Abstract
The emergence of the swine-origin 2009 influenza pandemic illustrates the need for improved vaccine production and delivery strategies. Skin-based immunization represents an attractive alternative to traditional hypodermic needle vaccination routes. Microneedles (MNs) can deliver vaccine to the epidermis and dermis, which are rich in antigen-presenting cells (APC) such as Langerhans cells and dermal dendritic cells. Previous studies using coated or dissolvable microneedles emphasized the use of inactivated influenza virus or virus-like particles as skin-based vaccines. However, most currently available influenza vaccines consist of solubilized viral protein antigens. Here we test the hypothesis that a recombinant subunit influenza vaccine can be delivered to the skin by coated microneedles and can induce protective immunity. We found that mice vaccinated via MN delivery with a stabilized recombinant trimeric soluble hemagglutinin (sHA) derived from A/Aichi/2/68 (H3) virus had significantly higher immune responses than did mice vaccinated with unmodified sHA. These mice were fully protected against a lethal challenge with influenza virus. Analysis of postchallenge lung titers showed that MN-immunized mice had completely cleared the virus from their lungs, in contrast to mice given the same vaccine by a standard subcutaneous route. In addition, we observed a higher ratio of antigen-specific Th1 cells in trimeric sHA-vaccinated mice and a greater mucosal antibody response. Our data therefore demonstrate the improved efficacy of a skin-based recombinant subunit influenza vaccine and emphasize the advantage of this route of vaccination for a protein subunit vaccine.
INTRODUCTION
The skin acts as a mechanical barrier against the environment and provides the first line of defense against pathogens. The skin-associated lymphoid tissue (SALT), which was first described by Streilein (34), represents an ideal target for skin-based vaccinations because it contains keratinocytes, Langerhans cells (LC), dermal dendritic cells (DDC), and T cells. Skin-associated antigen-presenting cells (APC) and keratinocytes have been shown to express several pattern recognition receptors (PRRs), including TLR9, TLR2, and TLR3 (19, 20), which are important enhancers of the immune response. The LC and DDC present in the epidermis and dermis, respectively, have been shown to take up antigen, migrate to draining lymph nodes, and induce an antigen-specific adaptive immune response (36). Therefore, targeting vaccine to the skin has been shown to enhance immunogenicity (2, 3, 15, 21).
The 2009 swine-origin influenza pandemic illustrates the need for rapid and effective vaccination. Skin-based influenza vaccines have utilized approaches including tape stripping (32), epidermal powder immunization (4), and microneedles (MNs) (9). These strategies have used diverse antigens including virus-like particles (VLP) (25), inactivated influenza virus (24), and hemagglutinin (HA) DNA vaccines (1). However, a recombinant HA subunit vaccine, which has the advantage of rapid, high-yield production in an expression system with a high level of purity, has not been evaluated.
Microneedle arrays are designed to penetrate the stratum corneum, the outer layer of the skin, and deposit vaccine or drug into the epidermis and dermis. Using this approach, vaccine is applied as a coating to the surface of metal microneedles or encapsulated in a polymer making up the microneedle (22). Delivery of soluble protein via coated microneedles suggests that antigen can be delivered quickly into the skin. Furthermore, this immunization method generated an antigen-specific antibody response that was superior to those induced by subcutaneous (s.c.) and intramuscular (i.m.) routes (17, 18, 35, 41).
We previously demonstrated that a modified form of soluble HA (sHA) derived from the H3N2 influenza virus A/Aichi/2/68 containing the GCN4pII trimerization repeat stabilized the trimeric structure of the HA protein (20). In the current study, we tested the hypothesis that MN delivery of the recombinant vaccine would induce levels of protective immune responses superior or at least equivalent to those induced by subcutaneous immunization. Specifically, we investigated the efficacy of skin delivery of stabilized trimeric influenza virus HA from the H3 virus A/Aichi/2/68 via coated microneedles. In addition we determined whether the stabilized trimeric sHA microneedle vaccination induces improved humoral and cellular responses compared with those induced by s.c. immunization. To compare the effects of immunization on postchallenge virus clearance, we determined virus lung titers after challenge infection. The work presented here illustrates the first analysis of transdermal delivery of a recombinant influenza virus subunit HA vaccine using microneedle technology.
MATERIALS AND METHODS
Recombinant trimeric soluble influenza virus hemagglutinin (sHA).
The HA gene derived from the H3N2 influenza virus A/Aichi/2/68 was truncated, and the trimeric GCN4pII sequence from Saccharomyces cerevisiae, encoding the trimerization motif, was fused to the C terminus and cloned into the recombinant baculovirus (rBV) pFastBac1 expression vector as previously described (37). rBVs carrying genes for the sHA and sHA.GCN4pII proteins were generated, and recombinant proteins were expressed and purified as previously described (37). For purification, a His tag was added to the C terminus of each protein construct.
Microneedle fabrication and coating.
Microneedles were fabricated from stainless steel sheets (Trinity Brand Industries, Georgia; SS 304; 50 μm thick) by wet etching. Individual microneedles had a length of 750 μm and a width of 200 μm.
The coating solution was composed of 1% (wt/vol) carboxymethyl cellulose sodium salt (low viscosity, USP grade; Carbo-Mer, San Diego, CA), 0.5% (wt/vol) Lutrol F-68 NF (BASF, Mt. Olive, NJ), and soluble HA protein at 5 mg/ml. In order to reach a high vaccine concentration in coating solution, we used evaporation for 5 to 10 min at room temperature (+23°C) at the final step of preparation (Vacufuge; Eppendorf, New York). The coating step was performed by a dip coating process (12). The apparatus had a chamber with coating solution and a microneedle holder which was attached to a linear stage that allowed the microneedle array to move in two dimensions with 0.4-μm accuracy. The coating was performed automatically and was monitored by a video camera (Prosilica, Massachusetts) attached to a computer.
To measure the amount of vaccine applied as a coating per row of microneedles, three rows out of each batch of coated microneedles were each submerged into 200 μl of phosphate-buffered saline (PBS) buffer for 5 min. The concentration of protein in the solution was measured by bicinchoninic acid (BCA) protein assay and was consistent within each batch (Pierce, Rockford, IL).
BS3 cross-linking.
The oligomeric status of purified recombinant proteins was determined using the water-soluble BS3 (bis[sulfosuccinimidyl] suberate) cross-linker (Pierce, Rockford, IL). Cross-linking was performed as described by De Fillette et al. (8), with the following modifications. Briefly, 1 μg of recombinant protein was incubated at room temperature in the presence of BS3 (final concentration, 3 mM) for 30 min. Cross-linking was stopped by the addition of 1 M Tris-HCl, pH 8.0, to a final concentration of 50 mM. After cross-linking, proteins were separated on a 5 to 15% SDS-PAGE gel under reducing conditions (1% mercaptoethanol) and then blotted and analyzed by Western blotting using anti-six-His antibody and developed using ECL-Plus.
Vaccinations.
Female BALB/c mice (6 to 8 weeks old) were anesthetized with a xylazine-ketamine cocktail intraperitoneally (i.p.), and hair on the lower back was removed with a hair-removal cream (Nair; Church & Dwight Co.) 2 days prior to microneedle vaccination. Mice were anesthetized again, and microneedle arrays were inserted into the skin on days 0 (prime) and 28 (boost) and left in place for 5 min to allow the vaccine coating to dissolve.
