Abstract
The objective of this study was to retrospectively evaluate the utility of serum neopterin as a diagnostic marker of hemophagocytic lymphohistiocytosis (HLH). The medical records of patients diagnosed with HLH (familial and secondary) between January 2000 and May 2009 were reviewed retrospectively, and clinical and laboratory information related to HLH criteria, in addition to neopterin levels, was recorded. A group of 50 patients with active juvenile dermatomyositis (JDM) (who routinely have neopterin levels assessed) served as controls for the assessment of the accuracy, sensitivity, and specificity of neopterin as a diagnostic test for HLH. The Pearson correlation was used to measure the association between serum neopterin levels and established HLH-related laboratory data. Serum neopterin levels were measured using a competitive enzyme immunoassay. During the time frame of the study, 3 patients with familial HLH and 18 patients with secondary HLH were identified as having had serum neopterin measured (all HLH patients were grouped together). The mean neopterin levels were 84.9 nmol/liter (standard deviation [SD], 83.4 nmol/liter) for patients with HLH and 21.5 nmol/liter (SD, 10.13 nmol/liter) for patients with JDM. A cutoff value of 38.9 nmol/liter was 70% sensitive and 95% specific for HLH. For HLH patients, neopterin levels correlated significantly with ferritin levels (r = 0.76, P = 0.0007). In comparison to the level in a control group of JDM patients, elevated serum neopterin was a sensitive and specific marker for HLH. Serum neopterin has value as a diagnostic marker of HLH, and prospective studies are under way to further evaluate its role as a marker for early diagnosis and management of patients.
INTRODUCTION
Hemophagocytic lymphohistiocytosis syndrome (HLH) is a rare clinical condition characterized by prolonged fevers in association with hepatosplenomegaly, cytopenias, coagulopathy, and central nervous system (CNS) manifestations. HLH results from a pathological activation of macrophages leading to hyperproduction of cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (9), that is believed to be the cause of many of the clinical symptoms. HLH is currently classified into a familial form, affecting primarily infants and young children, and a secondary form, which usually occurs in older children. The secondary form of HLH is associated with autoimmune disorders, infections, and malignancies. Macrophage activation syndrome (MAS) is a term that has been used by rheumatologists and refers to the secondary form of HLH seen in the context of rheumatic disorders (13). Familial HLH is an invariably fatal disease curable only with bone marrow transplant. MAS or the secondary form of HLH also has a relatively high mortality rate (8 to 22%) even if treated appropriately (16).
The current diagnostic and therapeutic guidelines were recently reviewed in the Histiocyte Society Treatment and Guideline Protocol (HLH-2004) (8). These diagnostic criteria, summarized in Table 1, do not distinguish between familial or secondary HLH. The diagnosis requires that five of the following eight criteria are met: fever, splenomegaly, cytopenias, hypertriglyceridemia and/or hypofibrinogenemia, hemophagocytosis in bone marrow, spleen, or lymph nodes with no evidence of malignancy, low or absent natural killer (NK) cell activity, hyperferritinemia, and elevated soluble CD25 (i.e., soluble interleukin 2 receptor alpha [sIL-2Rα]) (8). Other findings, such as liver dysfunction with elevated serum transaminases, coagulopathy, and neurological symptoms, are often seen in patients with HLH (2–4) but are not included in the current HLH-2004 diagnostic guidelines (6, 8).
Table 1.
Diagnostic guidelines for HLH based on the HLH-2004 protocola
| Guideline | Description |
|---|---|
| 1 | Molecular diagnosis consistent with HLH |
| 2 | Diagnostic criteria for HLH fulfilled (≥5 of the 8 criteria below) |
| (a) Fever | |
| (b) Splenomegaly | |
| (c) Cytopenias (affecting at least 2 of the following 3 parameters in the peripheral blood) | |
| Hemoglobin, <90 g/liter (<100 g/liter in infants <4 wk old) | |
| Platelets, <100 × 109/liter | |
| Neutrophils, <1.0 × 109/liter | |
| (d) Hypertriglyceridemia and/or hypofibrinogenemia | |
| Fasting triglycerides, ≥3.0 mmol/liter (≥265 mg/dl) | |
| Fibrinogen, ≤1.5 g/liter | |
| (e) Hemophagocytosis in bone marrow, spleen, lymph nodes, or cerebrospinal fluid; no evidence of malignancy | |
| (f) Low or absent NK cell activity (according to local laboratory reference) | |
| (g) Elevated ferritin (≥500 U/liter) | |
| (h) Soluble CD25 (i.e., soluble IL-2R) above normal limits for age |
The diagnosis of HLH can be established by one or both of the guidelines described in the table, which has been adapted from reference 8 with permission of the publisher.
