Abstract
The clinical picture of herpes simplex virus type 2 (HSV-2) infection includes genital blisters and less frequently meningitis, and some individuals suffer from recurrent episodes of these manifestations. We hypothesized that adaptive and/or innate immune functional deficiencies may be a major contributing factor in susceptibility to recurrent HSV-2 meningitis. Ten patients with recurrent HSV-2 meningitis were studied during clinical remission. For comparison, 10 patients with recurrent genital HSV infections as well as 21 HSV-seropositive and 19 HSV-seronegative healthy blood donors were included. HSV-specific T cell blasting and cytokine secretion were evaluated in whole blood cultures. HSV-2-induced NK cell gamma interferon production, dendritic cell Toll-like receptor (TLR) expression, and TLR agonist-induced alpha interferon secretion were analyzed. Patients with recurrent HSV-2 meningitis had elevated T cell blasting and Th1 and Th2 cytokine production in response to HSV antigens compared to those of patients with recurrent genital infections. A somewhat increased NK cell response, increased dendritic cell expression of TLR3 and -9, and increased TLR-induced alpha interferon responses were also noted. Contrary to our expectation, recurrent HSV-2 meningitis patients have increased HSV-specific adaptive and innate immune responses, raising the possibility of immune-mediated pathology in the development of recurrent HSV2 meningitis.
INTRODUCTION
Benign recurrent aseptic meningitis of unknown cause, called Mollaret's syndrome, was first described in 1944 (30). The disease is characterized by recurrent periods of neck stiffness, headaches and fever, and the presence of large cells in cerebrospinal fluid (CSF). Since the development of the PCR technique, the causative agent of recurrent meningitis (RM) is generally considered to be herpes simplex virus type 2 (HSV-2) (22, 36, 43), which is readily detectable in CSF. The first episode of HSV-2 meningitis usually develops within 2 weeks following symptomatic or asymptomatic primary genital HSV-2 infection (8, 37). HSV-2 meningitis is more common in women than in men, with a reported incidence of 36% in women and 13% of men among patients with primary genital HSV-2 infection (8). After a first episode of HSV-2 meningitis, the incidence of recurrent meningitis varies between studies but is around 30% (1, 6, 37).
HSV infects submucosal nerve endings in oral or genital tissue and establishes latency in nerve ganglions such as trigeminal ganglion in oral HSV-1 infection and sacral dorsal root ganglion in genital HSV-2 infection. Viral reactivation occurs frequently and is induced by events such as hormonal changes, stress, or other diseases. It results in viral transport along the axon and shedding of virus at mucosal surfaces, in most cases without causing clinical symptoms (27, 47). In HSV-2 meningitis, viral entrance to CSF has assumed to be by seeding from infected ganglia, but a recent report of frequent viremia during primary genital infection suggests the possibility of viral spread to the central nervous system (CNS) from blood (15).
Immune suppression associates to symptomatic viral reactivation, and gamma interferon, produced mainly by CD4+ T cells (45) but also by CD8+ T cells (25), and granzymes (19) have been described to be of importance in combating viral production. HSV-specific cytotoxic T cells can be demonstrated in latently infected ganglia (46) as well as near infected nerve endings in mucosa (52). As for specific T cell responses in HSV-2 infections, a number of glycoproteins have been described as immunodominant, including envelope gB, gC, and gD (20, 21, 29), in some cases even in the absence of detectable antibodies in serum (34). Innate immune mechanisms important in preventing primary HSV infection have been identified, genetically and functionally defining naturally occurring immune deficiencies in familial HSV-1 encephalitis. NK cells (31), as well as Toll-like receptors (TLR) and alpha interferon (IFN-α) responses, play vital roles (50). However, there is no information available on HSV-specific adaptive or innate cellular immune responses in patients with recurrent HSV meningitis, and such data are important to better understand the pathogenesis of this disease. Interestingly, Aurelius found that 61 of 64 consecutive cases of primary and recurrent HSV-2 meningitis lacked antibodies to HSV-1 (2), suggesting that a major risk factor for developing meningitis was the absence of prior HSV-1 infection and, hence, absence of a prior immune response to HSV.
As literature on cell-mediated immune responses in patients with recurrent HSV-2 meningitis is lacking, we set forth to characterize immune responses in this disease with the aim to detect immune defects or deviations in patients with recurrent HSV-2 meningitis that may help to explain why some individuals get meningitis as a complication to viral reactivation. We investigated immune responses in 10 patients with recurrent and severe manifestations of HSV-2 meningitis, as well as in 10 patients with recurrent genital HSV-2 infection (RG) and in 21 HSV-seropositive and 19 HSV-seronegative healthy controls. Our data show increased expression of Toll-like receptors, increased IFN-α responses, and increased specific T cell responses in patients with HSV-2 meningitis. Thus, patients with recurrent meningitis have elevated rather than decreased immune responses. One interpretation of our data is that a strong antiviral immune response, such as high levels of cytokines, can contribute to pathology and viral spread to the meninges.
