Abstract
Chronic infection of cattle with Leptospira borgpetersenii serovar Hardjo reduces animal production through reproductive failure and presents a persistent health threat to workers in the animal industry. Cattle are maintenance hosts for serovar Hardjo, and development of vaccines that establish long-term protective immunity has been problematic; induction of high titers of anti-serovar Hardjo antibody does not appear to be protective. Rather, development of an antigen-specific Th1 response appears to be critical for limiting renal colonization and urinary shedding of bacteria. In this study we compared two monovalent killed bacterial cell vaccines to assess long-term (12 months) protection against live serovar Hardjo challenge. Although neither vaccine prevented infection, renal colonization and urinary shedding of bacteria were reduced compared to those of control animals. Increased proliferation of CD4+, CD8+, and γδ T cells from vaccinated, but not control, animals was detected. In addition, NK cells from vaccinated animals and from all animals following infection, when exposed to antigen ex vivo, demonstrated a gamma interferon (IFN-γ) recall response. We propose that programming NK cells to respond quickly to L. borgpetersenii serovar Hardjo infection may be an important step toward developing protective immunity.
INTRODUCTION
Leptospirosis is one of the most widespread zoonotic diseases in the world and significantly impacts livestock production. Infections with pathogenic Leptospira result in either an acute, potentially lethal infection or, in maintenance hosts, a chronic infection with few outward signs of disease (14). Cattle are maintenance hosts of L. borgpetersenii serovar Hardjo, and infected animals typically show no apparent signs of infection except during pregnancy. Reproductive failure (abortions, stillbirths, and birth of weak offspring) and reduced milk production due to serovar Hardjo infection have a significant impact on beef and dairy operations. Additionally, as a human pathogen, serovar Hardjo is a health threat to workers in the animal industry.
Current multivalent Leptospira vaccines contain killed whole cells from several different serovars and induce protective immunity against accidental infection with non-host-adapted strains, but development of an efficacious vaccine that protects cattle against serovar Hardjo infection has been more elusive. Standard serovar Hardjo vaccine formulations induce high antibody titers and may reduce but do not prevent chronic renal colonization or urinary shedding (6–8). Consequently, urine from infected animals presents a potential source of infection. Furthermore, during pregnancy, infected animals often experience reproductive failure. At least two commercial monovalent serovar Hardjo vaccines induce CD4+ and γδ T cell proliferation and production of gamma interferon (IFN-γ) in response to serovar Hardjo antigens (4, 18, 19). Induction of this Th1 response appears to provide short-term (4 months) protective immunity against urinary shedding or renal infection after live challenge (5). It is unknown if induction of antigen-specific Th1 responses in cattle also provides long-term protection (≥1 year) following vaccination against live challenge.
In this study, we tested two monovalent vaccines for long-term protection against live challenge and found that although vaccination with whole killed cells does not provide sterile protection against live challenge, it does reduce urinary shedding of bacteria. We also report that NK cells from vaccinated animals exhibit a recall response when exposed to antigen, and this in turn may be effective in bacterial clearance from kidneys of infected animals.
MATERIALS AND METHODS
Bacterial culture.
L. borgpetersenii serovar Hardjo strain 203 was propagated in semisolid medium as described previously (25). The initial infectious challenge was derived from a single-passage culture approximately 3 weeks after recovery from the frozen state. Urine from two steers inoculated with this culture was collected weekly and analyzed by fluorescent antibody (FA) analysis. Once urinary shedding was confirmed, the animals were euthanized, and bacterial cultures were obtained from kidney homogenates. Primary cultures were used for all animal infections in the live challenge studies.
Animals.
All animals were screened by the microscopic agglutination test (MAT) (11) to ensure that they were free of preexisting antibodies to Leptospira serovars Canicola, Grippotyphosa, Hardjo, Icterohaemorrhagiae, and Pomona before entering them into this study (titers from all animals, ≤50). Twenty-three Holstein steers ∼10 months of age were assigned to one of three groups in the 1-year duration of immunity study: adjuvant without antigen (control) (n = 7), a commercial monovalent serovar Hardjo vaccine (Spirovac; Pfizer, Groton, CT) (Mono1) (n = 8), and a monovalent U.S. reference vaccine prepared from L. borgpetersenii serovar Hardjo strain RZ33 (a colony-purified derivative of a reference field isolate, L. borgpetersenii serovar Hardjo isolate 93U) as described previously (5) (Mono2) (n = 8). Eight Holstein steers ∼10 months of age were also included in this study to test for induction of short-term immunity by Mono1. The control group was used to ensure that the challenge inoculum was adequate to infect all animals that were not previously exposed to Leptospira antigens. In addition to the cattle noted above, two mixed-breed steers were used as a source of the initial infectious inoculum. Animals were acclimated to the facility for at least 1 week before studies were initiated. Animals were housed in open barns during the preinfection stages of these studies. One week prior to infectious challenge, animals were moved to individual pens in a biosafety level 2 agriculture (BL-2 Ag) facility. All animal protocols had approval of the National Animal Disease Center Institutional Animal Care and Use Committee.