Tissue and mucosal secretion collection.
Vaginal washes were collected as previously described (7). For lung homogenates, mice were anesthetized with a xylazine-ketamine cocktail and perfused with sterile PBS to remove circulating blood. Lung tissue was removed, stored in PBS with a protease cocktail (Thermo Scientific, Rockford, IL), and homogenized.
Virus and challenge.
To determine vaccine efficacy, vaccinated mice were lightly anesthetized with isoflurane and challenged by slow intranasal inoculation of 50 μl containing 5 50% lethal doses (LD50) of live-mouse-adapted A/Aichi/2/68 (H3N2). Body weight loss and survival rates were monitored daily for 14 days postchallenge. Weight loss of ≥25% was used as the endpoint at which mice were euthanized according to IACUC guidelines.
Viral lung titers.
Mouse lungs were collected on day 4 postchallenge as previously described (17). MDCK cells were maintained at a low passage number in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (HyClone, ThermoFisher, Rockford, IL). Plaque assays were performed on lung homogenates from challenged mice as previously described (31).
ELISA.
IgG enzyme-linked immunosorbent assay (ELISA) was performed on serum and lung homogenates as previously described (17). All horseradish peroxidase (HRP)-conjugated secondary antibodies to mouse IgG, IgG1, IgG2a, IgG2b, and IgG3 were purchased from Southern Biotechnology Associates (Birmingham, AL). Briefly, 96-well immunoplates (Nunc Co., Rochester, NY) were coated overnight at 4°C with 4 μg of inactivated A/Aichi/2/68 virus per well. Plates were washed with PBS-Tween (0.05%) and blocked with PBS-Tween supplemented with 3% bovine serum albumin (BSA). Sera were diluted 1:100 and incubated for 1.5 h at 37°C. Plates were washed 3 times with PBS-Tween (0.05%) and incubated for 1.5 h at 37°C in a 1:1,000 dilution of goat, anti-mouse HRP-conjugated secondary antibody (Southern Biotechnology, Birmingham, AL). Plates were washed 3 times with PBS-Tween (0.05%), o-phenylenediamine (OPD) substrate (Invitrogen, Carlsbad, CA) was added to each well, and color was allowed to develop. Color development was stopped using 1 M phosphoric acid. Absorbances were read at 450 nm on a Bio-Rad Model 680 microplate reader. Immunoglobulin concentrations were determined by linear regression of a standard curve of known concentrations.
The IgA ELISA procedure was modified from the work of Rodriguez et al. (28). Briefly, 96-well immunoplates (Nunc Co., Rochester, NY) were coated as described above and blocked with 1% BSA. Vaginal washes were diluted 1:5, and sera were diluted 1:100 and then incubated overnight at 4°C. A 1:500 dilution of biotin-conjugated rat anti-mouse IgA was used, and streptavidin-HRP followed by OPD substrate was used as a detection method (obtained from BD Pharmingen, San Jose, CA).
HAI and microneutralization.
Hemagglutination inhibition (HAI) tests were performed on vaccinated animal sera based on the WHO protocol (38). Briefly, sera were treated with receptor-destroying enzyme (Denka Seiken Co. Ltd., Tokyo, Japan) for 16 h at 37°C and then heat inactivated for 30 min at 56°C. Treated sera were diluted to a final concentration of 1:10 in PBS and incubated with packed chicken erythrocytes (RBC) for 1 h at 4°C to remove cryoglobulins. Treated sera were serially diluted and incubated with 4 HA units of A/Aichi/2/68 virus for 30 min at room temperature. An equal volume of 0.5% chicken RBC was added to each well and incubated for 30 min at room temperature. The HAI titer was read as the reciprocal of the highest dilution of serum that inhibited hemagglutination. Values were expressed as the geometric mean with a 95% confidence interval.
The microneutralization assay was performed as described by Rowe et al. (29) with modifications. Briefly, mouse sera were heat inactivated for 30 min at 56°C and serially diluted in virus diluent (DMEM plus 1% BSA) in a 96-well tissue culture plate. Virus (200 50% tissue culture infective doses [TCID50]) was added to diluted serum in virus diluent supplemented with 2 μg/ml of tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin and incubated for 2 h at 37°C. Freshly trypsinized MDCK cells were added to all wells and incubated overnight at 37°C. Cells were then fixed in 80% acetone-PBS and washed 3 times with PBS-Tween (0.05% Tween 20). A 1:2,000 dilution of biotin-conjugated anti-influenza A virus nucleoprotein (clone A3) (Milipore, Billerica, MA) and streptavidin-HRP was added for the detection of infected cells. For each plate, the 50% specific signal value was calculated as follows: [(average of virus-only wells) − (average of cell-only well)]/2 + average of cell-only well. The 50% endpoint neutralization titer is reported as the last dilution to score below the 50% specific signal value.
Cellular immune responses.
Single-cell suspensions were prepared from spleens of mice 14 days postvaccination or from those of naïve mice, by mincing the tissue through a 70-μm cell strainer (BD Falcon), followed by incubation in red blood cell lysing buffer (Sigma) to remove red blood cells. CD4+ T cells were purified by negative selection using BD iMag magnetic cell separation (BD Biosciences, San Jose, CA). Naïve splenocytes were treated with mitomycin C for 30 min and used as accessory cells after incubation with 20, 5, or 0 μg of vaccine in complete RPMI medium overnight at 37°C in a 5% CO2 incubator. Purified CD4+ T cells were added to accessory cells at a ratio of 2:1 (responder/accessory cells) in complete RPMI medium supplemented with 30 U of recombinant human interleukin-2 (IL-2; BD Biosciences, San Jose, CA). In vitro antigen-specific stimulation was measured after incubation for 5 days by intracellular cytokine staining.
Antibodies and flow cytometry.
Cells were washed with PBS-1% BSA buffer and surface stained with fluorochrome-conjugated antibodies to CD4 and CD3, followed by intracellular staining of gamma interferon (IFN-γ) and IL-4. Antibodies were purchased from eBiosciences and BD Biosciences. For intracellular cytokine staining, cells were fixed and permeabilized using the BD Cytofix/Cytoperm manufacturer's protocol and reagents (BD Biosciences, San Jose, CA). The data were acquired on a BD LSR-II flow cytometer and analyzed with FlowJo Software (Tree Star, Inc.; v7.6.1).
Statistics.
Statistical analysis was done using the one-way analysis of variance (ANOVA) of grouped data with GraphPad Prism5 software. Survival curves were analyzed using the log rank test. All data followed normal Gaussian distributions unless otherwise noted. Viral lung titers were analyzed using one-way ANOVA. A P value of less than 0.05 was defined as a significant difference.
RESULTS
Trehalose stabilizes the trimeric structure of sHA.GCN4pII.
