Abstract
To enhance preclinical evaluation of serological immune responses to the individual diphtheria, tetanus, and pertussis (DTP) components of DTP combination vaccines, a fast hexavalent bead-based method was developed. This multiplex immunoassay (MIA) can simultaneously determine levels of specific mouse serum IgG antibodies to P antigens P.69 pertactin (P.69 Prn), filamentous hemagglutinin (FHA), pertussis toxin (Ptx), and combined fimbria type 2 and 3 antigens (Fim2/3) and to diphtheria toxin (Dtx) and tetanus toxin (TT) in a single well. The mouse DTP MIA was shown to be specific and sensitive and to correlate with the six single in-house enzyme-linked immunosorbent assays (ELISAs) for all antigens. Moreover, the MIA was expanded to include avidity measurements of DTP antigens in a multivalent manner. The sensitivities of the mouse DTP avidity MIA per antigen were comparable to those of the six individual in-house avidity ELISAs, and good correlations between IgG concentrations obtained by both methods for all antigens tested were shown. The regular and avidity mouse DTP MIAs were reproducible, with good intra- and interassay coefficients of variability (CV) for all antigens. Finally, the usefulness of the assay was demonstrated in a longitudinal study of the development and avidity maturation of specific IgG antibodies in mice having received different DTP vaccines. We conclude that the hexaplex mouse DTP MIA is a specific, sensitive, and high-throughput alternative for ELISA to investigate the quantity and quality of serological responses to DTP antigens in preclinical vaccine studies.
INTRODUCTION
Mouse serum IgG antibodies specific for individual components of multivalent vaccines are important immunogenicity markers used in preclinical testing of vaccines and are commonly evaluated by employing multiple enzyme-linked immunosorbent assays (ELISAs). Also, for monitoring murine serum responses to diphtheria, tetanus, and pertussis (DTP) antigens, ELISAs are broadly used (7, 29, 31, 49) and even required by the regulatory authorities for the batch release of combination vaccines with acellular pertussis components (DTaP) (13). ELISAs are time-consuming and may require substantial amounts of specific antigen for plate coating in large studies. Furthermore, since ELISAs are monovalent, preclinical evaluation of serological responses to multicomponent vaccines are labor-intensive and require considerable mouse serum sample volumes, especially as numbers of vaccine components to be tested increase or when avidity analysis is involved. As an alternative serological assay, many laboratories have successfully developed multiplex flow-cytometric immunoassays (MIAs) using fluorescent bead sets as carriers for different antigens, including DTP antigens (10, 11, 22, 23, 25, 35, 39, 41, 50). The most important advantage of serological MIAs over ELISAs is that antibody responses to multiple antigens can be determined simultaneously in a single well. Therefore, MIAs are considerably less labor-intensive, are serum saving, and usually require small amounts of bead-coated antigen. The available MIA systems can readily measure total human IgG antibody levels but are currently also being adapted to enable measurement of antibody quality as well (11, 18).
Recently, van Gageldonk et al. (50) developed a pentaplex MIA for the detection of human IgG responses to five antigens present in DTP combination vaccines as an important step toward replacing time-consuming ELISAs in immune surveillance studies and vaccine trials. To screen preclinical sera for DTP antibodies, as is required for regulatory purposes or in the framework of ongoing vaccine research and development (16, 31, 46), only mouse ELISAs are available. Therefore, we here adapted the human bead-based assay to a hexaplex MIA system to simultaneously determine mouse serum concentrations of IgG antibodies to six components of DTP combination vaccines, i.e., P.69 pertactin (Prn), filamentous hemagglutinin (FHA), pertussis toxin (Ptx), combined fimbria type 2 and 3 antigens (Fim2/3), diphtheria toxin (Dtx), and tetanus toxin (TT), saving time and requiring only small serum aliquots available from preclinical venipuncture samples. Moreover, we extended the serological MIA in order to determine the avidity of the mouse DTP antibody responses in a multivalent manner.
MATERIALS AND METHODS
Antigens and reagents.
Bordetella pertussis P.69 Prn was recombinantly expressed in Escherichia coli and purified as described elsewhere (47). Ptx, FHA, and Fim2/3 antigens were purified from B. pertussis biomass in-house according to procedures described in the literature (42–44). Mws and purity of these antigens were verified using SDS-PAGE, and the presence of detectable impurities of E. coli lipopolysaccharide (LPS) in recombinant P.69 Prn (rP.69 Prn) and of B. pertussis lipooligosaccharide (LOS) in Ptx, FHA, and Fim2/3 preparations was ruled out in a Limulus amebocyte lysate (LAL) test (hence endotoxin levels were <0.015 endotoxin units [EU]/ml). Corynebacterium diphtheriae Dtx and Clostridium tetani TT were purchased as toxins from Sigma-Aldrich. Mouse monoclonal antibodies (moAbs) directed at P antigens FHA (29E7), P.69 Prn (Pem72), and Fim3 (81H11) were all obtained as hybridoma culture supernatants from the Dutch National Institute of Public Health and the Environment (19, 38), and the moAb directed to Ptx S1 (3F10) was obtained as a dilution of ascitic fluid from the National Institute for Biological Standards and Control (NIBSC; code 99/520). Color-coded carboxylated microspheres representing distinct bead regions were obtained from Bio-Rad Laboratories. N-Hydroxysulfosuccinimide (sulfo-NHS) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) were purchased from Pierce. Bovine serum albumin (BSA), natrium thiocyanate (NaSCN), and (3,3′,5,5′)-tetramethylbenzidine (TMB) were purchased from Sigma-Aldrich. R-Phycoerythrin (RPE)-conjugated goat anti-mouse total IgG, IgG1, and IgG2a antibodies and horseradish peroxidase (HRP)–goat anti-mouse IgG were obtained from Southern Biotech. Phosphate-buffered saline (PBS), pH 7.2, was obtained from Invitrogen. Tween 20 and Tween 80 were purchased from Merck (Germany). Skim milk (Protifar) was obtained from Nutricia (Netherlands).
Mouse reference serum.
Mouse reference serum with standardized IgG concentrations for P antigens, P.69 Prn, FHA, Ptx, and Fim2/3 was obtained from NIBSC (code 97/642) (15). The concentrations of anti-Dtx and TT IgG antibodies in this reference serum were unknown, and therefore these concentrations were arbitrarily set at 100 U/ml. Concentrations of specific IgG1 and IgG2a antibodies in the reference serum were not standardized but measurable, albeit low for IgG2a. Consequently, the median fluorescence intensities (MFI) of the reference serum for IgG1 and IgG2a were checked for consistency between MIAs (coefficient of variance [CV] < 20%), and IgG1 and IgG2a levels were presented as MFI ± standard deviations (SD).