MATERIALS AND METHODS
Subjects.
Ten patients with recurrent HSV meningitis were included in the study (Table 1). Exclusion criteria were ongoing symptomatic herpes simplex infection, acute febrile illness, other chronic diseases, immunosuppressive treatment, or vaccination within a month prior to inclusion. All patients had at least three prior admissions with lymphocytic meningitis with HSV-2 etiology (≥5 × 106 mononuclear leukocytes per liter of cerebrospinal fluid; HSV-2 etiology verified with culture or with nested [5] or real-time [4] PCR in cerebrospinal fluid). Many of the diagnosed patients experienced a large number of meningitis episodes that were not confirmed by medical examination or analysis of cerebrospinal fluid, and these episodes were included as clinical recurrences in Table 1. Seven of the meningitis patients had a history of recurrent genital and/or skin blisters on the lower part of the body. As controls, 10 patients suffering from recurrent genital HSV infections were included (Table 2). The presence of HSV-2 in genital lesions was confirmed by PCR and/or culture. All patients included in the study were asymptomatic at the time of inclusion. In addition, 40 consecutive blood bank donors were included. Of these individuals, 21 (age 59 [range, 45 to 82] years) displayed HSV antibodies and 19 (age 68 [47 to 86] years) were seronegative for HSV. No information regarding clinical symptoms from the HSV infection was available from the seropositive donors, but they were healthy at the time of blood donation and we estimate that they follow the overall morbidity observed in Sweden (16). In addition, 21 extra blood donors (8 seronegative, 2 HSV-2 seropositive, 10 seropositive for HSV-1, and 1 seropositive for both HSV-1 and HSV-2) were included in the study to test the specificity of the immune response toward the HSV-2-infected whole-cell lysate. All patients gave their informed consent.
Table 1.
Clinical description of 10 patients with recurrent HSV-2 meningitis
| Gender | Age (yr) | HSV antibody(ies)a | Time (yr) since first meningitis | No. of recurrences |
Time since last meningitis | |
|---|---|---|---|---|---|---|
| Laboratory diagnosed | Clinically suspected | |||||
| Female | 49 | HSV-2 | 6 | 4 | 0 | 6 mo |
| Female | 29 | HSV-2 | 5 | 3 | 3 | 6 mo |
| Female | 41 | HSV-2 | 4 | 3 | 4 | 8 mo |
| Female | 29 | HSV-2 | 3 | 5 | 15 | 3 mo |
| Female | 51 | HSV-2 | 33 | 7 | 1 | 1 mo |
| Female | 39 | HSV-2 | 10 | 4 | 6 | 5 mo |
| Female | 62 | HSV-2 | 33 | 5 | 20 | 6 mo |
| Male | 38 | HSV-2 | 15 | 6 | 58 | 2 wk |
| Female | 49 | HSV-2 | 26 | 5 | 2 | 4 mo |
| Female | 51 | HSV-1, -2 | 13 | 7 | 18 | 10 mo |
The presence of HSV-1 or -2 was confirmed by PCR or culture of cerebrospinal fluid in the first three to seven episodes of each patient, as indicated. All patients included were asymptomatic at the time of inclusion.
Table 2.
Clinical description of 10 patients with recurrent HSV genitalis
| Gender | Age (yr) | HSV antibody(ies)a | Time (yr) since first episode | No. of recurrences |
|---|---|---|---|---|
| Female | 33 | HSV-2 | 4 | 4 |
| Male | 36 | HSV-2 | 6 | 4 |
| Male | 54 | HSV-2 | 18 | 4 |
| Male | 44 | HSV-1, -2 | 16 | 8 |
| Male | 34 | HSV-1, -2 | 6 | 2 |
| Female | 33 | HSV-2 | 5 | 11 |
| Male | 28 | HSV-1, -2 | 8 | 3 |
| Male | 33 | HSV-1, -2 | 8 | 3 |
| Female | 34 | HSV-2 | 12 | 12 |
| Male | 40 | HSV-2 | 17 | 4 |
The presence of HSV-1 or -2 was confirmed by PCR or culture of samples taken from the blisters. All patients included were asymptomatic at the time of inclusion.
Herpes simplex virus serology.
Anti-HSV antibodies were detected using the HerpeSelect enzyme-linked immunosorbent assay (ELISA) (Focus Technologies, Cypress, CA) for qualitative detection of type-specific IgG antibodies against recombinant HSV-1 gG-1 or HSV-2 gG-2 according to the manufacturer's instructions.
Enumeration of blood cells and FACS staining.
Cells were stained for CD3, CD4, CD8, CD16/56, TCR-α/β, TCR-γ/δ, HLA-DR, CD14, CD16, CD19, CD20, CD123, CD11c, CD19, and CD45 and analyzed by fluorescence-activated cell sorting (FACS) using TruCounts tubes (BD Biosciences, San Diego, CA), allowing determination of absolute cell counts in blood (CellQuest Pro and MultiSET software and all antibodies from BD Biosciences).
