyses were approved by the Research Review Committee and Ethical Review Committee of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh, and the IRB of the Massachusetts General Hospital.
Isolation of PBMC.
We separated peripheral blood mononuclear cells (PBMC) from plasma by centrifugation of diluted heparinized blood on Ficoll-Isopaque (Pharmacia, Piscataway, NJ), storing plasma at −70°C for future immunological analysis. We suspended freshly harvested PBMC at a concentration of 106 cells/ml in RPMI-complete medium (Gibco, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT). We used resuspended cells in a culture-based memory B-cell assay (described below) or used them immediately in an ELISPOT format to detecting circulating antigen specific IgG or IgA ASC.
Vibriocidal antibody assay.
We assessed vibriocidal antibody titers as previously described, using guinea pig complement and V. cholerae O1 Ogawa (X-25049) or Inaba (strain 19479) as the target organism (25). For patients, we used the homologous serotype of V. cholerae O1 as the target organism in the assay. For vaccinees, we used the Ogawa serotype (1). We defined the vibriocidal titer as the reciprocal of the highest dilution resulting in >50% reduction of the optical density compared to that of control wells without plasma (24). We considered individuals showing a ≥4-fold increase in vibriocidal titer as responders.
CtxB and LPS-specific IgG and IgA antibodies in plasma.
We assessed CtxB and LPS-specific IgG and IgA responses in plasma by using standardized enzyme-linked immunosorbent assay protocols (23, 25). We used 96-well polystyrene plates (Nunc F) coated with homologous V. cholerae O1 LPS for patients and V. cholerae O1 Ogawa LPS for the vaccinees (2.5 μg/ml) or coated with 0.3 nmol of ganglioside GM1/ml, followed by recombinant CtxB subunit (0.5 μg/ml; gifts from A. M. Svennerholm, University of Gothenburg, Gothenburg, Sweden) (1). We then added 100 μl of plasma (diluted 1:100 for CtxB and 1:25 for LPS in 0.1% bovine serum albumin in phosphate-buffered saline–Tween)/well and used horseradish peroxidase-conjugated secondary antibodies to human IgG or IgA (Jackson Immunoresearch, West Grove, PA), developing the samples with ortho-phenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.1% hydrogen peroxide. We read the plates kinetically at 450 nm for 5 min (12) and normalized the maximal rate of change in optical density in milli-absorbance units per minute across plates by calculating the ratio of the test sample to a standard of pooled convalescent-phase serum from previously infected cholera patients included on each plate. We considered individuals with a ≥2-fold increase in anti-CtxB and LPS responses as responders.
ELISPOT assay for circulating IgG and IgA ASC.
We performed the ASC assay as previously described (8, 25). Briefly, we coated nitrocellulose-bottom plates (MSHAN-4550; Millipore, Bedford, MA) with GM1 ganglioside (3 nmol/ml), followed by recombinant CtxB (2.5 μg/ml), or with LPS (25 μg/ml), or with keyhole limpet hemocyanin (KLH; Pierce Biotechnology, Rockford, IL) (2.5 μg/ml, as a negative control) or affinity-purified goat anti-human immunoglobulin to detect total immunoglobulin memory B cells (Jackson Immunology Research West Grove, PA) at 5 μg/ml. After the plates were blocked with RPMI 1640 containing 10% FBS, we added freshly isolated PBMC and incubated the plates for 3 h at 37°C in a 5% CO2 incubator. After incubation, we washed the plates and detected IgG ASC using alkaline phosphatase-conjugated goat anti-human IgG (Southern Biotech, Birmingham, AL) diluted 1:500 and IgA ASC using horseradish peroxidase-conjugated mouse anti-human IgA (Hybridoma Reagent Laboratory, Baltimore, MD) diluted 1:500. We developed the IgG plates with BCIP (5-bromo-4-chloro-3-indolylphosphate)/nitroblue tetrazolium (NBT) (Sigma), and we developed the IgA plates with 3-amino-9-ethyl carbazole (AEC). We visualized IgG-ASC as blue spots and IgA-ASC as red spots on the same nitrocellulose membranes. We quantified the number of ASC per well by using a stereomicroscope, and data were collected independently by two individuals and then averaged. We expressed the number of antigen-specific IgG and IgA ASC as the percentage of total circulating ASC of the same isotype.