Experimental design.
Animals were vaccinated twice with the appropriate preparation, 4 weeks apart. One year following the second inoculation, animals were moved to containment facilities, acclimated for 1 week, and then given three successive daily administrations of 1 × 107 low-passage L. borgpetersenii serovar Hardjo strain 203 using conjunctival instillation as described previously (7). Animals were followed for 6 weeks before euthanasia. To test for short-term immunological protection by Mono1, eight animals were vaccinated twice according to the manufacturer's directions and then challenged as described above, 12 weeks following the second inoculation of vaccine.
Sample collection.
Urine and blood samples were collected at weekly intervals following infectious challenge. Urine was collected from cattle by clean catch approximately 15 min after animals were injected with furosemide as described previously (25). Urine samples from all postchallenge time points were processed for culture, FA, and PCR detection. Blood samples were collected from the jugular vein and processed for evaluation of serum antibody titers and lymphocyte assays.
Sera were prepared and evaluated for the presence of antibodies to Leptospira using the MAT following well-established protocols (11). For lymphocyte assays, blood samples were placed into an acid-citrate-dextrose solution and either used directly for whole blood stimulation or processed for enrichment of peripheral blood mononuclear cells (PBMCs) as described previously (21).
Gross pathology of kidneys was noted at necropsy. When present, gross lesions consisted of multifocal, small (up to 0.5 cm), depressed areas of pallor on the capsular surface. On cut surfaces, multifocal small (up to 0.5 cm) areas of pallor were present within the cortex. Rarely, wedge-shaped areas of pallor (infarcts) extending from the cortex to capsular surface were present.
Samples of liver and kidney were collected at necropsy and fixed in 10% neutral buffered formalin for histopathology using standard techniques. Liver and kidney samples were also prepared for bacteriological culture by gently homogenizing the tissue, and two serial 10-fold dilutions of the tissue homogenate were used to inoculate Ellinghausen-McCullough-Johnson-Harris (EMJH) bacteriological medium (13, 15) as described previously (25). Cultures were periodically examined for the presence of bacteria using dark-field microscopy for up to 6 months before being designated negative.
Fluorescent antibody detection.
Detection of Leptospira in urine and tissue was done by fluorescence microscopy following direct staining of bacteria with high-titer rabbit anti-Leptospira sera conjugated with fluorescein isothiocyanate (FITC) as described previously (25). An FA sample was scored positive if at least one spirochete cell was detected in a sample. Samples that lacked detectable spirochetes were scored negative, even if there was fluorescent material present.
PCR detection of bacterial DNA.
Bacteria were enriched from 50 ml of urine by centrifugation, and total DNA was extracted using a kit (DNeasy, Qiagen Corp.). DNA was collected in 100 μl buffer and used as a template in PCRs using a modification of previously described methods (1, 26). Two primers (P1805, 5′-GCGGAAAGTGAACCGTATCGAG-3′, and P1809, 5′-CTGATATTCGCGGGTTGGAAGG-3′) were designed to amplify a 630-bp product from IS1533, an insertion element present in approximately 85 copies/genome of L. borgpetersenii serovar Hardjo. Each reaction mixture contained 7 μl of either eluted DNA, water (as a negative control), or serovar Hardjo DNA (as a positive control). Target DNA was amplified using a program of 94°C for 15 s melting, followed by 67°C for 2 min annealing and extension, repeated for a total of 50 cycles. The entire contents of each reaction were applied to 1.5% agarose gels (1% NuSieve, 0.5% agarose) buffered with 89 mM Tris, 89 mM boric acid, and 2 mM EDTA, and the products were separated by electrophoresis and visualized by standard techniques.
Histopathology.
Formalin-fixed paraffin-embedded kidney sections were stained with hematoxylin-eosin or Steiner silver stains (7) or processed for indirect immunofluorescence as described previously (17) using rabbit sera directed against LipL32 (a generous gift from David Haake, VA Medical Center, Los Angeles, CA). Tissue was assessed on the basis of morphological features and graded as stage 1 (little or no pathology, few monocytic cells), stage 2 (cords or sheets of mononuclear cells surrounding and dividing tubules or glomeruli), stage 3 (stage 2 with tubular degeneration or glomerular atrophy), or stage 4 (stage 3 with glomerular sclerosis and interstitial fibrosis, often with an irregular capsular surface).
