Abstract
Plasma loads of torque teno virus (TTV) among individuals differ extensively beginning early in life, suggesting a role for innate immunity. Here, congenital mannose-binding lectin deficiencies, but not deficiencies in respiratory ciliary function, correlated with increased TTV loads. Notably, however, the presence of either disorder was associated with particularly high TTV loads.
Torque teno virus (TTV) is a small, nonenveloped single-stranded DNA virus. Human TTV is classified in 29 genetically distinct species, which cluster in 5 branches (genogroups 1 to 5; http://www.ictvonline.org/virusTaxonomy.asp?version=2009) in phylogenetic trees. TTV causes chronic, possibly lifelong viremias in most people regardless of age, health status, and other variants. In fact, 70% to 90% of the general population worldwide is viremic, and the virus has not yet been associated with any disease with certainty, suggesting a remarkable adaptation to its host (2, 11).
Individual viremia loads (VLs) of TTV differ extensively for reasons that remain to be defined (17). Recently, the number of coinfecting viral variants was identified as a factor (16). However, VLs differed extensively also if this number was constant, suggesting the existence of a further determinant(s) of VL size. Because there are already wide differences between VLs in individuals early in life (18, 22), we reasoned that innate immunity might be involved. Therefore, we considered it interesting to investigate whether a mannose-binding lectin (MBL) deficit, probably the most common congenital deficit of innate immunity, may have an impact. MBL, encoded by gene MBL2 on chromosome 10, is a pattern recognition protein that exerts its protective action by activating the complement cascade through an antibody-independent pathway triggered by its binding to pathogen surfaces and other mechanisms (13). MBL is probably most important in antibacterial defenses (14) but also plays a significant role against viruses; indeed, MBL2 polymorphisms known to lead to lower-than-normal blood concentrations of functional MBL have been linked with an increased susceptibility to several enveloped and nonenveloped viruses (3, 4, 6, 8, 10, 12, 24, 25, 26). Furthermore, because the airways are colonized by TTV early in life and may represent a port of body entry (18), we conducted the investigation with patients being evaluated to confirm or exclude the presence of primary ciliary dyskinesia (PCD). This much less common (<1 case per 10,000 people) congenital disorder of innate immunity consists of a defective motion of the cilia lining the lower and upper respiratory tract that leads to significantly increased susceptibility to respiratory infections (15). Its diagnosis is often missed early in life, but the dysfunction is evident from the time of embryonic development (27).
After we received their informed consent, patients were consecutively enrolled at the Department of Pediatrics of the Pisa University Hospital from January 2008 to July 2010. Based on phenotypic criteria (genetic diagnosis is not recommended, due to the multiple existing and newly emerging phenotypes [1]), including results of ciliary motion analysis and ultrastructural assessment of the cilia, ciliary function after ciliogenesis in culture of nasal brushing samples, and other data, 52 patients (30 males and 22 females; median age, 11 years [95% confidence limits, 8 to 20 years]; 33 with situs inversus, an abnormality often accompanying PCD) were recognized to be affected by PCD, while in 46 (21 males and 25 females; median age, 14 years [95% confidence limits, 11 to 22 years]), the defect was attributed to viral or bacterial respiratory infections or pollution-induced irritant injury of the mucosa (secondary ciliary dyskinesia [SCD]). DNA extracted from 200 μl of whole blood by the BioRobot EZ1 (Qiagen GmbH, Hilden, Germany) was used for both MBL2 genotyping and TTV analysis. The four MBL2 single-nucleotide polymorphisms—3 in the first exon (codons 52, 54, and 57) and 1 in the promoter—that, alone or in combination, determine MBL deficiencies were investigated by specific PCR amplification and sequencing. The frequencies of MBL2 variants in the patients (data not shown) were consistent with results of previous surveys in the European population (21). Finally, based on MBL2 make-up, the patients were subdivided into three groups depending on expected blood level of MBL (normal, moderately low, and very low to undetectable) according to established criteria (Table 1) (9). TTV viremia was determined by a quantitative single-step TaqMan PCR targeting a highly conserved segment of the untranslated region. The analytical sensitivity was 100 copies/ml of blood, as determined using TTV-negative serum spiked with different numbers of a standard DNA template and extracted and amplified with a protocol studied to achieve this sensitivity (7).
Table 1.