Memory B-cell culture and ELISPOT assay.
We performed memory B-cell assays using PBMC recovered on days 0, 30, 90, 180, 270, and 360, using a previously described method (5, 6, 8, 12). For this assay, we placed 500,000 PBMC/well in 24-well cell culture plates (BD Biosciences, San Jose, CA) containing culture medium optimized to stimulate antigen-independent proliferation and differentiation of memory B cells into ASC. This medium consisted of RPMI 1640, 10% FBS, 200 U of penicillin/ml, 200 μg of streptomycin/ml, 2 mM l-glutamine, 50 μM β-mercaptoethanol, and a mixture of three B-cell mitogens: 6 μg of CpG oligonucleotide (Operon, Huntsville, AL)/ml, a 1/100,000 dilution of crude pokeweed mitogen extract, and a 1/10,000 dilution of fixed Staphylococcus aureus Cowan (Sigma). As a negative control, we also placed PBMC into wells containing this culture medium lacking mitogens. We incubated the plates at 37°C in a 5% CO2 incubator for 5 to 6 days, after which the cells were harvested and washed. We performed antigen-specific (CtxB and LPS) and total IgG and IgA ELISPOT assays on these cultured cells. Specifically, we used 20% of the cells from each well to assess total IgG and IgA-ASC and 80% to detect antigen-specific IgG and IgA-ASC. We detected IgG and IgA ASC using horseradish peroxidase-conjugated mouse anti-human IgG and IgA (Hybridoma Reagent Laboratory), respectively, developing them with AEC. We expressed ELISPOT counts as the percentage of antigen-specific memory B cells out of the total IgG or IgA memory B cells. We used wells coated with KLH and unstimulated samples as negative controls. We defined appropriate stimulation of PBMC in our assay as a ≥4-fold increase in the number of total immunoglobulin memory cells after stimulation compared to unstimulated cells. We excluded data from analysis for any of the following reasons: (i) the total immunoglobulin samples for each patient sample did not have appropriate stimulation, (ii) the study subject specimens had four or more antigen-specific ASC spots in the same sample prior to stimulation to exclude acute infection and exposure to V. cholerae O1 antigens and other related pathogens, or (iii) patient samples had three or more ASC spots to the negative control antigen KLH, as previously described (8). Antigen-specific memory B-cell response rates were also determined for participants at each time point. The limits of detection or responders were defined as 0.001% for IgG and 0.004% for IgA antigen-specific memory B cells per 5 × 105 PBMC after 6 days of stimulation (8). Comparison of the response rates for CTB in the IgA and IgG isotypes was also carried out for memory B-cell responses.
Statistical analyses.
We compared immunological responses based on the magnitude of the responses and/or response rates. Differences in the magnitude of the responses were assessed by using the Wilcoxon signed-rank test or the Mann-Whitney U test, as applicable. We compared response rates by using chi-square (χ2) tests. All reported P values are two tailed, with a cutoff of P < 0.05 considered a threshold for statistical significance. We performed analyses using GraphPad Prism 4.0, SigmaStat 3.1, and SPSS 14.0.
RESULTS
Study population.
We enrolled 60 healthy adult vaccinees (male, 48%; female, 52%) and 70 cholera patients in the present study. The cholera patients were adults presenting with diarrhea at the ICDDR,B hospital and with a positive microbiologic culture of stool for V. cholerae O1 (8, 12, 25). Of the vaccinees, 92% (n = 55) completed follow-up to day 360; of the cholera patients, 53% (n = 37) completed follow-up to day 360. The demographic characteristics of the study participants are presented in Table 1. The study groups had no significant differences in age, gender, or blood group distributions.
Table 1.