Lymphocyte proliferation and IFN-γ production.
PBMCs were enriched by centrifugation through Ficoll-sodium diatrizoate density gradients (Sigma Diagnostics, Inc., St. Louis, MO). Cells were diluted to 107 viable cells per ml in RPMI 1640 medium, and 50-μl aliquots (5 × 105 cells) were added to wells in 96-well flat-bottom microtiter plates. Lymphocyte proliferation was determined by measuring [3H]thymidine incorporation after 7 days incubation at 37°C under 5% CO2 in the presence of either 2 μg sonicated L. borgpetersenii serovar Hardjo strain RZ33 protein/ml, 5 μg pokeweed mitogen (PWM)/ml, or medium only as described previously (21). Stimulation indices were calculated from raw counts by comparing the counts per minute incorporated by cells in the presence of mitogen divided by the counts per minute incorporated in the absence of mitogen for statistical analysis.
In vitro production of IFN-γ by PBMCs (5 × 105 cells/well) was determined for each animal following vaccination. Cells were incubated for 48 h at 37°C under 5% CO2 in the presence of RPMI 1640 medium only or 1640 medium containing sonicated RZ33 antigen (5 μg/ml). IFN-γ levels were determined as described previously (21). Net antigen-specific IFN-γ production was calculated for each sample by subtracting the IFN-γ concentration produced in wells without antigen from IFN-γ concentrations produced in wells with antigen.
Flow cytometry.
At selected sampling times after vaccination and challenge, PBMC samples were prepared and cell suspensions adjusted to 1 × 107 viable cells per ml as described above and used for flow cytometry to characterize lymphocyte populations that produced IFN-γ and/or proliferated in response to Leptospira antigens. For these studies, 50 μl of each cell suspension (5 × 105 cells) was added to each of eight separate wells of 96-well flat-bottom microtiter plates that contained 100 μl of RPMI 1640 medium only or RPMI 1640 medium containing sonicated RZ33 antigen (5 μg/ml).
For measurement of intracellular IFN-γ, brefeldin (10 μg/ml; Sigma Chemical Company, St. Louis, MO), ionomycin (1 μg/ml; Sigma Chemical Co.), and phorbol myristic acetate (PMA) (1 μg/ml; Sigma Chemical Co.) were added to individual wells at 4 days of incubation. After incubation at 37°C and 5% CO2 for 4 h, approximately 2 × 105 pooled cells in 125 μl of culture medium were added to individual wells of round-bottom microtiter plates containing primary antibodies or wells containing medium alone. Primary antibodies (1 μg/well) included anti-CD4 (CATC138A-IgG1; VMRD, Pullman, WA), anti-CD8 (BAQ-111A-IgM; VMRD), anti-γδ T cell receptor (TCR) (CACT61A-IgM; VMRD), and anti-CD335 (AKS1-IgG1; Serotec, Raleigh, NC). Cells were incubated for 15 min and then centrifuged for 2 min at 400 × g. After removal of the supernatant, control and primary antibody-labeled cells were incubated with appropriate secondary antibodies (anti-IgM-Alexa 350 or anti-IgG1-peridinin chlorophyll protein [PerCP]). After centrifugation and removal of supernatants, cells were suspended in 100 μl of fixation buffer (Serotec) and incubated in the dark for 15 min. Cells were harvested by centrifugation and washed twice with phosphate-buffered saline (PBS). After suspension in 100 μl of permeabilization buffer (Serotec), anti-IFN-γ antibody (CC302-phycoerythrin [PE] conjugated; Serotec) was added. A control sample containing a concentration-matched PE-isotype control was also run. Cells were incubated for 30 min in the dark, washed twice in PBS, and suspended in 200 μl of FacsLyse buffer (Becton Dickinson, Franklin Lakes, NJ) before being analyzed on a three-laser LSR flow cytometer (Becton Dickinson). The resulting list mode data were analyzed using FlowJo (Treestar, Ashland, OR).
