Abstract
Diagnosis of pertussis by culture and PCR is most sensitive when performed on nasopharyngeal specimens collected <2 weeks and <3 weeks, respectively, after the onset of clinical disease. Conversely, serological testing allows the diagnosis of patients (mostly adults) with less typical whooping cough symptoms, for whom clinical samples are often collected at later time points. Here, we report on a 20-year serodiagnostic survey of pertussis in Belgium from 1990 to 2009. In total, 13,163 patients were analyzed for Bordetella pertussis-specific antibodies by agglutination, complement fixation, immunofluorescence, and ELISA. The number of positive pertussis cases detected by serodiagnosis ranged between 50 and 150 annually. The mean age of positive cases increased from 9.9 years in 1990 to 33.9 years in 2009. Whereas from 1990 to 2003, children and young adolescents made up the majority of cases, from 2004 onwards, cases were detected in all age groups and the distribution became bimodal, with a first peak at the age of 10 to 20 years and a second at the age of 35 to 50 years. In contrast, patients diagnosed since 2001 by PCR and/or culture were mostly children younger than 1 year of age. Despite extensive childhood vaccination campaigns, whooping cough is still present in Belgium. Our findings confirm the potential role of adults in the continued transmission of pertussis and strongly warrant booster or cocoon vaccinations in older age groups.
INTRODUCTION
Pertussis, or whooping cough, is a highly infectious and vaccine-preventable disease caused by Gram-negative Bordetella pertussis, a strict pathogen of the human respiratory tract (13). In young children, pertussis presents classically as a three-stage disease with a catarrhal, a paroxysmal, and a convalescence phase evolving over a period of 4 to 8 weeks. In adults, the clinical manifestations of the disease can be less characteristic and may be easily confounded with other respiratory tract infections. Most cases of serious disease and the majority of fatalities are observed in early infancy. The infection is highly contagious, and human-to-human transmission occurs mainly through respiratory droplets.
The clinical signs and pathogenicity are attributed to the presence of a wide variety of virulence factors, such as adhesins (e.g., filamentous hemagglutinin [FHA]), autotransporters (pertactin), toxins (pertussis toxin [PT], adenylate cyclase, and tracheal toxin), lipopolysaccharide, pili, the capsule, and flagellae (13). Complications include secondary infections like otitis media and pneumonia, the leading cause of death. Bronchiectasis and atelectasis can also develop. The prolonged intrathoracic raised pressure can provoke petechiae, epistaxis, subconjunctival and brain hemorrhages, and spontaneous pneumothorax. Convulsions are also noted.
Following the introduction of combined diphtheria, tetanus, and pertussis vaccination after World War II, the incidence of pertussis has dramatically decreased in industrialized countries. However, among the reportable vaccine-preventable bacterial diseases, pertussis is the least well controlled (18), and since the late eighties, the number of reported cases has again increased and the age distribution of reported cases has shifted. Whereas in the prevaccine period, pertussis was essentially an infection occurring in children under the age of 5 years (1), 53% of cases reported in 2006 to the National Disease Surveillance System of the United States were in persons more than 15 years of age (14). In Europe, a similar trend has occurred. Most outbreaks in industrialized countries are now described in adolescents and young adults, due to waning antibodies after vaccination [both after acellular (aP) and whole-cell (wP) vaccine] or declining naturally acquired immunity (4, 11, 20, 24). Waning is thought to occur 4 to 12 years after the last booster dose or 7 to 20 years after an episode of illness (22), although these figures have to be considered with caution as an actual antibody limit for seroprotection (such as the one described for tetanus and diphtheria) has not been determined for pertussis so far.
The laboratory diagnosis of pertussis is challenging (2, 23). Culture is highly specific, but the bacteria require a long incubation period and sensitivity can be low. Diagnosis based on PCR is rapid, specific, and sensitive when performed on nasopharyngeal specimens collected <3 weeks after cough onset but is less sensitive later on, suggesting that pertussis cases might be grossly underreported (3). Moreover, since pertussis is thought to be an uncommon disease in industrialized countries and the clinical presentation resembles that of other illnesses associated with prolonged cough, health care providers often do not consider pertussis in the differential diagnosis (5). Conversely, serological testing can allow the confirmation of clinical cases for patients with less typical pertussis symptoms, for whom clinical samples are generally collected at later points after the onset of disease (19). As already mentioned, no universally accepted serological correlates for protection and infection are available. Menzies et al. have recently reported on the development and analytical validation of an immunoassay for quantifying serum anti-pertussis toxin antibodies more specifically for serodiagnosis in adolescents and adults (15).
In this study, we present the results of a 20-year serological survey of pertussis diagnosis in Belgium, performed at the Federal Institute of Public Health (the former Pasteur Institute of Brabant). Whereas from 1990 to 2003, the majority of cases were detected in children and young adolescents, from 2004 onwards, cases were detected in all age groups and the distribution became more bimodal, with a first peak in the age group from 10 to 20 years old and a second one in the age group from 35 to 50 years old. These findings confirm the potential role of adults in the continued transmission of pertussis and strongly warrant the implementation of new vaccination strategies, such as universal vaccination of adults, selective vaccination of health care professionals and of those who work with infants and young children the so-called cocoon vaccination of mothers of newborns and of household contacts of infants and toddlers, or finally, selective vaccination of pregnant mothers.
