Abstract
The Mycobacterium bovis BCG vaccine is the only tuberculosis (TB) vaccine available, yet it provides limited protection against pulmonary TB in adults and fails to protect against TB reactivation. We hypothesized that immunity against Mycobacterium tuberculosis “resuscitation-promoting factors” (Rpfs), which are small bacterial proteins that promote proliferation of dormant mycobacteria, may be relevant in the human immune response to M. tuberculosis. In previous unpublished work, we found that Rpfs Rv0867c and Rv2389c induced gamma interferon (IFN-γ) production in the blood of TB patients' healthy household contacts in several different African populations. Here we examine these two dominant Rpf antigens in more detail and define the nature of the responding T-cell subsets. Multiparameter cytokine profiling showed that Rv2389c and, to a lesser extent, Rv0867c were recognized by mycobacterium-responsive healthy Dutch individuals; peptide-scanning revealed several epitopes, including a single immunodominant epitope in Rv2389c. Rv0867c and, to a lesser extent, Rv2389c Rpf-specific T-cell responses were maintained for decades in long-term M. tuberculosis nonprogressors. Prominent Rv0867c-specific double- and single-cytokine-producing CD8+ T-cell subset responses were found, including a large population of CD8+ effector memory and effector T-cell subsets. We conclude that M. tuberculosis Rpf antigens are important targets in the human immune response to M. tuberculosis and represent interesting TB vaccine candidate antigens.
INTRODUCTION
It is estimated that over 2 billion people are latently infected with Mycobacterium tuberculosis and that 5 to 10% of these individuals will develop active tuberculosis (TB) at one point in their lifetime, whereas the remainder are able to contain infection long term without developing clinical symptoms (28). During latency, the bacteria are thought to be in a dormant or slowly replicating state (16). The vast reservoir of individuals with latent infection is a major source of new TB cases due to reactivation and resuscitation of dormant bacilli (8, 18).
The term “dormancy” was first introduced by Joseph Warwick Bigger, who discovered that a culture of Staphylococcus pyogenes could not be sterilized after penicillin treatment since there was a small group of antibiotic-resistant bacteria that could be regrown from such cultures. Bigger proposed that these bacteria were dormant, nonreplicating, and thus insensitive to antimicrobials targeting bacterial metabolic pathways (5).
It is assumed that environmental factors are involved in inducing bacterial dormancy (40). M. tuberculosis enters a state of nonreplicating or slowly replicating persistence when grown under gradual oxygen depletion, which is thought to be one of the stress factors that M. tuberculosis encounters upon infection (39). Not only oxygen deprivation, but also low pH, NO, nutrient deprivation, and host immune pressure are stress factors M. tuberculosis is subjected to in the lung granulomatous lesions. In response to these stress factors, M. tuberculosis decreases its metabolic activity and alters its gene expression pattern (4, 38, 40). This adaptation results in increased resistance to environmental stress, by means of entering the nonreplicating or slowly replicating persisting state (35).
While several studies have addressed the bacterial transition from the replicating to the nonreplicating, slowly replicating, or dormant state, little is known regarding the cues that induce bacteria to reactivate and resume growth from dormancy. Mukamolova et al. were the first to discover a resuscitation-promoting factor (Rpf), a hormone-like protein secreted by Micrococcus luteus. Addition of this Rpf protein to dormant M. luteus resulted in resuscitation of M. luteus bacteria (6, 25). rpf genes were then found to be conserved throughout high-G+C Gram-positive bacteria, including M. tuberculosis. Five such genes were identified in the M. tuberculosis genome, notably Rv0867c (rpfA), Rv1009 (rpfB), Rv1884c (rpfC), Rv2389c (rpfD), and Rv2450c (rpfE). Each M. tuberculosis Rpf protein contains an ∼70-amino-acid Rpf-like domain similar to the M. luteus rpf-encoded protein (26). M. tuberculosis Rpfs showed similar properties to M. luteus Rpf, including their ability to resuscitate dormant mycobacteria (20, 26). M. tuberculosis Rpf protein expression was observed in vitro in actively growing M. tuberculosis and in Mycobacterium bovis bacillus Calmette-Guérin (BCG). Importantly, M. tuberculosis rpf gene expression was detected in infected murine and human tissue. Moreover, the presence of Rpf-like proteins was shown in M. tuberculosis-infected human tissue (11, 26, 30, 36). Recently, rpf gene expression was analyzed in M. tuberculosis cells grown under different physiological stress conditions and growth factors. All five rpf genes were expressed during actively replicating early log-phase growth of M. tuberculosis, confirming previous findings. Of note, M. tuberculosis rpf genes displayed differential expression patterns when analyzed in cultures grown under hypoxia, nutrient starvation, and acidic conditions and stationary-, nonculturable-, and resuscitation-phase-like conditions. These differential adaptive rpf expression profiles indicate that M. tuberculosis Rpfs likely play different roles (18).
While M. luteus Rpf is essential for M. luteus growth, individual M. tuberculosis Rpfs were found to be redundant for growth in vitro and in vivo in single-gene-knockout mutants (12, 36). However, when multiple rpf deletions were introduced simultaneously in M. tuberculosis (ΔRv0867c ΔRv1009 ΔRv1884c and ΔRv0867c ΔRv1009 ΔRv2389c), a significant loss in the ability of M. tuberculosis to resuscitate was found, accompanied by in vitro growth attenuation (13).
