Abstract
The protective effect of DNA vaccines expressing the Arg-gingipain A domain against bone loss induced by Porphyromonas gingivalis infection was investigated in a murine model. phgp44, which expresses the 44-kDa adhesion/hemagglutinin domain of Arg-gingipain A, prevented P. gingivalis-induced alveolar bone loss. The results indicate that phgp44 could be a candidate antigen for a vaccine against P. gingivalis infection.
TEXT
Periodontitis, a highly prevalent chronic inflammatory disease which causes irreversible destruction of the supporting tissue of the teeth, affects more than 30% of the adult population (19). Periodontitis has also been reported to be involved in the development of systemic diseases such as bacterial endocarditis, atherosclerosis, and diabetes (13).
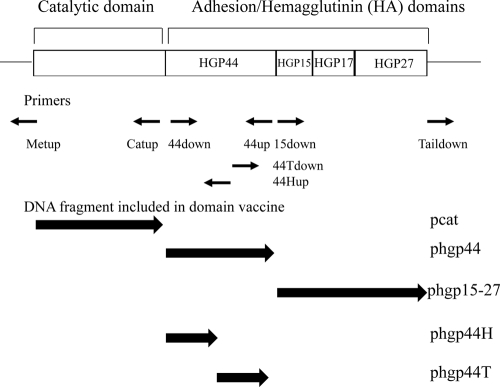
Porphyromonas gingivalis is a major pathogen of chronic periodontitis (5, 21). P. gingivalis expresses several virulence factors, including proteases, fimbriae, and endotoxin (1, 9, 18). Arg-gingipain A (RgpA) is a major virulence factor of P. gingivalis (16). RgpA is involved in activation of complement and bradykinin and degradation of C3b, interleukin 8 (IL-8), IgG, and monocyte chemoattractant protein 1 (MCP-1) (9). These activities may play an important role in the virulence of P. gingivalis. RgpA consists of a preproprotein, a catalytic domain, and an adhesin/hemagglutinin (HA) domain, which consists of HGP44, HGP15, HGP17, and HGP27 (Fig. 1). This HA domain has similarity to hemagglutinin A (HagA) genes and the HA domains of the Lys-gingipain (Kgp) (16). Antibody against gingipain was reported to have a protective effect against infection by P. gingivalis (7, 15, 23). In the present study, we investigated the protective effect of rgpA domain DNA vaccines against P. gingivalis-induced alveolar bone loss, with the aim of clarifying their potential as candidate antigens for a novel vaccine.
Fig. 1.
Map of cloned rgpA in the rgpA DNA vaccine and primers used to construct the rgpA domain DNA vaccines. Small arrows below the map indicate the locations of primers used in this study. Large arrows indicate fragments expressed by the DNA vaccines.
We investigated the protective effect of immunization with rgpA domain vaccines (Fig. 1) containing the rgpA catalytic domain (pcat), the HGP44 domain-coding region (phgp44), the HGP15-27 domain-coding region (phgp15-27), or the N-terminal (phgp44H) or C-terminal (phgp44T) half of the HGP44 domain-coding region against infection by P. gingivalis. All vaccines were constructed by self-ligation of amplified fragments from the rgpA DNA vaccine (23) with the primers described in Table 1, using LA Taq DNA polymerase (Takara Bio, Inc., Shiga, Japan). The plasmid used for construction of these vaccines, pVAX1 (Invitrogen, Carlsbad, CA), was used as a control.
Table 1.
Primers used in this study
| Primer name | Sequencea |
|---|---|
| Metup | 5′-CATGGTCGCTAGCTAGCCAGCTTGGGT-3′ |
| Taildown | 5′-TAATTCTGTCTTGGACTCGGAGCTCGAGTCTAG-3′ |
| 44down | 5′-AGCGGTCAGGCCGAGATTGTTCTTGAA-3′ |
| Catup | 5′-GCGAAGAAGTTCGGGGGCATCGCTGACTGACA-3′ |
| 15down | 5′-CGCAGACTTCACGGAAACGTTCGAGTCTTCTAC-3′ |
| 44up | 5′-CGCTTGCCGTTGGCCTTGATCTCAACCTCATCA-3′ |
| 44Tdown | 5′-CAGAACCTGACCGGTAGTGCAGTCGGCCAGA-3′ |
| 44Hup | 5′-TACAGGAGCAAATTCATTGGATCCTTCTACC-3′ |
Underlining indicates the start codon and the termination codon in Metup and Taildown, respectively.