(This work was presented in part as a selected oral presentation at the 9th International Bordetella Symposium, Baltimore, MD, 30 September to 3 October, 2010.)
MATERIALS AND METHODS
Patients.
Serum samples were sent to the laboratory of WIV-ISP for bacterial serology as part of the routine diagnosis of pertussis. These sera were obtained on a voluntary basis from medical practitioners, including general practitioners, pediatricians, and lung disease specialists, throughout the country. A standard questionnaire was sent to the medical practitioners to obtain information on birth date, gender, vaccination status, geographic origin, type of symptoms, duration of coughing, and possible contacts. A second serum sample, collected after a 2- to 3-week interval, was also requested. Nasopharyngeal aspirate specimens were sent to the Universitair Ziekenhuis Brussel. Different patients were tested by serology than by PCR/culture.
Serological tests.
Sera were heated at 56°C for 30 min and stored at 4°C prior to analysis. For the serological diagnosis of pertussis, four different tests were used. Agglutination assay was performed in round-bottom 96-microwell plates (Greiner). Two-fold serial dilutions of serum (starting at 1:10) were incubated for 24 h at 37°C with in-house-made B. pertussis antigen, and agglutination was scored visually. Complement fixation assay was performed in round-bottom 96-microwell plates (Greiner). Serial 2-fold serum dilutions (starting at 1:2) were tested using in-house-made B. pertussis antigen and sheep red blood cells (SRBC), complement, and rabbit anti-SRBC serum (all three from bioMérieux). The last serum dilution that fixed complement was scored visually. Immunofluorescence (IF) for IgG, IgM, and IgA was performed on Cel-Line diagnostic microscope slides (18 wells, 5 mm; Thermo-Scientific). The slides were coated with whole, killed B. pertussis bacteria (Difco Bordetella pertussis antigen; Becton Dickinson), and serial 2-fold dilutions of serum (starting at 1:20) were added and incubated for 35 min at 37°C. The slides were washed, and fluorescein isothiocyanate-conjugated rabbit antiserum to human IgG, IgM, and IgA (Nordic Immunological Laboratories, Netherlands) was added before they were read using a fluorescence microscope. IgG enzyme-linked immunosorbent assay (ELISA) for PT and FHA was performed in flat-bottom 96-microwell plates (Greiner) using purified PT and FHA antigens, kindly provided by GlaxoSmithKline, at serum dilutions of 1:200 and alkaline phosphatase-labeled anti-human IgG (Sigma) antibodies for development. The results were expressed as the percentage of an internal laboratory standard from pooled sera of positive cases. Serum samples were considered positive when a positive IgM and/or IgA response was found in the immunofluorescence test. In the absence of IgM/IgA antibodies in the IF test, a serum sample was considered positive when a second serum sample obtained 2 to 3 weeks after the first sample showed a doubling of the titer in the agglutination, complement fixation, or IgG immunofluorescence test, coupled with an increase in ELISA values. Alternatively, a positive serodiagnosis was made when very high titers were present in the agglutination (≥1/320), complement fixation (≥1/32), or IgG immunofluorescence (≥1/160) test. For 68 of the 94 serum samples diagnosed as positive in 2009 by these in-house tests, anti-pertussis IgG titers were also determined using a commercial ELISA (Virotech) and converted to international units (IU)/ml using World Health Organization (WHO) International Standard Pertussis Antiserum (NIBSC code 06/140) (25).
PCR and culture.
PCR and culture were mostly performed using nasopharyngeal aspirate specimens, although nasal or throat swab specimens and bronchoalveolar lavage fluid samples were occasionally used (8). Nasopharyngeal aspirate specimens were obtained by instilling 10 ml of 0.9% saline in one nostril, followed immediately by reaspiration of the fluid through a catheter (female bladder catheter CH08; Pharma-Plast) in the other nostril. Samples were cultivated on Regan-Lowe charcoal agar containing 10% horse blood and cephalexin (Bordetella selective supplement; Oxoid) and incubated at 35°C for 7 days. At the same time, part of the sample was used for the detection of the B. pertussis-specific IS481 insertion element and the Bordetella parapertussis-specific IS1001 insertion element by PCR (19). To monitor PCR inhibition, the pRRP100 plasmid was added as an internal positive control, as described elsewhere (19).
RESULTS
Number of positive pertussis cases diagnosed from 1990 to 2009 using serological testing.
Table 1 shows a summary of the serodiagnostic results obtained from 1990 to 2009. During this period, we performed 15,522 analyses corresponding to a total of 13,163 individual patients. The number of annual analyses increased from 408 in 1990 to 1,606 in 2009. A pronounced increase in the number of analyses was observed in 2004, when 1,424 sera (from 1,204 patients) were tested, compared to 633 sera (from 521 patients) in 2003. The number of positive cases throughout the 20-year survey period varied from 45 in 2002 to 154 in 2004. Positive cases were generally more frequent for the Flanders region (ranging from 36.2% to 68.8% of the cases) than for the Walloon region (ranging from 22.1% to 47.4%) and for the Brussels Capital Region (ranging from 4.6% to 20.0%). This regional difference was a reflection of the higher number of clinical samples received from Flanders than from Wallonia or from Brussels. In general, the positive cases from females outnumbered those of males. A second serum sample was solicited if a first sample, collected early after the onset of clinical signs, was negative in the IgM or IgA test. Unfortunately, only a low percentage of these second samples were received, and therefore, most cases diagnosed in this survey were based on the presence of IgM or IgA antibodies (in a first sample) and only a few were based on increased IgG titers in a second sample. Until 2003, most cases were diagnosed based on positive IgM antibodies. From 2004 onwards, the presence of IgA antibodies or very high titers present in the agglutination (≥1/320), complement fixation (≥1/32), or IgG immunofluorescence (≥1/160) test became a more important criterion.