Mouse immune sera.
BALB/c mice were housed and used for animal experiments according to the medical and ethical guidelines for animal experiments at the Netherlands Vaccine Institute.
Mouse immune sera were generated by immunization of groups of 6- to 8-week-old BALB/c mice (n = 24 mice/group) at day 0 and day 28 with 0.25 human dose (HD) of either a Dtx, TT, and acellular pertussis combination vaccine (DTaP; Infanrix; GlaxoSmithKline, United Kingdom) or a Dtx, TT, and whole-cell pertussis combination vaccine, as was formally used in the national immunization program in Netherlands (DTP Netherlands Vaccine Institute, Netherlands). These vaccines contain different amounts of pertussis antigens (48, 51) but equal amounts of Dtx and TT. Both vaccines contain aluminum hydroxide as an adjuvant. Orbital serum punction samples (200 μl) were taken at days 0, 28, 42, 98, and 308 after primary immunization.
ELISA.
IgG antibodies against individual DTP antigens were determined in six parallel ELISAs as described before (3) with minor modifications. Briefly, antigens were diluted in PBS at 2 μg/ml and absorbed in 100-μl volumes to high-protein-binding polystyrene 96-well microtiter plates (Nunc) by incubation overnight at room temperature (RT) and successively washed with distilled water containing 0.03% Tween 80. Six 3-fold dilutions of reference serum (starting at 1/100) or serum samples (starting at 1/100 for all antigens tested except for Dtx and TT, which started at 1/300) in PBS containing 0.1% Tween 80 (PBST) were added to the microtiter plates (100 μl). Dilutions of reference serum and blanks (without serum) were included in every plate. Following a 1-h incubation at 37°C, plates were washed and a 1:5,000 dilution of HRP–goat anti-mouse IgG in PBST containing 0.5% skim milk was added to each well (100 μl) and incubated for another hour at 37°C. After the final wash step, the plates were developed with 100 μl/well peroxidase substrate (0.1 mg/ml TMB with 0.012% H2O2 in 0.11 M sodium acetate buffer [pH 5.5]) and the reaction was stopped with 2 M H2SO4 after 10 min. Optical density at 450 nm (OD450) was read using an ELISA reader (Bio-Tek). For each serum sample, OD values were plotted against the serum dilution. OD values within the linear part of the curve were converted to U/ml by interpolation from a 4-parameter logistic (4-PL) standard curve of the reference serum and averaged.
IgG avidity measurement using ELISA.
Avidity of DTP-specific IgG antibodies in mouse reference serum was evaluated in six parallel ELISAs using different concentrations of the chaotropic reagent NaSCN in PBS (40), ranging from 0 M (untreated condition) to 3 M, as described elsewhere (28). NaSCN concentrations resulting in a reduction of 50% of the OD450 of the untreated controls were determined to be 1.5 M for P.69 Prn and Dtx, 2 M for FHA and Fim2/3, and 2.5 M for Ptx and TT (data not shown) and were considered the optimal conditions for avidity analysis of specific IgG antibodies in mouse immune sera. Briefly, for each antigen the ELISA was performed in duplicate plates, one treated as for a standard ELISA and one treated as follows. After the serum incubation step and subsequent washing, NaSCN was added to the wells (100 μl/well) at the optimized concentration per antigen. After 15 min of NaSCN treatment, wells were washed with PBST and the procedure was continued as described for the standard ELISA (i.e., incubation with HRP-IgG, etc.). IgG concentrations with NaSCN treatment are expressed as U/ml.
Conjugation of vaccine antigens to carboxylated microspheres.
Purified pertussis antigens P.69 Prn, FHA, Ptx, and Fim2/3 and Dtx and TT were coupled to distinct activated carboxylated microspheres (beads) of bead regions 2, 11, 60, 24, 45, and 28, respectively, essentially as described by van Gageldonk et al. (50). Briefly, 6.25 × 106 beads (500 μl) of each region were activated by incubation with 2.5 mg/ml EDC and 2.5 mg/ml sulfo-NHS in PBS for 20 min at RT in the dark under constant rotation at 25 rpm. Subsequently, beads were washed twice with PBS and resuspended in PBS with antigen-to-bead ratios of 5 μg/6.25 × 106 activated beads for P antigens and of 25 μg/6.25 × 106 activated beads for DT antigens. After incubation for 2 h at RT in the dark under constant rotation, beads were washed three times and stored in PBS containing 0.05% (wt/vol) sodium azide and 1% (wt/vol) BSA at 4°C in the dark until used.
Hexaplex MIA.
Eight steps of 3-fold dilutions (1/100 to 1/218,700) of the NIBSC reference serum were prepared in PBS containing 0.1% Tween 20 and 3% bovine serum albumin (PBS-T20-BSA). Immune sera were diluted 1/1,250 and 1/12,500 in PBS-T20-BSA. Each dilution of the reference or immune serum samples (25 μl) was mixed 1:1 with conjugated beads (25 μl containing 4,000 beads/region/well) in a 96-well Multiscreen HTS filter plate (Millipore Corporation) and incubated for 45 min at RT in the dark on a plate shaker at 600 rpm. Dilutions of reference serum and blanks (without serum) were included in every plate. The beads were washed three times with PBS by filtration using a vacuum manifold. Subsequently, 50 μl of a 1/200 dilution of RPE-conjugated anti-mouse total IgG (or IgG1 or IgG2a) in PBS was added to each well, and plates were incubated for 30 min under continuous shaking. After a washing, beads were resuspended in 100 μl PBS and flow cytometrically analyzed on a Bio-Plex 100 in combination with Bio-Plex Manager software (Bio-Rad Laboratories). Each bead is classified by its signature fluorescent pattern and then analyzed for the MFI of the signal of the reporter antibody. For each analyte, MFI was converted to U/ml by interpolation from a 5-PL standard curve (log-log) for every bead region/standard.
MIA cross-reactivity.