The GCN4pII trimerization repeat has been used to investigate the immunogenicity and protein structure for several viral proteins, including HIV gp120/41, parainfluenza virus 5 F protein, and influenza virus HA (6, 39, 40). We have previously demonstrated that modification of the sHA at the C terminus with the GCN4pII trimerization repeat generates a stabilized trimeric sHA (37). Because the trimeric structure was important for enhanced immunogenicity, we determined if this structure of sHA.GCN4pII was disrupted by the microneedle coating process. We coated solid metal microneedle arrays with 3 μg of recombinant protein in the presence or absence of 15% trehalose, allowed the coating to dry, and then redissolved the vaccine coating. As demonstrated by SDS gel electrophoresis, when redissolved samples were analyzed after chemical cross-linking, the sHA.GCN4pII protein dissociated into dimeric and monomeric species (Fig. 1, lane 3). However, the trimeric structure is maintained when 15% trehalose is added to the coating buffer (Fig. 1, lane 4). In contrasts, the unmodified sHA remains a mixture of trimers, dimers, and monomers independent of the presence of trehalose (Fig. 1, lanes 1 and 2). These data suggest that the trimeric sHA protein structure was disrupted during the microneedle coating and drying process; however, the structure was preserved by the addition of trehalose to the coating solution.
Fig. 1.
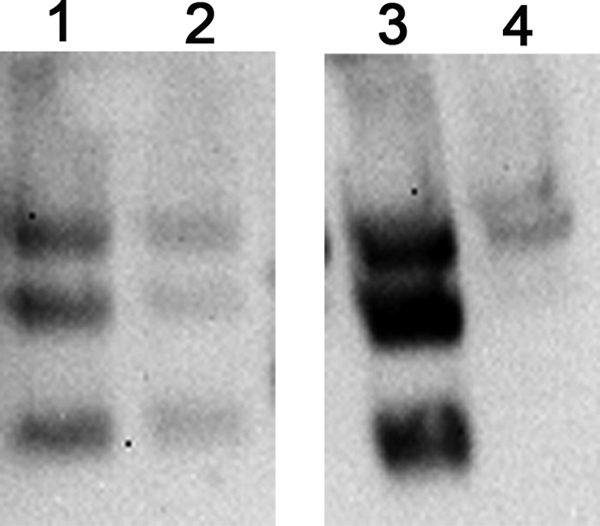
Trehalose-supplemented microneedle coating solution preserves the sHA.GCN4pII trimeric structure. BS3 cross-linking of recombinant baculovirus produced sHA and sHA.GCN4pII after dissolution of the coating from the microneedles coated in the presence or absence of 15% trehalose. Lane 1, sHA without 15% trehalose; lane 2, sHA with 15% trehalose; lane 3, sHA.GCN4pII without 15% trehalose; lane 4, sHA.GCN4pII with 15% trehalose. Cross-linked proteins were separated on 5 to 15% SDS-PAGE gels, and Western blotting was performed using mouse anti-His primary antibody.
Microneedle delivery of sHA.GCN4pII induces antigen-specific humoral responses.
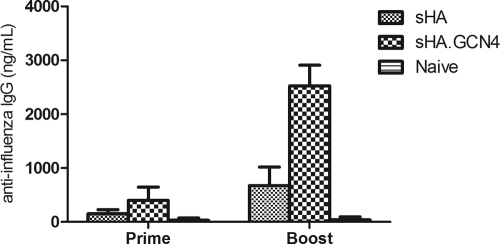
To test the immunogenicity of recombinant soluble HA delivered to the skin by coated microneedles, female BALB/c mice (6 to 8 weeks old) were vaccinated with 3 μg of sHA or sHA.GCN4pII on day 0 and boosted on day 28. After priming, mice vaccinated with sHA.GCN4pII had approximately 2.6-fold-higher A/Aichi/2/68-specific serum IgG titers than did mice boosted with sHA (P = 0.0032), indicating the enhanced immunogenicity of the HA when in its trimeric native form. In addition, 28 days later, boosting with sHA.GCN4pII induced approximately 3.7-fold-higher A/Aichi/2/68-specific serum IgG titers than did boosting with sHA (P < 0.0001) (Fig. 2).
Fig. 2.
Microneedle vaccination with sHA.GCN4pII induces higher serum levels of A/Aichi/2/68-specific IgG. Mice were primed and boosted 3 weeks later with 3 μg of sHA or sHA.GCN4pII or blank microneedles (naïve). Blood was collected 28 days after priming (prime) and 28 days after boosting (boost). ELISA plates were coated with 4 μg/ml of A/Aichi/2/68 virus, and antigen-specific serum IgG for prime and boost was measured by ELISA. HRP-conjugated goat anti-mouse IgG was used for detection (n = 12).
The influenza virus-specific IgG subtype profile in the serum of MN-vaccinated mice indicates that sHA.GCN4pII induced higher IgG1 (1.4-fold, P < 0.0001), IgG2a (2.2-fold, P = 0.0053), and IgG2b (2-fold, P = 0.0148) levels than did the use of sHA or uncoated microneedles (Fig. 3). No significant differences were found in the proportions of IgG1 to IgG2a/b between the groups, which indicates that the nature of the antigen did not influence the expression of the IgG subtype. There was no detectable antigen-specific IgG3 in either vaccinated group (data not shown). The serum immunoglobulin levels indicate that the transdermal delivery of recombinant trimeric sHA induces a robust IgG response and enhances the levels of IgG2a, IgG2b, and IgG1 subtypes over those in sHA-vaccinated mice.
Fig. 3.
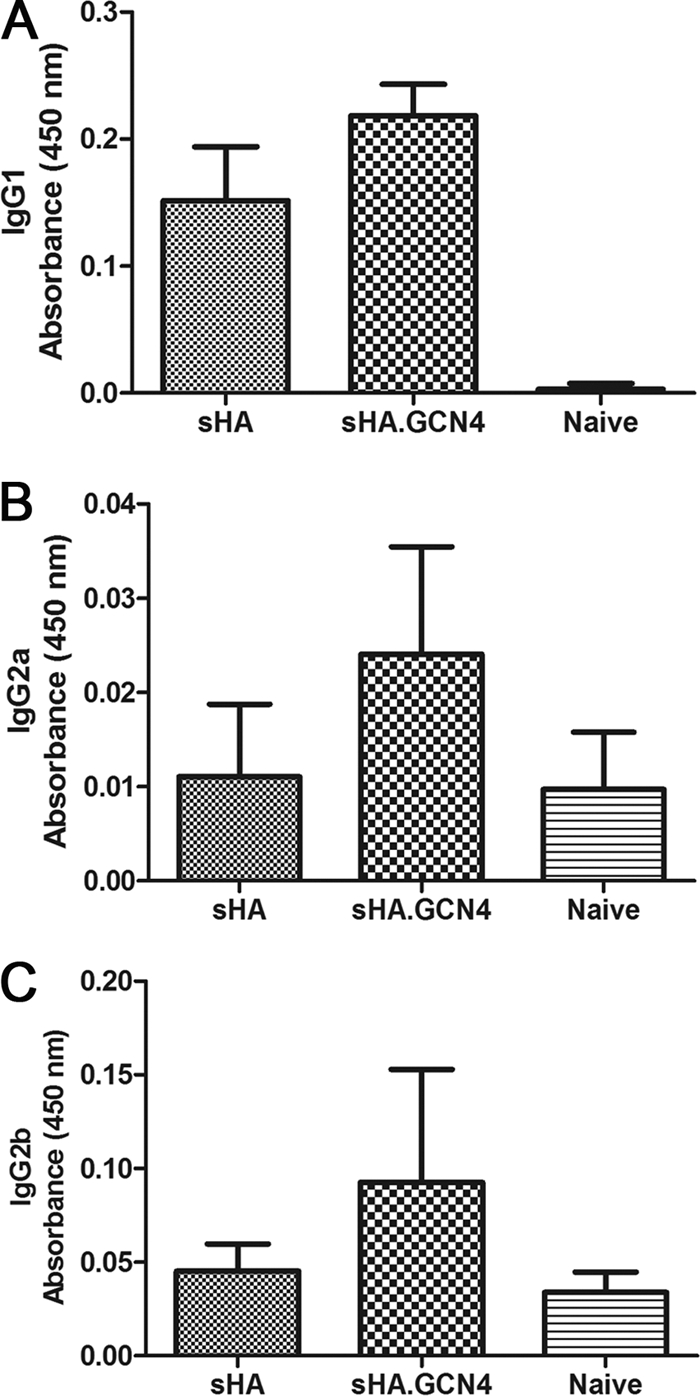
IgG subtype as determined by ELISA. Sera collected 21 days after boosting with antigen-coated microneedles were tested for antigen-specific IgG subclasses by ELISA as described in Materials and Methods. ELISA plates were coated with 4 μg/ml of A/Aichi/2/68, and antigen-specific IgG subclasses were detected using HRP-conjugated goat anti-mouse IgG1 (A), IgG2a (B), or IgG2b (C) (n = 6 per group).