Cellular responses.
The FASCIA method (40) was employed for the detection of CD4+ T cell blasting and cytokine production in whole-blood cultures (whole blood diluted 1/8 in medium) stimulated with HSV-1 nucleocapsid proteins (39) or with lysates from HSV-2-infected cells (19.5 μg/ml and 8 μg/ml, respectively; Microbix Biosystems Inc., Ontario, Canada), which includes proteins from the nucleocapsid, the viral envelope, and the tegument. RPMI medium supplemented with penicillin and streptomycin (Invitrogen, Carlsbad, CA) alone was used as a negative control. All cultures were run in duplicate, and supernatants were removed for Luminex analysis of cytokine concentrations: interleukin 2 (IL-2), eotaxin, IP-10, IFN-α, tumor necrosis factor alpha (TNF-α) (day 3), and IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, IL-17, MIP-1β, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IFN-γ (day 7) using a Bio-Plex assay (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions, employing a Bio-Plex suspension array system (Bio-Rad Laboratories) and the Bio-Plex manager software (version 4.1.1.). Cells were stained for CD3 and CD4 on day 7, and the number of blasting CD4+ T cells was determined by combining immunophenotyping and forward versus side scatter profiles during FACS analysis. For NK cell responses to live HSV-2 virus, 5 million/ml peripheral blood mononuclear cells (PBMCs) were grown overnight in RPMI plus 10% fetal calf serum (Sigma Aldrich) with or without HSV-2 strain Plummer (0.5 million PFU/ml). GolgiStop (BD Biosciences) was added during the last 4 h, and cells were stained for CD3, CD56, and, following permeabilization, intracellular IFN-γ (BD Biosciences) and analyzed by FACS. Toll-like receptor (TLR) expression was evaluated on gated pDC (CD3− 14− 19− 56− = Lin−, HLA-DR+ CD123+; BD Biosciences), gated mDC (CD3− 14− 19− 56−, HLA-DR+; BD Biosciences; CD11c+; Milteney Biotech, Bergisch Gladbach, Germany), and monocytes (gated by forward versus side scatter) using TLR3 (clone 40C1285.6; Abcam, Cambridge, United Kingdom)-, TLR7-, and TLR9 (rabbit polyclonal antibody and clone 26C593.2; Imgenex, San Diego, CA)-specific antibodies in intracellular stainings. For TLR stimulations, agonists to TLR3 [poly(I:C), 10 μg/ml; Invitrogen, San Diego, CA], TLR4 (lipopolysaccharide [LPS], 100 ng/ml; Sigma Aldrich), TLR7 (Imiquimod R837, 10 μg/ml; Invitrogen), TLR9 (CpG, 3 μg/ml, ODN2216; Metabion, Martinsried, Germany), live HSV-2 strain Plummer (1 million PFU/ml), or medium only was added to PBMCs (2.5 million cells/ml). Supernatants were collected after 24 h stimulation and assayed for IFN-α by ELISA (Bender Medsystems, Burlingame, CA).
Statistical methods.
The Mann-Whitney U test was applied, and P values of <0.05 were considered significant. In the box plot figures, the 25th and 75th percentiles define boxes and the whiskers indicate the nonoutlier minimum and maximum; outliers are defined as values above or below the 1.5 box length range from the upper and lower values of the box; outliers and extremes are not shown in the graph, and the median values are indicated within the boxes.
RESULTS
HSV-specific adaptive immune responses are elevated in patients with recurrent meningitis.
We analyzed HSV-specific immune responses to two different antigen preparations in patients with RM. One preparation consisted of HSV-1 nucleocapsid, and the other was a whole-cell lysate of HSV-2-infected cells, which includes nucleocapsid but also viral envelope antigens. The two antigen preparations were shown to elicit responses in HSV-1- as well as HSV-2-infected persons. Seronegative specimens did not respond (Fig. 1, Table 3). As controls, patients with recurrent genital HSV infection were included. Patients with recurrent HSV infections (RM or RG) had a highly significant increased production of Th1 cytokines such as IL-12, IL-10, and TNF-α compared to those of seropositive blood donors (Table 3). Interestingly, RM patients responded to both antigen preparations with higher levels of IL-4 and IL-13 than those of seropositive blood donors and RG patients (Table 3). In addition, the T cell blast response as well as the production of several cytokines upon stimulation with the whole-cell lysate was higher for the RM group than for the RG group. These data show that patients with recurrent HSV-2 meningitis develop stronger rather than weaker responses to HSV antigens than seropositive patients without a documented history of meningitis or patients with a history of recurrent genital manifestations.
Fig. 1.
