Abstract
We previously showed that recombinant (r) Listeria monocytogenes carrying ΔactA and a selected prfA* mutation (r-Listeria ΔactA prfA*) secreted >100-fold more immunogen in broth culture than wild-type r-Listeria or r-Listeria ΔactA and elicited much greater cellular and humoral immune responses than r-Listeria ΔactA after intravenous vaccination of mice. Here, we conducted comparative studies evaluating vaccine-elicited immune responses in systemic and mucosal sites after intranasal, intravenous, intraperitoneal, or subcutaneous immunization of mice with r-Listeria ΔactA prfA* vaccine candidates. Intranasal vaccination of mice with r-Listeria ΔactA prfA* vaccine candidates elicited a robust gamma interferon-positive (IFN-γ+) cellular response in systemic sites, although intravenous or intraperitoneal immunization was more efficient. Surprisingly, intranasal vaccination elicited an appreciable pulmonary IFN-γ+ cellular response that was nonstatistically higher than the magnitude induced by the intravenous route but was significantly greater than that elicited by subcutaneous immunization. Furthermore, although intranasal r-Listeria ΔactA prfA* delivery induced poor systemic IgG responses, intranasal vaccination elicited appreciable secretory immunogen-specific IgA titers that were similar to or higher in mucosal fluid than those induced by subcutaneous and intravenous immunizations. Thus, intranasal vaccination with r-Listeria ΔactA prfA* appears to be a useful approach for eliciting robust systemic and pulmonary cellular responses and measurable secretory mucosal IgA titers.
INTRODUCTION
Listeria monocytogenes is an intracellular bacterium with the unique ability to live in the cytoplasm and escape to the cytosol of antigen-presenting cells (APC). Listeria therefore can serve as a useful bacterial vaccine vector to deliver immunogens and to elicit strong CD8+ T-cell immune responses (5, 7, 23, 26, 31). Since the deletion of the actA gene in L. monocytogenes results in remarkable attenuation without affecting immune potency, Listeria lacking actA have been explored as vaccine candidates for delivering immunogens against cancers (3, 4, 24). We recently showed that recombinant (r) Listeria ΔactA vectors carrying a selected prfA(G155S) mutation (Listeria ΔactA prfA*) secreted >100-fold-higher levels of vaccine immunogens in broth culture than wild-type r-Listeria or r-Listeria ΔactA and consistently elicited much greater cellular and humoral immune responses than r-Listeria ΔactA vectors after intravenous (i.v.) vaccination of mice (32). Importantly, r-Listeria ΔactA prfA* vaccine vectors remained as attenuated as r-Listeria ΔactA without any detectable side effects (32). The PrfA*(G155S)-enhanced immune response was also reported by another group (16). Therefore, there is a sound rationale to further explore r-Listeria ΔactA prfA* vectors as potential vaccine candidates against infectious agents and cancers.
Many pathogens invade humans through mucosal interfaces, and it is therefore of central importance to elicit cellular and humoral responses in mucosae through optimized routes of vaccination. It has recently been shown that immunization via different routes can elicit qualitatively differing immune response patterns (3, 6, 10, 17, 21, 30). Nasopharynx-associated lymphoid follicles in the nasal cavity and gut-associated lymphoid tissues (GALT) are part of the common mucosal immune system, capable of effectively inducing antigen-specific T helper (Th) cell, cytotoxic T lymphocytes (CTLs), and IgA B-cell responses (8, 12). Interestingly, of all the mucosal routes tested in humans and macaques, only vaccination via the nasal route stimulates disseminated cellular and antibody (Ab) mucosal responses (11, 13, 14). Although L. monocytogenes has been studied as a vector to deliver immunogens against pathogens (2, 19, 22, 25, 27), direct comparisons of intranasal and other parenteral routes have not been reported. Given the potential of Listeria ΔactA prfA* vectors to elicit potent immune responses, we extended our previous work to conduct comparative studies evaluating vaccine-elicited immune responses in systemic and mucosal sites after intranasal (i.n.), i.v., intraperitoneal (i.p.), and subcutaneous (s.c.) vaccination of mice with r-Listeria ΔactA prfA* vaccine candidates. We found that the nasal mucosal route was suitable for delivering r-Listeria ΔactA prfA* vaccine vectors and that intranasal vaccination elicited robust systemic and pulmonary cellular responses and secretory mucosal IgA.
MATERIALS AND METHODS
Plasmids, bacterial strains, and media.
The shuttle integration vector pPL2, which can replicate autonomously in Escherichia coli and integrate into a single location within the chromosome of L. monocytogenes (15), was used as the parent plasmid in this study. pPL2 and all derivative recombinant plasmids were maintained in E. coli strain DH5α in Luria-Bertani broth (LB; Fisher Biotech) under chloramphenicol (25 μg/ml) selection. E. coli SM10 was used as the donor strain for transforming recombinant plasmids from E. coli to L. monocytogenes ΔactA prfA(G155S) strain NF-L974 (Listeria ΔactA prfA*). Listeria ΔactA prfA* was grown at 30°C with shaking at 280 rpm in brain heart infusion (BHI) medium (BD), and recombinant Listeria ΔactA prfA* cells containing the pPL2 plasmid derivatives were selected under 7.5 μg/ml of chloramphenicol and 200 μg/ml of streptomycin in BHI medium.
Recombinant Listeria monocytogenes constructs.