sHA.GCN4pII induces improved HAI and neutralizing antibody responses.
The HAI test is used to measure antibodies which bind to the HA receptor binding domain, blocking binding of HA to sialic acid receptors. In general, an HAI titer of ≥40 is correlated with vaccine-induced protection in humans (13). Therefore, serum from microneedle-vaccinated mice was tested for HAI titers. At 21 days after priming, no detectable HAI titer was observed in the vaccinated mice. However, after boosting, the sHA.GCN4pII-vaccinated mice had an HAI titer of 190, while sHA-vaccinated mice had no detectable HAI titer (P < 0.0001) (Fig. 4 A). We previously observed similar enhancement of HAI titers when the stabilized trimeric HA was administered by subcutaneous immunization (37).
Fig. 4.
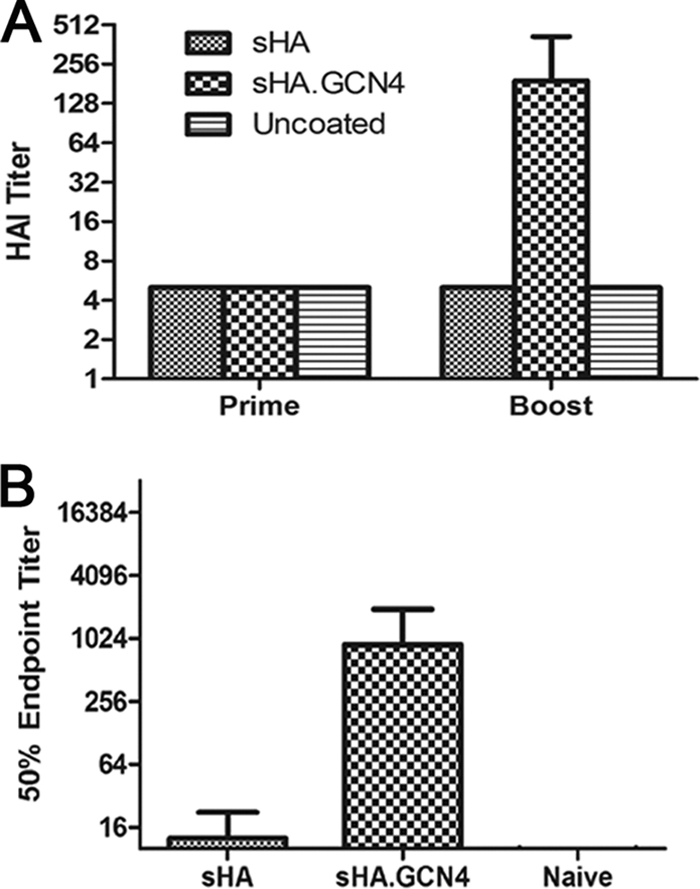
Transdermal vaccination with sHA.GCN4pII induces higher HAI and microneutralization titers. (A) Hemagglutination inhibition testing of prime and boost sera was performed as described in Materials and Methods using MDCK cell-grown A/Aichi/2/68 virus. HAI titers were reported as the reciprocal of the last dilution of serum to inhibit agglutination of RBC. (B) A microneutralization assay using boost serum was performed as described in Materials and Methods with 200 TCID50 of MDCK cell-grown A/Aichi/2/68. Virus infection was detected using biotin-conjugated antinucleoprotein monoclonal antibody and streptavidin-HRP. Fifty percent endpoint titers were reported as the dilution of serum able to inhibit 50% of signal observed in virus-infected MDCK cells.
In order to measure the ability of serum antibodies to neutralize virus infectivity, we performed a microneutralization assay. sHA.GCN4pII-vaccinated mice had an approximately 70-fold-higher neutralization endpoint titer than did mice vaccinated with sHA (P < 0.0001) (Fig. 4B). Therefore, MN vaccination with the sHA.GCN4pII protein induced high levels of functional antibodies but similar immunization with the sHA protein did not induce such responses.
Microneedle vaccination with sHA.GCN4pII induces a robust antibody response at mucosal sites.
Influenza virus is a respiratory pathogen that infects the airway epithelia. A strong mucosal antibody response has been associated with prevention of viral pathology in the upper respiratory tract of mice (27). Previous studies using intradermal or transdermal influenza vaccinations have investigated the induction of secretory IgA (sIgA) at mucosal sites (32). Therefore, to determine the induction of secretory IgA by microneedle vaccination with recombinant soluble HA, we measured sIgA in vaginal washes by ELISA. On day 28 after boosting, sHA.GCN4pII-vaccinated mice had approximately 6.7-fold-higher sIgA levels than did mice vaccinated with sHA (P = 0.0105) (Fig. 5 A). When serum IgA levels were measured, we found that the levels in the sHA.GCN4pII microneedle-vaccinated mice were approximately 3.3-fold higher than those in the sHA-vaccinated mice (P < 0.0001), correlating with the higher mucosal IgA levels that we observed (Fig. 5B).
Fig. 5.