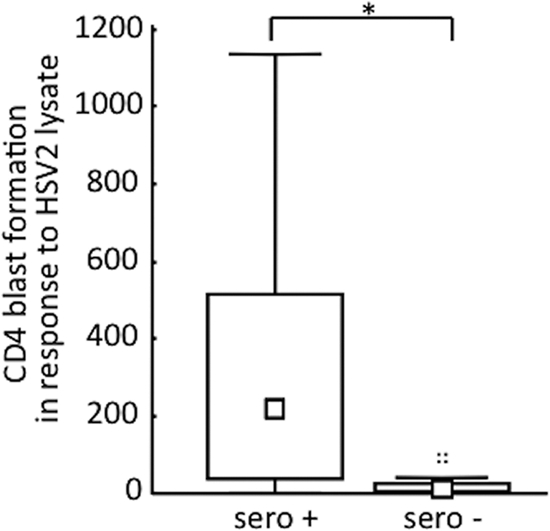
Specific response to HSV-2 whole-cell lysate stimulation. Median and 25th and 75th percentiles of the numbers of CD4+ T cell lymphoblasts generated per μl of whole blood from HSV-seropositive (sero+) and -seronegative (sero−) donors cultured 7 days in the presence of HSV-2-infected whole-cell lysates. The Mann-Whitney U test was used for statistical analysis. *, P < 0.05.
Table 3.
HSV-specific cell-mediated immune responses
| Cytokine response | Result from stimulation with HSV-1 nucleocapsida |
Result from stimulation with HSV-2 lysatea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HSV− | HSV+ (D) | HSV genitalis (RG) | HSV meningitis (RM) | D vs RG | D vs RM | RG vs RM | HSV genitalis (RG) | HSV meningitis (RM) | RG vs RM | ||
| CD4+ T-blast | 1.8 (0.0–5.7) | 126 (51–491) | 588 (226–1,143) | 723 (306–2,190) | NS | ** | NS | 830 (654–971) | 2,250 (1,579–2,738) | * | |
| IL-12 | 0 (0–0) | 0 (0–0) | 4.9 (1.1–5.5) | 5.1 (4.2–5.6) | *** | *** | NS | 4.0 (0.7–4.1) | 5.4 (4.4–5.6) | ** | |
| IL-10 | 0 (0–0) | 1.9 (0.0–4.5) | 7.3 (5.7–16) | 16 (10–19) | ** | *** | NS | 8 (5–17) | 15 (11–22) | NS | |
| TNF-α | 0 (0–0) | 14 (2.8–45) | 96 (59–213) | 74 (59–153) | *** | *** | NS | 92 (62–138) | 141 (109–180) | NS | |
| IL-4 | 0 (0–0) | 0.6 (0.0–1.8) | 0.5 (0.1–0.6) | 1.3 (0.9–2.1) | NS | *** | ** | 0.3 (0.1–0.4) | 0.9 (0.6–1.6) | * | |
| IL-13 | 0 (0–0) | 12 (2.4–20) | 11 (2–30) | 45 (18–85) | NS | ** | * | 4.5 (0.7–5.2) | 26 (17–51) | ** | |
| IL-5 | 0 (0–0) | 4 (0.0–12) | 0.0 (0.0–16) | 13 (7–20) | NS | NS | NS | 1.4 (0.0–5.2) | 18 (6–40) | * | |
| IL-2 | 0 (0–0) | 43 (16–102) | 57 (27–97) | 67 (47–146) | NS | NS | NS | 40 (32–54) | 112 (64–149) | * | |
| GM-CSF | 0.0 (0.0–1.3) | 11 (3.1–62) | 11 (4–64) | 42 (23–66) | NS | NS | NS | 4.3 (0.8–9.2) | 58 (27–125) | * | |
| IFN-γ | 0 (0–0) | 301 (97–1740) | 226 (58–1278) | 816 (542–1,467) | NS | NS | NS | 221 (81–654) | 1879 (936–2,956) | * | |
| IFN-α | 0.0 (0.0–0.0) | 4.2 (0.0–9.9) | * | 0 (0–0) | 0 (0–0) | NS | |||||
| IL-6 | 2.4 (0.0–3.5) | 121 (63–463) | 421 (167–1,296) | 252 (215–377) | NS | NS | NS | 1,907 (844–2,571) | 3,636 (1,875–5,671) | NS | |
| IL-17 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.0 (0.0–1.4) | NS | NS | NS | 0 (0–0) | 3.0 (0.0–10.7) | NS | |
| IP-10 | 8,744 (5,863–10,942) | 7,319 (6,595–8,426) | NS | 2,081 (763–2,669) | 4,142 (1,592–6,375) | NS | |||||
| IL-9 | 0 (0–0) | 2.3 (0.0–3.1) | NS | 2.7 (0.0–3.6) | 5.2 (3.9–7.5) | NS | |||||
Median and 25th to 75th percentiles of the numbers of CD4+ T cell lymphoblasts generated per ml of blood and cytokines were measured (pg/ml, net results) in supernatants of whole blood cultured 3 days (IP-10, IL-2, TNF-α, IFN-α) or 7 days in the presence of HSV-1 nucleocapsid or HSV-2-infected whole-cell lysates. The Mann-Whitney U test was used for statistical analysis. NS, not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Patient groups: patients with recurrent HSV-2 meningitis [HSV meningitis (RM); n = 10] or recurrent genital HSV-2 infection [HSV genitalis (RG); n = 10] and blood donors seropositive [HSV+ (D); n = 21] or seronegative (HSV−; n = 19) for HSV antibodies.