Antigen specificity and cross contamination of the P antigen-coupled bead sets in the hexaplex MIA were determined by incubating hexavalent bead mixtures with dilutions of individual mouse moAbs directed to purified P antigens that yielded maximal reporter signal for the specific bead set. To pick up E. coli LPS as a contaminant of rP.69 Prn, bead mixtures were also incubated with undiluted culture supernatant of an anti-E. coli LPS moAb. The maximal MFI of a particular bead set with its specific moAb, e.g., “A”-coupled beads with moAb “anti-A,” was assayed as 100%. Cross contamination of another bead set, e.g., “B”-coupled beads, was expressed as the following percentage: (MFI of moAb “anti-A” to bead set “B”/MFI of moAb “anti-A” to antigen “A”)·100%. None of the bead sets in the hexavalent bead mixtures incubated with anti-E. coli LPS give any detectable reporter signal. These responses were set at 0%.
MIA specificity.
The NIBSC reference serum was diluted 1:4,000 in PBS and preincubated with each of the vaccine antigens (5 μg/ml). Following a 1-hour preincubation at room temperature, aliquots were incubated with beads and handled as described above. A nontreated 1:4,000 dilution of the reference serum was assayed as a control. The percentage of homologous and heterologous inhibition of the preincubated sample was calculated as follows: 100% − {[(U/ml obtained in the presence of inhibitor)/(U/ml obtained in the absence of inhibitor)] ·100%}.
MIA reproducibility.
A serum panel, taken from DTP antigen-immune mice at different time intervals after vaccination, was used to assess the reproducibility of both the regular and the avidity MIAs. For each antigen, intra-assay variation (within a plate and from plate to plate) was determined by assaying duplicates of the serum panel in the same MIA, either on the same plate or on different plates. Interassay variation for each antigen was assessed by testing the serum panel in two separate MIAs on different days. For each (intra-assay or interassay) duplicate result, the coefficient of variation (%CV) was calculated, and calculated %CVs for multiple duplicates (n ≥ 20 per antigen) were averaged.
IgG avidity measurements using MIA.
Conditions for evaluating avidity of mouse DTP-specific IgG antibodies in MIA were maximized for each DTP antigen using mouse reference serum and different NaSCN concentrations as described for ELISA. NaSCN concentrations resulting in a 40 to 60% reduction of the mean untreated fluorescence could be optimized at 0.5 and 2 M NaSCN for all antigens except FHA. For FHA the introduction of NaSCN in MIA induced an elevated background signal in the absence of serum (MFI > 1,200 for 0.5 M NaSCN). This was caused by elevated nonspecific binding of the anti-IgG part of the conjugate (and not of the phycoerythrin [PE] label of the conjugate) to the NaSCN-treated FHA beads and could not be circumvented by preincubating the conjugate with FHA or by using other conjugates (data not shown). As an alternative chaotropic reagent for thiocyanate ions, conditions were optimized for avidity evaluation in MIA using urea (8, 14, 37), known to also unfold proteins through a combination of direct binding to proteins and altering their solvent environment (4, 20, 30). Briefly, to determine optimal urea conditions for avidity measurement, i.e., after reducing antigen specific IgG levels by minimally 30% and maximally 80%, trends were obtained from serial incubations of day 42 postvaccination immune sera with beads in replicate plates. One plate was developed according to standard MIA conditions; the others were treated with urea as follows. After the 45-min incubation step of serum and beads and subsequent washing, 50 μl of a 6, 7, 8, or 9 M urea solution in PBS was added to the wells of the replicate plates and incubated for 10 min in the dark at room temperature. Then, plates were washed 3 times with PBS and the standard MIA procedure was followed again, i.e., incubation with RPE-anti-total IgG. Two optimized urea concentrations were determined, one for IgG avidity assessment for the combined P antigens as well as for TT (9 M urea) and one for Dtx-specific IgG (6 M urea). These two concentrations were used when determining avidity of IgG antibodies as indicated in Results. Urea treatment IgG concentrations are expressed as U/ml. The avidity index (AI) is calculated as the following percentage: (U/ml obtained in the presence of urea/U/ml obtained in the absence of urea)·100%.
Statistical analysis.
Determination of Pearsons's correlation coefficient and linear regression were applied to analyze correlation of variables between assays and the linearity of the relationship between data sets. A two-way analysis of variance (ANOVA) with a Bonferroni posttest was used to assess the significance of differences between levels and AIs of IgG antibodies or subclasses in differently vaccinated mice, and a one-way ANOVA with a Bonferroni posttest was used to evaluate differences in antibody levels versus time in DTaP- or DTP-vaccinated mice. P values <0.05 are considered to indicate significant difference.
RESULTS
Development of a hexaplex MIA system to quantify mouse DTP-specific IgG antibodies. (i) Bead specificity and serum dilutions.
DTP antigens were conjugated to individual bead sets at an optimized antigen concentration as described by van Gageldonk et al. to obtain six monovalent bead sets (50). Using mixed bead sets and monoclonal antibodies directed to the individual in-house-purified P antigens, bead specificity, cross-reactivity, and absence of antigen cross contamination through copurified antigen impurities were assessed in MIA. Specificity of the monovalent P bead components was confirmed, and cross-reactivity and heterologous P antigen contamination of beads were ruled out (see Table S1 in the supplemental material). To assess the range of reporter fluorescence intensities generated using a positive mouse serum, eight steps of 3-fold dilutions of a mouse reference serum were tested in the MIA. Standard curves for all six vaccine antigens, i.e., P.69 Prn, FHA, Ptx, Fim2/3, Dtx, and TT, were linear over approximately seven 3-fold dilutions of the reference serum (data not shown). Sample dilutions were optimized to cover a broad concentration range of specific serum IgG in pre- and postvaccination sera. With a minimum of two single serum dilutions (1:2,500 and 1:25,000), over 99% of the specific IgG antibody levels to the six DTP antigens could be determined in a multiplex manner (n = 200 sera tested; data not shown).
(ii) MIA specificity.
To further assess the specificity of the mouse DTP MIA, homologous and heterologous inhibition of binding of reference serum antibodies to antigen-coupled beads was determined through absorption. Dilutions (1:4,000) of reference serum were preincubated with each of the DTP antigens at a concentration of 5 μg/ml. The preabsorbed reference serum samples were then tested for reactivity to the pooled antigen-coupled beads in the MIA. As shown in Table 1, addition of the inhibitors resulted in a high inhibition of homologous signals by >79% for Ptx, Fim2/3, Prn P.69, Dtx, and TT and in a more moderate homologous inhibition for FHA (58%). Heterologous inhibition was ≤10% for all variables, except for inhibition of the Fim2/3 signal with preincubation of reference serum with P.69 Prn, which resulted in 17% heterologous inhibition. Using higher concentrations of antigens in this assay increased homologous inhibition of all antigens but was also found to increase heterologous inhibition of the signals of Fim2/3, FHA, and P.69 Prn (data not shown), probably related to their adhesin properties.