BCG vaccination is widely used and affords protection from severe forms of TB in children, but it provides only limited and highly variable protection against pulmonary TB in adults and does not protect against reactivation. Better TB vaccines are clearly needed (14). As the M. tuberculosis Rpf proteins are associated with resuscitation of mycobacteria, we hypothesized that immunity directed against these proteins may play a role in sensing actively replicating M. tuberculosis organisms and possibly play a role in host immune control of reactivating bacteria. Only two reports have investigated the immunogenicity of the M. tuberculosis Rpf antigens. One study with mice showed that Rv0867c, Rv1009, Rv2389c, and Rv2450c were immunogenic (42). Recently we identified the first human M. tuberculosis Rpf-specific T-cell responses against a subset of the five M. tuberculosis Rpfs in a larger antigen T-cell screening (33). Gamma interferon (IFN-γ) production was detected in tuberculin skin test (TST)-positive individuals in response to Rv1009, Rv1884c, Rv2450c, and, to a lesser extent, to Rv0867c, whereas limited to no IFN-γ production was found in TST-negative individuals. Rv2389c, however, was not included in this study (33).
These two studies indicate a possible role for T-cell responses in detecting M. tuberculosis Rpf antigen during M. tuberculosis infection. We have, therefore, performed a more detailed analysis studying the immunogenicity of the Rv0867c and Rv2389c M. tuberculosis Rpf proteins in several groups of mycobacterium-exposed individuals, including long-term nonprogressors. These two antigens were selected based on the highest recognition of all 5 Rpfs in a cross-sectional cohort study of HIV-negative, TST- and/or ESAT6/CFP10-positive household contacts from the Gambia, Uganda, and South Africa (BMGF GCGH GC6#74 Biomarkers for TB Consortium; http://www.biomarkers-for-tb.net/; unpublished results). Of interest, both Rv0867c and Rv2389c are predicted to be secreted proteins (SignalP and TMMHM server, Technical University of Denmark) (17), which may enhance availability of the antigen to the innate immune system. Indeed, Rv0867c protein is present in M. tuberculosis culture filtrate (24). Rv0867c and Rv2389c are both expressed in early log-phase-grown M. tuberculosis, but Rv2389c expression is induced in the stationary and noncultivatable phases of M. tuberculosis growth and during acidic conditions, whereas Rv0867c expression appears to be higher in nutrient-starved M. tuberculosis culture. Both genes are also highly induced during early resuscitation (18). In the work reported here, we (i) identify frequent and significant human T-cell responses against Rv0867c and Rv2389c; (ii) identify a series of novel M. tuberculosis Rpf epitopes, including a single dominant peptide epitope in Rv2389c; and (iii) describe M. tuberculosis Rpf-specific polyfunctional memory CD4+ and particularly CD8+ T-cell memory responses to M. tuberculosis Rv0867c and Rv2389c Rpf proteins in long-term naturally protected M. tuberculosis nonprogressors. Based on these data, we propose that Rpf antigens are potentially interesting new TB vaccine candidates.
MATERIALS AND METHODS
Study subjects.
Blood samples were collected by venipuncture from a group of Dutch individuals, including 9 tuberculosis (TB) patients, 10 tuberculin skin test (TST)-positive individuals (indurations of ≥10 mm), 10 BCG-vaccinated individuals, and 10 non-BCG-vaccinated, TST-negative, healthy individuals, as well as 12 Norwegian TST-positive individuals. The Norwegian donor group consisted of elderly people (average age, 70 years) exposed to TB transmission several decades ago without developing any clinical disease and without receiving any treatment. Previously recorded TST indurations associated with natural conversion ranged from 12 to 60 mm (average of 18 mm). Recent IFN-γ release assay (IGRA) testing with the Quantiferon Gold in-tube test showed that 9 of 12 donors in this category were positive. All donors gave written consent before blood donation. The study protocol (P027/99) was approved by the Institutional Review Board of the Leiden University Medical Center and the Regional Committees for Medical and Health Research Ethics in Norway. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by Ficoll density gradient centrifugation and stored in liquid nitrogen until further use.
Recombinant proteins.
Recombinant proteins were produced as previously described (15). Briefly, M. tuberculosis genes were amplified by PCR from genomic H37Rv DNA and cloned by Gateway technology (Invitrogen, Carlsbad, CA) in a bacterial expression vector containing a histidine tag at the N terminus. Vectors were overexpressed in Escherichia coli BL21(DE3) and purified. The size and purity of recombinant proteins were analyzed by gel electrophoresis and Western blotting with an anti-His antibody (Invitrogen, Carlsbad, CA) and an anti-E. coli polyclonal antibody (a kind gift from the Statens Serum Institute [SSI]). Endotoxin contents were below 50 IU/mg recombinant protein, as tested using a Limulus Amebocyte Lysate (LAL) assay (Cambrex, East Rutherford, NJ). All proteins were tested in lymphocyte stimulation assays in order to exclude antigen-nonspecific T-cell stimulation and cellular toxicity by using PBMCs of in vitro purified protein derivative (PPD)-negative healthy Dutch donors (22). PPD of M. tuberculosis was purchased from SSI, Copenhagen, Denmark.
Synthetic peptides.
Peptides (20-mers overlapping 10 amino acids) were produced at the Leiden University Medical Center (LUMC) facility by simultaneous multiple-peptide synthesis as described previously (19). Homogeneity and purity were confirmed by analytical reversed-phase high-pressure liquid chromatography, and mass spectrometry showed the expected masses. Peptide purity was ≥75%.
Lymphocyte stimulation assay.
PBMCs (1.5 × 105/well) were cultured in triplicate in 96-well round-bottom plates (Nunc, Roskilde, Denmark) and incubated with or without protein (10 μg/ml) in AIM-V medium (Invitrogen, Breda, Netherlands) at 37°C in 5% CO2. After 6 days, supernatants were harvested and used for cytokine and chemokine profiling.