To identify lymphocyte populations that proliferated in response to Leptospira antigen exposure, cells were stained with the green fluorescent dye PKH-67 (Sigma Chemical Co.) in accordance with the manufacturer's instructions. Following PHK-67 staining, cells were adjusted to a concentration of 1 × 107 viable cells per ml and 50 μl of each cell suspension (5 × 105 cells) was added to each of eight separate flat-bottom wells of 96-well microtiter plates that contained 100 μl of RPMI 1640 medium only or RPMI 1640 medium containing sonicated RZ33 (5 μg/ml). After incubation for 7 days at 37°C under 5% CO2, approximately 2 × 105 pooled cells in 200 μl of culture medium were added to individual wells of round-bottom microtiter plates. After centrifugation (15 min, 400 × g), cells were suspended in 100 μl in PBS containing 1% fetal bovine serum and 0.1% sodium azide (fluorescence-activated cell sorter[FACS] buffer) containing the primary antibody(ies) (1 μg/well) described above. After 15 min incubation at room temperature, cells were centrifuged (15 min, 400 × g) and suspended in 100 μl each of PerCP (1 μg/ml)-conjugated rat anti-mouse IgG1 (Becton Dickinson) and PE (1 μg/well)-conjugated goat anti-mouse IgM (Southern Biotechnology Associates, Birmingham, AL). Cells in secondary antibody were incubated for 15 min at room temperature in the dark, washed with FACS buffer, suspended in 200 μl of PBS containing 0.04% sodium azide, and analyzed on a FacScan flow cytometer (Becton Dickinson). Data were analyzed using commercially available software (CellQuest [Becton Dickinson]; Modfit [Verity Software House Inc., Topsham, ME]).
Statistical analysis.
Cellular proliferation and IFN-γ data were analyzed as the logarithms of their values. Differences between treatments in flow cytometric, [3H]thymidine incorporation, and net IFN-γ data at each sampling time were compared by a general linear model procedure (SAS Institute Inc., Cary, NC). Means for individual treatments were separated by use of a least significant difference procedure (P < 0.05). NK cell flow data were subjected to statistical analysis with SAS software 9.2 (SAS Institute, Inc., Cary, NC). A mixed model for repeated measures (PROC MIXED) using compound symmetry covariance was used (16). The model utilized the fixed effects of time, treatment, and the interaction of time with treatment. Prior to analysis, several covariance structures were tested, and the structure with the lowest score (−2 log residual maximum likelihood [REML], Akaike's information criterion, and Schwarz's Bayesian information criterion) was used in the final analysis. A P value of <0.05 was considered significant.
RESULTS
Vaccination strategy and infectious challenge.
Twenty-three Holstein steers were inoculated twice, 4 weeks apart, with one of three different vaccine formulations: adjuvant alone (control animals) or either of two monovalent serovar Hardjo vaccines, Mono1 and Mono2 (see Materials and Methods). One year following the second vaccination, 18 animals (Mono1 [n = 8], Mono2 [n = 7], and control [n = 3]) were challenged with low-passage L. borgpetersenii serovar Hardjo strain 203, a strain found previously to consistently infect and persist in cattle (5–8). Animals were followed for 6 weeks following challenge before euthanasia. Examination at necropsy failed to detect gross lesions in any (0/8) of the Mono1-vaccinated animals. Some (3/7) Mono2-vaccinated animals had gross lesions, while all (n = 3) control animals analyzed at necropsy had multiple gross lesions (data not shown).
A second study was done to test Mono1 for the ability to induce short-term immunity to infection. In this portion of the study, eight animals were immunized twice with Mono1 and then challenged 12 weeks following the second vaccination. The remaining control animals (n = 4) from the long-term protection study described above were used as infection controls for the short-term protection study. Infection and histopathology data for control animals shown in Tables 1 to 3 are combined from both challenge studies, whereas analysis of the immune response to infection was restricted to the three infection control animals challenged simultaneously with animals vaccinated 12 months earlier.
Table 1.
Detection of bacterial infection among vaccinated and control animals
| Vaccine and challenge time | No. of infections detected |
No. of infected animals/total no. of animals | ||||||
|---|---|---|---|---|---|---|---|---|
| Urine |
Kidney |
|||||||
| Culture | FA | PCR | Culture | FA | PCR | Histoa | ||
| Mono1 | ||||||||
| 3 mo | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 6/8 |
| 12 mo | 0 | 1 | 6 | 0 | 0 | 1 | 1 | 6/8 |
| Mono2, 12 mo | 0 | 4 | 6 | 0 | 5 | 0 | 0 | 7/7 |
| Control, 12 mob | 7 | 7 | 7 | 7 | 7 | 5 | 6 | 7/7 |
Results of microscopic examination of silver-stained tissue sections.
The same controls were used for 3-month and 12-month challenge studies.
Table 3.