Table 1.
Specificity of the hexaplex mouse DTP MIA
| Inhibitor | % inhibitiona for: |
|||||
|---|---|---|---|---|---|---|
| FHA | Ptx | Fim2/3 | P.69 Prn | Dtx | TT | |
| FHA | 58 | 0 | 0 | 0 | 0 | 0 |
| Ptx | 0 | 89 | 2 | 0 | 0 | 0 |
| Fim2/3 | 0 | 0 | 85 | 3 | 2 | 3 |
| P.69 Prn | 10 | 0 | 17 | 79 | 0 | 0 |
| Dtx | 0 | 1 | 0 | 0 | 95 | 0 |
| TT | 0 | 0 | 3 | 0 | 0 | 90 |
Percentage of homologous and heterologous inhibition after preincubation of the mouse reference serum with different antigens. Shown is one representative experiment out of three.
(iii) Comparison of DTP MIA and ELISA.
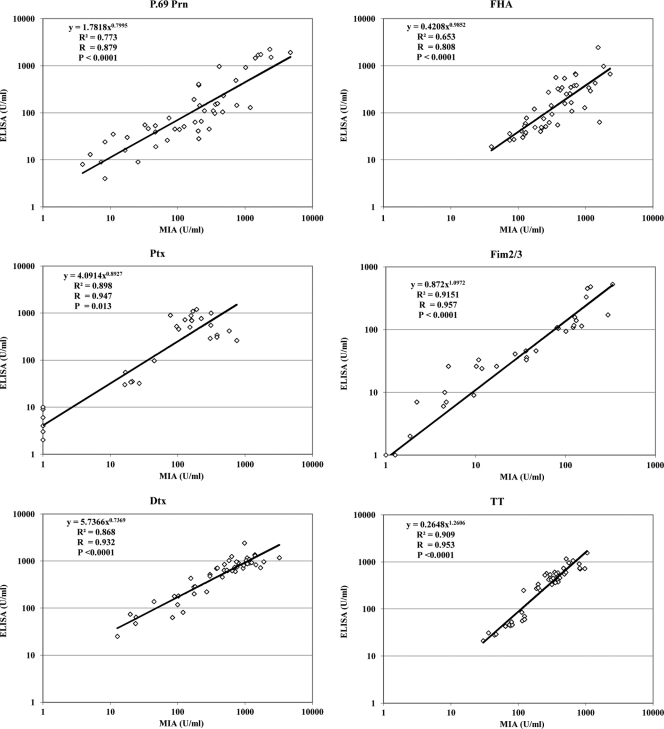
The compare the performance of the DTP MIA to that of ELISA, serum samples from mice taken at different time points after immunization representing a range of concentrations of the antibodies to the various vaccine antigens were tested both in MIA and in six parallel in-house ELISAs. The concentrations of antibodies measured in MIA were plotted in scatter plots against the ELISA values (Fig. 1). Results were compared by linear regression, and coefficients of correlation (R) were calculated. The mouse MIA had good correlation with mouse ELISA for all DTP antigens tested, with individual R values between 0.808 for FHA and 0.957 for Fim2/3 and P values lower than 0.0001 for all antigens except for Ptx (P = 0.013) (Fig. 1), which in part might be due to a poor low-end reproducibility of the in-house mouse Ptx ELISA.
Fig. 1.
Comparison of concentrations of specific mouse IgG antibody to DTP antigens obtained with the multiplex immunoassay (MIA) or with the ELISA. Sera (n = 27 to 47 sera/antigen) from DTP- or DTaP-vaccinated mice were tested for the presence of IgG antibodies specific for P.69 Prn, FHA, Ptx, Fim2/3, Dtx, and TT.
(iv) Assay sensitivity.
The sensitivity of the IgG MIA was determined for each DTP vaccine antigen. From 72 blank wells, MFI values were collected and mean MFI and SD were calculated for each analyte. The lower limit of detection (LLOD) was determined by interpolation of the value of 2 SD in the 5-PL curve for the NIBSC reference serum and represented as concentration (in mU/ml). LLODs for total IgG MIA were between 0.1 and 4.7 mU/ml (Table 2). For comparison, in each of the parallel IgG ELISAs the OD values from 24 blank wells were measured and means and SD were calculated. The LLODs for the parallel IgG ELISAs were then calculated by interpolating the mean OD plus 2 SD in the relevant 4-PL curve of the NIBSC reference serum (in mU/ml) (Table 2). Antigen-specific LLOD values (and derived lower limits of quantification [LLOQ]) for both methods are in the same order of magnitude (with a minor exception for TT) (Table 2, upper rows) and well below values measured in prevaccination sera (Fig. 1). Hence, the hexaplex MIA and the parallel ELISAs are equally sensitive for detection of DTP-specific IgG.
Table 2.
Calculated lower limits of detection and quantitation of the regular and avidity hexaplex mouse DTP MIA in comparison to ELISA
| Assay | LLOD (LLOQa) (mU/ml) for: |
|||||
|---|---|---|---|---|---|---|
| FHA | Ptx | Fim2/3 | P.69 Prn | Dtx | TT | |
| IgG MIA | 4.7 (14.1) | 0.1 (0.3) | 0.2 (0.6) | 0.7 (2.1) | 0.2 (0.6) | 1.9 (5.7) |
| IgG ELISA | 1.2 (3.6) | 0.1 (0.3) | 0.3 (0.9) | 0.7 (2.1) | 0.1 (0.3) | 0.1 (0.3) |
| Avidity MIA | 5.4 (16.2) | 0.2 (0.6) | 0.3 (0.9) | 0.5 (1.5) | 0.2 (0.6) | 0.1 (0.3) |
| Avidity ELISA | 3.5 (10.5) | 0.1 (0.3) | 0.3 (0.9) | 0.7 (2.1) | 0.1 (0.3) | 0.1 (0.3) |
The LLOQ of the assays can be calculated by multiplying the LLOD by a factor of 3 [in accordance with the guideline ICH Topic 2 (R1) of the European Medicines Agency, Validation of analytical procedures: text and methodology (CPMP/ICH/381/95) (htttp://www.ema.europa.eu)].