Familial HLH is inherited in an autosomal recessive pattern. Three genes have been found to underlie more than 50% of the familial HLH cases worldwide: PRF1, encoding perforin, a major cytotoxic protein; UNC13D, encoding the MUNC 13-4 protein, which is involved in the exocytosis of perforin-bearing cytotoxic granules; and STX11, encoding the protein t-SNARE syntaxin 11 involved in vesicular transport (6, 12). HLH has been associated with other autosomal recessive immunodeficiencies, including Chediak-Higashi syndrome caused by mutations in the LYST gene, Griscelli syndrome type 2 caused by mutation of RAB27A (6, 12), Hermansky-Pudlak syndrome type 2 caused by mutations of AP3B1 (5), and X-linked lymphoproliferative syndrome (XLP) caused by mutations in the SH2D1A and XIAP genes (5, 6, 12, 19).
An early diagnosis is fundamental for the rapid initiation of aggressive treatment, such as cyclosporine, corticosteroids, and etoposide in patients with familial HLH and Epstein-Barr virus (EBV)-related HLH (11), where a rapidly fatal course has been observed before the introduction of protocol-based therapy. There is no standardized protocol for secondary forms of HLH or MAS, but the introduction of early high doses of corticosteroid and cyclosporine is essential in the therapeutic treatment of these patients (based on unpublished personal experiences of M.F.I. and M.K.-G.). Delayed diagnosis, multiorgan failure, and CNS involvement have all been suggested as poor prognostic factors for HLH (10, 11, 16). Early diagnosis is, however, very challenging due to the nonspecific nature of the clinical and laboratory criteria in the HLH guidelines. Many of these criteria are observed in patients with other conditions, such as sepsis, multisystem organ failure, autoimmune disorders, and malignancy (6). While soluble IL-2Rα and NK cell activity are important criteria, assays for these factors are neither specific nor sensitive for HLH and are performed only in a small number of specialized laboratories (11).
Neopterin is a product secreted by activated macrophages and dendritic cells and can be detected in serum and other fluids using routine immunoassay methods (3). It is known to be a marker of inflammation associated with cell-mediated immunity in various diseases (7, 14, 15, 18, 20). In our institution, we had observed that patients with a confirmed diagnosis of HLH had very high serum neopterin levels. The latter observation led us to begin measuring neopterin levels in patients suspected of a diagnosis of HLH. The subject of this report is an evaluation of the utility of serum neopterin as a diagnostic marker of HLH in a retrospective study.
MATERIALS AND METHODS
Study population.
Patients with MAS or HLH were identified retrospectively by extracting and reviewing all electronic medical records of patients seen at Children's Memorial Hospital (CMH) between January 2000 and May 2009 that satisfied the search strategy for both the appropriate diagnostic codes (ICD-9 code 288.4 for hemophagocytic syndromes or 277.89 for histiocytosis) and neopterin results. For consistency, the returned records were reviewed independently by M.F.I. and M.R.G.O. (both principal investigators) in order to identify those patients who satisfied the criteria for HLH or MAS. A comparison/control population of 50 untreated children with active definite/probable juvenile dermatomyositis (JDM), as defined by the criteria of Bohan and Peter (2), was chosen, since untreated patients routinely have neopterin measurements performed at diagnosis. Approval to perform this study was obtained from the Children's Memorial Research Center Institutional Review Board (IRB 2009-13784).
Data collection.
The serum neopterin levels used in the study were those obtained at the time that a diagnosis of HLH/MAS was suspected and at the time that the diagnosis of active untreated disease was confirmed in the JDM group. In all HLH patients, the following data (corresponding to the neopterin time point) were also collected: liver enzymes, complete blood count and differential, erythrocyte sedimentation rate, C-reactive protein, triglycerides, and coagulation panel, including prothrombin time, fibrinogen, and D-dimer. Serum neopterin levels were measured according to the manufacturer's instructions by competitive enzyme immunoassay, using <10 nmol/liter as a normal value (ALPCO diagnostic neopterin kit).
Statistical analysis.