Table 1.
Diagnostic criteria used for pertussis diagnosis in serum samples from 1990 to 2009
| Parameter | Result for: |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
| No. of analyses | 408 | 389 | 414 | 364 | 375 | 326 | 566 | 796 | 644 | 831 | 783 | 598 | 682 | 633 | 1,424 | 1,514 | 1,353 | 1,691 | 1,721 | 1,606 |
| No. of patients | 343 | 319 | 336 | 302 | 306 | 271 | 470 | 656 | 555 | 691 | 754 | 546 | 593 | 521 | 1,204 | 1,329 | 1,151 | 1,495 | 1,578 | 1,430 |
| No. of positive patients | 91 | 80 | 56 | 54 | 61 | 75 | 101 | 97 | 82 | 79 | 88 | 46 | 45 | 57 | 154 | 125 | 130 | 85 | 108 | 94 |
| No. of paired positive samples | 23 | 25 | 18 | 20 | 23 | 15 | 41 | 15 | 25 | 26 | 26 | 16 | 16 | 18 | 57 | 38 | 41 | 27 | 32 | 24 |
| No. of IgM-positive samples | 84 | 60 | 36 | 17 | 31 | 46 | 66 | 81 | 44 | 51 | 72 | 21 | 22 | 45 | 79 | 33 | 33 | 7 | 38 | 48 |
| No. of IgA- and/or IgG-positive samples | 2 | 6 | 11 | 27 | 21 | 24 | 32 | 9 | 16 | 19 | 7 | 11 | 2 | 1 | 43 | 79 | 72 | 59 | 53 | 44 |
| No. diagnosed by rise in IgG titer | 0 | 1 | 1 | 3 | 1 | 0 | 1 | 3 | 3 | 3 | 3 | 3 | 7 | 5 | 6 | 2 | 5 | 4 | 5 | 0 |
| No. diagnosed by clinical data | 2 | 7 | 6 | 6 | 8 | 4 | 3 | 4 | 17 | 4 | 4 | 11 | 8 | 6 | 25 | 11 | 18 | 15 | 7 | 2 |
Age distribution of pertussis cases diagnosed from 1990 to 2009 using serological testing.
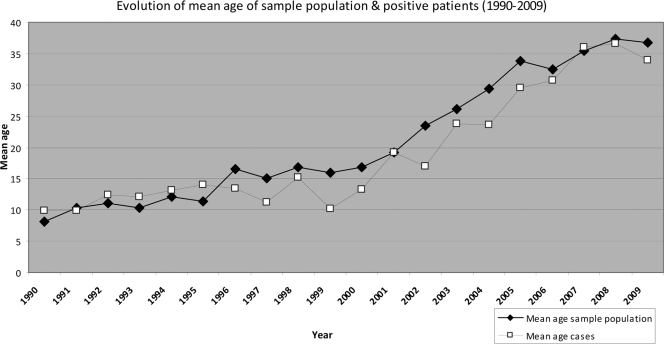
The mean age of the positive pertussis cases increased steadily over the 20-year study period. The mean age shifted from 9.9 years in 1990 to 15.2 years in 1995, 20.6 years in 2001, and 33.9 years in 2009 (Fig. 1). From 1990 to 2003, most positive cases were detected in children and young adolescents. As an example, at the beginning of this survey in 1990, 84% of the positive cases were diagnosed in children and adolescents younger than 20 years. It is important to note that in 1990, this age group also was the source of 81% of the serum samples received. From 2004 onwards, the age distribution of positive cases gradually became bimodal, with a first peak at the age of 10 to 20 years and a second one in the age group from 35 to 50 years. Table 2 shows a detailed age distribution for the serum samples received and positive pertussis cases identified by serodiagnosis in 1990, 1995, and from 2001 to 2009. As already mentioned, positive diagnosis in 1990 was almost exclusively based on the presence of IgM antibodies: 84 out of 91 positive samples, i.e., 92%, were diagnosed on this basis (Table 1). In 2002, the mean age of the sample population had increased to 23.4 years and the mean age of the positive cases had increased to 17.0 years. Children in the age group younger than 15 years still represented 66% of the total cases, and diagnosis was mostly based on the detection of IgM. During the last year of this survey, 2009, the mean age of the sample population had increased to 36.7 years and the mean age of the positive cases to 33.9 years. Diagnosis in 2009 was based in half of the patients on the presence of IgM antibodies and in half of the cases on the presence of IgA (Table 1). More specifically, for the 48 cases diagnosed in 2009 as positive on the basis of IgM, 12 were also positive in IgA, 21 had an agglutination titer of >160, and 5 had an agglutination titer of >320. For the 44 cases diagnosed on the basis of IgA, 24 showed agglutination titers of >160 and 8 had agglutination titers of >320.
Fig. 1.
The mean ages of positive pertussis cases but also of the sample populations increased from 1990 to 2009.
Table 2.