Cytokine and chemokine profiling.
Levels of cytokines (IFN-γ, interleukin-1β [IL-1β], IL-6, and tumor necrosis factor alpha [TNF-α]) and chemokines (IFN-γ-induced protein, 10 kDa [IP-10]; and macrophage inflammatory protein 1β [MIP-1β]) were analyzed with the Lincoplex kit (Millipore) according to the Milliplex Map protocol. Plates were analyzed with a BioPlex array reader with Bio-Plex software (Bio-Rad Laboratories, Veenendaal, Netherlands).
Generation of antigen-specific T-cell lines.
T-cell lines were generated as previously described (29). PBMCs (2 × 106 cells/well) from cured TB patients or TST-positive or BCG-vaccinated individuals were cultured in 24-well plates in the presence of protein (2 to 10 μg/ml) in IMDM medium (Gibco, Paisley, United Kingdom) supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin (Gibco, Paisley, United Kingdom), and 10% pooled human serum at 37°C in 5% CO2. At day 6, recombinant IL-2 (rIL-2) (Cetus, Emeryville, CA) was added to the cell cultures in a final concentration of 25 U/ml. Cultures were maintained for an additional 2 to 3 weeks in the presence of rIL-2. T cells were harvested and stored in liquid nitrogen until further use.
T-cell proliferation of antigen-specific T-cell lines.
Thawed T-cell lines were cultured in triplicate in 96-well flat-bottom plates (1.5 × 104/well) together with HLA-DR-matched irradiated (2,000 rads) PBMCs (5 × 104/well) with or without protein (10 μg/ml) or peptide (10 μg/ml) at 37°C in 5% CO2. After 3 days, supernatants were harvested and stored at −20°C. Cells were pulsed for an additional 18 h with [3H]thymidine (0.5 μCi/well), harvested, and counted on a Microbetaplate counter (Wallac, Turku, Finland) (3). A stimulation index (SI) of ≥3 was considered positive.
IFN-γ ELISA.
The concentration of IFN-γ in supernatants was measured by enzyme-linked immunosorbent assay (ELISA) (U-CyTech, Utrecht, Netherlands) according to the manufacturer's instructions. The detection limit of the assay was 20 pg/ml IFN-γ. Samples were tested in duplicate. An IFN-γ response of ≥100 pg/ml was considered positive.
Flow cytometric analysis.
PBMCs were thawed and rested. After 24 h, PBMCs were stimulated for 16 h with protein (10 μg/ml) in the presence of costimulatory antibodies anti-CD28 and anti-CD49d (Sanquin, Netherlands, and BD Biosciences, respectively). Brefeldin A (3 μg/ml; Sigma) was added after the first 4 to 6 h. Cells were stained for 30 min at 4°C with the following surface markers: anti-CD3-Pacific blue (PB), anti-CD4-peridinin chlorophyll protein (PerCP)-Cy5.5, anti-CD8-AmCyan, anti-CD25-allophycocyanin (APC)-Cy5, anti-CD45RA-phycoerythrin (PE)-Cy5, and anti-CCR7-PE-Cy7, and intracellular staining was performed with anti-IFN-γ–Alexa 700, anti-TNF-α–APC, anti-IL-2–PE, and anti-CD69–fluorescein isothiocyanate (FITC) (BD Biosciences) by using the Intrastain kit (Dako Cytomation, Denmark). Samples were acquired on an LSR II flow cytometer and analyzed with SPICE (software provided by M. Roederer, National Institute of Allergy and Infectious Disease) and FlowJo software (Treestar, Inc., Ashland, OR). Cell populations should contain at least 100 events.
Statistical analyses.
Differences between groups were analyzed with the nonparametrical Kruskal-Wallis test in GraphPad Prism (version 4). P values were corrected for multiple comparisons. The statistical significance level used was P < 0.05.
RESULTS
Recognition of M. tuberculosis Rpf antigens by T cells from M. tuberculosis-responsive individuals.
To determine the immunogenicities of both Rv0867c and Rv2389c, we investigated whether these two M. tuberculosis Rpf proteins were recognized by PBMCs of mycobacterium-exposed individuals. PBMCs from four groups of Dutch individuals were tested: (i) HIV-negative, treated tuberculosis patients (TB; n = 9); (ii) tuberculin skin test (TST)-positive individuals (TST; n = 10); (iii) BCG-vaccinated individuals (BCG; n = 10); and (iv) non-BCG-vaccinated, TST-negative and in vitro purified-protein derivative (PPD)-negative healthy controls (HCs; n = 10). The secretion of four cytokines (IFN-γ, IL-1β, IL-6, and TNF-α) was analyzed as a multiparameter readout of antigen-specific responses.
As expected, IFN-γ production in response to PPD was low in healthy individuals, whereas the TST+ individuals and TB patients showed high responses to PPD, by both IFN-γ and the other cytokines analyzed. The BCG-vaccinated individuals showed intermediate levels of responses (Fig. 1 A to D).
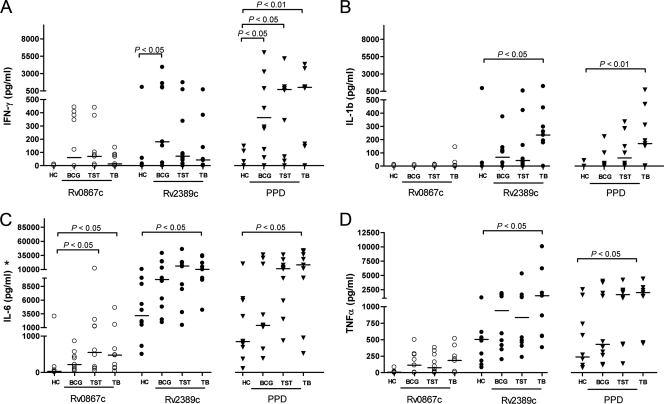
Fig. 1.