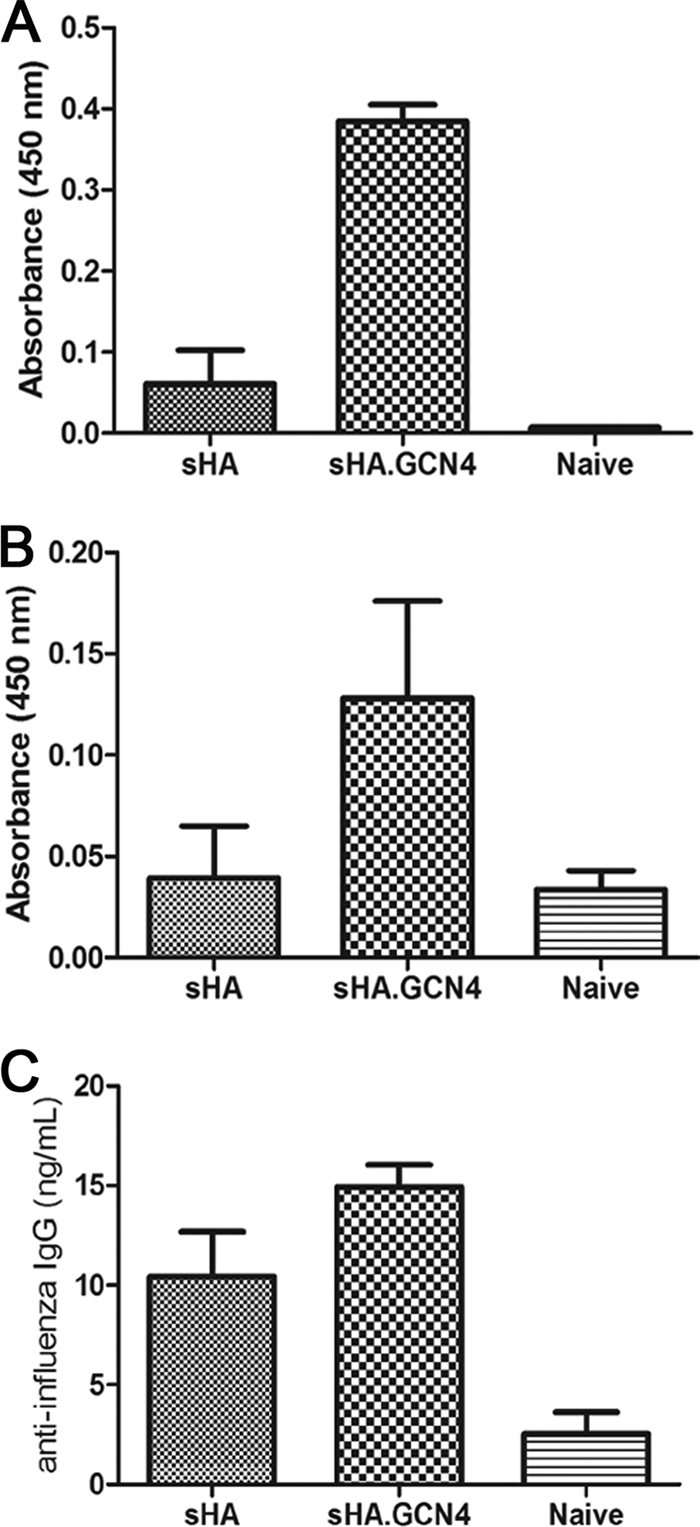
sHA.GCN4pII induces higher serum IgA levels and mucosal antibody responses. IgA ELISA was performed as described in Materials and Methods. ELISA plates were coated with 4 μg/ml of A/Aichi/2/68, and IgA was detected using biotin-conjugated rat anti-mouse and streptavidin-HRP. IgG ELISA was performed as described previously. IgA was assayed in vaginal washes (A) and serum (B). IgG was assayed in lung homogenate 21 days after boosting (C).
It has been demonstrated previously that IgG antibodies have an important role in neutralizing viruses after infection has been established in addition to preventing lung pathology (27). Therefore, we measured the total virus-specific IgG in the lung homogenates of microneedle-vaccinated mice. Lung homogenates from mice vaccinated with sHA.GCN4pII had 1.4-fold-higher levels of virus-specific IgG than did mice vaccinated with sHA (P = 0.0002) (Fig. 5C). These data suggest that skin delivery by microneedle vaccination with the trimeric HA, sHA.GCN4pII, induces improved humoral mucosal immune responses compared to those induced by sHA.
Microneedle vaccination with sHA.GCN4pII induces complete protection against challenge infection.
To determine the efficacy of microneedle vaccination with recombinant soluble HA, we challenged vaccinated mice intranasally with 5 LD50 (lethal) or 0.1 LD50 (sublethal) of mouse-adapted A/Aichi/2/68. Body weights and survival were monitored for 14 days postchallenge. Following lethal challenge, mice vaccinated with sHA.GCN4pII maintained their body weight for the duration of the challenge while mice vaccinated with sHA lost approximately 20% of their body weight (Fig. 6 A). Microneedle vaccination with sHA.GCN4pII confers 100% protection (6/6 mice) while sHA confers 50% protection (3/6 mice), although this difference was not significantly different (P = 0.0559) (Fig. 6B). These data were further supported in an independent study following sublethal infection with the mouse-adapted virus in which both vaccinated groups maintained their body weight over the course of the infection (Fig. 6C). Thus, transdermal vaccination with microneedles coated with soluble trimeric HA is an effective route of inducing protective immunity against influenza virus. We previously observed that s.c. immunization with this antigen was also effective in conferring complete protection against live virus challenge (37).
Fig. 6.
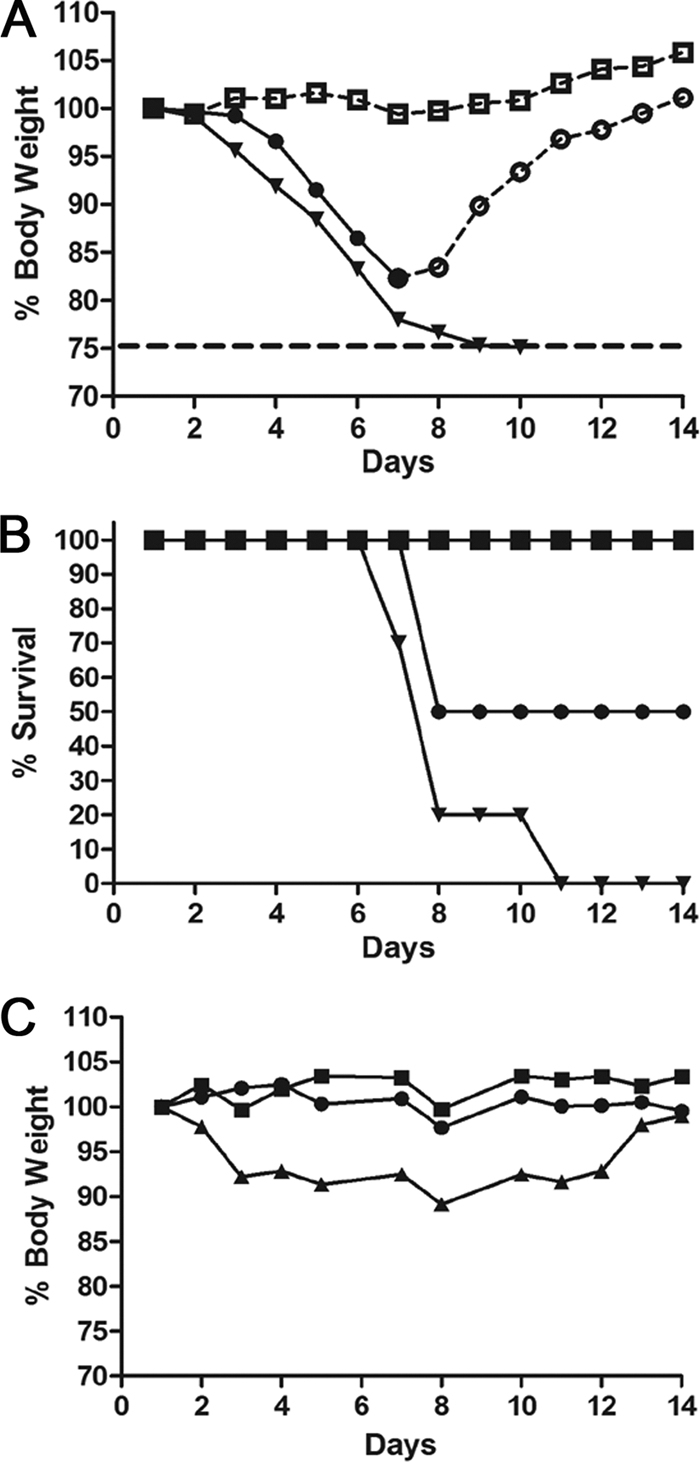
MN immunization with trimeric sHA induces improved protective immune responses against lethal challenge with mouse-adapted A/Aichi/2/68. (A and B) Mice primed and boosted were challenged with 5 LD50 of mouse-adapted H3N2 A/Aichi/2/68. Body weights and survival were followed for 14 days postchallenge. Symbols: circles, sHA; squares, sHA.GCN4; inverted triangles, PBS. Open symbols with a dashed line indicate percent mean initial body weight of surviving mice for each group. Closed symbols with a solid line indicate percent mean initial body weight of mice showing signs of morbidity (n = 6). (C) Sublethal infection of microneedle-vaccinated mice. Body weights were followed for 14 days postinfection (n = 6 per group). Symbols: circles, sHA; squares, sHA.GCN4; triangles, PBS.