Increased innate immune responses in patients with meningitis.
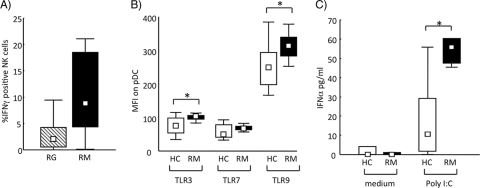
RM and RG patients had comparable numbers of NK cells (cells/μl blood: RG, 139 [range, 6 to 437]; RM, 218 [110 to 312]) in peripheral blood. Freshly analyzed NK cells did not show any phenotypical differences between patient groups as defined by the expression of NKG2D, DNAM-1, NKp30, NKp44, NKp46, and CD69, and NK cell cytotoxicity against the classical target cell line K562 was similar in all groups (data not shown). However, NK cells from RM patients, compared to those from RG patients, presented a somewhat increased IFN-γ production when cultured in the presence of HSV-2 live virus, although this difference was not statistically significant (Fig. 2 A). Since virally induced NK cell production of IFN-γ is most probably a consequence of accessory cell activation, we also characterized the dendritic cell (DC) phenotype of RM patients. The numbers of plasmacytoid DCs (pDC) and myeloid DCs (mDC) did not differ between RM and RG patients (cells/μl blood for RM: pDC, 8 [3 to 20], and mDC, 11 [5 to 22]; for RG: pDC, 6 [1 to 15], and mDC, 14 [9 to 23]). To further characterize differences in DC function, we examined by flow cytometry the expression of intracellular TLR3, TLR7, and TLR9. Unfortunately, we did not have access to blood from RG patients in this characterization, and therefore a comparison of RM was done only with healthy HSV-2-seropositive controls. We found an increased expression of TLR3 and TLR9 on pDC in RM patients compared to that for healthy controls (Fig. 2B), while the percentages of pDC among gated PBMC were comparable between the two groups (RM: 0.6% [0.4 to 0.9%]; HC: 0.6% [0.2 to 0.9%]). Similar results were seen for mDC but not for monocytes (data not shown). To examine whether this increased expression of TLR associates to increased function, we also analyzed IFN-α production from PBMCs cultured in the presence of different TLR agonists [lipopolysaccharide (LPS), CpG ODN2216, R837, poly(I:C), and HSV-2]. In accordance with elevated TLR3 levels in RM patients, higher IFN-α levels could be detected in RM samples than in those from healthy seropositive controls when stimulated with poly(I:C) (Fig. 2C). There were no significant differences in responsiveness to the other TLR agonists used (data not shown).
Fig. 2.
Innate immune function in patients with recurrent meningitis (RM) compared to patients with recurrent genitalis (RG) and healthy seropositive controls (HC). (A) PBMCs from RG (striped bar, n = 7) and RM (filled bar, n = 8) patients were cultured overnight in the presence of live HSV-2. The percentage of gated NK cells positive for IFN-γ as determined by intracellular FACS analysis is shown. P = 0.06. (B) PBMCs from healthy seropositive controls (unfilled bars, n = 10) and from RM patients (filled bars, n = 10) were freshly stained for indicated TLRs. TLR median fluorescence intensity (MFI) of gated pDC as determined by intracellular FACS analysis is shown. The Mann-Whitney U test was used for statistical analysis. *, P < 0.05. (C) IFN-α production by PBMCs from healthy seropositive controls (unfilled bars, n = 10) and from RM patients (filled bars, n = 10) cocultured overnight with or without poly(I:C) as indicated. The Mann-Whitney U test was used for statistical analysis. *, P < 0.05.
DISCUSSION
It is not known why some patients with HSV-2 infection develop recurrent meningitis. Data on innate and adaptive immune responses in patients affected are scarce, and ours is the first study of HSV-specific immune responses in patients with recurrent HSV-2 meningitis. We show for the first time that these patients have increased systemic immune responses compared to those of patients with recurrent genital HSV infections and to those of healthy seropositive individuals. The difference includes both innate and adaptive immune responses. We describe higher antigen reactivity, measured as CD4+ T cell blasting and cytokine production. In particular, the HSV lysate induced elevated Th1 and Th2 cytokine responses. In addition, a higher level of expression of TLR3 and TLR9, along with increased IFN-α production in response to a TLR3 agonist, was detected in meningitis patients than in seropositive healthy controls.