Histopathological evaluation of lesion formation following infection
| Vaccine and challenge time | No. of lesions found with histopathology |
|||
|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | |
| Mono1 | ||||
| 3 mo | 7 | 1 | 0 | 0 |
| 12 mo | 4 | 0 | 1 | 3 |
| Mono2, 12 mo | 1 | 1 | 0 | 5 |
| Control, 12 moa | 0 | 1 | 1 | 5 |
The same controls were used for 3-month and 12-month challenge studies.
Establishment of infection was measured using four methods: bacteriological culture, direct immunofluorescence analysis, PCR analysis of urine and kidney homogenates, and microscopic analysis of silver-stained tissue sections. Bacteriological culture from urine was attempted weekly from all animals following challenge. All urine samples from cattle vaccinated with Mono1 or Mono2 were negative for bacteriological culture, whereas urine samples from all control animals yielded positive bacterial cultures at various times following infectious challenge (Table 1). Fluorescent antibody analysis of urine from infected cattle showed that control animals were routinely positive, whereas Leptospira was detected in a few of the urine samples collected from vaccinated animals (Table 1). PCR analysis was used to detect bacterial DNA in urine and tissue to provide a sensitive assay for the presence of bacteria. This method was modified from a previously published assay (1) using primers designed to anneal to a consensus sequence of IS1533 derived from the L. borgpetersenii serovar Hardjo genome sequence (10). All control animals and most animals vaccinated with either Mono1 or Mono2 were positive for urinary shedding of L. borgpetersenii using PCR (Table 1), but shedding was less frequently detected in Mono1-vaccinated animals at later sampling dates (Table 2), and although the PCR method used was not quantitative, less product was produced from vaccinated animals compared to infection controls (data not shown). The same rate of infection (6/8) occurred in animals receiving infectious challenge 3 or 12 months following the second Mono1 vaccination (Table 1). None of the animals tested for short-term duration of immunity had PCR-detectable Leptospira in the urine or kidney tissue at either the last sampling or following necropsy, respectively (Table 2).
Table 2.
Number of animals PCR positive for urinary shedding following infection and positive for kidney infection at necropsy
| Vaccine and challenge time | No. of animals positive for: |
||||||
|---|---|---|---|---|---|---|---|
| Urinary shredding (at weeks p.i.) |
Kidney infection at necropsy | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Mono1 | |||||||
| 3 mo | 1 | 3 | 1 | 1 | 4 | 0 | 0 |
| 12 mo | 1 | 4 | 3 | 1 | 3 | 2 | 1 |
| Mono2, 12 mo | 1 | 5 | 0 | 5 | 4 | 3 | 5 |
| Control, 12 moa | 3 | 3 | 6 | 7 | 7 | 7 | 7 |
The same controls were used for 3-month and 12-month challenge studies.
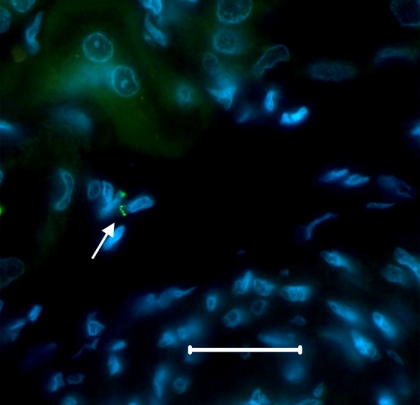
Histopathological analysis of kidney tissue taken from animals that were challenged 3 months following Mono1 vaccination showed little evidence of lesion formation (Table 3) and no detectable Leptospira by microscopic examination of tissue sections (Table 1). While 50% of Mono1-vaccinated animals challenged 12 months following vaccination had little or no detectable pathology, the remaining Mono1-vaccinated animals, the majority of Mono2-vaccinated animals (5/7), and almost all control animals (6/7) had stage 3 or 4 lesions (Table 3). Spirochetes were detected by microscopic examination of silver-stained tissue in only one of the vaccinated animals and 6 of the 7 control animals (Table 1). Confirmation that spirochetes observed by silver stain in kidney tissue from a Mono1-vaccinated animal were Leptospira was obtained by immunofluorescent microscopy using antisera directed against the major outer membrane protein, LipL32 (Fig. 1).
Fig. 1.
Detection of L. borgpetersenii within kidney tissue of a Mono1-vaccinated animal. Immunofluorescent detection of Leptospira in a paraffin-embedded, formalin-fixed section of kidney from animal 624, vaccinated with Mono1 prior to infectious challenge with serovar Hardjo strain 203. Leptospira is stained green (arrow), and host cell nuclei are stained blue. The bar represents 25 μm for reference.
Humoral immunological response to vaccination and infection.