Demographic and serologic characteristics of study subjects
| Characteristic | One-dose vaccine cohort (n = 30) | Two-dose vaccine cohort (n = 30) | Cholera patients (n = 70) |
|---|---|---|---|
| Median subject age in yr (50th, 75th percentiles) | 28 (25, 40) | 32 (27, 39) | 30 (24, 36) |
| No. (%) of female subjects | 18 (60) | 13 (43) | 32 (46) |
| No. (%) of subjects with blood type: | |||
| O | 11 (37) | 12 (40) | 27 (39) |
| A | 6 (20) | 7 (23) | 15 (21) |
| B | 11 (37) | 10 (33) | 25 (36) |
| A/B | 2 (7) | 1 (3) | 3 (4) |
| No. of patients with V. cholerae O1 serotype: | |||
| Ogawa | 55 | ||
| Inaba | 15 |
Vibriocidal responses.
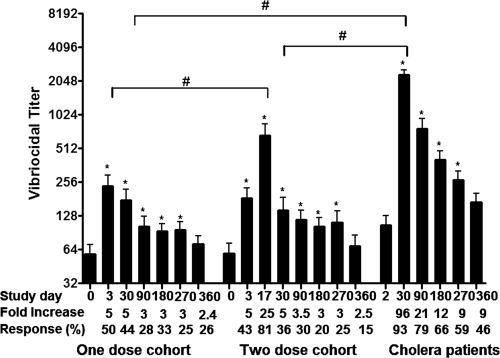
Prior to immunization (day 0), the geometric mean (GM) reciprocal vibriocidal titer (GMT) in the one- and two-dose vaccine cohorts were 27 (95% confidence interval [CI] = 17 to 42) and 26 (95% CI = 15 to 45), respectively (Fig. 1). Vibriocidal responses increased in vaccinees by day 3 in both the one-dose (GMT = 82, 95% CI = 37 to 197; P < 0.001) and two-dose (GMT = 68, 95% CI = 25.3 to 106.9, P < 0.001) cohorts, and response rates were comparable (P = 0.85). In two-dose recipients, the magnitude of the response (GMT = 306, 95% CI = 170 to 550) and the response rates increased further within 3 days of receiving the second dose of vaccine (study day 17) (P < 0.001). Vibriocidal antibody responses in both cohorts persisted up to day 270, before decreasing to baseline levels at day 360. Other than the day 17 increases in the two-dose cohort, the vibriocidal responder frequency and the magnitude of the responses were comparable in both one- and two-dose vaccine cohorts.
Fig. 1.
Vibriocidal responses in vaccinees and cholera patients. The vibriocidal antibody responses in plasma in Bangladeshi adults who received one or two doses of WC-rBS cholera vaccine separated by 2 weeks (days 0 and 14) and in adult cholera patients were graphed. The columns indicate mean reciprocal end titers, and error bars represent the standard errors of the mean. The Wilcoxon signed-rank test was used for analyses of the data. An asterisk denotes a statistically significant difference (P < 0.05) from the baseline (day 0 or 2) titer. Mean fold changes and responder frequencies are also listed. #, Statistically significant difference among the study groups (P < 0.05).
We assessed vibriocidal responses in cholera patients at the acute stage of disease (day 2) and at convalescence on days 30, 90, 180, 270, and 360. We observed an ∼90-fold increase in vibriocidal antibodies on day 30 compared to day 2. The level remained elevated up to day 270, before declining to baseline by day 360. However, responder frequency and magnitude of responses 30, 90, 180, 270, and 360 days after disease onset were higher in patients than vaccinees at similar time periods after immunization (P < 0.04).
CtxB and LPS-specific antibody and ASC responses.