(v) Assay reproducibility.
Reproducibility of the IgG MIA was assessed for each DTP vaccine antigen by determination of the level of both intra-assay and interassay variation using a panel of mouse immune sera. The mean %CVs for the intra-assay variation, determined for multiple sera tested in duplicate within one plate, ranged from 4 to 9% for all antigens and, tested in duplicate on different plates, from 4 to 15% (Table 3, untreated condition). Interassay variation was addressed by comparing duplicates of sera tested in two separate assays. The mean %CVs for the interassay variation for the different antigens ranged from 7 to 14%. Hence good intra- and interassay reproducibility was shown for all antigens in the MIA.
Table 3.
Reproducibility of the regular and avidity mouse DTP MIA
| Analyte | Mean %CV of duplicate results at indicated urea concn for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intra-assay (within plate) |
Intra-assay (between plates) |
Interassay (between assays) |
|||||||
| −a | 6 M | 9 M | − | 6 M | 9 M | − | 6 M | 9 M | |
| FHA | 9 | 15 | 13 | 10 | 13 | 14 | 13 | 13 | 18 |
| Ptx | 5 | 4 | 6 | 5 | 5 | 10 | 9 | 6 | 9 |
| Fim2/3 | 6 | NPb | 6 | 11 | NP | 9 | 14 | NP | 19 |
| P.69 Prn | 4 | 6 | 5 | 4 | 7 | 16 | 8 | 6 | 11 |
| Dtx | 6 | 5 | NP | 6 | 9 | NP | 7 | 7 | NP |
| TT | 5 | 2 | 2 | 15 | 2 | 4 | 10 | 3 | 2 |
−, untreated condition (regular).
NP, condition not preferable.
Development of a hexaplex avidity MIA to monitor avidity of mouse DTP-specific IgG antibodies. (i) Use of urea for dissociation of low-avidity IgG populations.
To be able to measure avidity of mouse IgG responses to single DTP antigens in a multiplex manner, the MIA was then extended to include an incubation step with a chaotropic reagent for elution of low-avidity IgG antibodies, prior to adding conjugate. With thiocyanate, an efficient eluting reagent in the in-house DTP antigen-specific avidity ELISAs as well as in a human MIA system (18), avidity indexes of postvaccination mouse IgG antibodies against Dtx, TT, P.69 Prn, Ptx, and Fim2/3 but not FHA could be measured in MIA (see Materials and Methods). To be able to include the FHA antigen in the avidity MIA, urea, also known as a chaotropic reagent releasing weakly binding antibody-antigen interactions (8, 14, 37), was used as an alternative. This reagent demonstrated a low background signal in the absence of serum when tested at optimized concentrations for all antigens, including FHA (MFI < 200). Relevant reduction of specific IgG antibody levels was observed at 9 M urea for the collective P antigens, P.69 Prn, FHA, Ptx, and Fim2/3, and for TT and at 6 M urea for Dtx. Therefore, using urea the DTP MIA can be adapted to multivalently release low-avidity IgG populations for DTP antigens, including FHA, although two different urea concentrations are needed to cover all six DTP antigens.
(ii) Correlation and sensitivity of avidity MIA and avidity ELISA.
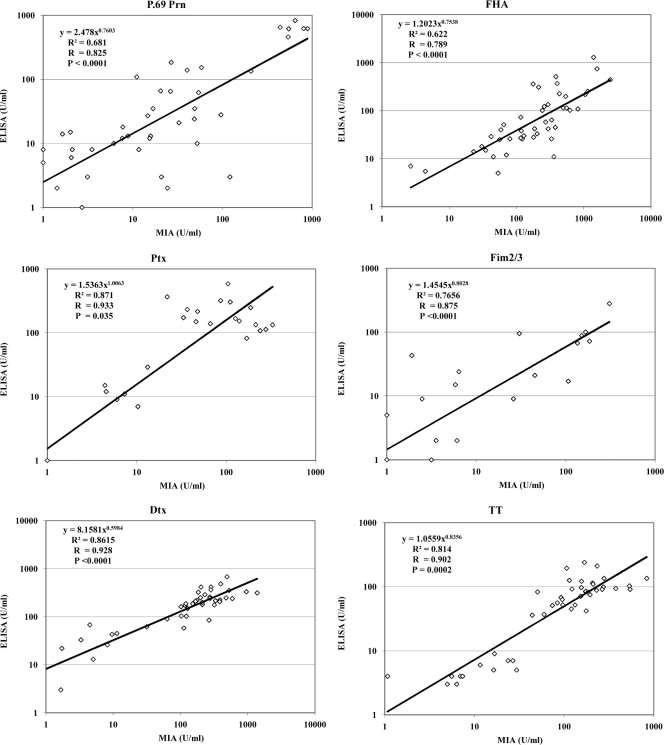
The performance of the urea-based avidity DTP MIA to assess serum antibody avidity, compared to the six in-house NaSCN-based avidity ELISAs, was evaluated using sera from mice at various time points after immunization. Figure 2 shows the correlation between antigen-specific IgG concentrations obtained after dissociation of low-avidity antibody interactions by both methods. Although the populations of IgG antibodies released by urea or thiocyanate may not fully overlap, good correlations were found, with P values lower than 0.0001 for all antigens, except for TT (P = 0.002) and Ptx (P = 0.0351). Addition of urea in MIA and NaSCN in ELISA did not alter the LLODs of the avidity assays (Table 2, lower part). Intra-assay variation of the avidity MIA within a plate ranged from 2 to 15% and between plates ranged from 2 to 16%, and interassay variation ranged from 2 to 19% for all antigens (Table 3, treated conditions).
Fig. 2.
Comparison of concentrations of specific mouse IgG antibody to DTP antigens obtained after treatment with NaSCN in ELISA or urea in MIA. Sera available from DTP- or DTaP-vaccinated mice (n = 18 to 41 sera/antigen) were tested in the avidity MIA for the presence of bound IgG antibodies specific for P.69 Prn, FHA, Ptx, Fim2/3, Dtx, and TT.
(iii) Detection of subclass distribution and avidity maturation of DTP-specific antibodies longitudinally after vaccination.