Patients with recurrent HSV-2 meningitis as well as patients with recurrent genital HSV-2 infection displayed stronger HSV-specific cell-mediated immune responses than healthy seropositive subjects, in particular regarding production of cytokines IL-12, TNF-α, and IL-10. One likely explanation is that frequent symptomatic recurrences provide repeated and high-dose exposure of HSV to the adaptive immune system, inducing an increased response, including cytokines such as IL-12 and TNF-α associated with Th1 profiles. This proinflammatory response may be one explanation for the more severe clinical symptoms seen in our two patient groups than in the seropositive donor group. Although seropositive healthy blood donors have frequent periods of viral secretion (27) and we expect that a minority of the healthy controls included have symptomatic infections, the total and cumulative viral dose may be lower in these donors than in the patient groups selected for frequent symptomatic reactivations.
Another possible cause of a higher T cell response in patients with meningitis than in healthy seropositive donors is a higher inherent innate function in the patients. To investigate this possibility, we analyzed TLR expression and responses in blood myeloid cells, since the production and function of IFN (9, 13) and functional TLRs (7) have been shown to play essential roles in protection against primary HSV-1 encephalitis. We show that in addition to elevated T cell responses and cytokine production, expression of TLR3 and -9 is increased in DCs from meningitis patients sampled during clinical remission compared to that in DCs from healthy HSV-2-seropositive donors. There are several reports showing changes in TLR expression levels during infectious disease and inflammation (24, 26, 33). Several animal studies document an importance of TLRs in controlling antiviral responses against, for example, murine cytomegalovirus (41, 42). Moreover, TLR3 and related molecules (51) have been shown to be indispensable in the control of herpes simplex encephalitis. The increased innate responses may indicate that patients developing recurrent meningitis are genetically different from those who do not develop recurrent meningitis, and we cannot exclude that TLR3 deficiencies are present in our group of patients, as such defects might be underestimated in our experimental setup. In fact, in a study characterizing genetic defects in IFN-α production, the TLR3 defect could be detected only by using fibroblasts and not peripheral blood cells (7). Another possibility would be that these differences of innate immunity could be the result of the persisting and recurrent HSV-2 meningitis, and to analyze this, one would need to characterize innate immunity in persons before they acquire HSV-2 meningitis. Additionally, NK cell function was somewhat elevated in meningitis patients compared to that in patients with genital recurrences, and it is possible that this reflects the elevated TLR reactivity of meningitis patients' DCs. Alternatively, it may be a reflection of a recently described long-lasting priming of NK cell function by viral activation (38).
We have found no support in the literature that immune mechanisms affected by, for example, medical immune suppression or HIV infection are involved in protection against HSV meningitis. However, the pathology of genital HSV-2 is clearly controlled by such mechanisms, since genital symptoms are increased on immune suppression (35). We speculate that the type of immune response elicited upon viral reactivation affects clinical outcome, with a strong response inducing pronounced immunopathological symptoms. An increased local IL-13 production in the dorsal ganglia during viral reactivation may perhaps result in tissue remodeling by IL-13-induced disruption of tight junctions (12). This could allow virus and T cells to pass through the blood-brain barrier. Clearly, oligoclonal T cells are present in infected ganglia (14, 46) even during latency (44), and virus may enter either via neuron-neuron interactions or from circulation during viremia (15). A higher incidence in women may, in this view of immune-mediated pathology, reflect a generally higher immune reactivity in females (8, 11). Accordingly, healthy women have been shown to respond more efficiently upon stimulation through TLR7 (28), with a subsequent higher T cell response, than men. An example of a viral infection in the CNS with proposed immune-mediated pathology is the lymphocytic choriomeningitis virus (LCMV). Here, cytotoxic T cells promote destruction of the blood-brain barrier through the release of cytokines and chemokines that facilitates recruitment of myeolomonocytic cells with consequent vascular leakage and disease progression (18). It is thus possible that such immune-mediated pathology could be more prominent in women than in men.
Johnston et al. recently demonstrated that HSV viremia is common during primary genital infection (15) and thus virus may spread to the central nervous system from blood during primary infection and establish latency in extrasacral neurons from which reactivated HSV repeatedly could be seeded to the subarachnoidal space and cause recurrent meningitis. Furthermore, development of meningitis when contracting HSV-2 is very rare in persons with already established latent HSV-1 infection (3) (Table 1), indicating that adaptive immunity to type-common HSV antigens can provide protection against development of HSV meningitis disease. The prevalence of HSV-1 and HSV-2 varies with age and geographical region. In Europe, HSV-1 is generally acquired during childhood, reaching a prevalence of up to 80 to 90% in adults, whereas HSV-2 is acquired mostly in adolescence and early adulthood, with a prevalence of up to 25% (32). A declining prevalence of HSV-1 has been recorded in adolescents in the United States during the last decades (49). This changing epidemiology of HSV infections suggests an increasing incidence of primary HSV-2 infections in HSV-1-negative persons, which has been reported to increase the likelihood of symptomatic genital infection (2, 23, 48) as well as meningitis (3). The cohort of recurrent meningitis patients included in our study confirms that HSV-2 meningitis is more common in patients without prior HSV-1 infection (Table 1). It could be that the absence of HSV antibodies in a subject newly exposed to HSV-2 can result in a higher viral titer and a stronger immune response that promotes viral spread to the CNS, thus contributing to meningitis. Based on this, we expect an increasing prevalence of HSV-2 meningitis in the future, making a better understanding of the pathogenesis of HSV-2 meningitis even more important.