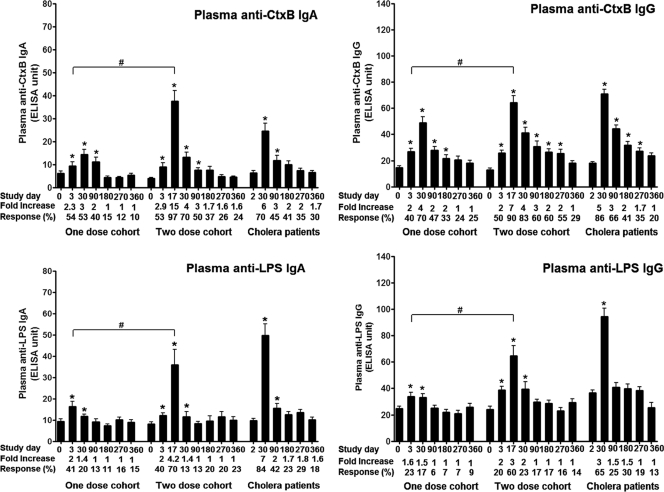
Plasma anti-CtxB and LPS-specific IgG and IgA antibody levels increased within 3 days of vaccination in both one- and two-dose cohorts (P < 0.001) (Fig. 2), and responses after one dose were comparable in both groups (response rate, P > 0.79; magnitude of response, P > 0.32). Antigen-specific responses increased further following the second dose of vaccine in the two-dose cohort (study day 17) (P < 0.001). In both vaccine cohorts, comparable levels of CtxB-specific IgA antibodies persisted at study day 90 but returned to baseline by day 180. CtxB-specific IgG antibody responses in the two-dose cohort persisted up to day 360 but decreased to baseline by day 270 in the one-dose cohort. In comparison, plasma IgG and IgA responses to LPS increased significantly by day 30 but returned to baseline by day 90 in both vaccine groups. Other than the day 17 responses, IgA and IgG responder frequencies and magnitude of responses against LPS and CtxB were comparable in both vaccine cohorts throughout the evaluation period.
Fig. 2.
Plasma anti-CtxB and LPS responses in adult vaccinees and cholera patients. The CtxB and LPS antibody responses in plasma in Bangladeshi adults who received one or two doses of WC-rBS cholera vaccine separated by 2 weeks (days 0 and 14) and in adult cholera patients were assessed. The columns indicate mean responses, and the error bars represent standard errors of the mean. The Wilcoxon signed-rank test was used for analyses of the data. An asterisk denotes a statistically significant difference (P < 0.05) from the baseline (day 0 or 2) titer. Mean fold changes and responder frequencies are also listed. #, statistically significant difference between the two vaccine cohorts (P < 0.05).
Compared to responses in both one- and two-dose vaccine cohorts, the anti-CtxB and anti-LPS IgG and IgA responses were higher in cholera patients, peaking 30 days after onset of disease (P = <0.001 to 0.032). Plasma anti-CtxB and anti-LPS responses returned to baseline by day 360 in patients. Anti-CtxB IgG and IgA response rates were comparable among vaccinees and patients (P > 0.1), but patients were more likely to develop anti-LPS plasma responses compared to vaccinees by day 30 (P < 0.001), and anti-LPS plasma IgA responses remained elevated in patients until day 90 (P = 0.018).
We also assessed ASC responses to CtxB and LPS at the first time point (day 0, vaccinees; day 2, patients) and at days 30, 90, 180, 270, and 360 of the study. Most participants did not have measurable CtxB or LPS-specific (≥4 ASC/5 × 105 PBMC) IgG or IgA ASC levels at day 0 (mean = 0.036 to 0.087 for both vaccine cohorts) or day 2 (mean = 0.082 to 0.102 for the patient group) of the study. We did not detect any measurable increases in anti-CtxB or LPS ASC responses in vaccinees or patients at any time point. In assessing memory B-cell responses, we excluded specimens with four or more antigen-specific ASC spots to minimize the effect that reexposure to V. cholerae or related antigens could have; in all, we excluded 4.4% (112/2,552) of samples for this reason.
Antigen-specific IgG and IgA memory B-cell responses.