Detailed age distribution of whole sample population and positive pertussis cases as serodiagnosed in 1990, 1995, and 2002 to 2009a
| Age group (yr) | 1990 |
1995 |
2002 |
2003 |
2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients (8.11b) | No. positive (9.95) | No. of patients (11.43) | No. positive (14.04) | No. of patients (23.4) | No. positive (17.0) | No. of patients (26.1) | No. positive (23.74) | No. of patients (29.3) | No. positive (23.6) | No. of patients (33.85) | No. positive (29.57) | No. of patients (32.54) | No. positive (30.64) | No. of patients (35.42) | No. positive (36.03) | No. of patients (37.4) | No. positive (36.67) | No. of patients (36.7) | No. positive (33.9) | |
| Unknown | 33 | 7 | 6 | 2 | 16 | 1 | 14 | 0 | 32 | 2 | 32 | 1 | 13 | 1 | 12 | 0 | 19 | 0 | 16 | 0 |
| 0–4 | 173 | 29 | 145 | 24 | 173 | 11 | 122 | 7 | 235 | 17 | 172 | 5 | 152 | 6 | 148 | 2 | 155 | 3 | 120 | 2 |
| 5–9 | 60 | 23 | 41 | 21 | 59 | 8 | 54 | 11 | 130 | 44 | 71 | 6 | 55 | 2 | 69 | 3 | 52 | 4 | 54 | 0 |
| 10–14 | 29 | 16 | 18 | 8 | 61 | 11 | 61 | 12 | 84 | 21 | 134 | 34 | 138 | 34 | 142 | 15 | 99 | 11 | 89 | 10 |
| 15–19 | 20 | 9 | 8 | 2 | 30 | 1 | 19 | 4 | 50 | 6 | 72 | 15 | 74 | 10 | 110 | 9 | 125 | 18 | 120 | 19 |
| 20–24 | 1 | 1 | 1 | 0 | 15 | 0 | 18 | 2 | 36 | 5 | 43 | 3 | 39 | 5 | 64 | 4 | 72 | 7 | 61 | 3 |
| 25–29 | 4 | 0 | 11 | 2 | 21 | 2 | 17 | 0 | 42 | 4 | 53 | 2 | 49 | 7 | 66 | 5 | 66 | 3 | 67 | 6 |
| 30–34 | 7 | 1 | 8 | 4 | 33 | 3 | 27 | 5 | 64 | 8 | 89 | 10 | 72 | 6 | 89 | 2 | 105 | 2 | 106 | 4 |
| 35–39 | 1 | 0 | 7 | 5 | 30 | 3 | 33 | 4 | 102 | 13 | 106 | 8 | 94 | 18 | 113 | 3 | 123 | 9 | 109 | 10 |
| 40–44 | 7 | 4 | 10 | 4 | 32 | 1 | 32 | 2 | 98 | 8 | 117 | 14 | 100 | 11 | 130 | 14 | 125 | 9 | 139 | 14 |
| 45–49 | 1 | 0 | 3 | 1 | 31 | 1 | 29 | 2 | 82 | 6 | 87 | 3 | 87 | 9 | 124 | 8 | 126 | 10 | 117 | 11 |
| 50–54 | 1 | 0 | 3 | 1 | 19 | 1 | 26 | 3 | 57 | 4 | 75 | 7 | 62 | 5 | 102 | 1 | 103 | 4 | 101 | 4 |
| 55–59 | 0 | 0 | 2 | 0 | 17 | 2 | 10 | 1 | 45 | 1 | 83 | 6 | 76 | 4 | 106 | 5 | 129 | 8 | 118 | 4 |
| 60–64 | 2 | 1 | 4 | 1 | 20 | 0 | 16 | 1 | 50 | 4 | 61 | 3 | 46 | 3 | 74 | 2 | 99 | 11 | 77 | 2 |
| 65–69 | 3 | 0 | 0 | 0 | 16 | 0 | 14 | 1 | 30 | 3 | 49 | 4 | 37 | 6 | 53 | 3 | 68 | 5 | 40 | 3 |
| 70–74 | 0 | 0 | 0 | 0 | 9 | 0 | 15 | 1 | 34 | 6 | 36 | 2 | 27 | 1 | 39 | 5 | 45 | 0 | 37 | 1 |
| >75 | 1 | 0 | 4 | 0 | 11 | 0 | 14 | 1 | 33 | 2 | 49 | 2 | 30 | 2 | 54 | 4 | 67 | 4 | 59 | 1 |
| Total | 343 | 91 | 271 | 75 | 593 | 45 | 521 | 57 | 1,204 | 154 | 1,329 | 125 | 1,151 | 130 | 1,495 | 85 | 1,578 | 108 | 1,430 | 94 |
Age groups with highest prevalence and total numbers of positive cases are indicated in boldface.
Mean age in years is shown in parentheses.
The number of positive cases in children younger than 5 years decreased significantly and continuously, from 32.0% in 1990 to 24.4% in 2002 and 2% in 2009 (P < 0.001). This tendency was paralleled by an increase of positive cases in the age group older than 25 years. In 1990, only 5.5% of the cases were detected in adults, in 2002, 28.8% of the positive cases were found in this age group, and in 2009, this figure had increased to 64% (P < 0.001).