In conclusion, we have characterized HSV-specific immune responses in patients with recurrent meningitis, in comparison to patients with recurrent genital infections, and found a higher innate function and HSV-specific T cell reactivity. The incidence of HSV-2 meningitis has probably been underestimated (2, 10, 17) and may increase in the future. Although local immune responses in CNS may be different to responses in peripheral blood, our data provide new information on the agent-host interaction in recurrent HSV-2 meningitis and may be valuable for designing future strategies for antiviral and/or immunological treatment of patients with this disease.
ACKNOWLEDGMENTS
Financial support was provided through the regional agreement on clinically oriented medical research and development (ALF) between Stockholm County Council and the Karolinska Institutet and from the Swedish Research Council, the Karolinska research network for inflammation and immunology KiiM, Clas Groschinsky's foundation, Karolinska Institutet, the Swedish Society of Medicine, and the Swedish Foundation for Strategic Research.
We report no conflicts of interest.
Footnotes
Published ahead of print on 16 February 2011.
REFERENCES
- 1. Afonso N., et al. 2007. Appropriate use of polymerase chain reaction for detection of herpes simplex virus 2 in cerebrospinal fluid of patients at an inner-city hospital. Diagn. Microbiol. Infect. Dis. 57:309–313 [DOI] [PubMed] [Google Scholar]
- 2. Aurelius E. 2006. Neurological disease in herpes simplex virus type 2 infection, p. 317–338 In Studahl M., Cinque P., Bergström T. (ed.), Herpes simplex viruses. Taylor and Francis Group, New York, NY [Google Scholar]
- 3. Aurelius E., Forsgren M., Gille E., Skoldenberg B. 2002. Neurologic morbidity after herpes simplex virus type 2 meningitis: a retrospective study of 40 patients. Scand. J. Infect. Dis. 34:278–283 [DOI] [PubMed] [Google Scholar]
- 4. Aurelius E., Johansson B., Skoldenberg B., Forsgren M. 1993. Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2 as determined by type-specific polymerase chain reaction and antibody assays of cerebrospinal fluid. J. Med. Virol. 39:179–186 [DOI] [PubMed] [Google Scholar]
- 5. Aurelius E., Johansson B., Skoldenberg B., Staland A., Forsgren M. 1991. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet 337:189–192 [DOI] [PubMed] [Google Scholar]
- 6. Bergstrom T., et al. 1990. Primary and recurrent herpes simplex virus type 2-induced meningitis. J. Infect. Dis. 162:322–330 [DOI] [PubMed] [Google Scholar]
- 7. Casrouge A., et al. 2006. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314:308–312 [DOI] [PubMed] [Google Scholar]
- 8. Corey L., Adams H. G., Brown Z. A., Holmes K. K. 1983. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann. Intern. Med. 98:958–972 [DOI] [PubMed] [Google Scholar]
- 9. Dupuis S., et al. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33:388–391 [DOI] [PubMed] [Google Scholar]
- 10. Franzen-Rohl E., et al. 2008. High diagnostic yield by CSF-PCR for entero- and herpes simplex viruses and TBEV serology in adults with acute aseptic meningitis in Stockholm. Scand. J. Infect. Dis. 40:914–921 [DOI] [PubMed] [Google Scholar]
- 11. Franzen-Rohl E., Tiveljung-Lindell A., Grillner L., Aurelius E. 2007. Increased detection rate in diagnosis of herpes simplex virus type 2 meningitis by real-time PCR using cerebrospinal fluid samples. J. Clin. Microbiol. 45:2516–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heller F., et al. 2005. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129:550–564 [DOI] [PubMed] [Google Scholar]
- 13. Hochrein H., et al. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. U. S. A. 101:11416–11421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoshino Y., Pesnicak L., Cohen J. I., Straus S. E. 2007. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J. Virol. 81:8157–8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnston C., et al. 2008. Herpes simplex virus viremia during primary genital infection. J. Infect. Dis. 198:31–34 [DOI] [PubMed] [Google Scholar]
- 16. Jonsson M. K., Levi M., Ruden U., Wahren B. 2006. Minimal change in HSV-2 seroreactivity: a cross-sectional Swedish population study. Scand. J. Infect. Dis. 38:357–365 [DOI] [PubMed] [Google Scholar]
- 17. Kallio-Laine K., et al. 2009. Recurrent lymphocytic meningitis positive for herpes simplex virus type 2. Emerg. Infect. Dis. 15:1119–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim J. V., Kang S. S., Dustin M. L., McGavern D. B. 2009. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457:191–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knickelbein J. E., et al. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koelle D. M., Abbo H., Peck A., Ziegweid K., Corey L. 1994. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J. Infect. Dis. 169:956–961 [DOI] [PubMed] [Google Scholar]
- 21. Koelle D. M., et al. 1994. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68:2803–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kupila L., Vainionpaa R., Vuorinen T., Marttila R. J., Kotilainen P. 2004. Recurrent lymphocytic meningitis: the role of herpesviruses. Arch. Neurol. 61:1553–1557 [DOI] [PubMed] [Google Scholar]