The foreign gene encoding either the anthrax protective antigen (PA) or the HIV Gag protein was amplified downstream of the 799-bp LLO promoter and signal sequence by gene splicing by overlapping extension-PCR (SOE-PCR) and subcloned into the KpnI and BamHI sites of pPL2 (Table 1). The pPL2 integration plasmids pPL2-PA and pPL2-Gag were individually transformed into CaCl2-competent E. coli SM10. The protocol for SM10-L. monocytogenes conjugation has been described in detail elsewhere (32). The resulting r-Listeria ΔactA prfA*/PA and r-Listeria ΔactA prfA*/Gag colonies were selected on BHI agar plates containing 200 μg/ml streptomycin and 7.5 μg/ml chloramphenicol and verified by PCR using previously described primers (15) and by Western blotting using specific anti-PA (ab63325; Abcam) or anti-Gag (ab36551; Abcam) antibodies.
Table 1.
PCR primers for constructing recombinant DNA fragments containing the LLO promoter/signal sequence and the SIV Gag or PA coding sequence
| Name of fragment | Primer sequence (5′→3′)a |
|
|---|---|---|
| Sense | Antisense | |
| Hly promoter | CGGGGTACCGATAATCAAAACTATCGTTGCTGTTTTGC (KpnI) | CCTGACAAGACGGAGTTTCTCACGCCATCCTTTGCTTCAGTTTGTTGCGC |
| Gag | GCGCAACAAACTGAAGCAAAGGAT-GGCGTGAGAAACTCCGTCTTGTCAGG | TCCCCCCGGGCTACTGGTCTCCTCCAAAGAGAGAATTGAGGTG (XmaI) |
| Hly/Gag recombinant | CGGGGTACCGATAATCAAAACTATCGTTGCTGTTTTGC (KpnI) | TCCCCCCGGGCTACTGGTCTCCTCCAAAGAGAGAATTGAGGTG (XmaI) |
| Hly promoter | GGGGTACCGATAATCAAAACTATCGTTGC (KpnI) | GTTTAACTTCTGCCTGAATCACCTCATCCTTTGCTTCAGTTTGTTGGCGC |
| PA | GCGCAACAACAAACTGAAGCAAAGGATGAGGTGATTCAGGCAGAAGTTAAAC | CGCGGATCCTTATCCTATCTCATAGCCTTTTTTAG (BamHI) |
| Hly/PA recombinant | GGGGTACCGATAATCAAAACTATCGTTGC (KpnI) | CGCGGATCCTTATCCTATCTCATAGCCTTTTTTAG (BamHI) |
Underlined portions indicate the restriction site, when present (the specific restriction enzyme is shown in parentheses).
Western blot analyses.
Western blotting assays were performed essentially as previously described (32). Briefly, Listeria culture supertanants were electrophoresed on denaturing polyacrylamide gels, electrotransferred onto nitrocellulose membranes, and probed with anti-Gag or anti-PA monoclonal antibodies (NIAID AIDS Research and Reference Reagent Program) at a 1:1,000 dilution (Genesis Biotech Inc.). Following incubation with peroxidase-labeled goat anti-mouse IgG (Pierce), protein was visualized by staining with West Pico chemiluminscent substrate (Pierce).
Immunization and sampling.
Eight- to 10-week-old female BALB/C mice were used in the immunogenicity studies. For comparisons of immunogenicity under different vaccination routes, the experiments were divided into two subsets, with six to eight mice per group for comparisons. In subset a, mice were randomly divided into three groups and vaccinated with 1 × 107 CFU r-Listeria ΔactA prfA*/Gag via the i.n. or i.v. route or with 107 CFU nonrecombinant Listeria ΔactA prfA* (control) via the intranasal route. Subset b included five groups of mice. Four groups of mice were vaccinated with 1 × 107 CFU r-Listeria ΔactA prfA*/PA either i.p., s.c., i.v., or i.n. The fifth group served as a control and received intravenously the same dose of nonrecombinant Listeria ΔactA prfA*. We chose 107 CFU for vaccination because earlier experiments showed this dose elicited better responses than 105 and 106 CFU but only a slightly lower response than a 108 CFU dose in mice. For intranasal immunization, mice were held on their backs and 20 μl of vaccine or control was gently applied onto the nostrils by using a standard pipette tip, so that the vaccine was inhaled automatically. For parenteral vaccination, vaccine was resuspended in 100 μl of phosphate-buffered saline (PBS) and injected accordingly. The i.v and s.c. injections were performed as previously described (32). The handling of mice was facilitated by intraperitoneally injecting 0.4 mg/kg of Fentanyl (32). All mice were primed at week 0 and boosted at week 4 with the same amount of Listeria, using the same inoculation route.
A 300- to 400-μl volume of heparinized blood per mouse was collected by retro-orbital bleeding and pooled from a group of six to eight mice to isolate peripheral blood mononuclear cells (PBMC) and plasma. Plasma and PBMC were collected for enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunospot (ELISPOT) analyses at weeks 0, 2, 4, and 6. Plasma isolated before and after immunization was stored at −20°C for subsequent ELISA analysis. At week 6, all mice were euthanatized and sacrificed by CO2 inhalation. Lymphocytes were isolated from spleens for ELISPOT and intracellular staining assays. To measure cellular and Ab responses in respiratory mucosa, whole lungs, with tracheal/bronchus, were collected from each mouse for performing bronchoalveolar lavages (BAL). This was done through making a small incision in the trachea and gently instilling 1 ml of PBS (containing 0.1% bovine serum albumin [BSA] and 1 mM phenylmethylsulfonyl fluoride as a protease inhibitor) three times with an 18-gauge needle. Two-thirds of the BAL fluid was used for measuring cellular gamma interferon (IFN-γ) responses and one-third was frozen at −20°C for measuring Ab responses. To collect intestinal mucosal fluid, intestines were collected from each animal, and intestinal washes (IW) were performed by repeated flushing and aspiration of 5 ml of the same buffer inside the intestine. Collected IW fluids were kept frozen at −20°C and were examined for the presence of immunogen-specific Ab by ELISA.