Immunization with rgpA domain DNA vaccines was carried out as described previously (23). Briefly, 6-week-old female BALB/c mice were separated into 8 groups of 4 mice each: a nonimmunized group and groups immunized with 2.5 μg rgpA DNA vaccine, pcat, phgp44, phgp15-27, phgp44H, phgp44T, or pVAX1 alone via the skin of the abdomen by a Gene Gun (Bio-Rad Laboratories, Hercules, CA) weekly for 5 weeks. Additional immunization with phgp44H and phgp44T was performed at 6 weeks, as the antibody titers for the mice immunized with the DNA vaccines had not reached a plateau at 5 weeks. The levels and reactivity of antibodies against RgpA at days 0, 28, 35, and 42 after immunization were determined by an enzyme-linked immunosorbent assay (ELISA) and immunoblotting. Approval to conduct these studies was obtained from the Animal Use Committee of Tokyo Dental College (Chiba, Japan).
The protective effect of the vaccinations against P. gingivalis infection was investigated according to the method of Baker et al. (2). Briefly, the mice were challenged with P. gingivalis at 6 weeks after the first immunization. Initially, the mice were given 5 mg each of kanamycin and ampicillin by gavage once a day for 4 days. After a 3-day antibiotic-free period, all mice except the nonimmunized control mice were orally infected with 1 × 109 CFU P. gingivalis ATCC 33277 in 2% carboxymethylcellulose. Challenge was carried out 3 times at 2-day intervals. Forty-two days after the last challenge, the mice were sacrificed and alveolar bone loss at the defined landmark sites on the maxillary molars was assessed as described previously (8). We performed measurements (14 sites) on each skull from the cemento-enamel junction (CEJ) to the alveolar bone crest (ABC) with a stereomicroscope. Measurements were made under a dissecting microscope fitted with a video image maker measurement system, MS-803 (Moritex Co., Tokyo, Japan), standardized to yield measurements in millimeters. The 4 noninfected and nonimmunized mice were used to determine the baseline value for the region from the CEJ to the ABC in normal mice. The experiments were repeated to confirm reproducibility, and a one-way analysis of variance (ANOVA) and the Turkey post hoc test were used to make multiple comparisons between groups in terms of antibody titers and protective effects against bone loss using the pooled data from the experiments.
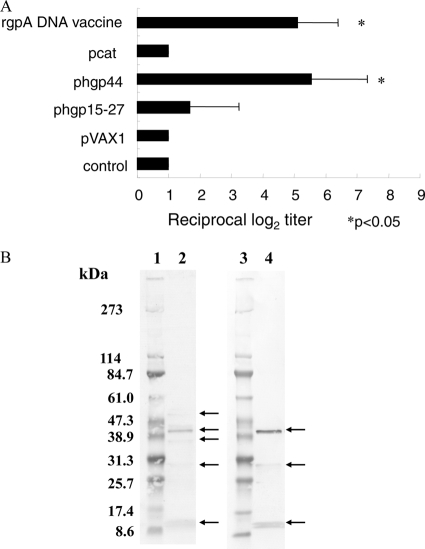
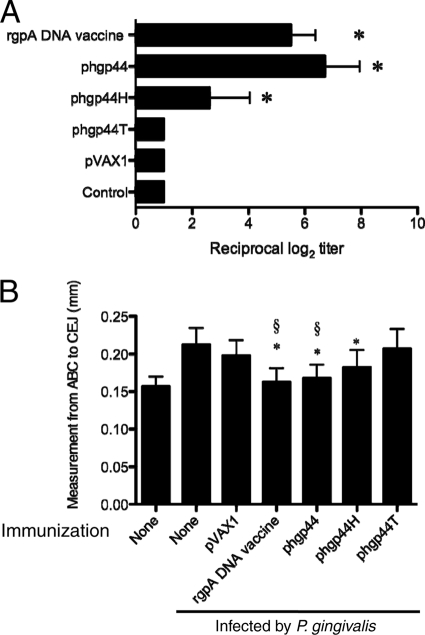
Antibody titers elicited by the pcat, phgp44, or phgp15-27 DNA vaccine plasmid are shown in Fig. 2A. Significant elevation of specific IgGs against P. gingivalis was observed, reaching similar levels in mice immunized with the rgpA DNA vaccine and in those immunized with phgp44. Only a small increase was seen in antibody levels obtained with phgp15-27, and the level obtained with pcat was almost the same as that in the controls. As shown in Fig. 2B, the specificities of IgG in mice immunized with phgp44 or the rgpA DNA vaccine were evaluated by immunoblot analyses. In sera from mice immunized with the rgpA DNA vaccine, 52.6-, 43.8-, 40.8-, 33.5-, and 14.5-kDa bands were observed. In sera from mice immunized with phgp44, 43.8-, 33.5-, and 14.5-kDa bands were observed. These multiple protein bands may have been degraded fragments of RgpA, Kgp, and HagA, which share antigenicity with HGP44. In both groups, the predominant band was the 43.8-kDa band, suggesting high immunogenicity for HGP44. This agrees with the results for earlier reports (10, 12). The epitope for the protective antibody was reported to be located within HGP44 (12). Twenty-one of 25 amino acid residues of the epitope were contained in phgp44H. The antibody titer for mice immunized with phgp44H was significantly high at week 5 (2.6 ± 1.43 log2) (see Fig. 4A), and the reciprocal titer reached 3.9 ± 1.64 log2 at week 6. These results suggest that the N-terminal half of HGP44 is potentially a potent epitope in the induction of protective antibody by RgpA, although further study is required to confirm this.