Patient demographic and baseline characteristics were summarized by descriptive statistics. Area under the curve (AUC) based on logistic regression modeling was measured to assess the accuracy of neopterin as a diagnostic marker. Receiver operating characteristic (ROC) analysis was used to examine the tradeoffs in sensitivity and specificity at various diagnostic neopterin cutoff levels. The best possible cutoff would yield a point in the upper left corner or coordinate (0, 1) of the ROC space, representing 100% sensitivity (no false negatives) and 100% specificity (no false positives). The Pearson correlation (at the time of diagnosis) was used to determine the association between neopterin levels and the other HLH-associated laboratory parameters. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). The level of significance used was an α of 0.05.
RESULTS
During the study period, there were 3 patients with primary HLH (i.e., familial HLH) and 18 patient with secondary HLH, 12 with underlying rheumatic disease and 6 with no underlying gene mutation or underlying autoimmune disorder. Patients with familial HLH had mutations in the UNC13D, RAB27A, and SH2D1A genes. Patients with secondary HLH included seven with systemic juvenile idiopathic arthritis, two with systemic lupus erythematosus, one with mixed connective tissue disorder, one with undifferentiated juvenile arthritis, and one with sarcoidosis. Only two out of the six patients with no underlying disorder were tested for the three known HLH genes (known and available during the study period), and the results were negative for both patients (Table 2). We acknowledge that some of four remaining patients could have familial HLH, since sequencing for all appropriate genetic mutations was not done for all of them (and more genes were found to be associated with familial HLH after our study concluded). For this study, both familial and secondary HLH patients were grouped together: 47% were female (n = 11) with a mean age of 9.7 years (±6.01 years standard deviation [SD]). The mean age of the JDM control group was 7.5 years (±3.73 years), and 74% were female. The majority of patients in both groups were white (Table 3), which represents the demographic of our patient population more than an increased susceptibility in any particular ethnicity.
Table 2.
Clinical data for patients with HLHa
| Patient | Primary diagnosis | Neopterin level at diagnosis (nmol/liter) | Fever | Splenomegaly | Cytopenias | Increased triglycerides | Decreased fibrinogen | Increased ferritin | Increased D-dimer | Hemophagocytosis | sCD25 | Decreased NK cell activity | Gene mutationb | Stem cell transplant | Statusc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AHLH | 26.4 | + | + | + | + | − | + | + | + | + | + | − | + | A |
| 2 | AHLH | 39.7 | − | + | + | + | − | − | + | + | + | − | − | 0 | A |
| 3 | SJIA | 87 | + | + | + | + | + | + | + | NA | NA | NA | NA | 0 | A |
| 4 | SLE | 23.2 | + | + | − | NA | NA | + | NA | NA | NA | NA | NA | 0 | A |
| 5 | AHLH | 250 | + | − | + | + | + | + | + | + | − | + | − | 0 | A |
| 6 | XLP | 71.5 | + | + | + | + | − | + | + | + | + | − | SH2D1A | + | A |
| 7 | AHLH | 72 | + | + | + | + | + | + | NA | NA | + | + | − | + | A |
| 8 | SJIA | NA | + | + | + | NA | − | + | + | NA | + | NA | − | 0 | A |
| 9 | SJIA | 34.4 | − | − | + | NA | NA | NA | − | − | NA | NA | NA | 0 | A |
| 10 | SLE | 36 | + | − | + | + | − | + | + | NA | NA | NA | NA | 0 | A |
| 11 | SJIA | 86.2 | + | + | + | + | − | + | + | NA | NA | NA | NA | 0 | A |
| 12 | JIA | 34 | + | − | + | + | − | + | + | + | NA | NA | NA | 0 | A |
| 13 | SJIA | 48.2 | + | − | + | − | NA | + | NA | NA | NA | NA | NA | 0 | A |
| 14 | MCTD | 32.2 | + | − | − | + | NA | + | + | NA | NA | NA | NA | 0 | A |
| 15 | AHLH | 63.9 | + | + | + | + | − | + | NA | + | − | + | − | 0 | A |
| 16 | SJIA | 347.7 | + | + | + | + | + | NA | NA | − | NA | NA | NA | 0 | A |
| 17 | Sarcoid | 94.7 | + | + | + | + | − | + | + | + | NA | NA | − | 0 | D |
| 18 | SJIA | 96 | + | − | + | + | + | + | + | NA | NA | NA | NA | 0 | A |
| 19 | KD/FHLH | 98.5 | + | + | + | + | − | − | + | + | + | + | RAB27A | + | A |
| 20 | FHLH | 71.8 | + | − | + | + | − | + | + | NA | NA | NA | UNC13D | 0 | D |
| 21 | AHLH | 63.8 | + | − | + | NA | + | + | + | − | + | − | − | 0 | A |
Characteristics are indicated as present (+) or absent (−). Abbreviations: NA, not available; AHLH, acquired HLH; SJIA, systemic juvenile idiopathic arthritis; SLE, systemic lupus erythematous; XLP, X-linked lymphoproliferative syndrome; JIA, juvenile idiopathic arthritis; MCTD, mixed connective tissue disorder; KD, Kikuchi disease; FHLH, familial HLH.