TTV detection and VLs in the study patients, subdivided by expected MBL level and type of ciliary dyskinesia
| MBL levela | Ciliary defect | TTV in whole blood |
|
|---|---|---|---|
| No. of patients positive/no. examined (%) | Median VL (95% CL)b | ||
| Any | PCD | 36/52 (69) | 397 (140–2,068) |
| SCD | 33/46 (72) | 2,305 (1,096–8,340) | |
| Normal | PCD | 11/19 (58) | 182 (100–1,175) |
| SCD | 10/14 (71) | 2,410 (100–10,580) | |
| Moderately low | PCD | 19/26 (73) | 710 (125–6,540) |
| SCD | 16/22 (70) | 5,000 (430–14,160) | |
| Very low/undetectable | PCD | 6/7 (86) | 14,830 (240–2,347,000)c |
| SCD | 7/10 (70) | 1,964 (100–21,094) | |
Following the current nomenclature, homozygosity for the wild-type first exon is denoted genotype A/A, homozygosity for low-MBL first exon is denoted O/O, and heterozygosity is denoted A/O. Similarly, homozygosity for the wild-type promoter is denoted Y/Y, homozygosity for the low-MBL promoter is denoted X/X, and heterozygosity is denoted Y/X. The normal-MBL-level category corresponded to genotypes A/A plus Y/Y and A/A plus Y/X. The moderately-low-MBL-level category corresponded to genotypes A/O plus Y/Y and A/A plus X/X. The very-low-to-undetectable-MBL-level category corresponded to genotypes O/O plus Y/Y and A/O plus X/Y.
DNA copies per ml. The patients who tested negative were arbitrarily assigned a value of 100. CL, confidence limits.
Significantly different from the normal-MBL-level PCD group at a P value of 0.02 (Mann-Whitney test). No other significant differences were detected.
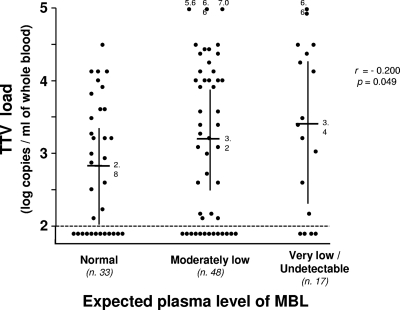
Sixty-nine of the 98 total patients (70%) had TTV-positive reactions, with VLs between 125 and 11,000,000 DNA copies per ml of whole blood (Table 1). These values were somewhat lower than those in previous reports by our group (23), likely due to the different DNA extraction method used (19, 20). However, since all samples were processed in the same manner, they permitted comparison of the various subgroups of patients. Figure 1 shows that, although there was a low degree of significance, the TTV VLs were inversely correlated to the expected blood levels of MBL, indicating that this effector of innate immunity is likely to exert a protective role against TTV. In contrast, PCD patients showed no significant differences in infection rates relative to SCD and, although the difference was not statistically different, had slightly greater VLs (Table 1), indicating that a congenital defect of respiratory ciliary motion, per se, does not lead to especially elevated VLs, at least relative to SCD. However, when the patients were sorted by MBL level and type of ciliary dyskinesia, the small group (7 patients) with both PCD and very low or undetectable MBL stood out for particularly high VLs (Table 1).
Fig. 1.
Individual TTV VLs in the blood of the 98 study patients, subdivided by expected MBL level. Horizontal bars, median VLs; vertical bars, 95% confidence limits. The broken line represents the lower limit of sensitivity of the quantitative detection method used. Symbols under this line represent patients who tested TTV negative. In statistical analyses, these patients were arbitrarily assigned a value of 2.0. The three groups of patients were not significantly different in terms of age and gender distribution.
In conclusion, consistent with findings from other viral infections (4, 6, 8, 10, 12, 24–26), MBL2 polymorphisms known to lead to MBL deficiencies were associated with higher TTV VLs than was wild-type MBL2, supporting the view that innate immunity efficiency contributes to determining the TTV VL size. The increase was modest but might have been greater had we examined subjects earlier in infancy, when the role of MBL in antimicrobial resistance is most important (5, 28). Since MBL exerts its protective activity through a variety of biological effects, including complement activation, phagocytosis stimulation, and cytokine synthesis modulation (28), future experiments will have to investigate their relative importance in this effect. Also, that the few patients congenitally deficient in both MBL levels and respiratory ciliary function had particularly elevated VLs suggests that the coexistence of different flaws in innate immunity may have a cooperative facilitating effect on TTV. Thus, it would be important to clarify whether and how extensively other defects of innate immunity, but also adaptive immunity, impact TTV viremia size and stability over time.
Footnotes
Published ahead of print on 16 February 2011.