Fig. 2.
(A) Induction of P. gingivalis-specific IgGs in mice immunized with rgpA domain DNA vaccines. Titers of IgG against sonicates of P. gingivalis in sera from mice were determined on day 42 after primary immunization, and endpoint titers were evaluated by measuring serially diluted sera. Results represent means ± standard deviations for log2 ELISA antibody titers. *, P < 0.05 by one-way ANOVA for comparison with mice immunized with pVAX1. (B) Immunoblot analysis with sera from mice immunized with the rgpA DNA vaccine or phgp44. Sonicates of P. gingivalis were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Blotted membranes were immunostained using sera from mice immunized with the rgpA DNA vaccine or phgp44. Lanes 1 and 3, molecular size markers; lane 2, sera from mice immunized with the rgpA DNA vaccine; and lane 4, sera from mice immunized with phgp44. Molecular mass markers are shown in kilodaltons.
Fig. 4.
(A) Induction of P. gingivalis-specific IgGs in mice immunized with rgpA domain DNA vaccines. Titers of IgG against sonicates of P. gingivalis in sera from mice were determined on day 43 after primary immunization, and endpoint titers were evaluated by measuring serially diluted sera. Results represent means ± standard deviations for log2 ELISA antibody titers. *, P < 0.05 by one-way ANOVA for comparison with mice immunized with pVAX1. (B) Levels of alveolar bone loss elicited following P. gingivalis oral challenge after immunization with the phgp44 derivative. BALB/c mice were immunized with rgpA DNA or rgpA domain DNA vaccines. The control groups consisted of age-matched, nonvaccinated mice and pVAX1-immunized mice. After immunization, mice were orally challenged with P. gingivalis ATCC 33277. At 42 days after oral challenge, all mice were sacrificed. *, P < 0.05 by one-way ANOVA for comparison with mice infected by P. gingivalis without immunization. §, P < 0.05 by one-way ANOVA for comparison with mice infected by P. gingivalis and immunized with pVAX1.
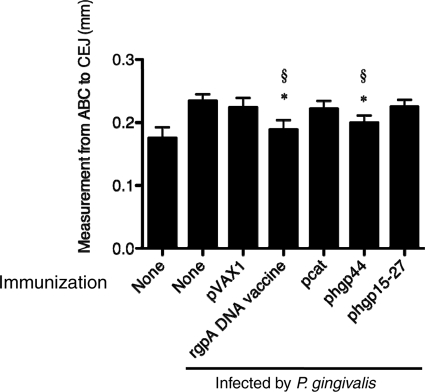
The effects of immunization with rgpA domain vaccines on P. gingivalis-induced alveolar bone loss are shown Fig. 3. The infected mice showed significantly greater alveolar bone loss in the maxillary molar area than did the uninfected control mice. Alveolar bone loss was reduced significantly in both the rgpA DNA vaccine-immunized group and the phgp44-immunized group, whereas the pcat- and phgp15-27-immunized groups showed no protection against alveolar bone loss. These results suggest that the HGP44 domain-coding region plays a predominant role in the rgpA DNA vaccine. The pattern of protection observed in the present study is in accordance with earlier results showing that passive immunization with monoclonal antibody against P. gingivalis protected against recolonization by P. gingivalis (3). Specific IgGs against P. gingivalis RgpA protected against colonization and alveolar bone loss in a murine model (3, 6, 7, 15, 23). The HGP44 domain was involved in adherence to epithelial cells (4) and Treponema denticola (11). Moreover, antibody elicited by an rgpA DNA vaccine inhibited binding of P. gingivalis to collagen sponges and hemagglutination of P. gingivalis (23). Antibody induced by a repeated sequence in the HGP44 domain inhibited binding of the RgpA-Kgp complex to fibrinogen, fibronectin, and collagen type IV (17). Antibody against anti-HGP44 also enhanced opsonization and killing of both invasive and noninvasive strains of P. gingivalis (22). These results agree with the protective effects we demonstrated with phgp44.