Cytokine and chemokine profiling. PBMCs from healthy individuals (HC; n = 10), BCG-vaccinated individuals (BCG; n = 10), TST-positive individuals (TST; n = 10), and TB patients (TB; n = 9) were stimulated with Rv0867c and Rv2389c proteins and PPD for 6 days. Levels of IFN-γ (A), IL-1β (B), IL-6 (C), and TNF-α (D) were measured and corrected for background levels. Horizontal bars represent median cytokine production levels. Antigen stimuli: ○, Rv0867c; •, Rv2389c; and ▾, PPD. *, background not corrected.
While minor IFN-γ levels were detectable in the three mycobacterium-exposed groups in response to Rv0867c, higher levels were found in response to Rv2389c (HCs and BCG; P < 0.05). The healthy controls did not respond to Rv0867c and Rv2389c (Fig. 1A). No IL-1β was produced upon Rv0867c stimulation in any of the four groups analyzed; however, Rv2389c stimulation induced production of IL-1β in all mycobacterium-exposed groups (HCs and TB; P < 0.05), but not in the healthy controls (Fig. 1B). Rv0867c stimulation also induced IL-6 in all three mycobacterium-exposed groups, but not in the healthy individuals (HCs, BCG, and TB; P < 0.05). In contrast to Rv0867c, Rv2389c induced high levels of IL-6 in the HC group but much higher levels in the mycobacterium-exposed individuals (HCs and TB; P < 0.05) (Fig. 1C). Rv2389c stimulation induced also high levels of TNF-α, especially within the mycobacterium-exposed groups (HCs and TB; P < 0.05), while Rv0867c induced only limited levels of TNF-α in all four groups (Fig. 1D).
Overall, the mycobacterium-exposed individuals produced the highest levels of cytokines upon Rv0867c and Rv2389c stimulation, and TB patients were often the highest responders to Rv2389 (HCs and TB; P < 0.05 for IL-1β, IL-6, and TNF-α). Rv2389c was more strongly recognized than Rv0867c, which induced no or low levels of cytokines.
Identification of immunogenic peptides of M. tuberculosis Rpf proteins.
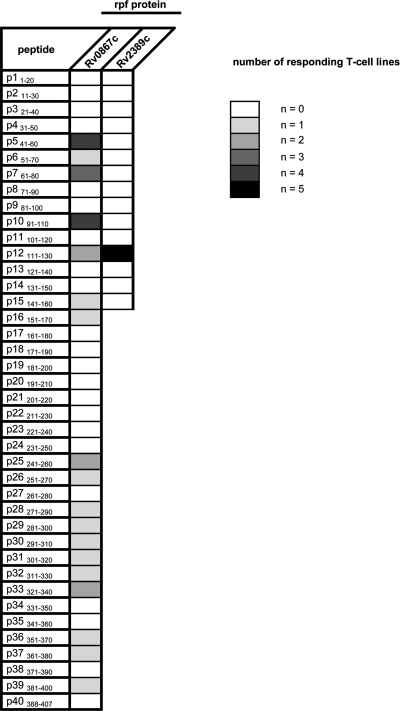
To further characterize the Rpf-specific responses observed above, five CD4+ T-cell lines specific for Rv0867c and five CD4+ T-cell lines specific for Rv2389c were generated. These T-cell lines were tested for recognition of all individual 20-mer peptides (overlapping by 10 amino acids) (see Table S1A and B in the supplemental material), and both IFN-γ production and T-cell proliferation were measured. Table 1 shows the recognition pattern of the Rpf peptides by the Rv0867c and Rv2389c antigen-specific T-cell lines examined. All T-cell lines responded to the corresponding Rpf protein and to one or more of the proteins' corresponding individual peptides. The Rv0867c-specific T-cell lines recognized many different peptides ranging from 1/5 to 4/5 of the donors. Four out of the five T-cell lines recognized peptides P541–60 and P1091–110, and three of the five lines recognized peptide P761–80. In contrast to Rv0867c-specific T cells, all Rv2389c-specific T-cell lines recognized only a single peptide, P12111–130.
Table 1.
CD4+ antigen-specific T-cell responses to single peptides of M. tuberculosis Rpf proteinsa
Peptide responses with a proliferation SI of ≥3 and IFN-γ responses of ≥100 pg/ml were considered positive.
Characterization of M. tuberculosis Rpf-specific polyfunctional T cells in long-term M. tuberculosis nonprogressors.
We next investigated T-cell responses to Rv0867c and Rv2389c antigens in a cohort of long-term M. tuberculosis nonprogressors which had been infected several decades ago but had never developed any signs of active TB. We analyzed responsive CD4+ and CD8+ T-cell subsets producing IFN-γ, TNF-α, and/or IL-2 by multiparameter flow cytometry. Polyfunctional T cells have been associated with protection following vaccination (10), but the situation in human infection is more complex, as polyfunctional T cells are also found in active TB patients (8). In any case, the memory T-cell subsets responding to Rv0867c or Rv2389c were analyzed with PBMCs from long-term nonprogressors (n = 12) and PPD-negative HCs (n = 11).