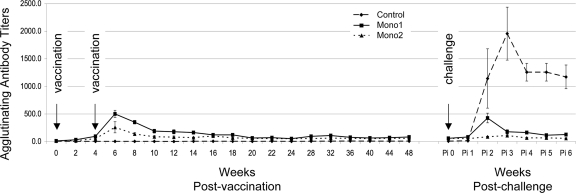
All animals used in this study had negative antibody titers (<100) to five common leptospiral serovars before initiating vaccinations. All animals vaccinated with either serovar Hardjo vaccine developed agglutinating antibodies; agglutinating antibody titers to serovar Hardjo peaked (mean titers, 500 [Mono1] and 257 [Mono2]) 4 weeks postvaccination (p.v.) and then slowly returned to a background titer of approximately 100 until after live challenge (Fig. 2). Control animals failed to develop antibody titers above background (12.5) before live challenge (Fig. 2).
Fig. 2.
Serum titers following vaccination and challenge. Group mean serum titers determined by the MAT are shown for each sampling. Arrows indicate the timing of each vaccination and live bacterial challenge. Pi, postinfection. The error bars represent standard deviations.
Animals previously vaccinated with Mono1 produced an anamnestic response that peaked at 2 weeks postinfection (p.i.) (mean peak titer, 425). Animals vaccinated with Mono2 had no detectable increase in agglutinating antibodies following infection. Control animals mounted a strong humoral response that peaked by week 3 p.i. (mean peak titer, 1,957). Mono1 and Mono2 animals failed to develop positive cross-reacting antibody titers to serovars Canicola, Grippotyphosa, Icterohaemorrhagiae, or Pomona, as a result of either vaccination or exposure to live L. borgpetersenii serovar Hardjo. Two of three control animals developed minimally positive titers (peak titer was 100) to serovar Canicola following infectious challenge with serovar Hardjo, but no positive titers were detected for serovars Grippotyphosa, Icterohaemorrhagiae, or Pomona (data not shown).
Lymphocyte proliferation in response to antigen.
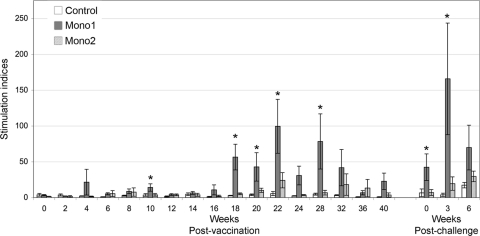
Lymphocytes were stimulated ex vivo with antigen prepared from high-passage L. borgpetersenii serovar Hardjo strain RZ33, providing uniformity with previous studies (3, 4, 9, 18, 19) and consistency within this experiment; RZ33 was used to prepare the Mono2 reference vaccine in this study and previous studies. PBMCs obtained from Mono1-vaccinated animals mounted a significantly higher proliferation response after exposure to antigen than either Mono2-vaccinated or control animals (Fig. 3). Mono2-treated animals showed a trend toward a stronger, but nonsignificant, proliferation response to antigen than control animals (Fig. 3).
Fig. 3.
Proliferation response to serovar Hardjo antigen. Cattle PBMCs were incubated at 37°C under 5% CO2 for 7 days and pulsed with [3H]thymidine, and incorporation was determined by liquid scintillation. Group mean stimulation indices were calculated as described in Materials and Methods. Significant differences (P ≤ 0.05) between Mono1 and either Mono2 or control animals are indicated by asterisks. The error bars represent standard errors.
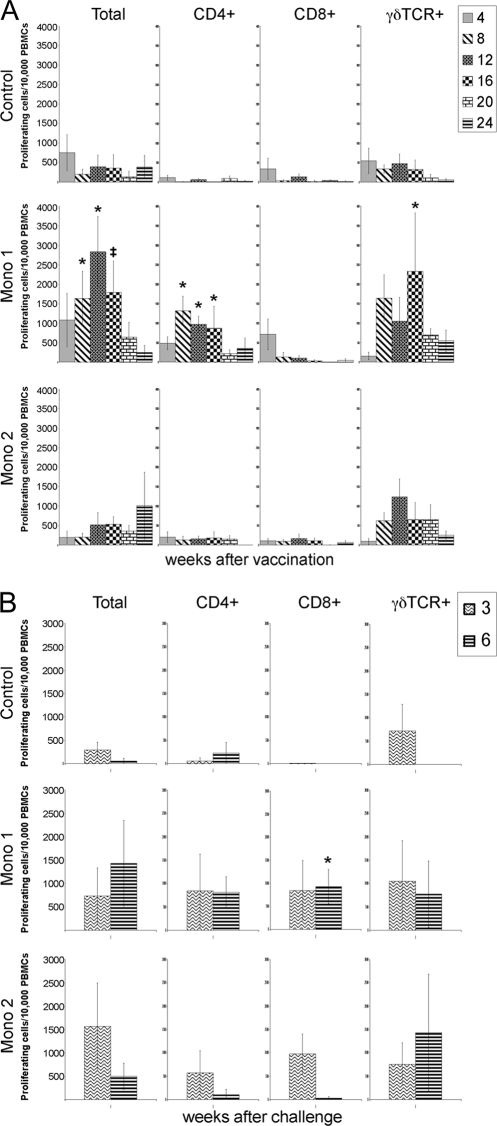
The identity of lymphocytes replicating in response to antigen was determined by flow cytometry. Consistent with the stimulation indices, cattle vaccinated with Mono1 showed a significant increase in overall lymphocyte populations starting at 8 weeks p.v. and continuing through week 16 over samples from both control and Mono2-treated animals (Fig. 4 A). Between weeks 8 and 16 p.v. there was a significant increase of CD4+ and a trend for higher γδ T cell populations in both sets of vaccinated animals.