Fig. 3.
Levels of alveolar bone loss elicited following P. gingivalis oral challenge after immunization with the rgpA domain DNA vaccine. BALB/c mice were immunized with rgpA DNA or rgpA domain DNA vaccines. The control groups consisted of age-matched, nonvaccinated mice and pVAX1-immunized mice. After immunization, mice were orally challenged with P. gingivalis ATCC 33277. At 42 days after oral challenge, all mice were sacrificed. *, P < 0.05 by one-way ANOVA for comparison with mice infected by P. gingivalis without immunization. §, P < 0.05 by one-way ANOVA for comparison with mice infected by P. gingivalis and immunized with pVAX1.
In this study, phgp44H was observed to exert a protective effect in comparison with the level for the nonimmunized mice but not in comparison with the level for pVAX-immunized mice (Fig. 4B). The antibody titer for phgp44H-immunized mice was lower than that for phgp44-immunized mice (Fig. 4A). Further study is required to determine the dose and rate of immunization required to induce protection against infection by P. gingivalis. Taken together with the results of an earlier study, the present results suggest that antibody against the N-terminal half of the HGP44 domain has a major protective effect.
The protective effect of phgp44 in the present experiments was somewhat lower than that of the rgpA DNA vaccine, although the difference was not significant. Kuboniwa et al. (14) reported that a DNA vaccine encoding the catalytic subunits of Rgp and Kgp elicited antibody production. The antibody attenuated protease activity and showed a protective effect against lethal challenge by P. gingivalis. Genco et al. (6) reported that an N-terminal peptide of the RgpA catalytic domain showed a protective effect against colonization by P. gingivalis. One study reported that a T cell epitope in the catalytic domain of Kgp induced Th2 responses (20). It is possible that an additional effect in response to the catalytic subunit is necessary to elicit the same protective effect as that obtained with the rgpA DNA vaccine. HGP44 was reported to be involved in the adherence by this microorganism (4, 11). Colonization by P. gingivalis was also reported to occur in the mouse model (2). It is possible that the protection against colonization by P. gingivalis plays a major role in the inhibition of bone loss in the present study. Based upon our results, we cannot exclude the possibility that the proteolytic activity of RgpA plays an important role in bone loss since protection mediated by the adhesive and proteolytic domains may not be additive. Therefore, further analysis is required to investigate the effects of antibody against the catalytic subunit on bone loss.
In the present study, the HGP44 domain-coding DNA vaccine could account for the protective effect of the rgpA DNA vaccine. The results of the present study indicate that phgp44 has the ability to induce protective immunity against P. gingivalis-induced alveolar bone loss and that the HGP44-coding region is a candidate antigen for a DNA vaccine.
Acknowledgments
We thank Howard K. Kuramitsu for critical review of the manuscript and Jeremy Williams for his assistance with the development of the manuscript.
This study was supported by grant HRC7 from the Oral Health Science Center of Tokyo Dental College and a High-Tech Research Center Project for Private Universities Matching Fund Subsidy from MEXT (2006 to 2010).