No measurable CFU of Listeria bacteria were recovered in tissue homogenates from liver/spleen, lungs, or brain at days 5 and 14 after vaccination with 107 CFU r-Listeria ΔactA prfA* vaccine strains via the nasal route (data not shown). We also found no evidence that r-Listeria ΔactA prfA* induces brain lesions after i.v. or i.n. vaccination, based on complete necropsy at day 14 after vaccination (data not shown).
IFN-γ ELISPOT assays.
The IFN-γ ELISPOT assay was designed to enumerate antigen-specific IFN-γ-secreting T cells in single-cell suspensions. This method was modified from our protocols described for previous studies (32). Briefly, the murine IFN-γ ELISPOT kit (Diaclone, France), consisting of capture and detection antibodies, was used according to the manufacturer's instructions with several modifications. Ninety-six-well polyvinylidene difluoride (PVDF) plates (Millipore, Billerica, MA) were coated under sterile conditions with 100 μl of capture antibody overnight at 4°C. After washing with PBS, plates were blocked with PBS containing 2% skimmed dry milk for 6 h at 4°C. The plate was then seeded with 5 × 105 PBMC or 5 × 105 splenocytes or lung lavage cells per well, and each sample was evaluated in triplicate. Cells were incubated with a pool of 15-mer peptides overlapped by a 12-mer spanning the entire PA or Gag peptide (synthesized by GenScript; 2.5 μg/ml for each peptide) at 37°C in 5% CO2 for 16 to 20 h. Positive and negative controls consisted of 10 μg/ml of concanavalin A (Sigma) and 10% fetal bovine serum in RPMI 1640, respectively. The plates were then incubated with biotinylated detection antibody for 1.5 h at 37°C, washed, and thereafter developed with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium buffer for 10 min at room temperature. The area in which a single cell was stimulated to secrete IFN-γ were detected as spots on the PVDF membrane. Measurements were obtained in triplicate for each stimulant. The plates were counted using software as described previously (32). The mean number of spots from triplicate wells was expressed as the mean ± standard deviation per 1 × 106 PBMC, lung lavage cells, or splenocytes. Data were obtained in two to three separate experiments and subjected to statistical analyses.
Intracellular cytokine staining.
Intracellular cytokine staining was done essentially as previously described (1). A total of 1 × 106 splenocytes were stimulated in 96-well flat-bottom plates with 15-mer pooled PA peptides or medium alone for 1 h and then in the presence of brefeldin A (GolgiPlug; BD Biosciences) for an additional 5 h at 37° in 5% CO2. Cells were washed with staining buffer (phosphate-buffered saline with 3% fetal bovine serum and 0.09% sodium azide), pretreated with anti-Fc receptor Ab for 10 min, and then stained with anti-CD4–Pacific blue, anti-CD8–phycoerythrin (PE)-Cy7, and anti-CD3–fluorescein isothiocyanate (Pharmingen) at a 1:100 final concentration on ice for 20 to 30 min. Cells were then permeabilized, fixed with Cytofix/CytoPerm (Pharmingen), and stained for intracellular IFN-γ with anti-IFN-γ–PE or a PE-labeled isotype control MAb (1:100). Stained cells were fixed in 2% formalin–PBS and then run on a CyAn ADP flow cytometer (DakoCytomation, Carpinteria, CA). Data were analyzed using the Summit data acquisition and analysis software created by DakoCytomation.
ELISA.
Antigen-specific IgG antibody titers were determined by ELISA as previously described (32) by using the plasma pooled from each group of vaccinated mice. Briefly, high-binding-capacity 96-well ELISA plates (Costar) were coated with PA (0.25 μg/well; Alpha Diagnostic International) in coating buffer overnight at 4°C. After blocking, plates were incubated with serial dilutions of plasma for 1 h at 37°C and washed. Horseradish peroxidase-conjugated anti-mouse IgG (KPL) was incubated for 1 h at a dilution of 1:3,000. A colorimetric reaction was obtained by addition of the ABTs 1-component microwell peroxidase substrate (KPL) for 8 to 10 min. Optical densities at 405 nm (OD405) were read using an ELISA plate reader (model 550; Bio-Rad). Plasma IgG Ab end point titers were defined as the reciprocal plasma dilution that gave an optical density that was three times the average value obtained with bovine serum albumin.
Secretory anti-PA IgA and IgG antibody responses in bronchoalveolar lavage fluid and intestinal washing fluid were measured by ELISA as previously described (18). Briefly, 96-well plates were coated overnight at 4°C with 0.25 μg/well PA (Alpha Diagnostic International). The intestinal washing fluid (1:1 dilution) and lung lavage fluid (1:5 dilution) were added in triplicate into wells of the plates and incubated for 1 h at 37°C. After washing, horseradish peroxidase-conjugated goat anti-mouse IgA (1:1,000 dilutions; KPL) or goat anti-mouse IgG (1:3,000 dilutions; KPL) were added, and incubated for 1 h, and then reactions were completed by addition of the substrate as described above. Concentrations of PA-bound IgA or IgG Ab were calculated from calibration curves prepared with purified mouse IgA (eBioscience) and IgG (Sigma).