Fig. 4.
Proliferating lymphocytes identified by surface marker. PKH-67-stained cells were surface labeled for CD4, CD8, and γδ TCR and then separated and analyzed by flow cytometry. Proliferating cells before (A) and after (B) live challenge with serovar Hardjo were identified by a reduction in PKH-67 staining using standard techniques. Significant differences (P ≤ 0.05) between Mono1 and both Mono2 and control animals are indicated by asterisks. ‡, significant differences (P ≤ 0.05) between Mono1 and control animals but not between either group and Mono2 animals. The error bars represent standard errors.
Following infectious challenge, PBMCs from Mono1-vaccinated animals responded with a strong proliferation response (Fig. 3). CD4+, CD8+, and γδ T cells from both Mono1- and Mono2-vaccinated animals proliferated in response to antigen following challenge (Fig. 4B). In contrast, only limited proliferation of γδ T cells was seen in samples from control animals at 3 weeks p.i. Aside from the Mono1-vaccinated CD8+ T cell population at week 6 p.i., no significant differences were detected in lymphocyte proliferation profiles among the three animal groups (Fig. 4B).
Lymphocyte IFN-γ production.
PBMCs collected from Mono1-vaccinated animals produced significantly higher levels of IFN-γ in response to antigen than PBMCs obtained from control or Mono2-vaccinated animals (data not shown). IFN-γ production by PBMCs from Mono1-vaccinated animals peaked at 8 weeks p.v. and returned to background by week 24 p.v. (data not shown). PBMCs from control animals did not produce significantly elevated levels of IFN-γ in response to antigen exposure.
NK cells respond to antigen.
Previous studies have provided strong evidence that a Th1 response primarily involving CD4+ and γδ T cells is important for protection against live serovar Hardjo challenge. In this study we observed results (Fig. 4) similar to those that we (18, 19) and others (3, 4, 9) have reported previously over the course of vaccination and postchallenge.
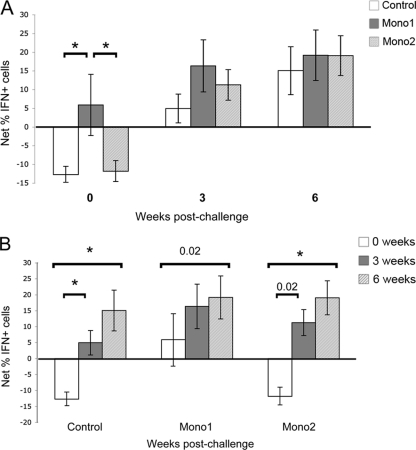
The role of bovine NK cells has not previously been examined in relation to Leptospira vaccination or infection. Therefore, we tested NK cells (those carrying the CD335 marker) for the capacity to express an IFN-γ recall response when exposed to antigen. Before infectious challenge, a significantly higher percentage of NK cells from Mono1-vaccinated animals expressed IFN-γ than either control or Mono2-vaccinated animals (Fig. 5 A). Following challenge, NK cells from animals in all groups showed significant increases in the percentage of IFN-γ-positive CD335+ cells (Fig. 5B) and the mean fluorescence intensity of IFN-γ in IFN-γ-positive CD335+ cells (data not shown). While Mono1-vaccinated animals had a higher level of IFN-γ-positive NK cells at 3 weeks postchallenge than either control or Mono1-vaccinated animals, by 6 weeks, all groups were equivalent (Fig. 5A). Additionally, NK cells from Mono2-vaccinated animals showed a trend of higher levels of IFN-γ-positive cells than those of controls at 3 weeks, although the levels were not statistically significant (Fig. 5A).