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Amano A., Nakagawa I., Okahashi N., Hamada N. 2004. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal. Res. 39:136–142 [DOI] [PubMed] [Google Scholar]
- 2. Baker P. J., Evans R. T., Roopenian D. C. 1994. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch. Oral Biol. 39:1035–1040 [DOI] [PubMed] [Google Scholar]
- 3. Booth V., Ashley F. P., Lehner T. 1996. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect. Immun. 64:422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen T., Nakayama K., Belliveau L., Duncan M. J. 2001. Porphyromonas gingivalis gingipains and adhesion to epithelial cells. Infect. Immun. 69:3048–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darveau R. P. 2010. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 8:481–490 [DOI] [PubMed] [Google Scholar]
- 6. Genco C. A., Odusanya B. M., Potempa J., Mikolajczyk-Pawlinska J., Travis J. 1998. A peptide domain on gingipain R which confers immunity against Porphyromonas gingivalis infection in mice. Infect. Immun. 66:4108–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gibson F. C., III, Genco C. A. 2001. Prevention of Porphyromonas gingivalis-induced oral bone loss following immunization with gingipain R1. Infect. Immun. 69:7959–7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez D., Tzianabos A. O., Genco C. A., Gibson III F. C. 2003. Immunization with Porphyromonas gingivalis capsular polysaccharide prevents P. gingivalis-elicited oral bone loss in a murine model. Infect. Immun. 71:2283–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo Y., Nguyen K. A., Potempa J. 2010. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol. 2000 54:15–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inagaki S., Ishihara K., Yasaki Y., Yamada S., Okuda K. 2003. Antibody responses of periodontitis patients to gingipains of Porphyromonas gingivalis. J. Periodontol. 74:1432–1439 [DOI] [PubMed] [Google Scholar]
- 11. Ito R., Ishihara K., Shoji M., Nakayama K., Okuda K. 2010. Hemagglutinin/adhesin domains of Porphyromonas gingivalis play key roles in coaggregation with Treponema denticola. FEMS Immunol. Med. Microbiol. 60:251–260 [DOI] [PubMed] [Google Scholar]
- 12. Kelly C. G., et al. 1997. The relationship between colonization and haemagglutination inhibiting and B cell epitopes of Porphyromonas gingivalis. Clin. Exp. Immunol. 110:285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kinane D., Bouchard P. 2008. Periodontal diseases and health: consensus report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 35:333–337 [DOI] [PubMed] [Google Scholar]
- 14. Kuboniwa M., Amano A., Shizukuishi S., Nakagawa I., Hamada S. 2001. Specific antibodies to Porphyromonas gingivalis Lys-gingipain by DNA vaccination inhibit bacterial binding to hemoglobin and protect mice from infection. Infect. Immun. 69:2972–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyachi K., Ishihara K., Kimizuka R., Okuda K. 2007. Arg-gingipain A DNA vaccine prevents alveolar bone loss in mice. J. Dent. Res. 86:446–450 [DOI] [PubMed] [Google Scholar]
- 16. Nakayama K. 2003. Molecular genetics of Porphyromonas gingivalis: gingipains and other virulence factors. Curr. Protein Pept. Sci. 4:389–395 [DOI] [PubMed] [Google Scholar]
- 17. O'Brien-Simpson N. M., et al. 2005. An immune response directed to proteinase and adhesin functional epitopes protects against Porphyromonas gingivalis-induced periodontal bone loss. J. Immunol. 175:3980–3989 [DOI] [PubMed] [Google Scholar]
- 18. Ogawa T., Asai Y., Makimura Y., Tamai R. 2007. Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A. Front. Biosci. 12:3795–3812 [DOI] [PubMed] [Google Scholar]
- 19. Pihlstrom B. L., Michalowicz B. S., Johnson N. W. 2005. Periodontal diseases. Lancet 366:1809–1820 [DOI] [PubMed] [Google Scholar]
- 20. Tam V., O'Brien-Simpson N. M., Pathirana R. D., Frazer L. T., Reynolds E. C. 2008. Characterization of T cell responses to the RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis in BALB/c mice. J. Immunol. 181:4150–4158 [DOI] [PubMed] [Google Scholar]
- 21. Teles R. P., Haffajee A. D., Socransky S. S. 2006. Microbiological goals of periodontal therapy. Periodontol. 2000 42:180–218 [DOI] [PubMed] [Google Scholar]
- 22. Yasaki-Inagaki Y., Inagaki S., Yamada S., Okuda K., Ishihara K. 2006. Production of protective antibodies against Porphyromonas gingivalis strains by immunization with recombinant gingipain domains. FEMS Immunol. Med. Microbiol. 47:287–295 [DOI] [PubMed] [Google Scholar]
- 23. Yonezawa H., Ishihara K., Okuda K. 2001. Arg-gingipain a DNA vaccine induces protective immunity against infection by Porphyromonas gingivalis in a murine model. Infect. Immun. 69:2858–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]