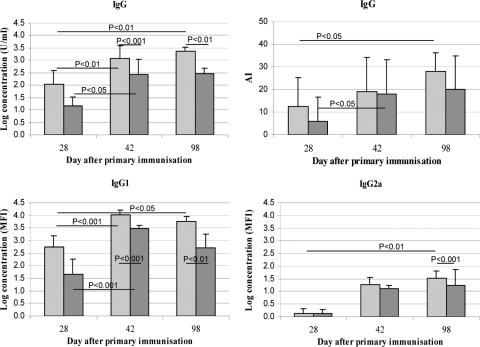
The usefulness of the hexaplex regular and avidity MIAs for monitoring the in vivo development and avidity maturation of DTP-specific antibody responses was assessed. Longitudinal orbital serum samples from individual BALB/c mice were taken at different time intervals after vaccination with a combined DTaP or a combined DTP vaccine. Levels and AIs of DTP-specific IgG antibodies were assessed, as well as levels of specific IgG1 and IgG2a subclasses. As is illustrated for the P.69 Prn-specific response, the DTP MIA could reveal trends in IgG responses in time and between differently vaccinated mice (Fig. 3). Significantly higher anti-P.69 Prn total IgG, IgG1, and IgG2a end concentrations were observed at various postbooster time points (day 42 and day 98) in mice vaccinated with the DTaP vaccine, compared to mice vaccinated with the DTP vaccine. In addition, significant longitudinal increase of responses was observed in the DTaP-vaccinated group for total IgG, IgG1, and IgG2a (between days 28 and 98) and in the DTP-vaccinated group for total IgG and IgG1 (between days 28 and 42). Furthermore, the avidity DTP MIA showed significant progress in avidity maturation between days 28 and 42 for DTP-vaccinated mice and between days 28 and 98 for DTaP-vaccinated mice. Likewise, trends in IgG levels and avidity maturation for other DTP vaccine antigens could be revealed using MIA (data not shown). Hence, the regular and avidity mouse DTP MIAs are able to reveal trends in levels and AIs of DTP antigen-specific IgG antibodies in experimental mouse models.
Fig. 3.
Levels, avidity, and isotypes of the mouse P.69 Prn-specific IgG antibody response after vaccination. BALB/c mice (n ≥ 6 sera/vaccine type) were vaccinated at day 0 and day 28 with 1/4 human dose of either a DTaP (light gray) or a DTP (dark gray) vaccine, and longitudinal orbital serum samples were taken at the indicated time points. Shown are total IgG concentrations (mean ± SD), IgG1 and IgG2a concentrations (mean ± SD) in MFI, and AIs in % of the original IgG levels.
DISCUSSION
Recently several laboratories developed human MIA systems to measure IgG antibodies to DTP antigens (39, 41, 50), and Hendrikx et al. (18) described avidity measurements of specific human IgG antibodies to two pertussis antigens using MIA (P.69 Prn and Ptx). Here, a mouse counterpart of these MIA systems was developed and extended to enable the assessment of levels, isotypes, and avidity of antigen-specific mouse IgG antibodies to six DTP antigens. Both the regular and avidity mouse DTP MIAs were reproducible and specific and had LLODs comparable to the in-house regular and avidity ELISAs, respectively. In addition, a good correlation between all IgG concentrations retrieved from the regular MIA and ELISAs, as well as between those retrieved from the avidity MIA and ELISAs, was obtained. In general, MIA systems are flexible, fast, and robust assays rapidly entering the field of immunosurveillance, disease diagnostics, and vaccinology (10, 11, 22, 23, 25, 35, 50). Their most important advantages over parallel monovalent ELISAs are illustrated by a theoretical example of the main requirements for testing levels and avidity of IgG antibodies to all six antigens in 75 serum samples in our hexaplex mouse DTP MIA or ELISAs (Table 4). Clearly, the advantages of MIA systems will become more substantial when novel targets of interest are included with no extra sample volume being required and hardly any extra human labor to run samples. The investment for individual laboratories to set up a serological MIA is to manufacture vaccine antigen-conjugated bead sets, since these are not commercially available, and to validate their individual specificities and sensitivities in monovalent and multivalent usage. Successful serodiagnostic MIA systems for protein (18, 23, 50), polysaccharide (5, 10, 11, 24, 25), and lipopolysaccharide (22) vaccine antigens, involving up to 14 different specificities, have been developed (5). Nevertheless, some troubleshooting may be necessary depending on the system or serum samples in use (34), or, as was the case in our regular and avidity mouse DTP MIA, on the antigen, i.e., FHA. Usage of NaSCN as a chaotropic agent in the hexavalent avidity MIA worked well for five DTP antigens but induced an elevated background MFI signal in the absence of serum only for FHA. This was caused by a nonspecific interaction of the conjugate with NaSCN-treated FHA-coupled beads (data not shown). Notably, applying a human avidity DTP MIA based on thiocyanate ions, Hendrikx et al. showed data for P antigens Ptx and P.69 Prn only and not for FHA (18). In our hands, the solution for FHA was to use urea, although 2 different urea concentrations were needed to evaluate the avidity of the IgG antibodies specific for all six DTP antigens. Furthermore, although the specificity, sensitivity, and reproducibility of FHA measurements in both regular and avidity MIAs were acceptable, they were lowest of those for all DTP antigens. All these points related to FHA could be due to its size and biological function. FHA is a major attachment factor for bacterial adherence to ciliated epithelial cells and is the biggest of the six DTP antigens (26, 32); these characteristics may result in more nonspecific interactions or increased sensitivity to conformational changes upon covalent coupling to beads. Once a MIA system is optimized, however, the time investment is paid back by preparation of stocks of antigen-coupled beads that are stable for at least 6 months (25, 50), reducing conjugation time but also the variability of bead batches.
Table 4.
Analysis of requirements for a set of regular and avidity mouse DTP MIAs and ELISAs
| Requirement | Levela for: |
|
|---|---|---|
| MIA | ELISA | |
| 96-well plates | 3 low-protein-binding filter platesc | 60 high-protein-binding platesh |
| Microbeads | 1.2 × 106 beads/regiond | None |
| Antigen | 1 μg/pertussis antigen; 5 μg/diphtheria or tetanus antigene | 200 μg/antigeni |
| Serum per mouse (μl) | 0.3 μlf | 24 μlj |
| Conjugate | 75 μl anti-IgG-PEg | 125 μl anti-IgG-HRPk |
| Human laborb | 0.5 day coupling + 1 day assay | 3 days |
Calculations are made for an experiment in which levels and avidity of IgG antibodies to 6 DTP antigens are determined in 75 mouse sera.
Time needed for one operator with a maximum of handling 20 plates per day.
Based on accommodating 75 sera (and controls) per plate and testing under 3 MIA conditions (0, 6, and 9 M urea).