Table 3 shows a more detailed age distribution of pertussis cases serodiagnosed in children younger than 5 years old in 1990, 1995, 2002, and 2009. The number of pertussis cases detected in children by serodiagnosis decreased progressively. In 2009, only two pertussis cases were serodiagnosed in the 120 serum samples from children younger than 5 years, and both children were among the oldest in this age group (4 to 5 years).
Table 3.
Detailed age distribution of the whole population supplying samples and of positive pertussis cases for children younger than 5 years as serodiagnosed in 1990, 1995, 2002, and 2009
| Age | 1990 |
1995 |
2002 |
2009 |
||||
|---|---|---|---|---|---|---|---|---|
| No. of samples | No. positive | No. of samples | No. positive | No. of samples | No. positive | No. of samples | No. positive | |
| <6 mo | 22 | 5 | 17 | 2 | 23 | 0 | 30 | 0 |
| 6 mo–1 yr | 42 | 0 | 49 | 1 | 69 | 3 | 13 | 0 |
| 1–2 yr | 47 | 11 | 32 | 5 | 44 | 3 | 29 | 0 |
| 2–3 yr | 13 | 3 | 13 | 5 | 14 | 0 | 11 | 0 |
| 3–4 yr | 27 | 5 | 19 | 5 | 13 | 1 | 17 | 0 |
| 4–5 yr | 22 | 5 | 15 | 6 | 10 | 4 | 20 | 2 |
| Total | 173 | 29 | 145 | 24 | 173 | 11 | 120 | 2 |
Age distribution of pertussis cases identified by the pertussis reference laboratory using culture and PCR.
The exact number of patients diagnosed with pertussis annually in Belgium by culture and/or PCR is difficult to know. Only 70% of recognized clinical laboratories in Belgium are part of the network of sentinel laboratories that report their data to the Federal Scientific Institute of Public Health, through the pertussis reference center. Whereas from 1990 to 1996, an average 4 to 11 pertussis cases were reported annually in Belgium, this number has increased dramatically since 1997, and it reached a peak of 315 cases in 2007 (an incidence of 3.0/105 inhabitants). This number is certainly an underestimation of the total national figure, as certain districts in Flanders and Wallonia had problems with the reporting. The increase since 1997 may have been related to the introduction of the diagnosis by PCR in that year, but growing awareness among clinicians and waning of immune protection in a progressively ageing population (which is no longer boosted by frequent exposure to B. pertussis) may be other factors. Concerning the age distribution of pertussis cases identified by microbiology laboratories, the majority of cases diagnosed since 2001 by PCR and/or culture were children younger than 1 year old, although this proportion decreased from 57% to 38% between 2001 and 2009, while the proportion of patients younger than 19 years decreased from 81 to 56%. The number of adult cases detected by PCR and/or culture increased from 7.8% in 2001 to 27.7% in 2009.
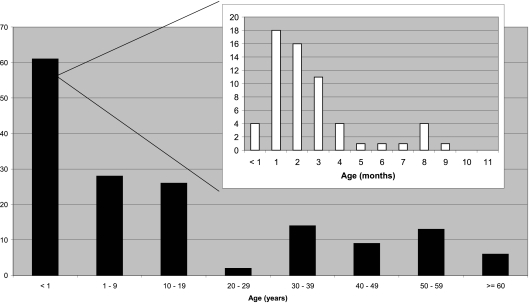
In 2009, the Pertussis Reference Center of the University Hospital-Brussels identified by culture 55 patients with B. pertussis and one patient with B. parapertussis. One patient was positive in culture for both B. pertussis and B. parapertussis. Moreover, 99 patients with a negative culture were identified by PCR as positive for B. pertussis and 5 for B. parapertussis. Thus, in total, the Reference Center identified 160 patients, 154 for B. pertussis, 1 for B. pertussis and B. parapertussis, and 6 for B. parapertussis. Ninety-nine patients were females and 61 males, an expected male/female ratio, considering the known predominance of pertussis in girls and women. Figure 2 shows the age distribution of patients diagnosed by the Reference Center by culture and/or PCR in 2009. A majority of cases (58/154, i.e., 38%) were diagnosed in children younger than 1 year old. These were essentially unvaccinated or incompletely vaccinated children younger than 3 months old (Fig. 2, inset).
Fig. 2.
Age distribution of pertussis cases identified in 2009 by the pertussis reference laboratory using culture and/or PCR.
Antibody levels against pertussis toxin among 68 subjects with a positive serodiagnosis of pertussis in 2009.
Using the newly available National Institute for Biological Standards and Control (NIBSC) standard and the commercial Virotech ELISA for the detection of pertussis toxin IgG, we calculated the anti-PT antibody titers (expressed in IU/ml) in 68 of the 94 patients diagnosed as positive with our routine assays in 2009. Table 4 shows the mean antibody titers detected in the different age groups. The mean overall anti-PT antibody titer for the 68 patients was 285 IU/ml. More than 70% had antibody titers of >200 IU/ml (cutoff value utilized by Massachusetts State Laboratory [12]), and 24% had antibody titers of >400 IU/ml. The strongest antibody responses were detected in the age groups from 15 to 19 years and 45 to 49 years. In these two groups, more than 40% showed anti-PT levels above 400 IU/ml.
Table 4.