Figure 2 A shows that the Rv0867c protein induced polyfunctional T cells with different cytokine profiles (triple-positive [IFN-γ+ TNF-α+ IL-2+] or double-positive [IFN-γ+ TNF-α+, TNF-α+ IL-2+, and IFN-γ+ IL-2+] cells). Such polyfunctional T cells were detected in 9 out of the 12 long-term latently infected individuals. Strikingly, higher frequencies of polyfunctional CD8+ T cells (ranging between 0.20 and 9.72%) were observed, exceeding those of CD4+ T cells (ranging between 0.21 and 1.29%). Only 2 out of 12 donors showed polyfunctional CD8+ T cells recognizing Rv2389c, typically with a lower frequency (ranging between 0.24 and 0.82%) (Fig. 2C). Single-cytokine-producing CD4+ and CD8+ T cells were observed for both Rv0867c and Rv2389c, where IFN-γ+ CD4+ T cells are the most prominent T-cell subset of all single-cytokine-producing T cells. No polyfunctional T cells were induced within the negative healthy control population upon Rv0867c stimulation, although for unknown reasons, low numbers of IL-2+ single-positive cells were seen (Fig. 2B). Some low frequencies of polyfunctional and single-positive T cells were observed upon Rv2389c stimulation within the healthy control group (Fig. 2D), but lower than those in the latently infected group.
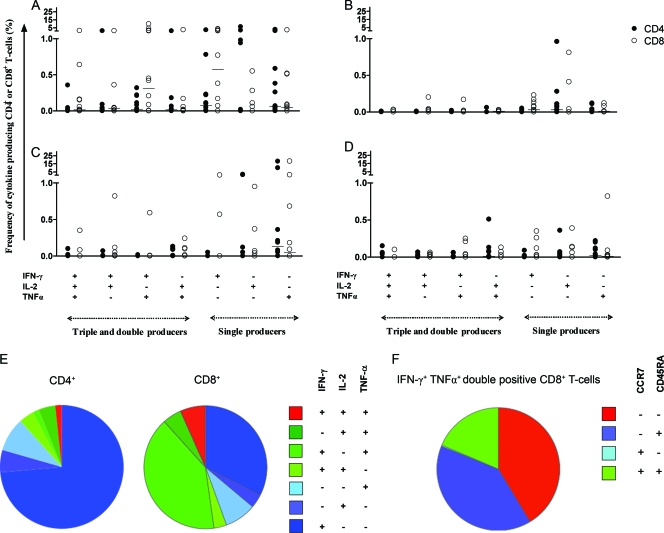
Fig. 2.
Frequency of antigen-specific polyfunctional T cells in long-term M. tuberculosis nonprogressors. Shown is the frequency of antigen-specific CD4+ and CD8+ T cells in long-term latently infected elderly subjects (n = 12) producing combinations of IFN-γ, TNF-α, and IL-2 after stimulation for 16 h with Rv0867c (A) or Rv2389c (C) protein. Healthy individuals were also analyzed for their polyfunctional responses to Rv0867c (B) and Rv2389c (D). CD4+ T cells are indicated as closed circles and CD8+ T cells as open circles. Horizontal bars represent the median frequency of antigen-specific CD4+ and CD8+ T cells. Slices in the pie chart represent the fraction of single (blue)-, double (green)-, or triple (red)-positive CD4+ and CD8+ T cells for Rv0867c (E). Expression of T-cell memory markers CCR7 and CD45RA was analyzed and is shown for the largest T-cell population identified: Rv0867c-specific IFN-γ and TNF-α double-positive CD8+ T cells (F). Effector memory T cells are CCR7− and CD45RA−, central memory T cells are CCR7+ and CD45RA−, naive T cells are CCR7+ and CD45RA+, and effector T cells are CCR7− and CD45RA+.
Figure 2E shows the proportions of polyfunctional and single-positive CD4+ and CD8+ T cells for Rv0867c, as this antigen was the best recognized in this cohort. IFN-γ+ TNF-α+ double-cytokine-producing CD8+ polyfunctional T cells were the most prominent polyfunctional T-cell subset identified.
In addition, we analyzed the expression of the T-cell memory markers CCR7 and CD45RA in these IFN-γ+ TNF-α+ double-cytokine-producing CD8+ T cells in the Rv0867c responders. After dividing the responding T cells into central memory (Tcm) and effector memory (Tem) T-cell subsets (according to Seder et al. [31, 34]), we found that Rv0867c-responding IFN-γ+ TNF-α+ CD8+ T cells consisted mostly of effector memory (CCR7− and CD45RA−) and effector (Teff) (CCR7− and CD45RA+) T-cell subsets (Fig. 2F). These data show the presence of Rv0867c-specific CD8+ memory and effector T cells in long-term M. tuberculosis nonprogressors.
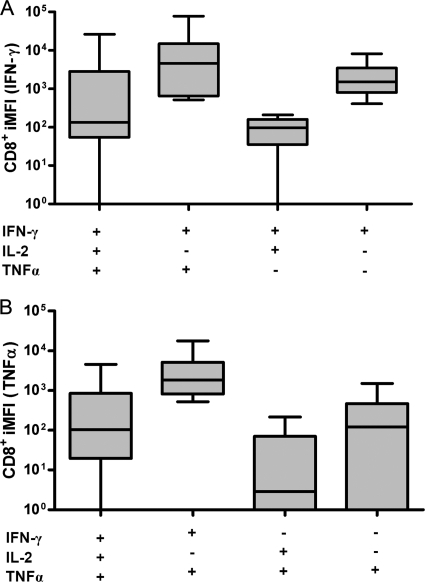
Besides the quantity of the T-cell response, the quality of the T-cell response plays an important role in protection. We therefore also measured the median fluorescence intensity (MFI) of each cytokine produced by the Rv0867c-responsive T cells. Multiplication of the frequency by the MFI results in an integrated MFI (iMFI) value, which was introduced previously as a quantitative parameter of the overall functionality of the T-cell response analyzed (10). The MFI values showed that the triple-positive T cells had the highest MFI values, followed by the double-cytokine-producing T cells, whereas single-positive T cells showed only minor MFI values (data not shown). However, when iMFI values were analyzed, the IFN-γ iMFI was the highest intensity within the IFN-γ+ TNF-α+ double-positive CD8+ T-cell population, followed by IFN-γ+ single-cytokine-producing T cells (Fig. 3 A). This was also observed for TNF-α iMFI (Fig. 3B). Taken together, these results indicate that double- and single-positive T cells contribute quantitatively more to the M. tuberculosis Rpf Rv0867c antigen response in long-term nonprogressors than triple-positive T cells.