Based on the use for each bead region of 4,000 microbeads per well in 3 plates.
Based on the use of approximately 20% of a standard bead stock (i.e., 6.25 × 106 beads) requiring 5 μg of pertussis antigens or 25 μg of diphtheria or tetanus antigens for conjugation.
The minimal serum volume that can be accurately pipetted. This makes up 375 μl of a 1:1,250 serum dilution, which is largely sufficient to be analyzed in MIA in 3 wells (50 μl/well, 3 conditions; 0, 6, and 9 M urea).
Used as a 1:200 dilution at 50 μl/well, in 3 plates.
Based on accommodating 15 sera and controls (in six dilutions) and one antigen per plate and testing under 2 ELISA conditions (without and with optimized [NaSCN]).
Amount per antigen needed to coat 10 ELISA plates at 2 μg/ml, 100 μl/well.
Based on using 100 μl of serum dilution per well and making 200 μl of a 1:100 starting dilution per antigen (n = 6) per ELISA condition (n = 2).
Used as a 1:5,000 dilution at 100 μl/well, in 60 plates.
The usefulness of our mouse DTP MIA systems in an experimental vaccination model was confirmed by showing longitudinal trends in total levels, isotype distribution, and avidity maturation of specific IgG antibodies in differently vaccinated mice. Monitoring of AIs or isotype distribution of antigen-specific IgG antibodies is important, since quality in combination with or rather than quantity of antibody responses seems to define the efficacy of an immune response (1, 6, 21, 27, 33, 36, 45). Although still largely unpredictable, variables such as formulation and antigen composition of multicomponent vaccines do greatly influence the qualitative outcome of B cell responses (2, 9, 12, 17, 18). It is therefore our expectation that, in view of the exponentially growing demand for new or improved vaccines and vaccine combinations, high-throughput serological MIA systems, by being specific, sensitive, flexible, and cost-effective and by enabling the assessment of qualitative Ig parameters in a multiplex manner, will rapidly increase in number and acceptance in vaccinology. MIAs will simplify the preclinical phase of vaccine development, in which hundreds or even thousands of immune sera are being compared when testing multiple vaccine candidates, combinations, schedules, or batches in mice. Our hexavalent mouse DTP MIA may be a first step toward introducing such a high-throughput serological assay as a replacement of ELISA in the development phase, efficacy analysis, and batch release of new combination vaccines containing DTP antigens.
Supplementary Material
ACKNOWLEDGMENT
We gratefully thank Pieter van Gageldonk for his advice on setting up a mouse MIA system involving DTP antigens.
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 16 February 2011.
REFERENCES
- 1. Aase A., et al. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 63:3531–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arigita C., et al. 2003. Restored functional immunogenicity of purified meningococcal PorA by incorporation into liposomes. Vaccine 21:950–960 [DOI] [PubMed] [Google Scholar]
- 3. Banus S., et al. 2008. The role of Toll-like receptor-4 in pertussis vaccine-induced immunity. BMC Immunol. 9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bennion B. J., Daggett V. 2003. The molecular basis for the chemical denaturation of proteins by urea. Proc. Natl. Acad. Sci. U. S. A. 100:5142–5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borgers H., et al. 2010. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin. Immunol. 134:198–205 [DOI] [PubMed] [Google Scholar]
- 6. Borrow R., Andrews N., Goldblatt D., Miller E. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corbel M. J., et al. 2008. WHO Working Group on Revision of the Manual of Laboratory Methods for Testing DTP Vaccines—report of two meetings held on 20–21 July 2006 and 28–30 March 2007, Geneva, Switzerland. Vaccine 26:1913–1921 [DOI] [PubMed] [Google Scholar]
- 8. Delgado M. F., et al. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 15:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denoel P. A., Goldblatt D., de Vleeschauwer I., Jacquet J. M., Pichichero M. E., Poolman J. T. 2007. Quality of the Haemophilus influenzae type b (Hib) antibody response induced by diphtheria-tetanus-acellular pertussis/Hib combination vaccines. Clin. Vaccine Immunol. 14:1362–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Voer R. M., Schepp R. M., Versteegh F. G., van der Klis F. R., Berbers G. A. 2009. Simultaneous detection of Haemophilus influenzae type b polysaccharide-specific antibodies and Neisseria meningitidis serogroup A, C, Y, and W-135 polysaccharide-specific antibodies in a fluorescent-bead-based multiplex immunoassay. Clin. Vaccine Immunol. 16:433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Voer R. M., et al. 2008. Development of a fluorescent-bead-based multiplex immunoassay to determine immunoglobulin G subclass responses to Neisseria meningitidis serogroup A and C polysaccharides. Clin. Vaccine Immunol. 15:1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eskola J., et al. 1999. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 354:2063–2068 [DOI] [PubMed] [Google Scholar]
- 13. European Pharmacopoeia Commission 2005. European pharmacopoeia 5.0. Assay of pertussis vaccine (acellular); document 01/2005:20716. European Directorate for the Quality of Medicines, Strasburg, France [Google Scholar]
- 14. Fenoy S., Rodero M., Pons E., Aguila C., Cuellar C. 2008. Follow-up of antibody avidity in BALB/c mice infected with Toxocara canis. Parasitology 135:725–733 [DOI] [PubMed] [Google Scholar]
- 15. Gaines Das R., Xing D., Rigsby P., Newland P., Corbel M. 2001. International collaborative study: evaluation of proposed International Reference Reagent of pertussis antiserum (mouse) 97/642. Biologicals 29:137–148 [DOI] [PubMed] [Google Scholar]
- 16. Geurtsen J., et al. 2008. Supplementation of whole-cell pertussis vaccines with lipopolysaccharide analogs: modification of vaccine-induced immune responses. Vaccine 26:899–906 [DOI] [PubMed] [Google Scholar]
- 17. Gylca R., et al. 2000. A new DTPa-HBV-IPV vaccine co-administered with Hib, compared to a commercially available DTPw-IPV/Hib vaccine co-administered with HBV, given at 6, 10 and 14 weeks following HBV at birth. Vaccine 19:825–833 [DOI] [PubMed] [Google Scholar]
- 18. Hendrikx L. H., Berbers G. A., Veenhoven R. H., Sanders E. A., Buisman A. M. 2009. IgG responses after booster vaccination with different pertussis vaccines in Dutch children 4 years of age: effect of vaccine antigen content. Vaccine 27:6530–6536 [DOI] [PubMed] [Google Scholar]