Statistical analysis.
All data were analyzed by using Student's t test for statistical significance. The differences between groups were evaluated for statistical significance by calculating the P value (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
RESULTS
Construction and characterization of r-Listeria ΔactA prfA* expressing SIV Gag or anthrax PA.
To assess vaccination routes for optimum delivery of r-Listeria ΔactA prfA* immunogens, we constructed two r-Listeria ΔactA prfA* vaccine candidates expressing the immunogens SIV Gag or anthrax PA. Our PCR-based approach confirmed the integration of the foreign genes encoding the immunogen in the recombinant Listeria strain, and the culture showed r-Listeria ΔactA prfA* shared similar growth kinetics with the parental strain (data not shown). To characterize the ability of recombinant r-Listeria ΔactA prfA*/Gag or r-Listeria ΔactA prfA*/PA to secrete immunogen protein, the recombinant was grown for 16 to 18 h in BHI medium and culture supernatants were collected for Western blot analysis using monoclonal anti-Gag and anti-PA antibodies as described previously (32). Gag or PA protein with a predicted molecular size in the supernatant was detected by Western blot analysis (Fig. 1) and indicated that recombinant r-Listeria ΔactA prfA*/Gag and r-Listeria ΔactA prfA*/PA were able to produce and secret Gag and PA, respectively.
Fig. 1.
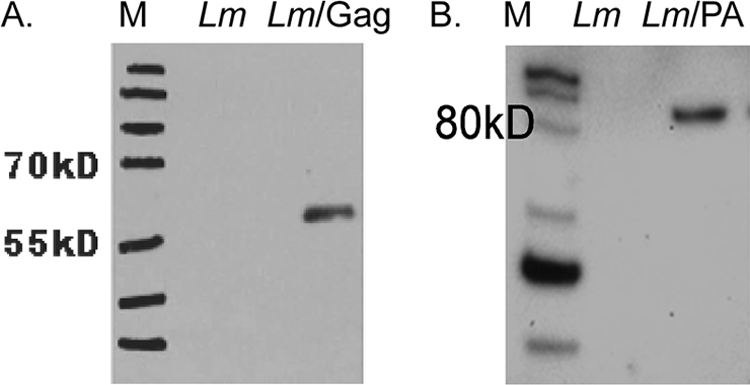
Characterization of r-Listeria ΔactA prfA* expressing SIV Gag or anthrax PA. Shown are Western blot analyses results for protein expression in the culture supernatants of r-Listeria ΔactA prfA* expressing Gag (A) and r-Listeria ΔactA prfA* expressing PA (B). The supernatants were collected after 16 to 18 of culturing at 30°C. Monoclonal anti-PA or anti-Gag Ab was used in the blot assay. The expressed full-length protein sizes can be judged based on the markers on the left of each blot. The nonrecombinant Listeria ΔactA prfA* strain (Lm) served as a negative control in the assay.
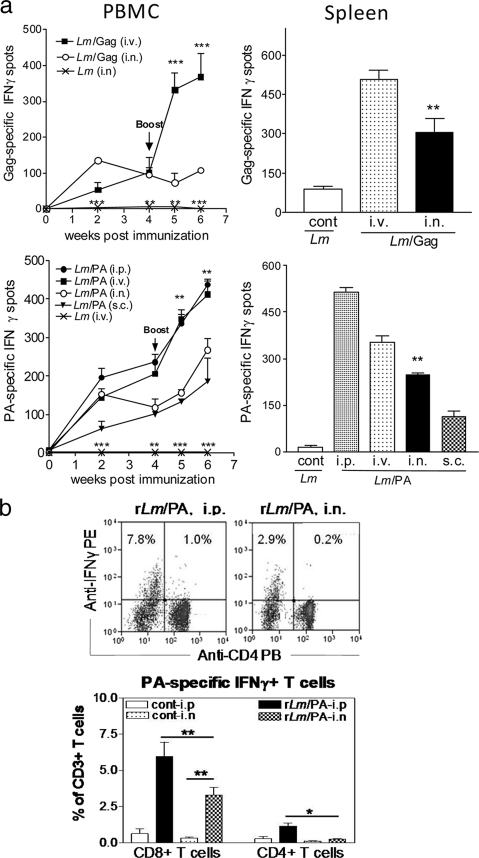
Intranasal vaccination of mice with r-Listeria ΔactA prfA* vaccine candidates elicited robust cellular response in systemic sites, but the intraperitoneal or intravenous immunizations were more efficient for elicitation.
We recently demonstrated that intravenous immunization of mice with r-Listeria ΔactA prfA* vaccine candidates can induce robust cellular and humoral immune responses while remaining remarkably safe (32). In the current study, we sought to compare nasal mucosal vaccination and other vaccination routes to determine whether they would elicit similar r-Listeria vaccine immune responses. In our initial efforts to address this question, three groups of mice were primed and boosted at weeks 0 and 4 with 1 × 107 CFU r-Listeria ΔactA prfA*/Gag intranasally, 1 × 107 CFU r-Listeria ΔactA prfA*/Gag intravenously, or 1 × 107 CFU Listeria ΔactA prfA*/Sham (control) intranasally. Intranasal r-Listeria ΔactA prfA*/Gag prime vaccination elicited significantly higher IFN-γ cellular responses in PBMC than the sham control prime immunization (Fig. 2 a, upper left) (P < 0.01). Although the second intranasal r-Listeria ΔactA prfA*/Gag vaccination did not boost the cellular response in PBMC, up to 280 IFN-γ+ cells were detected in splenocytes after the boost (Fig. 2a, upper right) (P < 0.01). However, intravenous prime and boost vaccination with r-Listeria ΔactA prfA*/Gag elicited significantly greater numbers of Gag-specific IFN-γ+ cells in PBMC and splenocytes than did intranasal immunization (Fig. 2a, upper panel).