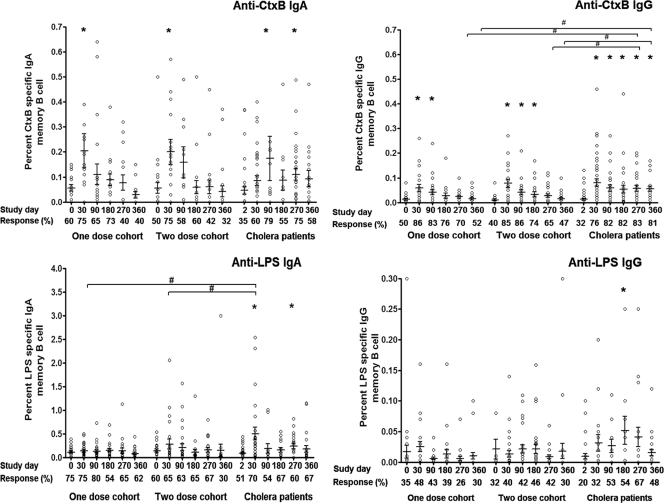
We assessed memory B-cell responses at study initiation (days 0 and 2 in vaccinees and patients, respectively) and on days 30, 90, 180, 270, and 360. Only samples that showed stimulation of PBMC as a ≥4-fold increase in the number of total immunoglobulin memory cells following stimulation, compared to unstimulated cells, were considered for analyses. We detected statistically significant increases in anti-CtxB specific IgA memory B-cell responses in day 30 samples compared to day 0 samples in both vaccine cohorts (P = 0.01); these responses returned to baseline by day 90 (Fig. 3). We similarly detected statistically significant increases in anti-CtxB specific IgG memory B-cell responses in day 30 samples compared to day 0 samples in both vaccine cohorts (P < 0.001). These responses persisted longer than IgA responses, returning to baseline by day 180 in the one-dose vaccine cohort and by day 270 in the two-dose vaccine cohort. The magnitudes of antigen-specific anti-CtxB memory B-cell responses were comparable in both one- and two-dose vaccine cohorts. In comparison, anti-CtxB IgA memory responses were detectable in cholera patients through day 270, and IgG memory responses were still present on day 360, the last day assessed. Of the study participants who had CtxB specific IgG or IgA memory, both IgG and IgA isotype-specific responses were observed in 58% of one-dose vaccinees, 55% of two-dose vaccinees, and 66% of patients.
Fig. 3.
Anti-CtxB and LPS memory B-cell responses in adult vaccinees and cholera patients. The CtxB and LPS-specific memory B-cell responses in Bangladeshi adults who received one or two doses of WC-rBS cholera vaccine separated by 2 weeks (days 0 and 14) and in adult cholera patients, expressed as the percent antigen-specific responses of total isotype-specific memory B cells, were assessed. Bars represent mean responses and standard errors of the mean. The Wilcoxon signed-rank test was used for analyses of the data. An asterisk denotes a statistically significant difference (P < 0.05) from the baseline (day 0 or 2) titer. #, statistically significant difference between vaccinees and cholera patients (P < 0.05).
Cholera patients developed anti-LPS IgA memory B-cell responses by day 30, and although these levels fell, they were still significantly elevated over day 2 levels on day 270 (P < 0.05) (Fig. 3). We also detected anti-LPS IgG memory B-cell responses on day 180 in cholera patients. In comparison, we never detected any significant anti-LPS IgA or IgG memory B-cell response in either vaccine cohort at any time point.
DISCUSSION
In this study, we found both a significantly higher magnitude and a longer duration of memory B-cell responses in adult Bangladeshi patients recovering from cholera than in Bangladeshi adults receiving a currently licensed oral killed cholera vaccine (WC-rBS), including in individuals receiving two doses of vaccine. These differences were particularly pronounced when considering anti-LPS memory B-cell responses: we did not detect memory B-cell responses against LPS in vaccinees, although we detected both IgA and IgG anti-LPS memory B-cell responses in individuals recovering from cholera. These observations are of particular interest when considering that protection against cholera may largely be mediated by anti-LPS responses. For instance, the current surrogate marker of protection against cholera, the vibriocidal response, is largely adsorbed by LPS. Also, anti-LPS IgA responses have been associated with protection against cholera (11), and protection from infection with V. cholerae O1 and O139 is serotype specific, even though these two serotypes share similar cholera toxins and in many respects are identical except for substitution of a different O antigen region of LPS and a capsule in V. cholerae O139 compared to V. cholerae O1 (36).