The gene mutation is identified if present.
A, alive; D, dead.
Table 3.
Demographics of patients with HLH versus JDM
| Parameter | Value for patients with: |
P value | |
|---|---|---|---|
| HLH | JDM | ||
| Total no. of patients | 21 | 50 | |
| Gender [no. (%) of patients] | 0.08 | ||
| Female | 11 (52.38) | 37 (74) | |
| Male | 10 (47.62) | 13 (26) | |
| Race/ethnicity [no. (%) of patients] | 0.042 | ||
| White | 13 (61.90) | 36 (72) | |
| African-American | 4 (19.05) | 1 (2) | |
| Latin-American | 2 (9.52) | 10 (20) | |
| Asian | 1 (4.76) | 2 (4) | |
| Other/unknown/none | 1 (4.76) | 1 (2) | |
| Age (yr) | 0.1 | ||
| Mean | 9.5 | 7.55 | |
| SD | 6.01 | 3.73 | |
| Range | 0.36–17.98 | 2.57–16.41 | |
The patients with familial HLH were treated with a combined steroid/immunosuppression protocol, followed by stem cell transplant, with the exception of one child who had UNC13D mutations and who died prior to transplant. Patients with HLH and a rheumatic condition were treated with corticosteroids and cyclosporine and went into remission, except for the patient with sarcoidosis, who died secondary to multiorgan failure. Two of the six patients with no known underlying disorder underwent stem cell transplant due to lack of control of HLH symptoms following immunosuppressant medication.
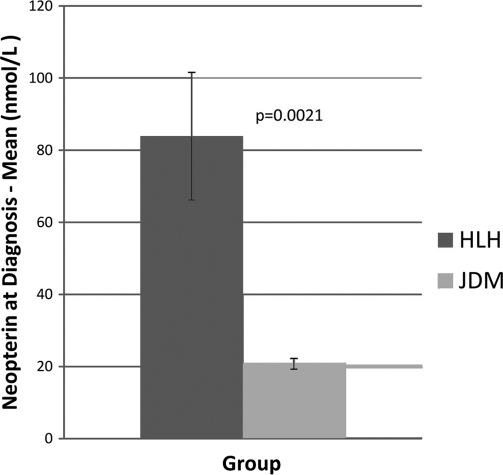
Serum neopterin levels in the HLH group were significantly higher than those observed in the JDM control group (mean, 83.9 nmol/liter versus 20.8 nmol/liter, respectively) (Fig. 1). The minimum level of serum neopterin in the HLH group was 23.2 nmol/liter, and the maximum level was 347.7 nmol/liter. In comparison, the minimum level in the JDM group was 5.7 nmol/liter, and the maximum level was 43 nmol/liter. In the HLH patients, there were no significant associations with any of the HLH laboratory criteria (data not shown) other than the level of ferritin, which showed a significant correlation with the neopterin level at the time of diagnosis (P = 0.0007, r = 0.76, n = 16).
Fig. 1.
Serum neopterin levels in the HLH group at the time of diagnosis were significantly higher than those observed in the JDM control group.
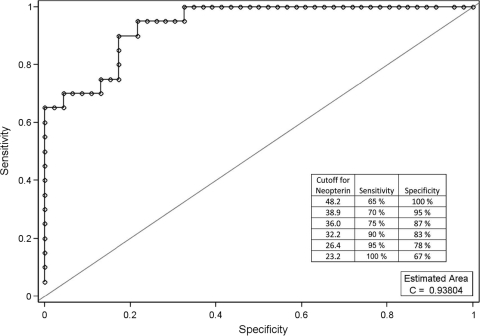
A nonparametric ROC curve using the serum neopterin level at the time of initial diagnosis of HLH was generated. The AUC for serum neopterin levels was 0.93804 (95% confidence interval, 0.86 to 1.00). The ROC analyses indicated that a cutoff value of 38.9 nmol/liter was 70% sensitive and 95% specific for HLH (Fig. 1). At the time of diagnosis (according to the standard criteria [8]), the accuracy of serum neopterin levels ranged from 86% to 100%. The sensitivity and specificity measured at different neopterin cutoff levels ranged from 65% and 100%, respectively, at a cutoff of 48.2 nmol/liter to 100% and 67%, respectively, at the lower cutoff level of 23.2 nmol/liter (summarized in Fig. 2). A prospective study is required to more accurately define a neopterin level appropriate for clinical applications.