IgG antibody levels against pertussis toxin among 68 subjects with a positive diagnosis of pertussis in 2009a
| Age group (yr) | Mean titer (IU/ml) | SD | Total no. of cases | No. (%) of cases with indicated titer (IU/ml) |
|
|---|---|---|---|---|---|
| >200 | >400b | ||||
| 0–4 | 232 | NA | 1 | 1 (100) | 0 (0) |
| 5–9 | NA | NA | 0 | 0 (0) | 0 (0) |
| 10–14 | 285 | 103 | 7 | 6 (86) | 1 (14) |
| 15–19 | 340 | 128 | 12 | 10 (83) | 5 (42) |
| 20–24 | 293 | 107 | 3 | 2 (67) | 0 (0) |
| 25–29 | 252 | 162 | 5 | 3 (60) | 1 (20) |
| 30–34 | 192 | 124 | 2 | 1 (50) | 0 (0) |
| 35–39 | 226 | 130 | 7 | 4 (57) | 1 (14) |
| 40–44 | 287 | 144 | 9 | 6 (67) | 2 (22) |
| 45–49 | 328 | 127 | 10 | 8 (80) | 4 (40) |
| 50–54 | 302 | 112 | 4 | 3 (75) | 1 (25) |
| 55–59 | 164 | 161 | 3 | 1 (33) | 0 (0) |
| 60–64 | 317 | NA | 1 | 1 (100) | 0 (0) |
| 65–69 | 281 | 170 | 2 | 2 (100) | 0 (0) |
| 70–74 | 56 | NA | 1 | 0 (0) | 0 (0) |
| >75 | 427 | NA | 1 | 1 (100) | 1 (100) |
| Unknown | NA | NA | 0 | 0 (0) | 0 (0) |
| Total | 285 | 129 | 68 | 49 (72) | 16 (24) |
Results were obtained by using NIBSC 06/140 standard antiserum and the Virotech anti-PT ELISA. NA, not applicable.
The groups with the highest percentages of stronger antibody responses are highlighted in boldface.
DISCUSSION
Pertussis, or whooping cough, is an upper respiratory tract infection caused by the Bordetella pertussis or Bordetella parapertussis bacterium. It is a serious disease that can cause permanent disability and even death in infants. The infection usually lasts 6 weeks, and the initial diagnosis is generally based on clinical symptoms, such as prolonged coughing with paroxysms and/or whooping or choking. In infants, older vaccinated children, adolescents, and adults, the clinical course may not be typical, and prolonged coughing may be the only symptom (10). Laboratory diagnosis is essentially based on a culture of nasal secretions; however, the results can take up to 10 to 14 days, after which time the person has likely passed the infection to other community members. PCR is both faster and more accurate than culture. However, microbiological diagnosis of pertussis by culture and PCR is most sensitive when performed on nasopharyngeal specimens collected <2 weeks and <3 weeks, respectively, after the onset of disease. Conversely, serological testing can allow the diagnosis of patients with less typical whooping cough symptoms, for whom clinical samples are generally collected at later time points after the onset of disease. Although serological monitoring of pertussis antibodies has mostly been used in epidemiological studies and vaccination monitoring, it is also important in an immunodiagnostic setting.
Here, we present results from a 20-year serological survey of pertussis in Belgium. Our results show that serological diagnosis can be complementary to diagnosis by PCR and culture, particularly for the adult population. Indeed, about 40% of the pertussis cases reported in recent years to the Belgian Pertussis Reference Center (by laboratories using PCR and culture) were in the age group of children younger than 1 year old (and more particularly in infants younger than 3 months). In contrast, since 2005, positive cases detected by serodiagnosis were found in all age groups. The most remarkable evolution was the significant decrease in cases in children younger than 10 years. Whereas in 1990, 52 of 91 (57%) positive cases were detected in this age group, the figure decreased to 19 of 45 (42%) in 2002, 11 of 125 (9%) in 2005, and 2 of 94 (2%) in 2009.
There are many reasons for the change in age distribution that we have observed during this 20-year serodiagnostic follow-up study. First and most importantly, as reported in neighboring countries, the number of pertussis cases in adults has undoubtedly increased in recent years. The waning of immune protection in an adult population that is no longer boosted by frequent exposure to B. pertussis may play a role. Second, a growing awareness of the problem among clinicians has resulted in an increased demand for analysis of adult serum samples. In this respect, it is interesting to note that the mean age of our sample population also increased during this period. Thus, in 1990, the mean age of the sample population was 8.1 years and 82% of the samples received came from children and young adolescents <20 years old, whereas in 2009, the mean age of the sample population was 36.7 years, only 26.8% of the samples came from the subjects in the young age group, and the age distribution of samples became more widespread. Finally, it is very likely that the introduction of PCR for the microbiological diagnosis of pertussis since 1997 has modified the type of samples sent to the laboratory for immunodiagnostic testing.
A recent study by de Greeff et al. analyzed a group of 560 household contacts of 164 infants younger than 6 months hospitalized for pertussis (6). The most likely source of infection of the infant was established for 96 households (60%), being a sibling (41%), mother (38%), or father (17%). A smaller study, performed in Belgium from February 1999 to January 2002 by the Pertussis Reference Center, showed similar evidence for a pertussis reservoir in adults (8). Thus, among 63 household contacts of 28 index patients, PCR and culture for B. pertussis identified 25 B. pertussis-positive persons, and more importantly, 19 of these 25 household contacts were asymptomatic (8). It is clear that an integrated approach combining bacteriological and serological results provided by the microbiological and the immunodiagnostic laboratories, respectively, could improve the performance of pertussis diagnosis and reduce the underreporting of cases in Belgium.