- 23. Langenberg A. G., Corey L., Ashley R. L., Leong W. P., Straus S. E. 1999. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N. Engl. J. Med. 341:1432–1438 [DOI] [PubMed] [Google Scholar]
- 24. Lester R. T., et al. 2008. Toll-like receptor expression and responsiveness are increased in viraemic HIV-1 infection. AIDS 22:685–694 [DOI] [PubMed] [Google Scholar]
- 25. Liu T., Khanna K. M., Chen X., Fink D. J., Hendricks R. L. 2000. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lun S. W., Wong C. K., Ko F. W., Hui D. S., Lam C. W. 2009. Expression and functional analysis of Toll-like receptors of peripheral blood cells in asthmatic patients: implication for immunopathological mechanism in asthma. J. Clin. Immunol. 29:330–342 [DOI] [PubMed] [Google Scholar]
- 27. Mark K. E., et al. 2008. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J. Infect. Dis. 198:1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meier A., et al. 2009. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 15:955–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mikloska Z., Cunningham A. L. 1998. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J. Gen. Virol. 79(pt. 2):353–361 [DOI] [PubMed] [Google Scholar]
- 30. Mollaret P. 1944. La meningite endothelio-leucocytaire multirecurrente benigne. Syndrome nouveau ou maladie nouvelle. Documents clinique. Rev. Neurol. Paris 76:57–76 [Google Scholar]
- 31. Orange J. S., Ballas Z. K. 2006. Natural killer cells in human health and disease. Clin. Immunol. 118:1–10 [DOI] [PubMed] [Google Scholar]
- 32. Pebody R. G., et al. 2004. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex. Transm. Infect. 80:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peri P., et al. 2008. Herpes simplex virus type 1 Us3 gene deletion influences Toll-like receptor responses in cultured monocytic cells. Virol. J. 5:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Posavad C. M., Remington M., Mueller D. E., Zhao L., Magaret A. S., Wald A., Corey L. 2010. Detailed characterization of T cell responses to herpes simplex virus-2 in immune seronegative persons. J. Immunol. 184:3250–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramaswamy M., Geretti A. M. 2007. Interactions and management issues in HSV and HIV coinfection. Expert Rev. Anti Infect. Ther. 5:231–243 [DOI] [PubMed] [Google Scholar]
- 36. Schlesinger Y., Tebas P., Gaudreault-Keener M., Buller R. S., Storch G. A. 1995. Herpes simplex virus type 2 meningitis in the absence of genital lesions: improved recognition with use of the polymerase chain reaction. Clin. Infect. Dis. 20:842–848 [DOI] [PubMed] [Google Scholar]
- 37. Skoldenberg B., Jeansson S., Wolontis S. 1975. Herpes simplex virus type 2 and acute aseptic meningitis. Clinical features of cases with isolation of herpes simplex virus from cerebrospinal fluids. Scand. J. Infect. Dis. 7:227–232 [DOI] [PubMed] [Google Scholar]
- 38. Sun J. C., Beilke J. N., Lanier L. L. 2009. Adaptive immune features of natural killer cells. Nature 457:557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sundqvist V. A., Linde A., Wahren B. 1984. Virus-specific immunoglobulin G subclasses in herpes simplex and varicella-zoster virus infections. J. Clin. Microbiol. 20:94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Svahn A., et al. 2003. Development and evaluation of a flow-cytometric assay of specific cell-mediated immune response in activated whole blood for the detection of cell-mediated immunity against varicella-zoster virus. J. Immunol. Methods 277:17–25 [DOI] [PubMed] [Google Scholar]
- 41. Tabeta K., et al. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. U. S. A. 101:3516–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tabeta K., et al. 2006. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7:156–164 [DOI] [PubMed] [Google Scholar]
- 43. Tedder D. G., Ashley R., Tyler K. L., Levin M. J. 1994. Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Ann. Intern. Med. 121:334–338 [DOI] [PubMed] [Google Scholar]
- 44. Theil D., et al. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 163:2179–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tilton J. C., et al. 2008. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J. Virol. 82:3997–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verjans G. M., et al. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. U. S. A. 104:3496–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wald A., Zeh J., Selke S., Ashley R. L., Corey L. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 333:770–775 [DOI] [PubMed] [Google Scholar]
- 48. Xu F., et al. 2002. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J. Infect. Dis. 185:1019–1024 [DOI] [PubMed] [Google Scholar]
- 49. Xu F., et al. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973 [DOI] [PubMed] [Google Scholar]
- 50. Zhang S. Y., et al. 2007. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol. Rev. 220:225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang S. Y., et al. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522–1527 [DOI] [PubMed] [Google Scholar]
- 52. Zhu J., et al. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 204:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]