Each dose of WC-rBS vaccine is supplemented with 1 mg of recombinant CtxB, and we did detect anti-CtxB IgA and IgG memory B-cell responses in vaccinees in the present study. However, these responses were relatively short-lived, falling to baseline within 30 to 180 days after vaccination, while remaining elevated through the last study day evaluated (day 360) in patients recovering from wild-type cholera.
Interestingly, previous studies have noted a boosting of anti-CtxB and vibriocidal responses and an earlier peaking of serologic responses following WC-rBS reimmunization months after primary oral immunization (13, 14), suggesting some degree of anamnestic recall, but protective efficacy rapidly falls after oral immunization with WC-rBS (4). Our findings suggest a possible mechanism for the relatively short-lived protection afforded by current oral killed cholera vaccination compared to that which develops following cholera. Natural infection with V. cholerae O1 induces protective immunity that lasts for at least 3 years in volunteer challenge studies (the last period evaluated) (20), and mathematical modeling of over 4 decades of epidemiologic data from rural Bangladesh indicates that although protective immunity following infection with V. cholerae O1 El Tor begins to decline after 3 years, substantial protective immunity persists for over a decade after infection (17). In comparison, although two- and three-dose regimens of WC-rBS have a protective efficacy of ca. 85% in Bangladesh (3) and Peru (29) within 6 months of vaccination, this protection falls to 50% within 3 years and to baseline within 24 to 36 months in young children who may not have been previously exposed (4).
The immunologic mechanism of protection against cholera is currently unknown, although available evidence suggests it probably involves anti-LPS responses, as described above. Vibriocidal responses are often used to predict protection, but this complement-binding serum-based assay is presumably a surrogate marker of protection, since V. cholerae is a noninvasive luminal pathogen, there is no known vibriocidal titer above which protection is complete (28), and vibriocidal antibody levels fall to baseline within several months after infection (8). Similarly, serum and mucosal anti-V. cholerae antibody responses fall to baseline within months of infection, despite the presence of ongoing protection from disease (8). These observations suggest that long-term protection following cholera may be mediated through memory and anamnestic responses.
We have recently shown that cholera patients develop memory B-cell responses of both IgG and IgA isotypes to CtxB and the major colonization factor toxin coregulated pilus (TcpA), that these responses are detectable for at least 1 year after infection, and that these memory responses persist even after antigen-specific ASC and plasma antibody levels have returned to baseline (8). We have also recently shown that LPS-specific memory B-cell responses develop after natural cholera (8), although these responses were somewhat shorter lived than those targetting protein antigens. Our present results suggest that recipients of the oral killed cholera vaccine WC-rBS do not develop comparable memory B-cell responses, especially those targeting the T-cell-independent antigen LPS.
Our study also compared one- and two-dose regimens of WC-rBS among adults presumably previously exposed to V. cholerae. Cholera is endemic in Bangladesh, including in the capital city of Dhaka (9, 30), and cholera is now endemic in approximately 50 countries (10, 15, 39, 41). In areas of the world where cholera is endemic, it is uncertain whether one or two doses of oral cholera vaccine are needed for adequate protection from cholera. One dose may be sufficient to boost previous anamnestic responses. Although our study did not compare protective efficacy, it did compare a number of immunologic responses. Of note, in this area of endemicity, we observed significant increases in vibriocidal and antigen-specific plasma anti-V. cholerae responses within 3 days of vaccination, findings consistent with previous exposure. Although all of these responses increased further following a second dose of vaccine, the response magnitude, responder frequency, and duration of response were generally comparable in the one- and two-dose cohorts over the 1 year of the study, although plasma IgA and IgG anti-LPS and CtxB responses reached their highest level immediately after receipt of a second oral dose of vaccine. Whether this transient increase in IgA and IgG is of clinical significance is currently unknown. The memory B-cell responses were also comparable and poor in both vaccine cohorts, even in these previously exposed adults. These results suggest that a single dose of vaccine in adults residing in an zone where cholera is endemic induces an immune response that is in many ways comparable to that seen in two-dose recipients over the course of a year and suggests that evaluation of one-dose vaccine regimens in areas of endemicity may be warranted.