The vaccination status of the patients in our study was recorded based on questionnaires completed by the practitioners. From 1990 to 1999, many of the positive cases had a record of complete vaccination status. Later, reports of complete vaccination status decreased (37.7% in 1999 and 14.9% in 2008 [P < 0.001]). However, these figures should be considered with some caution, as the mean ages of the sample population and positive cases increased, and therefore, the accuracy of vaccination records was likely to be lower. Universal vaccination against pertussis (coupled to tetanus and diphtheria) started in Belgium in 1959. Polio is the only mandatory vaccine in Belgium, but the diphtheria-tetanus-pertussis (DTP) vaccine is included in the recommended schedule of childhood vaccinations. At the end of the 1990s, the cellular pertussis component in DTP vaccine based on whole bacteria was replaced by the acellular component in DTaP. A random cluster sampling according to the WHO Expanded Program on Immunization (EPI) cluster sampling technique was conducted in Flanders (North Belgium) in 1999 to ascertain the vaccination coverage of 18- to 24-month-old children (21). The coverage level for the full schedule for DTP in this 1999 study was 89% (21). In 2005, children of the same birth year as surveyed in 1999 were enrolled in a similar survey study to evaluate possible catch-up of primary vaccine doses after the age of 24 months, as well as the uptake of vaccines recommended between 2 and 6 years of age. Coverage rates were 84.2% for recommended DTP primary vaccine doses. In addition, 88.3% received a DT-polio booster at 6 years of age (acellular pertussis vaccine was subsequently added to this 6-year booster vaccine) (17). Since 2004, the average number of Infanrix (DTaP) vaccine doses sold in Belgium has fluctuated around 500,000 (Tuni Randall, Project Leader, GlaxoSmithKline Biologicals, personal communication).
In conclusion, despite extensive childhood vaccination campaigns, whooping cough is still present in Belgium and remains a disease of public health concern. The serodiagnostic findings presented here confirm the potential role of adults in the continued transmission of pertussis and strongly warrant the implementation of booster vaccinations. Since 2006, a lower-dose dTpa vaccine, adapted for adolescents and adults, is available in Belgium and recommended for young adolescents between 14 and 16 years of age. Data concerning safety and protection against pertussis following booster vaccination of adults are lacking for the moment, and so far, the Belgian Superior Health Council has not recommended these vaccinations in adults. However, it is clear that further reducing infant pertussis will require serious commitment and new vaccination approaches beyond infancy, such as vaccination of health care workers or “cocoon” vaccination of mothers of newborns and of household contacts of infants and toddlers (7, 9).
Because of the limited number of second serum samples received for retesting, most of our positive diagnoses were based on the presence of IgM or IgA antibodies against whole bacteria in immunofluorescence. High IgG titers in agglutination tests were also a parameter, particularly for the pertussis cases in adults. IgG antibodies against pertussis toxin were also determined by ELISA, but in the absence of an internationally accepted threshold for positivity and because of the limited number of second serum samples received for retesting, these results were only used if confirmed by the other tests. In 2009, anti-PT IgG titers, expressed in IU/ml, were also determined for 68 of 94 positive samples, using the commercial Virotech ELISA and the newly available WHO International Standard Pertussis Antiserum (NIBSC 06/140). More than 70% of the patients diagnosed by our routine assays had antibody titers of >200 IU/ml (cutoff value utilized by Massachusetts State Laboratory [12]), and 24% of them showed antibody titers of >400 IU/ml. The strongest antibody responses were detected in the age groups 15 to 19 years and 45 to 49 years. In these groups, more than 40% had anti-PT levels of >400 IU/ml.
Because of the limited number of second serum samples received for retesting, a number of patients diagnosed as negative (negative IgM or IgA antibodies) may have been falsely diagnosed as negative. On the basis of the IgG levels detected with the in-house ELISA for PT and conversion of these figures to IU/ml using the International Standard Pertussis Antiserum (NIBSC 06/140), we found that at least 20% of these sera had anti-PT IgG levels of >200 IU/ml. These figures are suggestive of an actual underestimation of the positive cases detected by our conventional assays. Recently, Guiso et al. have published recommendations from EU reference laboratories involved in serological diagnosis of pertussis (10). The use of ELISA or multiplex (16) immunoassays for the detection of IgG antibodies against PT is recommended, while the use of other antigens and other methods, such as microagglutination, complement fixation, and indirect immunofluorescence, are discouraged. The detection of pertussis-specific IgM antibodies is not recommended, and the detection of IgA anti-PT is recommended only with indeterminate IgG anti-PT levels or when a second sample cannot be obtained (10). Unfortunately, the comparison of paired serum samples is not always possible. As an example, less than 30% of the positive cases in our study could be diagnosed using the paired-sample approach. Continuously informing clinicians of the limitations of serological results based on single serum samples (particularly if collected late after the onset of coughing) may help to overcome this problem.
ACKNOWLEDGMENT
We are very grateful to GlaxoSmithKline Biologicals for generously providing us the purified pertussis toxin PT and filamentous hemagglutinin for our routine ELISAs.
Footnotes
Published ahead of print on 23 February 2011.