- 19. Hijnen M., et al. 2004. Epitope structure of the Bordetella pertussis protein P.69 pertactin, a major vaccine component and protective antigen. Infect. Immun. 72:3716–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hua L., Zhou R., Thirumalai D., Berne B. J. 2008. Urea denaturation by stronger dispersion interactions with proteins than water implies a 2-stage unfolding. Proc. Natl. Acad. Sci. U. S. A. 105:16928–16933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huber V. C., et al. 2006. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccine Immunol. 13:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iihara H., et al. 2007. Rapid multiplex immunofluorescent assay to detect antibodies against Burkholderia pseudomallei and taxonomically closely related nonfermenters. Jpn. J. Infect. Dis. 60:230–234 [PubMed] [Google Scholar]
- 23. Jones L. P., et al. 2002. Multiplex assay for detection of strain-specific antibodies against the two variable regions of the G protein of respiratory syncytial virus. Clin. Diagn. Lab. Immunol. 9:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lal G., Balmer P., Joseph H., Dawson M., Borrow R. 2004. Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W-135. Clin. Diagn. Lab. Immunol. 11:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lal G., et al. 2005. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J. Immunol. Methods 296:135–147 [DOI] [PubMed] [Google Scholar]
- 26. Locht C., Bertin P., Menozzi F. D., Renauld G. 1993. The filamentous haemagglutinin, a multifaceted adhesion produced by virulent Bordetella spp. Mol. Microbiol. 9:653–660 [DOI] [PubMed] [Google Scholar]
- 27. Lottenbach K. R., et al. 1999. Age-associated differences in immunoglobulin G1 (IgG1) and IgG2 subclass antibodies to pneumococcal polysaccharides following vaccination. Infect. Immun. 67:4935–4938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luijkx T. A., van Gaans-van den Brink J. A., van Dijken H. H., van Den Dobbelsteen G. P., van Els C. A. 2008. Hyperproliferation of B cells specific for a weakly immunogenic PorA in a meningococcal vaccine model. Clin. Vaccine Immunol. 15:1598–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahon B. P., Brady M. T., Mills K. H. 2000. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J. Infect. Dis. 181:2087–2091 [DOI] [PubMed] [Google Scholar]
- 30. Mason P. E., Neilson G. W., Dempsey C. E., Barnes A. C., Cruickshank J. M. 2003. The hydration structure of guanidinium and thiocyanate ions: implications for protein stability in aqueous solution. Proc. Natl. Acad. Sci. U. S. A. 100:4557–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mielcarek N., et al. 2006. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mills K. H. 2001. Immunity to Bordetella pertussis. Microbes Infect. 3:655–677 [DOI] [PubMed] [Google Scholar]
- 33. Mills K. H., Ryan M., Ryan E., Mahon B. P. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66:594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pickering J. W., Larson M. T., Martins T. B., Copple S. S., Hill H. R. 2010. Elimination of false-positive results in a Luminex assay for pneumococcal antibodies. Clin. Vaccine Immunol. 17:185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pickering J. W., et al. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589–596 [DOI] [PubMed] [Google Scholar]
- 36. Plotkin S. A. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47:401–409 [DOI] [PubMed] [Google Scholar]
- 37. Polack F. P., Hoffman S. J., Crujeiras G., Griffin D. E. 2003. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat. Med. 9:1209–1213 [DOI] [PubMed] [Google Scholar]
- 38. Poolman J. T., Kuipers B., Vogel M. L., Hamstra H. J., Nagel J. 1990. Description of a hybridoma bank towards Bordetella pertussis toxin and surface antigens. Microb. Pathog. 8:377–382 [DOI] [PubMed] [Google Scholar]
- 39. Prince H. E., Lape-Nixon M., Matud J. 2006. Evaluation of a tetraplex microsphere assay for Bordetella pertussis antibodies. Clin. Vaccine Immunol. 13:266–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pullen G. R., Fitzgerald M. G., Hosking C. S. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83–87 [DOI] [PubMed] [Google Scholar]
- 41. Reder S., Riffelmann M., Becker C., Wirsing von Konig C. H. 2008. Measuring immunoglobulin g antibodies to tetanus toxin, diphtheria toxin, and pertussis toxin with single-antigen enzyme-linked immunosorbent assays and a bead-based multiplex assay. Clin. Vaccine Immunol. 15:744–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robinson A., Gorringe A. R., Funnell S. G., Fernandez M. 1989. Serospecific protection of mice against intranasal infection with Bordetella pertussis. Vaccine 7:321–324 [DOI] [PubMed] [Google Scholar]
- 43. Sato Y., Cowell J. L., Sato H., Burstyn D. G., Manclark C. R. 1983. Separation and purification of the hemagglutinins from Bordetella pertussis. Infect. Immun. 41:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. 1983. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J. Biol. Chem. 258:14647–14651 [PubMed] [Google Scholar]
- 45. Siegrist C. A., et al. 2004. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine 23:615–622 [DOI] [PubMed] [Google Scholar]
- 46. Skerry C. M., et al. 2009. A live attenuated Bordetella pertussis candidate vaccine does not cause disseminating infection in gamma interferon receptor knockout mice. Clin. Vaccine Immunol. 16:1344–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stenger R. M., et al. 2009. Immunodominance in mouse and human CD4+ T-cell responses specific for the Bordetella pertussis virulence factor P.69 pertactin. Infect. Immun. 77:896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thalen M., van der Ark A., van den Ijssel J., van Straaten I., Jansen D., Beuvery C., Martens D., Tramper J. 2008. Improving the cellular pertussis vaccine: increased potency and consistency. Vaccine 26:653–663 [DOI] [PubMed] [Google Scholar]
- 49. Underwood J. R., Chivers M., Dang T. T., Licciardi P. V. 2009. Stimulation of tetanus toxoid-specific immune responses by a traditional Chinese herbal medicine. Vaccine 27:6634–6641 [DOI] [PubMed] [Google Scholar]
- 50. van Gageldonk P. G., van Schaijk F. G., van der Klis F. R., Berbers G. A. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 335:79–89 [DOI] [PubMed] [Google Scholar]
- 51. Westdijk J., van den Ijssel J., Thalen M., Beuvery C., Jiskoot W. 1997. Quantification of cell-associated and free antigens in Bordetella pertussis suspensions by antigen binding ELISA. J. Immunoassay. 18:267–284 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.