Fig. 3.
Integrated mean fluorescence intensity (iMFI) of IFN-γ-, TNF-α-, and/or IL-2-producing CD8+ T cells. Shown is a box and whisker plot representing the iMFI values of CD8+ T cells from long-term nonprogressors (n = 8) in response to Rv0867c protein. iMFI is the product value of multiplication of the frequency of CD8+ T cells with the indicated (poly- or mono-) cytokine profiles with the MFI of IFN-γ (A) and TNF-α (B) produced by these T-cell subsets. The horizontal line represents the median, the lower boundary of the box represents the 25th percentile, and the upper boundary represents the 75th percentile. Whiskers extend from the box to the highest and lowest values.
DISCUSSION
New TB vaccines are urgently needed to help control the TB pandemic. Both prophylactic and postexposure vaccines are considered important (2), but the antigens that can best be incorporated into such vaccines have been identified incompletely at best. Previously we have shown that dormancy-related or DosR regulon-encoded M. tuberculosis antigens, which are expressed during hypoxia and nitric oxide stress, were preferentially recognized by TST-positive individuals, suggesting an association with control of infection (7, 21, 33). Unexpectedly, BCG vaccination failed to induce responses to these antigens in humans and mice, even though BCG is able to express the DosR regulon under hypoxia conditions in vitro (22). In contrast to the DosR regulon-encoded antigens, rpf genes Rv0867c and Rv2389c are expressed by actively replicating early log-phase-grown M. tuberculosis (18). Rv0867c and Rv2389c share the M. luteus Rpf protein's property of being able to resuscitate dormant mycobacteria. We therefore hypothesized that immunity directed against these proteins may play a role in sensing and detecting actively resuscitating and replicating M. tuberculosis organisms and therefore possibly also in host immune control of reactivating bacteria.
We observed significant differences in levels of Rv0867c and Rv2389c recognition between mycobacterium-exposed versus PPD-negative individuals, yet some Rv2389c and, to a lesser extent, Rv0867c responses were found in the healthy PPD-nonresponding group. We have previously reported a similar recognition pattern for some M. tuberculosis DosR regulon-encoded antigens by PPD-nonresponding donors. We explained these results by cross-reactive immunity to nontuberculous mycobacteria (NTM) which share large parts of the DosR regulon with M. tuberculosis and M. bovis BCG (23). Antigen contaminants (E. coli products) are less likely to be involved, since our recombinant proteins are standard quality control (QC) tested on cells from 4 or 5 PPD-negative donors and released only when no IFN-γ is produced. A BLAST search (http://www.ncbi.nlm.nih.gov/blast/) of the M. tuberculosis Rv0867c and Rv2389c Rpf protein sequences indeed showed protein sequence identity in several NTM and nonmycobacterial environmental bacteria (Table 2). Part of the immunogenic peptides identified in our current study are indeed conserved in the identified protein sequences of the species shown in Table 2 (P541-60 of Rv0867c, CE*GGNW*INT; and P12111-130 of Rv2389c, TQG*GAWP*C), compatible with cross-reactivity at the polyclonal T-cell level.
Table 2.
M. tuberculosis Rpf protein sequence identity in NTM and nonmycobacterial environmental bacteria
| Strain | % protein sequence identity to M. tuberculosis Rpf antigena: |
|
|---|---|---|
| Rv0867c | Rv2389c | |
| NTM | ||
| Mycobacterium marinum M | 88 | 55 |
| Mycobacterium ulcerans Agy99 | 88 | 54 |
| Mycobacterium avium subsp. paratuberculosis K10 | 84 | 49 |
| Mycobacterium sp. strain MCS | 76 | 55 |
| Mycobacterium vanbaalenii PYR-1 | 76 | 52 |
| Mycobacterium smegmatis MC2 155 | 78 | 57 |
| Mycobacterium avium 104 | 83 | 59 |
| Mycobacterium bovis BCG | 100 | 100 |
| Nonmycobacterial environmental bacteria | ||
| Nocardia farcinica IFM 10152 | 62 | 58 |
| Rhodococcus jostii RHA1 | 61 | 56 |
| Streptomyces avermitilis MA-4680 | 56 | 45 |
| Streptomyces coelicolor A3(2) | 70 | 43 |
| Corynebacterium jeikeium K411 | 54 | 52 |
| Corynebacterium diphtheriae NCTC 13129 | 55 | 45 |
| Corynebacterium glutamicum ATCC 13032 | 55 | 54 |
H37Rv Rpf protein sequences were compared to nontuberculous mycobacteria and nonmycobacterial environmental bacterial strains by BLAST searches (http://www.ncbi.nlm.nih.gov/blast/). The percentage of protein sequence identity is given per antigen and species. For analysis, a cutoff value of 40% was used.