REFERENCES
- 1. Cherry J. D. 1999. Pertussis in the preantibiotic and prevaccine era, with emphasis on adult pertussis. Clin. Infect. Dis. 28:S107–S111 [DOI] [PubMed] [Google Scholar]
- 2. Cherry J. D., Grimprel E., Guiso N., Heininger U., Mertsola J. 2005. Defining pertussis epidemiology: clinical, microbiologic and serologic perspectives. Pediatr. Infect. Dis. 24:S25–S34 [DOI] [PubMed] [Google Scholar]
- 3. Crowcroft N. S., Andrews N., Rooney C., Brisson M., Miller E. 2002. Deaths from pertussis are underestimated in England. Arch. Dis. Child. 86:336–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crowcroft N. S., Pebody R. G. 2006. Recent developments in pertussis. Lancet 367:1926–1936 [DOI] [PubMed] [Google Scholar]
- 5. Deeks S., et al. 1999. Failure of physicians to consider the diagnosis of pertussis in children. Clin. Infect. Dis. 28:840–846 [DOI] [PubMed] [Google Scholar]
- 6. de Greeff S. C., et al. 2010. Pertussis disease burden in the household: how to protect young infants.. Clin. Infect. Dis. 50:1339–1345 [DOI] [PubMed] [Google Scholar]
- 7. Demaria A., Lett S. M. 2010. Vaccinate the village. Clin. Infect. Dis. 50:1346–1348 [DOI] [PubMed] [Google Scholar]
- 8. De Schutter I., et al. 2003. Molecular typing of Bordetella pertussis isolates recovered from Belgian children and their household members. Clin. Infect. Dis. 36:1391–1396 [DOI] [PubMed] [Google Scholar]
- 9. Forsyth K. D., et al. 2004. New pertussis vaccination strategies beyond infancy: recommendations by the Global Pertussis Initiative. Clin. Infect. Dis. 39:1802–1809 [DOI] [PubMed] [Google Scholar]
- 10. Guiso N., et al. 2011. What to do and what not to do in serological diagnosis of pertussis: recommendations from EU reference laboratories. Eur. J. Clin. Microbiol. Infect. Dis. 30:307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He Q. 1994. Outcomes of Bordetella pertussis infection in different age groups of an immunized population. J. Infect. Dis. 170:873–877 [DOI] [PubMed] [Google Scholar]
- 12. Marchant C. D., et al. 1994. Pertussis in Massachusetts, 1981-1991: incidence, serologic diagnosis and vaccine effectiveness. J. Infect. Dis. 169:1297–1305 [DOI] [PubMed] [Google Scholar]
- 13. Mattoo S., Cherry J. D. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella species. Clin. Microbiol. Rev. 18:326–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McNabb S. J., Jakosky R. A., Hall-Baker P. A. 2007. Summary of notifiable diseases—United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 54:1–92 [PubMed] [Google Scholar]
- 15. Menzies S. L., et al. 2009. Development and analytical validation of an immunoassay for quantifying serum anti-pertussis toxin antibodies resulting from Bordetella pertussis infection. Clin. Vaccine Immunol. 16:1781–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reder S., Riffelmann M., Becker C., Wirsing von König C. H. 2008. Measuring immunoglobulin G antibodies to tetanus toxin, diphtheria toxin, and pertussis toxin with single-antigen enzyme-linked immunosorbent assays and a bead-based multiplex assay. Clin. Vaccine Immunol. 15:744–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Theeten H., et al. 2009. Coverage of recommended vaccines in children at 7-8 years of age in Flanders, Belgium. Acta Paediatr. 98:1307–1312 [DOI] [PubMed] [Google Scholar]
- 18. Tondella M. L., et al. 2009. International Bordetella pertussis assay standardization and harmonization meeting report. Centers for Disease Control and Prevention, Atlanta, Georgia, United States, 19-20 July 2007. Vaccine 27:803–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Zee A., Agterberg C., Peeters M., Mooi F., Schellekens J. 1996. A clinical validation of Bordetella pertussis and Bordetella parapertussis polymerase chain reaction: comparison with culture and serology using samples from patients with suspected whooping cough from a highly immunized population. J. Infect. Dis. 174:89–96 [DOI] [PubMed] [Google Scholar]
- 20. Van Rie A., Wendelboe A. M., Englund J. A. 2005. Role of maternal pertussis antibodies in infants. Pediatr. Infect. Dis. J. 24:S62–S65 [DOI] [PubMed] [Google Scholar]
- 21. Vellinga A., Depoorter A. M., Van Damme P. 2002. Vaccination coverage estimates by EPI cluster sampling survey of children (18-24 months) in Flanders, Belgium. Acta Paediatr. 91:599–603 [DOI] [PubMed] [Google Scholar]
- 22. Wendelboe A. M., et al. 2007. Transmission of Bordetella pertussis to young infants. Pediatr. Infect. Dis. J. 26:293–299 [DOI] [PubMed] [Google Scholar]
- 23. Wendelboe A. M., Van Rie A. 2006. Diagnosis of pertussis: a historical review and recent developments. Expert Rev. Mol. Diagn. 6:857–864 [DOI] [PubMed] [Google Scholar]
- 24. Wendelboe A. M., Van Rie A., Salmaso S., Englund J. A. 2005. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 24:S58–S61 [DOI] [PubMed] [Google Scholar]
- 25. Xing D., et al. 2009. Characterization of reference materials for human antiserum to pertussis antigens by an international collaborative study. Clin. Vaccine Immunol. 16:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]