Fig. 2.
Intranasal vaccination of mice with r-Listeria ΔactA prfA* vaccine candidates elicited robust cellular responses in systemic sites, but i.p. or i.v. immunizations were more efficient for elicitation. (a) Numbers of Gag-specific IFN-γ+ cells (upper panels) or PA-specific IFN-γ+ cells (lower panels) in 1 × 106 PBMC (left) or 1 × 106 spleen lymphocytes (right) from individual groups of mice that were vaccinated i.n., i.v., i.p., or s.c. For the statistical analyses of PBMC results, significant differences (**, P < 0.01; ***, P < 0.001) were seen when the i.n. group was compared with the control group (bottom), the i.v. group, or the i.p. group. For statistical analysis of results with spleen lymphocytes, a significant difference (P < 0.01) was seen when the i.n. group was compared with control group, the s.c. group, or the i.v. group. Intramuscular vaccination was less immunogenic than the i.v. route (data not shown), and the intradermal route was not evaluated, as such results are often undistinguishable from s.c. immunizations in mice. (b) Representative flow cytometry histograms, indicating IFN-γ+ CD4+ T cells and IFN-γ+ CD4− (CD8+) populations in gated CD3 T cells of spleen lymphocytes from an intraperitoneally vaccinated or an intranasally vaccinated mouse (upper) as well as mean percentages of PA-specific IFN-γ+ CD4+ and CD8+ T cells detected in spleen lymphocytes from the i.p. and i.n. groups (bottom). Data were generated in an intracellular cytokine staining assay, using pooled 15-mer PA peptides for stimulation. *, P < 0.05; **, P < 0.01. Our previous studies in mice (32) and nonhuman primates (1) demonstrated that an increase in the frequency of Ag-specific T effector cells predicts increased absolute numbers of them in lymphoid tissues.
To extend our study, we conducted an additional immunogenicity experiment involving five groups of mice (six mice per group) that were vaccinated with r-Listeria ΔactA prfA*/PA via the intraperitoneal, intravenous, subcutaneous, or intranasal route. Interestingly, intranasal r-Listeria ΔactA prfA*/PA prime vaccination elicited a PA-specific cellular response comparable to that induced by the intraperitoneal or intravenous priming in PBMC at week 2, although the intraperitoneal and intravenous routes boosted much greater cellular responses (Fig. 2a, lower left). It was also noteworthy that the magnitude of PA-specific cellular response elicited by the intranasal route was as great as that induced by the subcutaneous parenteral route (Fig. 2a, lower panel). However, the intraperitoneal prime/boost vaccination of mice with r-Listeria ΔactA prfA*/PA appeared to elicit a greatest cellular immune response in PBMC and splenocytes than did the intravenous, subcutaneous, or intranasal routes (Fig. 2a, lower panel). An intracellular cytokine staining assay also showed that intraperitoneal vaccination elicited greater numbers of PA-specific IFN-γ+ T cells in spleens than did intranasal immunization (Fig. 2b). Interestingly, IFN-γ+ effector cells elicited by r-Listeria ΔactA prfA*/PA appeared comprised predominantly of CD8+ T cells (Fig. 2b), which might be driven by the efficient major histocompatibility complex class I (MHC-I)/peptide presentation due to the translocation of Listeria antigen (Ag) to the cytosol from endosomes. Thus, intranasal vaccination with r-Listeria ΔactA prfA* vaccine candidates elicited robust cellular responses in systemic sites, but the intraperitoneal and intravenous routes were more efficient for elicitation.
Intranasal vaccination of mice with r-Listeria ΔactA prfA*/PA elicited a greater pulmonary IFN-γ cellular immune response than subcutaneous immunization.
We next questioned whether intranasal vaccination with r-Listeria ΔactA prfA* was able to elicit a comparable cellular response in bronchoalveolar mucosa, compared with parenteral routes. To address this straightforward question, we collected the entire bronchus and lung organs at week 6 after prime/boost and performed bronchoalveolar lavage to isolate pulmonary mucosal cells for immune assays. Interestingly, intranasal vaccination with r-Listeria ΔactA prfA*/PA elicited significantly greater numbers of PA-specific IFN-γ+ cells in bronchoalveolar mucosa than the subcutaneous immunization (Fig. 3). The intranasal immunization also elicited a notably greater pulmonary cellular response than the intravenous vaccination, while the intraperitoneal route was more efficient for elicitation of pulmonary responses (Fig. 3). These results therefore demonstrated that intranasal vaccination of mice with r-Listeria ΔactA prfA* elicits a greater IFN-γ cellular immune response in brochoalveolar mucosa than does subcutaneous immunization.
Fig. 3.