Microneedle vaccination induces improved viral clearance after lethal challenge.
As a sensitive approach to determine the ability of vaccinated mice to reduce viral replication following lethal challenge, we examined the viral lung titers 4 days postchallenge. Mice vaccinated with sHA.GCN4pII-coated microneedles had no detectable viral lung titers at 4 days postchallenge, representing a reduction of over 106-fold compared with the unimmunized group (P < 0.001). In contrast, mice vaccinated subcutaneously with sHA.GCN4pII had a residual virus titer of about 102 PFU/g, demonstrating less efficient clearance. Independent of the route of immunization, the mice immunized with sHA-coated microneedles had less than a 1-log reduction in viral lung titers compared to those in unimmunized control mice (Fig. 7). These data suggest that the microneedle route of vaccination induces improved clearance of replicating virus for both sHA and sHA.GCN4pII compared to the s.c. route.
Fig. 7.
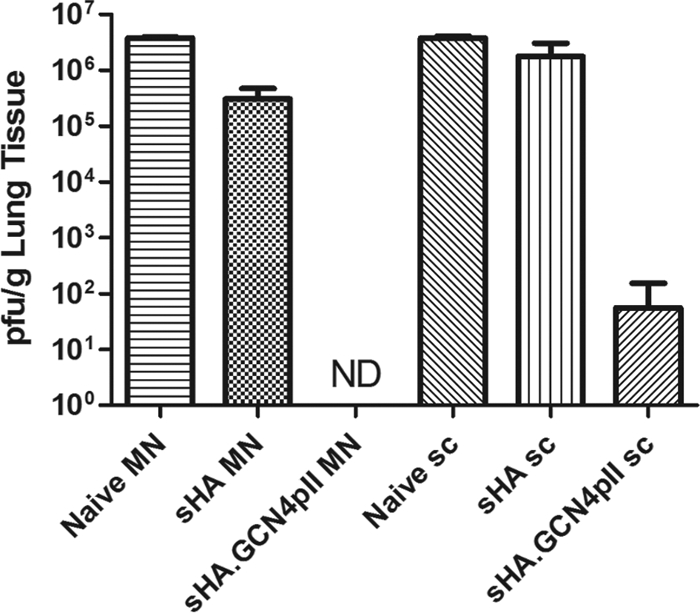
MN vaccination with trimeric sHA induces improved clearance of virus from the lung. Mice primed and boosted with 3 μg of recombinant soluble HA by the microneedle or subcutaneous routes were challenged with 5 LD50 of mouse-adapted A/Aichi/2/68. Lungs were removed on day 4 postchallenge and processed for virus plaque assays. Lung viral titers are reported as the arithmetic mean ± standard deviation (n = 3). ND, not detectable.
sHA.GCN4pII induces a type 1 helper T cell population after microneedle vaccination.
The effector phenotype of the helper T cell compartment can influence the B cell response and the CD8+ T cell response (14, 26). Therefore, to determine the helper T cell phenotype, we purified CD4+ T cells from vaccinated mice and restimulated the cells with vaccine being presented by accessory cells. The cytokines IFN-γ and IL-4 are known to be produced by helper T cell type 1 and helper T cell type 2, respectively. The frequency of IFN-γ+ CD4+ T cells was assayed by intracellular cytokine staining after in vitro restimulation. We observed that mice vaccinated with sHA.GCN4pII had an approximately 2-fold-higher frequency of IFN-γ+ CD4+ T cells than did mice vaccinated with sHA at both antigen concentrations (20 μg, P = 0.117, and 5 μg, P = 0.0262) (Fig. 8 A). Conversely, mice vaccinated with sHA had an approximately 1.3-fold-higher frequency of IL-4+ CD4+ T cells than did mice vaccinated with sHA.GCN4pII at both antigen concentrations, although this difference was not significant (Fig. 8B). These results indicated that microneedle vaccination with sHA.GCN4pII induced a more robust Th1 response in mice. The ratio of IFN-γ+ CD4+ T cells to IL-4+ CD4+ T cells suggests that the helper T cell phenotype is dominated by Th1 cells in mice vaccinated with sHA and sHA.GCN4pII (20 μg, P = 0.0563, and 5 μg, P = 0.0323) (Fig. 8C). These results indicated that microneedle vaccination with sHA.GCN4pII induced a more robust Th1 response in mice.
Fig. 8.