REFERENCES
- 1. Barbato A., et al. 2009. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur. Respir. J. 34:1264–1276 [DOI] [PubMed] [Google Scholar]
- 2. Bendinelli M., et al. 2001. Molecular properties, biology, and clinical implications of TT virus, a recently identified widespread infectious agent of humans. Clin. Microbiol. Rev. 14:98–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catano G., et al. 2008. Independent effects of genetic variations in mannose-binding lectin influence the course of HIV disease: the advantage of heterozygosity for coding mutations. J. Infect. Dis. 198:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eisen D. P., Minchinton R. M. 2003. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis. 37:1496–1505 [DOI] [PubMed] [Google Scholar]
- 5. Endeman H., et al. 2008. Mannose-binding lectin genotypes in susceptibility to community-acquired pneumonia. Chest 134:1135–1140 [DOI] [PubMed] [Google Scholar]
- 6. Filho R. M., et al. 2010. High frequency of variant alleles of the mannose-binding lectin 2 (MBL2) gene are associated with patients infected by hepatitis B virus. Viral Immunol. 23:449–453 [DOI] [PubMed] [Google Scholar]
- 7. Focosi D., et al. 2010. Torquetenovirus viremia kinetics after autologous stem cell transplantation are predictable and may serve as a surrogate marker of functional immune reconstitution. J. Clin. Virol. 47:189–192 [DOI] [PubMed] [Google Scholar]
- 8. Friborg J. T., et al. 2010. Mannose-binding lectin genotypes and susceptibility to Epstein-barr virus infection in infancy. Clin. Vaccine Immunol. 17:1484–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garred P., et al. 1999. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J. Clin. Invest. 104:431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hair P. S., et al. 2010. Human astrovirus coat protein binds C1q and MBL and inhibits the classical and lectin pathways of complement activation. Mol. Immunol. 47:792–798 [DOI] [PubMed] [Google Scholar]
- 11. Hino S., Miyata H. 2007. Torque teno virus (TTV): current status. Rev. Med. Virol. 17:45–57 [DOI] [PubMed] [Google Scholar]
- 12. Hu Y., Wu D., Tao R., Shang S. 2010. Association between mannose-binding lectin gene polymorphism and pediatric cytomegalovirus infection. Viral Immunol. 23:443–447 [DOI] [PubMed] [Google Scholar]
- 13. Ip W. K., Takahashi K., Ezekowitz R. A., Stuart L. M. 2009. Mannose-binding lectin and innate immunity. Immunol. Rev. 230:9–21 [DOI] [PubMed] [Google Scholar]
- 14. Kilpatrick D. C. 2002. Mannan-binding lectin and its role in innate immunity. Transfus. Med. 12:335–352 [DOI] [PubMed] [Google Scholar]
- 15. Leigh M. W., et al. 2009. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet. Med. 11:473–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maggi F., et al. 2005. Relationships between total plasma load of torquetenovirus (TTV) and TTV genogroups carried. J. Clin. Microbiol. 43:4807–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maggi F., Bendinelli M. 2009. Immunobiology of the torque teno virus and other anelloviruses. Curr. Top. Microbiol. Immunol. 331:65–90 [DOI] [PubMed] [Google Scholar]
- 18. Maggi F., et al. 2003. TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity. J. Virol. 77:2418–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michelin B. D., et al. 2008. Detection of cytomegalovirus (CMV) DNA in EDTA whole-blood samples: evaluation of the quantitative artus CMV LightCycler PCR kit in conjunction with automated sample preparation. J. Clin. Microbiol. 46:1241–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller S., Seet H., Khan Y., Wright C., Nadarajah R. 2010. Comparison of Qiagen automated nucleic acid extraction methods for CMV quantitative PCR testing. Am. J. Clin. Pathol. 133:558–563 [DOI] [PubMed] [Google Scholar]
- 21. Müller S., et al. 2007. MBL2 variants in relation to common childhood infections and atopy-related phenotypes in a large German birth cohort. Pediatr. Allergy Immunol. 18:665–670 [DOI] [PubMed] [Google Scholar]
- 22. Naganuma M., et al. 2008. TT virus prevalence, viral loads and genotypic variability in saliva from healthy Japanese children. Acta Paediatr. 97:1686–1690 [DOI] [PubMed] [Google Scholar]
- 23. Pifferi M., et al. 2008. Torquetenovirus infection and ciliary dysmotility in children with recurrent pneumonia. Pediatr. Infect. Dis. J. 27:413–418 [DOI] [PubMed] [Google Scholar]
- 24. Ribeiro L. Z., et al. 2008. Serum mannose-binding lectin levels are linked with respiratory syncytial virus (RSV) disease. J. Clin. Immunol. 28:166–173 [DOI] [PubMed] [Google Scholar]
- 25. Segat L., et al. 2009. MBL2 gene polymorphisms are correlated with high-risk human papillomavirus infection but not with human papillomavirus-related cervical cancer. Hum. Immunol. 70:436–439 [DOI] [PubMed] [Google Scholar]
- 26. Seppanen M., et al. 2009. Mannose-binding lectin 2 gene polymorphism in recurrent herpes simplex virus 2 infection. Hum. Immunol. 70:210–221 [DOI] [PubMed] [Google Scholar]
- 27. Sharma N., Berbari N. F., Yoder B. K. 2008. Ciliary dysfunction in developmental abnormalities and diseases. Curr. Top. Dev. Biol. 85:371–427 [DOI] [PubMed] [Google Scholar]
- 28. Thiel S., Gadjeva M. 2009. Humoral pattern recognition molecules: mannan-binding lectin and ficolins. Adv. Exp. Med. Biol. 653:58–73 [DOI] [PubMed] [Google Scholar]