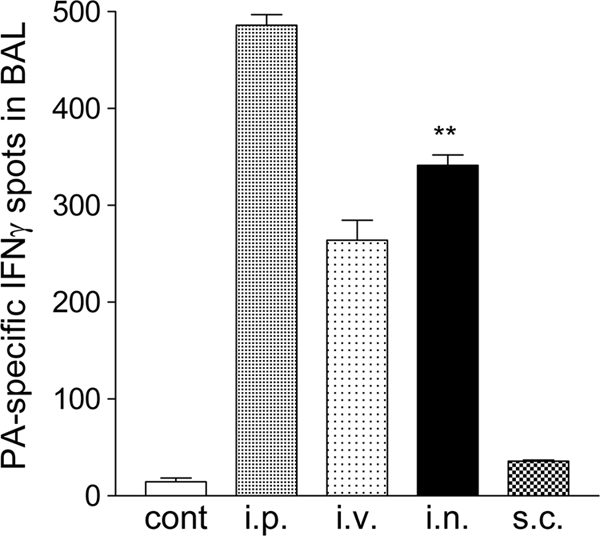
Intranasal vaccination of mice with r-Listeria ΔactA prfA*/PA elicited a greater pulmonary IFN-γ cellular immune response than subcutaneous immunization. Shown are mean ELISPOT data derived from two to three separate experiments, indicating the numbers of IFN-γ+ cells in 5 × 105 lymphocytes in bronchoalveolar lavage fluid pooled from two to three mice in individual groups vaccinated via the i.n., i.v., i.p., or s.c. route. **, P < 0.01 for the i.n. group compared with the control group or the s.c. group and P > 0.05 for i.n. compared to i.v.
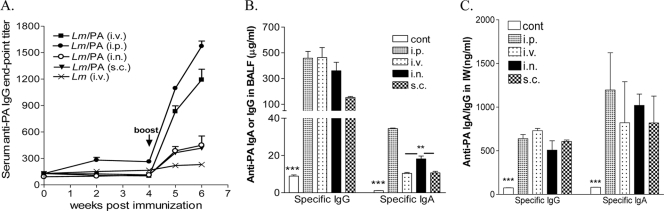
The i.n. vaccination with r-Listeria ΔactA prfA* appeared to elicit higher secretory mucosal IgA Ab titers than s.c or i.v. immunizations, but the i.v. route induced much greater systemic IgG responses.
The next question asked was whether intranasal or parenteral vaccination of mice with r-Listeria ΔactA prfA*/PA could elicit comparable immunogen-specific Ab in systemic and mucosal sites. The i.n. vacccination with r-Listeria ΔactA prfA*/PA was much less efficient than the parenteral routes in eliciting immunogen-specific IgG responses in the blood (Fig. 4). The i.n. vaccination with r-Listeria ΔactA prfA*/PA did not elicit higher levels of PA-specific IgG Ab in pulmonary or intestinal mucosa than via parenteral immunizations (Fig. 4). Interestingly, the i.n. vaccination elicited higher levels of secretory PA-specific IgA Ab in bronchoalveolar fluids, but not in intestinal wash fluids, compared to the i.v. or s.c. immunization routes (Fig. 4). Thus, intranasal vaccination with r-Listeria ΔactA prfA* appeared to elicit higher secretory mucosal IgA Ab titers than s.c or i.v. immunization, but the i.v. route induced much greater systemic IgG responses.
Fig. 4.
Intranasal vaccination with r-Listeria ΔactA prfA*/PA appears to elicit higher secretory mucosal IgA Ab titers than s.c. or i.v. immunizations, but the i.v. route induced much greater systemic IgG responses. (A) Mean anti-PA IgG Ab titers in sera of individual groups of mice vaccinated via different routes. A significant difference (P < 0.01) was found when the i.p. or i.v. group was compared with any one of other groups at weeks 5 and 6. (B) Mean anti-PA IgA or IgG Ab in bronchoalveolar lavage fluid. Note that IgG Ab levels in the i.n. group were not higher than those of the i.p. and i.v. groups, and IgA Ab levels in the i.n. group were significantly greater than those in the i.v. and s.c. groups. **, P < 0.01. (C) Mean anti-PA IgA or IgG Ab levels in IW fluid from the i.n. group were not higher than those in the i.p., i.v., or s.c. groups. The detection limit for IgA or IgG was ∼100 ng/ml. All vaccinated groups had significantly greater Ab titers than the control group (***, P < 0.001).
DISCUSSION
The current study represents the first comparative investigations of immunogen-specific humoral and cellular responses elicited by r-Listeria ΔactA prfA* vaccine candidates delivered by different immunization routes. Head-to-head comparisons of intranasal and parenteral routes for delivery of recombinant Listeria vectors have not been reported. The recombinant Listeria vaccine vectors for infections reported to date by other investigators have been based on either wild-type Listeria (2, 29) or Listeria deleted of the essential d-alanine synthesis pathway (9), thus raising safety concerns (with the wild type) or requiring special d-alanine supplemental treatments during vaccination of animals (dal dat mutants). We recently demonstrated that attenuated the Listeria ΔactA prfA*strain is a promising vaccine vector, since r-Listeria ΔactA prfA* secretes >100-fold-higher levels of vaccine immunogens in broth culture than wild-type r-Listeria or r-Listeria ΔactA, and it elicits much higher magnitudes of immunogen-specific humoral and cellular immune responses after intravenous vaccination of mice than those induced by r-Listeria ΔactA (32). The current study extends our previous work and compares immune responses elicited by r-Listeria ΔactA prfA* via different vaccination routes. We chose the i.n. as the mucosal r-Listeria ΔactA prfA* immunization route for comparisons instead of the well-described oral route, since i.n. mucosal r-Listeria vaccination has not been studied. Our data in the current studies allowed us to prove our concepts. Notably, this new study shows that intranasal r-Listeria ΔactA prfA* vaccination is able to elicit robust IFN-γ cellular responses in systemic sites and bronchoalveolar mucosa and induce appreciable titers of secretory immunogen-specific IgA Ab at mucosal interfaces.