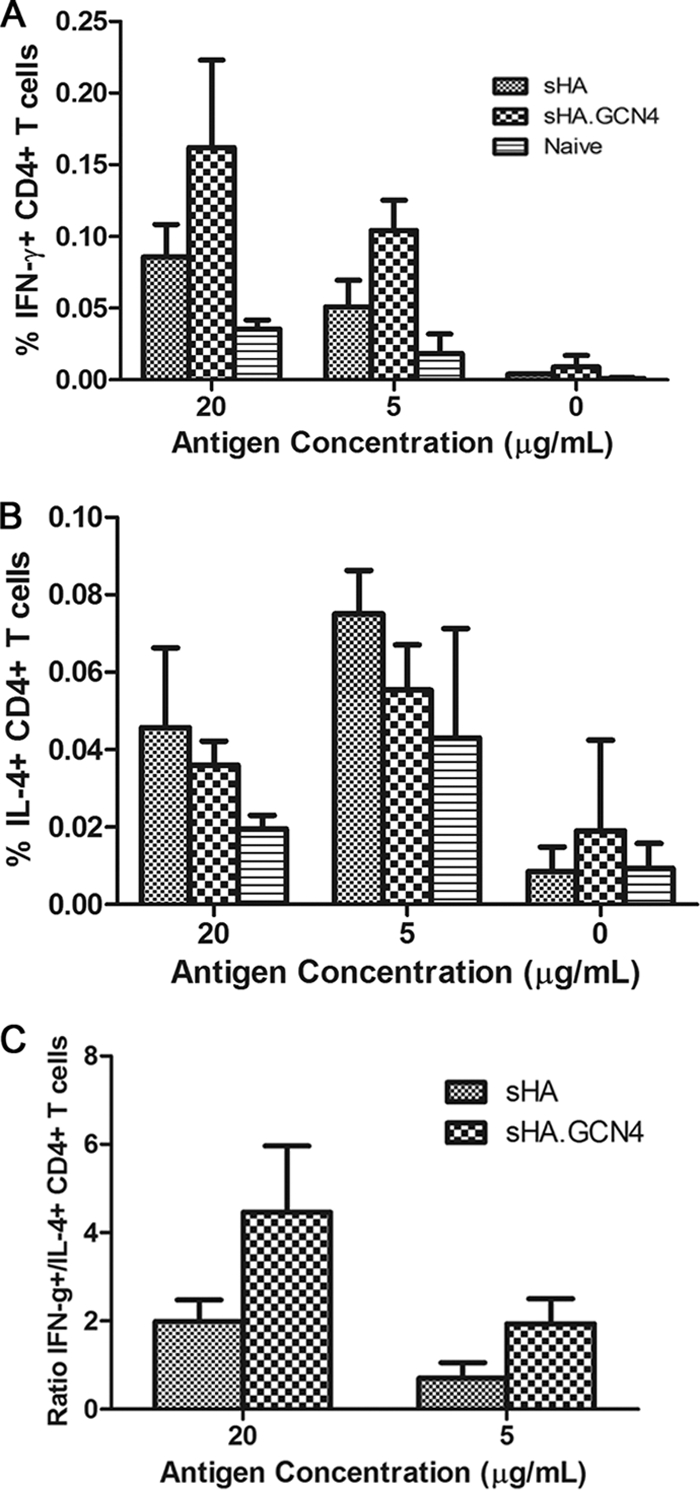
Microneedle vaccination with trimeric recombinant sHA induces greater Th1 responses. Purified CD4+ T cells were restimulated with 20, 5, or 0 μg/ml of vaccine for 5 days supplemented with 30 U/ml of recombinant human IL-2. (A and B) On day 5, restimulated cells were fixed and stained for surface markers and the indicated cytokines. (C) Ratios of frequencies of IFN-γ+ to IL-4+ helper T cells in microneedle-vaccinated mice.
DISCUSSION
The recent H1N1 influenza pandemic has illustrated the need for convenient alternatives to traditional influenza vaccine. Previous studies from our lab and others have demonstrated the successful use of both coated and dissolving microneedles for transdermal delivery of influenza vaccines using virus-like particles or inactivated virus (17, 23, 35). However, little information is available on using this approach to deliver a protein subunit vaccine. Microneedles have the advantage of delivering vaccine to an area rich in APC such as the epidermis and dermis without causing pain (11). These vaccinations induced robust antibody and cellular responses, resulting in protection against lethal challenge with several subtypes of influenza virus.
We have previously demonstrated that the influenza virus HA protein modified at the C terminus with the trimerization repeat, GCN4pII, generates stable trimeric soluble HA. Following subcutaneous vaccination, the modified trimeric sHA was able to induce higher virus-specific serum IgG and HAI titers (37). These enhanced humoral responses translated to a greater vaccine-induced protection following challenge with homologous virus. In the present study, we have tested the hypothesis that MN-mediated delivery via the transdermal route would be effective in enhancing the immune response to an influenza virus subunit vaccine. We demonstrated that the structure of the recombinant trimeric sHA was preserved when 15% (wt/vol) trehalose was included in the coating formulation. However, the unmodified sHA remained as a mixture of trimers, dimers, and monomers in the presence or absence of 15% trehalose. A stabilizing effect of trehalose on the HA activity of influenza virus was previously observed when coating microneedles with the whole inactivated virus (24).
Microneedle vaccination with the modified trimeric sHA induced an improved humoral systemic response compared with that induced by unmodified sHA as determined by ELISA. This improved response to trimeric sHA over that to unmodified sHA was particularly evident in HAI and microneutralization titers. These results strongly support the view that the trimeric sHA induces higher levels of functional antibodies than does the unmodified sHA, while both recombinant proteins induce binding antibodies as measured by ELISA. This difference in induction of functional antibodies could be attributed to the better presentation of native epitopes present in the stabilized trimeric structure of the modified sHA corresponding to those in the live virus. The unmodified sHA, in contrast, probably presents additional epitopes found at the interfaces between monomers, which are not exposed in the native virus. Importantly, sHA.GCN4pII immunization also induced 100% protection while sHA vaccination provided only partial protection.
The postchallenge viral lung titers observed in mice vaccinated with recombinant soluble HA-coated microneedles demonstrate that the soluble trimeric HA induced immune responses that were efficient at clearing replicating virus. In addition, comparing the postchallenge viral lung titers of mice vaccinated subcutaneously and of mice vaccinated with coated microneedles indicated that the skin-based immunization resulted in greater clearance of replicating virus independently of the recombinant protein used. These data support the conclusion that skin-based delivery of a recombinant protein antigen using microneedles increases the efficacy of such vaccines.
Further investigation of the vaccine-induced responses indicated that sHA.GCN4pII induced a balanced IgG subtype profile, contrasting with sHA, which induces a predominantly IgG1 profile. The cytokines expressed by CD4+ helper T cells influence the B cell isotype switch; therefore, we assessed the CD4 T cell phenotype in terms of a Th1 cytokine (IFN-γ) and a Th2 cytokine (IL-4) by intracellular cytokine staining. We observed that both recombinant sHA vaccines induced both IFN-γ-producing T cells and IL-4-producing T cells. Notably, sHA.GCN4pII induced a higher frequency of IFN-γ+ CD4+ T cells. Helper T cells which express IFN-γ have been shown to play an important role in inducing isotype switching to IgG2 in BALB/c mice (5). In addition, IFN-γ induces an antiviral state, upregulation of major histocompatibility complex (MHC) classes I and II, and costimulatory molecules on APC (30). Here, we showed that both Th1 and Th2 effector cells are generated after antigen delivery to the skin and that Th1 effector CD4 T cells are predominant in the sHA.GCN4pII group, correlating with the highest vaccine efficacy. The microneedle route for vaccination has the potential to enhance the CD4+ T cell response over that with traditional routes of immunization (10). Thus, the role of CD4+ T cells should be further examined to determine their significance in determining the efficacy of transdermal vaccination.