To more precisely investigate the function and phenotype of Rv0867c- and Rv2389c-specific T-cell subsets, we analyzed the presence of mono- and polyfunctional CD4+ and CD8+ T cells in PBMCs of latently infected individuals who had been infected decades ago without developing TB. In the Dutch cohort above, we had identified Rv2389c as the best-recognized antigen, whereas in the long-term M. tuberculosis nonprogressors, Rv0867c induced higher frequencies of polyfunctional and single-positive T cells. This difference might be attributable to the type and/or longevity of the infection as M. tuberculosis is more likely to be chronic than NTM infections, and M. tuberculosis might be a stronger immunogen than NTM.
Among the polyfunctional antigen-specific T cells present in the long-term nonprogressors, predominantly Rv0867c-specific CD8+ T cells were identified, particularly double-producing IFN-γ+ TNF-α+ Tem and T effector (Teff) cells with high iMFI. This suggests that these two Rpf-specific CD8+ T-cell subsets may play a significant role in TB infection, next to triple-positive T cells. Of interest, IFN-γ+ TNF-α+ double-positive CD8+ T cells were also found following vaccination with AERAS-402 vaccine, containing Ag85A, Ag85B, and TB10.4 antigens as a BCG boost, and these cells persisted over time (1). Overall, CD8+ T cells are important in controlling M. tuberculosis infection, and the identification of prominent populations of Rv0867c-specific CD8+ T cells supports this notion. Nevertheless, relatively little is known about these antigen-specific polyfunctional CD8+ T cells and their role in protection (9).
Rv0867c-specific, IFN-γ+ TNF-α+ double-cytokine-producing CD8+ T cells were mainly Tem and Teff cells. These high CD8+ Tem and Teff subset responses to Rv0867c in the long-term M. tuberculosis nonprogressors do not seem to correspond to the hypothesis that after clearance of infection Tem populations gradually wane, resulting mainly in long-lived Tcm cells (41). A recent study showed that reexposure to antigens increased the number of CD8+ Tem cells. Our latently infected individuals may be continuously reexposed to Rpf antigens expressed by either endogenous uncleared M. tuberculosis organisms or by other environmental bacteria expressing cross-reactive Rpf-like antigens, which boost Rv0867c-specific Tem cells (37). Regardless, Rv0867c M. tuberculosis Rpf antigen is recognized in long-term M. tuberculosis nonprogressors, indicating that Rpf-specific T-cell responses are maintained for decades after the initial M. tuberculosis infection. Of note, the immune system changes with aging. Fewer naïve T cells will be available during aging as a result of thymic involution. However, CD8+ Tcm, Tem, and Teff cells accumulate with age (27, 32). These data are in agreement with our Tem and Teff findings in our elderly population (average age, 70 years).
Besides CD8+ T cells, also a minor proportion of polyfunctional CD4+ T cells was observed (Fig. 2E). Although previous results have indicated that triple-positive polyfunctional CD4+ T cells can be induced by BCG vaccination, they are also clearly present in TB patients, such that they do not necessarily correlate with protection against developing disease (8). Minor but significant proportions of triple-positive CD4+ polyfunctional T cells were identified in our work reported here.
We conclude that M. tuberculosis Rv0867c and Rv2389c Rpf antigens are immunogenic in humans, as evidenced by antigen-specific cytokine production and high frequencies and iMFI values. Both single- and double-cytokine-producing Rv0867c-specific CD4+ and CD8+ T cells were identified in long-term latently infected individuals who did not develop TB. By directing T-cell responses to M. tuberculosis Rpf antigens, it may be possible to enhance immune surveillance of reactivating and resuscitating M. tuberculosis bacilli and thereby help to control TB reactivation, which is the major complication in latent TB that keeps fueling the TB pandemic.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the FP7 NEWTBVAC Project, the Bill and Melinda Gates Foundation Grand Challenges in Global Health (GC6#74), and TI Pharma (project D-101-1).
We thank Corine Prins and Sandra Arend for collecting and providing the PBMCs used in this study.
The text represents the authors' views and does not necessarily represent a position of the FP7 NEWTBVAC Project Commission, who will not be liable for the use made of such information.
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 19 January 2011.