It is not surprising that parenteral delivery of r-Listeria ΔactA prfA* vaccine candidates elicits greater cellular and antibody responses in systemic sites than intranasal vaccination. Intravenous or intraperitoneal vaccination provides a setting in which r-Listeria ΔactA prfA* vaccine vectors can be efficiently delivered to monocytes or tissue macrophages and therefore be readily phagocytosed by these professional antigen-presenting cells (APC). Thus, production/secretion, MHC processing, and presentation of vector immunogens in APC via the intravenous or intraperitoneal route would be expected to be more efficient for eliciting potent B-cell and T-cell responses. Such systemic vaccination with Listeria vectors may also readily provide access to dendritic cells for eliciting robust immune responses (28). It is noteworthy that subcutaneous injection delivering r-Listeria ΔactA prfA* does not elicit humoral and cellular immune responses as efficiently as intravenous or intraperitoneal vaccinations. This may be simply due to the fact that r-Listeria ΔactA prfA* vaccine vectors are attenuated and are readily contained in the subcutaneous tissue, with a limited capacity to spread to large populations of APC after vaccination.
Our findings suggest that the intranasal mucosa serves as an effective site for the delivery of r-Listeria ΔactA prfA* vectors, and it is suitable for eliciting secretory mucosal IgA Ab responses as well as systemic and pulmonary T-cell responses. Interestingly, intranasal vaccination of mice with r-Listeria ΔactA prfA* elicited greater IFN-γ cellular immune responses in brochoalveolar mucosa than subcutaneous or intravenous immunization, while mucosal delivery also induced an appreciable immunogen-specific T-cell response in systemic sites. Notably, intranasal vaccination elicited higher levels of secretory immunogen-specific IgA Ab at mucosal interfaces than subcutaneous or intravenous immunization, although the nasal r-Listeria ΔactA prfA* vaccination induced poor systemic IgG responses. These findings may reflect the presence of unique immune components in nasal mucosa. It has been demonstrated that nasopharynx-associated lymphoid follicles in the nasal cavity can confer induction of antigen-specific Th lymphocytes, CTLs, and IgA B-cell responses (8, 12). The appreciable vaccine-elicited cellular response in pulmonary mucosa after intranasal r-Listeria ΔactA prfA* vaccination appears consistent with an earlier observation that indicated that virulent Listeria monocytogenes bacteria can be detected more readily in the lungs after nasal infection than after intravenous infection (20). Most studies using the standard nasal vaccination (a 20-μl volume applied via pipette tip to mouse nostrils) do not indicate a technical bias that would lead to dominant lung responses. Lung T-cell responses and pulmonary mucosal Ab responses after nasal r-Listeria ΔactA prfA* vaccination did not appear to be due to the active lung infection or injuries, as we could not detect any lung lesions or active replication of r-Listeria ΔactA prfA*. Nasal vaccination may deliver r-Listeria to tracheal or bronchial mucosa but not directly the lung alveolae, because it is generally believed that only an ∼1-μm-diameter aerosol generated by aerosolization (i.e., via a collison nebulizer or coughing) can be delivered to lung alveolae.
The Listeria ΔactA prfA* vector is highly immunogenic and remarkably attenuated (32). It is not yet completely clear why prfA* strains elicit an enhanced immune response. The expression of immunogens in these studies was driven by the PrfA-dependent hly promoter, thus resulting in high-level expression of immunogens in prfA* strains immediately upon entry into the cytosol as well as within the vacuole. It is possible that the altered pattern and enhanced level of PrfA*-dependent immunogen expression contributed to the improved host response. We were unable to detect r-Listeria ΔactA prfA*-induced lesions in tissues, including brain, lung, liver, and spleen, after vaccination with the r-Listeria ΔactA prfA* vector (32).
The appreciable immune responses observed after intranasal r-Listeria ΔactA prfA* vaccination of mice merit further assessment of r-Listeria ΔactA prfA* vaccine candidates for immunogenicity and antimicrobial immunity. It is important to note that most infections with human pathogens are initiated at mucosal interfaces. Induction of both humoral and cellular immune responses in mucosal sites therefore appears to be of central importance for optimal immune defense against mucosal invasion by pathogens. From a mucosal immunity standpoint, intranasal vaccination with r-Listeria ΔactA prfA* would be an attractive or promising approach, based on the ease of vaccination (without needle injection) and the potency of vaccine-elicited immune responses at both systemic and mucosal sites. In future studies, it will be important to conduct comprehensive protection experiments and to determine if the measurable immune responses in mucosal and systemic compartments via different routes confer detectable protection against infections initiated at the corresponding anatomic sites. Particularly, it will be beneficial to investigate if nasal vaccination with r-Listeria ΔactA prfA* induces immunity against challenge with pulmonary pathogens by the respiratory route. These extensive protection experiments are beyond the scope of the current proof-of-concept study. Nevertheless, our results in the current study provide a sound rationale to conduct in-depth or finely tuned immunogenicity and efficacy studies of r-Listeria ΔactA prfA* vaccine candidates in animal models.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI070426, HL64560, and RR13601 (all to Z.W.C.).
Footnotes
Published ahead of print on 26 January 2011.