For influenza virus infection, secretory IgA (sIgA) has been shown to bind and neutralize virus in the upper respiratory tract and nasal cavity (27). In addition, transcytosis of influenza virus-specific IgG across the lung epithelial cell layer via FcRn plays an important role in preventing lung pathology (27, 33). We observed that mice receiving sHA.GCN4pII induced higher levels of tissue-localized virus-specific IgG and mucosal sIgA, suggesting that microneedle vaccination induced the critical antibody responses at the site of infection. The differences observed at the mucosal site could be the result of lower immunogenicity of sHA, or perhaps sHA results in a homing pattern different from that of sHA.GCN4pII.
These results demonstrate the efficacy of transdermal vaccinations using recombinant sHA derived from the A/Aichi/2/68 (H3N2) virus. Microneedle vaccination with the stabilized HA trimers induced protective immune responses in mice. It is noteworthy that in comparison to our previous work with subcutaneous vaccination, the MN immunization with the trimeric HA induced similar serum IgG and HAI titers and induced the same level of protection against lethal challenge as that in the s.c. vaccinated mice. Comparisons between intramuscular, intranasal, subcutaneous, and microneedle vaccinations using virus-like particles (VLP) and inactivated virus antigens have all demonstrated the enhanced immunogenicity of the transdermal route (16, 17, 23). Taken together, the results emphasize the conclusion that the delivery route as well as the nature of the vaccine antigen is important in determining the efficacy of an influenza vaccine.
ACKNOWLEDGMENTS
We thank Erin-Joi Collins for her valuable assistance in the preparation of the manuscript.
This project was supported in part by NIH grants EB006369 (M.R.P.) and AI074579 (R.W.C.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
M.R.P. serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. This possible conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
Footnotes
Published ahead of print on 2 February 2011.
REFERENCES
- 1. Alarcon J. B., Hartley A. W., Harvey N. G., Mikszta J. A. 2007. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin. Vaccine Immunol. 14:375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baldwin S. L., et al. 2009. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine 27:3063–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chau K. F., et al. 2004. Efficacy and side effects of intradermal hepatitis B vaccination in CAPD patients: a comparison with the intramuscular vaccination. Am. J. Kidney Dis. 43:910–917 [DOI] [PubMed] [Google Scholar]
- 4. Chen D., et al. 2003. Epidermal powder immunization of mice and monkeys with an influenza vaccine. Vaccine 21:2830–2836 [DOI] [PubMed] [Google Scholar]
- 5. Coffman R. L., Savelkoul H. F., Lebman D. A. 1989. Cytokine regulation of immunoglobulin isotype switching and expression. Semin. Immunol. 1:55–63 [PubMed] [Google Scholar]
- 6. Cornelissen L. A., et al. 2010. A single immunization with soluble recombinant trimeric hemagglutinin protects chickens against highly pathogenic avian influenza virus H5N1. PLoS One 5:e10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cottey R., Rowe C. A., Bender B. S. 2001. Influenza virus. Curr. Protoc. Immunol., chapter 19, unit 19.11 [DOI] [PubMed] [Google Scholar]
- 8. De Filette M., et al. 2008. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J. Biol. Chem. 283:11382–11387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duggan S. T., Plosker G. L. 2010. Intanza 15 microg intradermal seasonal influenza vaccine: in older adults (aged ≥60 years). Drugs Aging 27:597–605 [DOI] [PubMed] [Google Scholar]
- 10. Furio L., Briotet I., Journeaux A., Billard H., Peguet-Navarro J. 2010. Human Langerhans cells are more efficient than CD14(-)CD1c(+) dermal dendritic cells at priming naive CD4(+) T cells. J. Invest. Dermatol. 130:1345–1354 [DOI] [PubMed] [Google Scholar]
- 11. Gill H. S., Denson D. D., Burris B. A., Prausnitz M. R. 2008. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 24:585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gill H. S., Prausnitz M. R. 2007. Coated microneedles for transdermal delivery. J. Control. Release 117:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hobson D., Curry R. L., Beare A. S., Ward-Gardner A. 1972. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (Lond.) 70:767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janssen E. M., et al. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852–856 [DOI] [PubMed] [Google Scholar]
- 15. Kenney R. T., Frech S. A., Muenz L. R., Villar C. P., Glenn G. M. 2004. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 351:2295–2301 [DOI] [PubMed] [Google Scholar]
- 16. Kim Y. C., et al. 2009. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine 27:6932–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koutsonanos D. G., et al. 2009. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One 4:e4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matriano J. A., et al. 2002. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm. Res. 19:63–70 [DOI] [PubMed] [Google Scholar]
- 19. Miller L. S., Modlin R. L. 2007. Toll-like receptors in the skin. Semin. Immunopathol. 29:15–26 [DOI] [PubMed] [Google Scholar]
- 20. Nestle F. O., Di Meglio P., Qin J. Z., Nickoloff B. J. 2009. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9:679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osinubi M. O., et al. 2009. Enhancing comparative rabies DNA vaccine effectiveness through glycoprotein gene modifications. Vaccine 27:7214–7218 [DOI] [PubMed] [Google Scholar]
- 22. Prausnitz M. R., Langer R. 2008. Transdermal drug delivery. Nat. Biotechnol. 26:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quan F. S., et al. 2010. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J. Virol. 84:7760–7769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quan F. S., et al. 2009. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One 4:e7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quan F. S., Vunnava A., Compans R. W., Kang S. M. 2010. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS One 5:e9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reinhardt R. L., Liang H. E., Locksley R. M. 2009. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10:385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Renegar K. B., Small P. A., Jr., Boykins L. G., Wright P. F. 2004. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J. Immunol. 173:1978–1986 [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez A., et al. 2005. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine 23:2565–2572 [DOI] [PubMed] [Google Scholar]
- 29. Rowe T., et al. 1999. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 37:937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schroder K., Hertzog P. J., Ravasi T., Hume D. A. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163–189 [DOI] [PubMed] [Google Scholar]
- 31. Sha Z., Compans R. W. 2000. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J. Virol. 74:4999–5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skountzou I., Quan F. S., Jacob J., Compans R. W., Kang S. M. 2006. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine 24:6110–6119 [DOI] [PubMed] [Google Scholar]
- 33. Spiekermann G. M., et al. 2002. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 196:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Streilein J. W. 1983. Skin-associated lymphoid tissues (SALT): origins and functions. J. Invest. Dermatol. 80(Suppl.):12s–16s [DOI] [PubMed] [Google Scholar]
- 35. Sullivan S. P., et al. 2010. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 16:915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valladeau J., Saeland S. 2005. Cutaneous dendritic cells. Semin. Immunol. 17:273–283 [DOI] [PubMed] [Google Scholar]
- 37. Weldon W. C., et al. 2010. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One 5(9):e12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization 2002, posting date WHO manual on animal influenza diagnosis and surveillance. World Health Organization, Geneva, Switzerland: http://www.wpro.who.int/NR/rdonlyres/EFD2B9A7-2265-4AD0-BC98-97937B4FA83C/0/manualonanimalaidiagnosisandsurveillance.pdf [Google Scholar]
- 39. Yang X., et al. 2000. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J. Virol. 74:4746–4754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yin H. S., Wen X., Paterson R. G., Lamb R. A., Jardetzky T. S. 2006. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature 439:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu Q., et al. 2009. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. U. S. A. 106:7968–7973 [DOI] [PMC free article] [PubMed] [Google Scholar]