REFERENCES
- 1. Abel B., et al. 2010. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4 and CD8 T cells in adults. Am. J. Respir. Crit. Care Med. 181:1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abu-Raddad L. J., et al. 2009. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc. Natl. Acad. Sci. U. S. A. 106:13980–13985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arend S. M., et al. 2000. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect. Immun. 68:3314–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Betts J. C., Lukey P. T., Robb L. C., McAdam R. A., Duncan K. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717–731 [DOI] [PubMed] [Google Scholar]
- 5. Bigger J. W. 1944. Treatment of staphylococcal infections with penicillin. Lancet ii:497–500 [Google Scholar]
- 6. Biketov S., et al. 2000. Culturability of Mycobacterium tuberculosis cells isolated from murine macrophages: a bacterial growth factor promotes recovery. FEMS Immunol. Med. Microbiol. 29:233–240 [DOI] [PubMed] [Google Scholar]
- 7. Black G. F., et al. 2009. Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in Africa. Clin. Vaccine Immunol. 16:1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caccamo N., et al. 2010. Multifunctional CD4+T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 40:2211–2220 [DOI] [PubMed] [Google Scholar]
- 9. Caccamo N., et al. 2009. Analysis of Mycobacterium tuberculosis-specific CD8 T-cells in patients with active tuberculosis and in individuals with latent infection. PLoS. One 4:e5528. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Darrah P. A., et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850 [DOI] [PubMed] [Google Scholar]
- 11. Davies A. P., et al. 2008. Resuscitation-promoting factors are expressed in Mycobacterium tuberculosis-infected human tissue. Tuberculosis (Edinb.) 88:462–468 [DOI] [PubMed] [Google Scholar]
- 12. Downing K. J., et al. 2004. Global expression profiling of strains harbouring null mutations reveals that the five rpf-like genes of Mycobacterium tuberculosis show functional redundancy. Tuberculosis (Edinb.) 84:167–179 [DOI] [PubMed] [Google Scholar]
- 13. Downing K. J., et al. 2005. Mutants of Mycobacterium tuberculosis lacking three of the five rpf-like genes are defective for growth in vivo and for resuscitation in vitro. Infect. Immun. 73:3038–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fine P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339–1345 [DOI] [PubMed] [Google Scholar]
- 15. Franken K. L., et al. 2000. Purification of His-tagged proteins by immobilized chelate affinity chromatography: the benefits from the use of organic solvent. Protein Expr. Purif. 18:95–99 [DOI] [PubMed] [Google Scholar]
- 16. Garton N. J., et al. 2008. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 5:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gomez M., Johnson S., Gennaro M. L. 2000. Identification of secreted proteins of Mycobacterium tuberculosis by a bioinformatic approach. Infect. Immun. 68:2323–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta R. K., Srivastava B. S., Srivastava R. 2010. Comparative expression analysis of rpf-like genes of Mycobacterium tuberculosis H37Rv under different physiological stress and growth conditions. Microbiology 155:2714–2722 [DOI] [PubMed] [Google Scholar]
- 19. Hiemstra H. S., et al. 1997. The identification of CD4+ T cell epitopes with dedicated synthetic peptide libraries. Proc. Natl. Acad. Sci. U. S. A. 94:10313–10318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kell D. B., Young M. 2000. Bacterial dormancy and culturability: the role of autocrine growth factors. Curr. Opin. Microbiol. 3:238–243 [DOI] [PubMed] [Google Scholar]
- 21. Leyten E. M., et al. 2006. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect. 8:2052–2060 [DOI] [PubMed] [Google Scholar]
- 22. Lin M. Y., et al. 2007. Lack of immune responses to Mycobacterium tuberculosis DosR regulon proteins following Mycobacterium bovis BCG vaccination. Infect. Immun. 75:3523–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin M. Y., et al. 2009. Cross-reactive immunity to Mycobacterium tuberculosis DosR regulon-encoded antigens in individuals infected with environmental, nontuberculous mycobacteria. Infect. Immun. 77:5071–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malen H., Berven F. S., Fladmark K. E., Wiker H. G. 2007. Comprehensive analysis of exported proteins from Mycobacterium tuberculosis H37Rv. Proteomics 7:1702–1718 [DOI] [PubMed] [Google Scholar]
- 25. Mukamolova G. V., Kaprelyants A. S., Young D. I., Young M., Kell D. B. 1998. A bacterial cytokine. Proc. Natl. Acad. Sci. U. S. A. 95:8916–8921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mukamolova G. V., et al. 2002. A family of autocrine growth factors in Mycobacterium tuberculosis. Mol. Microbiol. 46:623–635 [DOI] [PubMed] [Google Scholar]
- 27. Naylor K., et al. 2005. The influence of age on T cell generation and TCR diversity. J. Immunol. 174:7446–7452 [DOI] [PubMed] [Google Scholar]
- 28. Ottenhoff T. H. 2009. Overcoming the global crisis: “yes, we can," but also for TB? Eur. J. Immunol. 39:2014–2020 [DOI] [PubMed] [Google Scholar]
- 29. Ottenhoff T. H., Elferink D. G., Hermans J., de Vries R. R. 1985. HLA class II restriction repertoire of antigen-specific T cells. I. The main restriction determinants for antigen presentation are associated with HLA-D/DR and not with DP and DQ. Hum. Immunol. 13:105–116 [DOI] [PubMed] [Google Scholar]
- 30. Rachman H., et al. 2006. Unique transcriptome signature of Mycobacterium tuberculosis in pulmonary tuberculosis. Infect. Immun. 74:1233–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712 [DOI] [PubMed] [Google Scholar]
- 32. Saule P., et al. 2006. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech. Ageing Dev. 127:274–281 [DOI] [PubMed] [Google Scholar]
- 33. Schuck S. D., et al. 2009. Identification of T-cell antigens specific for latent Mycobacterium tuberculosis infection. PLoS One 4:e5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seder R. A., Darrah P. A., Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258 [DOI] [PubMed] [Google Scholar]
- 35. Shleeva M. O., Salina E. G., Kaprelyants A. S. 2010. Dormant forms of mycobacteria. Microbiology 79:1–12 [Google Scholar]
- 36. Tufariello J. M., Jacobs W. R., Jr., Chan J. 2004. Individual Mycobacterium tuberculosis resuscitation-promoting factor homologues are dispensable for growth in vitro and in vivo. Infect. Immun. 72:515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vezys V., et al. 2009. Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457:196–199 [DOI] [PubMed] [Google Scholar]
- 38. Voskuil M. I., et al. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wayne L. G., Hayes L. G. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wayne L. G., Sohaskey C. D. 2001. Nonreplicating persistence of mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139–163 [DOI] [PubMed] [Google Scholar]
- 41. Woodland D. L., Kohlmeier J. E. 2009. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 9:153–161 [DOI] [PubMed] [Google Scholar]
- 42. Yeremeev V. V., et al. 2003. Proteins of the Rpf family: immune cell reactivity and vaccination efficacy against tuberculosis in mice. Infect. Immun. 71:4789–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.