In the present study, we also measured antibodies in plasma. Vibriocidal and serum or plasma antibodies have been used as surrogate markers for mucosal responses during cholera and have been shown to correlate with protection against cholera (11, 13, 14, 25). In the present study, we did not attempt to directly measure mucosal responses through analysis of endoscopically obtained small bowel biopsy specimens, nor did we assess fecal extracts because of the shorter duration of these latter responses and their variability (2, 23, 31, 38).
In summary, our study suggests that the memory B-cell response in patients with cholera is significantly different from that in vaccinees, despite both groups developing comparable serologic responses. These findings may explain in part the relatively short-term protection afforded by oral cholera vaccination compared to natural disease. Although the mechanism of this disparity is currently unclear, the absence of the potent immunoadjuvant cholera holo-toxin from the oral killed cholera vaccine but present during wild-type infection may play a role (7, 18, 34, 35). Of note, our study focused on adults residing in an endemic zone; memory responses in immunologically naive individuals, including children, have yet to be assessed following natural infection or vaccination. However, these results may suggest the need for either ongoing booster doses or improved vaccination strategies, even among previously exposed populations with on-going risk of cholera.
ACKNOWLEDGMENTS
This study was supported by the ICDDR,B and by grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (AI058935 [S.B.C., E.T.R., and F.Q.] and AI077883 [E.T.R. and F.Q.]), a training grant in vaccine development from the Fogarty International Center (TW05572 [M.M.A. and F.Q.], and a career development award (K01) from the Fogarty International Center (TW007409 [J.B.H.]). This study was also supported by the Swedish Sida (F.Q.).
Footnotes
Published ahead of print on 23 February 2011.
REFERENCES
- 1. Ahmed T., Svennerholm A. M., Al Tarique A., Sultana G. N., Qadri F. 2009. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine 27:1433–1439 [DOI] [PubMed] [Google Scholar]
- 2. Chowdhury F., et al. 2008. A comparison of clinical and immunologic features in children and older patients hospitalized with severe cholera in Bangladesh. Pediatr. Infect. Dis. J. 27:986–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clemens J. D., et al. 1986. Field trial of oral cholera vaccines in Bangladesh. Lancet ii:124–127 [DOI] [PubMed] [Google Scholar]
- 4. Clemens J. D., et al. 1990. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 335:270–273 [DOI] [PubMed] [Google Scholar]
- 5. Crotty S., Aubert R. D., Glidewell J., Ahmed R. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111–122 [DOI] [PubMed] [Google Scholar]
- 6. Crotty S., et al. 2003. Cutting edge: long-term B-cell memory in humans after smallpox vaccination. J. Immunol. 171:4969–4973 [DOI] [PubMed] [Google Scholar]
- 7. Elson C. O., Ealding W. 1984. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J. Immunol. 132:2736–2741 [PubMed] [Google Scholar]
- 8. Harris A. M., et al. 2009. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 77:3850–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris A. M., et al. 2008. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 79:708–714 [PMC free article] [PubMed] [Google Scholar]
- 10. Harris J. B., et al. 2010. Cholera's western front. Lancet 376:1961–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris J. B., et al. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jayasekera C. R., et al. 2008. Cholera toxin-specific memory B-cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J. Infect. Dis. 198:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jertborn M., Svennerholm A. M., Holmgren J. 1988. Five-year immunologic memory in Swedish volunteers after oral cholera vaccination. J. Infect. Dis. 157:374–377 [DOI] [PubMed] [Google Scholar]
- 14. Jertborn M., Svennerholm A. M., Holmgren J. 1994. Immunological memory after immunization with oral cholera B subunit–whole-cell vaccine in Swedish volunteers. Vaccine 12:1078–1082 [DOI] [PubMed] [Google Scholar]
- 15. Kanungo S., et al. 2009. Immune responses following one and two doses of the reformulated, bivalent, killed, whole-cell, oral cholera vaccine among adults and children in Kolkata, India: a randomized, placebo-controlled trial. Vaccine 27:6887–6893 [DOI] [PubMed] [Google Scholar]