Fig. 2.
Using a cutoff value of ≥38.9 nmol/liter for neopterin for HIH/MAS versus JDM patients gives a sensitivity of 70% and a specificity of 95%. AUC, 0.93804; 95% confidence interval, 0.86-1.00).
DISCUSSION
HLH is an aggressive and potential fatal syndrome with significant challenges related to both its diagnosis and treatment. It is characterized by an uncontrolled macrophage activation leading to hyperproduction of cytokines. Neopterin is a product of activated human monocyte-derived macrophages and dendritic cells.
Chemically, neopterin is an unconjugated pteridine, which is synthesized from GTP through the GTP cyclohydrolase I pathway. GTP cyclohydrolase I can be induced by IFN-γ in various cells. Neopterin has been reported as a sensitive marker of cell-mediated immune activity (3). Elevated plasma levels of neopterin have been detected in association with cancers (18), during sepsis (3) and numerous infections, including febrile neutropenia (15) and human immunodeficiency virus infection (14), hepatitis B-related chronic liver disease (7), and atherosclerosis (20). Serum neopterin levels have also been reported to correlate with disease activity in JDM patients (4). In addition, Shimizu et al. (17) recently found that serum neopterin concentrations in EBV-related HLH patients were significantly higher than those in patients with MAS/systemic juvenile idiopathic arthritis or Kawasaki disease. The purpose of our study was to evaluate the utility of neopterin as a potential diagnostic marker of HLH. We compared the levels observed in patients with HLH to levels observed in patients with active JDM. In addition, we measured the sensitivity and specificity of serum neopterin levels in the diagnosis of HLH and evaluated the association of neopterin levels with the laboratory parameters established for the diagnostic criteria.
In this study, the patients diagnosed with HLH had a mean neopterin level that was eight times higher than the cutoff value for normal and four times higher than the levels observed in the control group of patients with active JDM. Neopterin levels greater than 38.9 nmol/liter were very specific (95%) and relatively sensitive (70%) for a diagnosis of HLH when the JDM group was used as the comparator. Serum neopterin levels correlated significantly with serum ferritin levels at the time of diagnosis in our HLH patients. Studies have supported that hyperferritinemia, to the extent observed in HLH patients, is a typical characteristic and part of the diagnosis guidelines for HLH. An elevated plasma ferritin level above 500 μg/liter is a criterion for HLH diagnosis (Table 1). It is also suggested that ferritin levels above 10,000 μg/liter appear to be more specific and sensitive for an HLH diagnosis (1). However, there were 2 patients (out of 21 patients) who did not have elevated levels of ferritin at the time of diagnosis. In both patients, neopterin levels were significantly increased (98.5 and 39.7 nmol/liter).
Mutations in PRF1, STX11, and UNC13D are important in the diagnosis of familial HLH, but these mutations occur in only 50% of familial HLH. Soluble IL-2 receptor alpha (sIL-2Rα) levels are relatively sensitive and specific for HLH (1) but are not widely available and could not be compared to neopterin levels in our group since sIL-2Rα levels were not measured in all patients. We have established that neopterin levels are significantly elevated in patients with HLH. Prospective studies are being planned to establish the role of neopterin measurements as a criterion in the diagnosis and management of HLH.
As a retrospective review, this study has limitations, some of which have already been addressed. The control group included only JDM patients, which was the only patient group available that routinely had serum neopterin levels measured as an assessment of their disease activity. Elevated serum neopterin levels have been observed with posttraumatic complications among patients with multiple injuries, viral and bacterial infections, and malignancies (3, 7, 14, 15, 18, 20), and no patients with these complications were included in our study. Therefore, a prospective study should be designed to include other patient populations. We also must acknowledge that our patient sample may not represent all of the patients with HLH/MAS seen at CMH during the defined study period, since the patients were identified retrospectively by extraction of specific ICD-9 codes.