REFERENCES
- 1. Ali Z., et al. 2009. γδ T cell immune manipulation during chronic phase of simian HIV infection confers immunological benefits. J. Immunol. 183:5407–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyer J. D., et al. 2005. DNA prime Listeria boost induces a cellular immune response to SIV antigens in the rhesus macaque model that is capable of limited suppression of SIV239 viral replication. Virology 333:88–101 [DOI] [PubMed] [Google Scholar]
- 3. Brockstedt D. G., et al. 2004. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl. Acad. Sci. U. S. A. 101:13832–13837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brundage R. A., Smith G. A., Camilli A., Theriot J. A., Portnoy D. A. 1993. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 90:11890–11894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Busch D. H., Pilip I. M., Vijh S., Pamer E. G. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353–362 [DOI] [PubMed] [Google Scholar]
- 6. Cortes-Perez N. G., et al. 2007. Influence of the route of immunization and the nature of the bacterial vector on immunogenicity of mucosal vaccines based on lactic acid bacteria. Vaccine 25:6581–6588 [DOI] [PubMed] [Google Scholar]
- 7. Edelson B. T., Unanue E. R. 2000. Immunity to Listeria infection. Curr. Opin. Immunol. 12:425–431 [DOI] [PubMed] [Google Scholar]
- 8. Fukuyama S., et al. 2006. Cutting edge: uniqueness of lymphoid chemokine requirement for the initiation and maturation of nasopharynx-associated lymphoid tissue organogenesis. J. Immunol. 177:4276–4280 [DOI] [PubMed] [Google Scholar]
- 9. Jiang S., et al. 2007. Live attenuated Listeria monocytogenes expressing HIV Gag: immunogenicity in rhesus monkeys. Vaccine 25:7470–7479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johansson E. L., Bergquist C., Edebo A., Johansson C., Svennerholm A. M. 2004. Comparison of different routes of vaccination for eliciting antibody responses in the human stomach. Vaccine 22:984–990 [DOI] [PubMed] [Google Scholar]
- 11. Kantele A., et al. 1998. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect. Immun. 66:5630–5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiyono H., Fukuyama S. 2004. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat. Rev. Immunol. 4:699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kozlowski P. A., Cu-Uvin S., Neutra M. R., Flanigan T. P. 1997. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 65:1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kozlowski P. A., et al. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169:566–574 [DOI] [PubMed] [Google Scholar]
- 15. Lauer P., Chow M. Y., Loessner M. J., Portnoy D. A., Calendar R. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lauer P., et al. 2008. Constitutive activation of the PrfA regulon enhances the potency of vaccines based on live-attenuated and killed but metabolically active Listeria monocytogenes strains. Infect. Immun. 76:3742–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lefford M. J., Warner S., Amell L. 1979. Listeria pneumonitis: influence of route of immunization on resistance to airborne infection. Infect. Immun. 25:672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Medaglini D., Pozzi G., King T. P., Fischetti V. A. 1995. Mucosal and systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordonii after oral colonization. Proc. Natl. Acad. Sci. U. S. A. 92:6868–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miki K., et al. 2004. Induction of protective cellular immunity against Mycobacterium tuberculosis by recombinant attenuated self-destructing Listeria monocytogenes strains harboring eukaryotic expression plasmids for antigen 85 complex and MPB/MPT51. Infect. Immun. 72:2014–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizuki M., Nakane A., Sekikawa K., Tagawa Y. I., Iwakura Y. 2002. Comparison of host resistance to primary and secondary Listeria monocytogenes infections in mice by intranasal and intravenous routes. Infect. Immun. 70:4805–4811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mullins D. W., et al. 2003. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 198:1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orr M. T., Orgun N. N., Wilson C. B., Way S. S. 2007. Cutting edge: recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J. Immunol. 178:4731–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pamer E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4:812–823 [DOI] [PubMed] [Google Scholar]
- 24. Peters C., Domann E., Darbouche A., Chakraborty T., Mielke M. E. 2003. Tailoring host immune responses to Listeria by manipulation of virulence genes: the interface between innate and acquired immunity. FEMS Immunol. Med. Microbiol. 35:243–253 [DOI] [PubMed] [Google Scholar]
- 25. Rayevskaya M., Kushnir N., Frankel F. R. 2002. Safety and immunogenicity in neonatal mice of a hyperattenuated Listeria vaccine directed against human immunodeficiency virus. J. Virol. 76:918–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schoen C., et al. 2008. Listeria monocytogenes as novel carrier system for the development of live vaccines. Int. J. Med. Microbiol. 298:45–58 [DOI] [PubMed] [Google Scholar]
- 27. Shen H., et al. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc. Natl. Acad. Sci. U. S. A. 92:3987–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skoberne M., et al. 2008. KBMA Listeria monocytogenes is an effective vector for DC-mediated induction of antitumor immunity. J. Clin. Invest. 118:3990–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stevens R., Howard K. E., Nordone S., Burkhard M., Dean G. A. 2004. Oral immunization with recombinant Listeria monocytogenes controls virus load after vaginal challenge with feline immunodeficiency virus. J. Virol. 78:8210–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thatte J., Rath S., Bal V. 1995. Analysis of immunization route-related variation in the immune response to heat-killed Salmonella typhimurium in mice. Infect. Immun. 63:99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weiskirch L. M., Paterson Y. 1997. Listeria monocytogenes: a potent vaccine vector for neoplastic and infectious disease. Immunol. Rev. 158:159–169 [DOI] [PubMed] [Google Scholar]
- 32. Yan L., et al. 2008. Selected prfA* mutations in recombinant attenuated Listeria monocytogenes strains augment expression of foreign immunogens and enhance vaccine-elicited humoral and cellular immune responses. Infect. Immun. 76:3439–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]