- 16. Kendall E. A., et al. 2010. Development of immunoglobulin M memory to both a T-cell-independent and a T-cell-dependent antigen following infection with Vibrio cholerae O1 in Bangladesh. Infect. Immun. 78:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koelle K., Rodo X., Pascual M., Yunus M., Mostafa G. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436:696–700 [DOI] [PubMed] [Google Scholar]
- 18. Lee J. B., Jang J. E., Song M. K., Chang J. 2009. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS One 4:e5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Legros D., et al. 1999. Mass vaccination with a two-dose oral cholera vaccine in a refugee camp. Bull. World Health Organ. 77:837–842 [PMC free article] [PubMed] [Google Scholar]
- 20. Levine M. M., et al. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818–820 [DOI] [PubMed] [Google Scholar]
- 21. Mahalanabis D., et al. 2008. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS One 3:e2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Merrell D. S., et al. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417:642–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qadri F., et al. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6:812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qadri F., et al. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2:685–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qadri F., et al. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ryan E. T., Calderwood S. B. 2000. Cholera vaccines. Clin. Infect. Dis. 31:561–565 [DOI] [PubMed] [Google Scholar]
- 27. Ryan E. T., Calderwood S. B., Qadri F. 2006. Live attenuated oral cholera vaccines. Expert Rev. Vaccines 5:483–494 [DOI] [PubMed] [Google Scholar]
- 28. Saha D., et al. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318–2322 [DOI] [PubMed] [Google Scholar]
- 29. Sanchez J. L., et al. 1994. Protective efficacy of oral whole-cell/recombinant-B-subunit cholera vaccine in Peruvian military recruits. Lancet 344:1273–1276 [DOI] [PubMed] [Google Scholar]
- 30. Schwartz B. S., et al. 2006. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am. J. Trop. Med. Hyg. 74:1067–1073 [PMC free article] [PubMed] [Google Scholar]
- 31. Shamsuzzaman S., et al. 2009. Robust gut associated vaccine-specific antibody-secreting cell responses are detected at the mucosal surface of Bangladeshi subjects after immunization with an oral killed bivalent Vibrio cholerae O1/O139 whole-cell cholera vaccine: comparison with other mucosal and systemic responses. Vaccine 27:1386–1392 [DOI] [PubMed] [Google Scholar]
- 32. Sur D., et al. 2009. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 374:1694–1702 [DOI] [PubMed] [Google Scholar]
- 33. Trach D. D., et al. 2002. Investigations into the safety and immunogenicity of a killed oral cholera vaccine developed in Viet Nam. Bull. World Health Organ. 80:2–8 [PMC free article] [PubMed] [Google Scholar]
- 34. Vajdy M., Lycke N. 1993. Stimulation of antigen-specific T- and B-cell memory in local as well as systemic lymphoid tissues following oral immunization with cholera toxin adjuvant. Immunology 80:197–203 [PMC free article] [PubMed] [Google Scholar]
- 35. Vajdy M., Lycke N. Y. 1992. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology 75:488–492 [PMC free article] [PubMed] [Google Scholar]
- 36. Waldor M. K., Colwell R., Mekalanos J. J. 1994. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc. Natl. Acad. Sci. U. S. A. 91:11388–11392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weil A. A., et al. 2009. Memory T-cell responses to Vibrio cholerae O1 infection. Infect. Immun. 77:5090–5096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wenneras C., Qadri F., Bardhan P. K., Sack R. B., Svennerholm A. M. 1999. Intestinal immune responses in patients infected with enterotoxigenic Escherichia coli and in vaccinees. Infect. Immun. 67:6234–6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization 2010. Cholera in Pakistan: WHO global alert and response. World Health Organization, Geneva, Switzerland [Google Scholar]
- 40. World Health Organization 2010. Cholera vaccines: WHO position paper–recommendations. Vaccine 28:4687–4688 [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization 2009. Cholera annual report 2008. Wkly. Epidemiol. Rec. 84:309–32419645127 [Google Scholar]
- 42. Zuckerman J. N., Rombo L., Fisch A. 2007. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 7:521–530 [DOI] [PubMed] [Google Scholar]