In summary, we show that serum neopterin levels are significantly elevated in patients with HLH compared to normal ranges in healthy controls and levels in patients with active JDM. In comparison with levels in active JDM patients, we observed that levels of serum neopterin of >39.8 nmol/liter in patients suspected of HLH are very specific and sensitive for the diagnosis of HLH. We also demonstrated that the elevation of serum neopterin levels in patients with HLH was often very significant (one patient had levels more than 30 times the normal cutoff level), and although levels correlated with ferritin levels (hyperferritinemia), the neopterin levels in two HLH patients were significantly elevated in the absence of elevated (>500 U/liter) ferritin. This demonstrates that serum neopterin measurements warrant further evaluation as a diagnostic parameter for HLH. In addition to a diagnostic marker, the role of serial neopterin measurements in the management of HLH should be evaluated.
ACKNOWLEDGMENTS
This work was supported by NIH/NIAMS R01 AR48289, the Cure JM Foundation, and the Macy's Miracle Foundation (L.M.P.).
Footnotes
Published ahead of print on 26 January 2011.
REFERENCES
- 1. Allen C. E., Yu X., Kozinetz C. A., McClain K. L. 2008. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 50:1227–1235 [DOI] [PubMed] [Google Scholar]
- 2. Bohan A., Peter J. B. 1975. Polymyositis and dermatomyositis. N. Engl. J. Med. 292:344–403 [DOI] [PubMed] [Google Scholar]
- 3. Castillo L., Carcillo J. 2009. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr. Crit. Care Med. 10:387–392 [DOI] [PubMed] [Google Scholar]
- 4. De Benedetti F., De Amici M., Aramini L., Ruperto N., Martini A. 1993. Correlation of serum neopterin concentrations with disease activity in juvenile dermatomyositis. Arch. Dis. Child. 2:232–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Enders A., et al. 2006. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood 108:81–87 [DOI] [PubMed] [Google Scholar]
- 6. Filipovich A. H. 2006. Hemophagocytic lymphohistiocytosis and related disorders. Curr. Opin. Allergy Clin. Immunol. 6:410–415 [DOI] [PubMed] [Google Scholar]
- 7. Gulcan E. M., Tirit I., Anil A., Adal E., Ozbay G. 2008. Serum neopterin levels in children with hepatitis-B-related chronic liver disease and its relationship to disease severity. World J. Gastroenterol. 14(44):6840–6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henter J.-I., et al. 2007. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 48(2):124–136 [DOI] [PubMed] [Google Scholar]
- 9. Humber C., et al. 1984. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J. Exp. Med. 160:310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imashuku S., et al. 2002. Low natural killer activity and central nervous system disease as a high-risk prognostic indicator for young patients with hemophagocytic lymphohistiocytosis (HLH). Cancer 94:3023–3031 [DOI] [PubMed] [Google Scholar]
- 11. Imashuku S., et al. 2001. Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J. Clin. Oncol. 19(10):2665–2673 [DOI] [PubMed] [Google Scholar]
- 12. Janka G. E. 2007. Familial and acquired hemophagocytic lymphohystiocytosis. Eur. J. Pediat. 166:95–109 [DOI] [PubMed] [Google Scholar]
- 13. Kelly A., Ramanan A. V. 2007. Recognition and management of macrophage activation syndrome in juvenile arthritis. Curr. Opin. Rheumatol. 19:477–481 [DOI] [PubMed] [Google Scholar]
- 14. Mildvan D., et al. 2005. Serum neopterin, an immune activation marker, independently predicts disease progression in advanced HIV-1 infection. Clin. Infect. Dis. 40:476–479 [DOI] [PubMed] [Google Scholar]
- 15. Prat C., et al. 2008. Evaluation of procalcitonin, neopterin, C-reactive protein, IL-6 and IL-8 as a diagnostic marker of infection in patients with febrile neutropenia. Leuk. Lymphoma 49:1752–1761 [DOI] [PubMed] [Google Scholar]
- 16. Sawhney S., Woo P., Murray K. J. 2001. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch. Dis. Child. 85:421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shimizu M., et al. 14 May 2010, posting date Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology. doi: 10.1093/rheumatology/keq133 [DOI] [PubMed]
- 18. Sucher R., et al. 2010. Neopterin, a prognostic marker in human malignancies. Cancer Lett. 287:13–22 [DOI] [PubMed] [Google Scholar]
- 19. Sumegi J., Johnson J., Filipovich A., Zhan K., Marsh R. 27 February 2004, posting date Lymphoproliferative disease, X-linked. GeneReviews. http://www.ncbi.nlm.nih.gov/books/NBK1406/
- 20. Tatzber F., et al. 1991. Elevated serum neopterin levels in atherosclerosis. Atherosclerosis 2-3:203–208 [DOI] [PubMed] [Google Scholar]