Fig. 5.
Antigen-specific IFN-γ response by NK cells. Lymphocytes were labeled for intracellular IFN-γ and surface labeled to detect CD335+ and then analyzed by flow cytometry. The percentage of IFN-γ-positive CD335+ cells was determined in the presence and absence of serovar Hardjo antigen, and the net mean difference in percentage of IFN-γ-positive cells for each vaccination group was calculated. Data are shown comparing vaccination groups by time (A) and comparing each group over time (B). Significant differences (P ≤ 0.05) between samples are indicated by asterisks, unless otherwise noted. The error bars represent standard errors.
DISCUSSION
In this study, two monovalent serovar Hardjo vaccines, a commercial vaccine (Mono1) and a reference vaccine (Mono2), were tested for the ability to limit infection 1 year following vaccination. Both vaccines reduced renal colonization and urinary shedding of bacteria relative to sham-vaccinated control animals; however, neither vaccine provided sterile immunity. Instead, samples from vaccinated animals provided evidence that bacterial shedding was either reduced or of shorter duration than that of sham-vaccinated control animals. Reduced renal pathology was also seen in Mono1-vaccinated animals versus control or Mono2-vaccinated animals, a finding likely linked to reduced renal colonization. CD4+ and γδ T cells from Mono1-vaccinated animals exhibited strong recall responses, apparent by increased lymphocyte proliferation and IFN-γ production, before live challenge, compared to responses of either control or Mono2-vaccinated animals. A significant finding of this study was the discovery that NK cells exhibit a recall response in Mono1-vaccinated animals before challenge and that after challenge, NK cell production of IFN-γ in response to bacterial antigen exposure from vaccinated and nonvaccinated animals increases significantly.
Identification of Leptospira-infected animals is improved through the use of multiple methods to detect bacteria or bacterial DNA (24). In this study we used a combination of bacteriological culture, FA, PCR, and microscopic examination of tissue sections to evaluate the infection status of animals challenged with live L. borgpetersenii serovar Hardjo. Successful bacteriological culture of L. borgpetersenii serovar Hardjo following infectious challenge provides irrefutable evidence of infection. However, recovery of live serovar Hardjo is rarely successful when using samples from animals vaccinated prior to infectious challenge (6–8), even if the vaccine clearly fails to prevent infection (7). A lack of success in isolating viable serovar Hardjo from infected vaccinated animals may be due to inhibitory factors or low bacterial numbers (7). Our results were similar to those of previous studies where infection control animals yielded positive bacteriological cultures whereas vaccinated animals did not (Table 1). Therefore, we relied on more sensitive assays (PCR and FA) to identify potentially infected animals. Representative PCR results (data not shown) provide evidence that bacterial shedding in the urine is intermittent. While these data are not quantitative, preliminary data obtained using real-time PCR of urine samples from control and vaccinated animals indicates that control animals shed at least 10- to 100-fold more bacteria than vaccinated animals (data not shown).
We found that cattle were susceptible to infection 3 months after receiving two inoculations of Mono1 (Table 2), whereas previously we reported that a related vaccine provided short-term immunity to infection (5). However, we attribute the difference between these two studies to an enhanced ability to detect the presence of bacteria in clinical samples using PCR. In the previous study, detection methods were limited to bacteriological culture and FA analysis, both of which were negative for samples obtained from Mono1-vaccinated animals in the short-term immunity portion of this study. It is important to note that neither PCR nor FA analysis can discriminate between living or dead bacteria. However, we assume that detection of bacterial DNA in urine of animals more than 1 week following live challenge indicates that bacteria may at least transiently colonize the kidney. Persistent shedding of bacterial DNA over several weeks was interpreted as an indication that a chronic infection was established. Data presented in Tables 1 and 2 clearly show that few Mono1-vaccinated animals had persistent infections in either the short-term or long-term studies, whereas bacterial DNA was detected in kidney samples from all control animals and 5 of 7 Mono2-vaccinated animals (Table 2). The immune response that ensues following Mono1 vaccination may limit kidney colonization and frequently clear the infection over time; however, we also provide evidence that renal colonization may persist (Fig. 1).
By analyzing the immune response stimulated by Mono1, it may be possible to identify key factors that limit infection and may contribute to bacterial clearance. Previous studies showed that vaccination could prime CD4+ and γδ T cells to undergo a Leptospira antigen-induced Th1 response with IFN-γ production 3 to 4 months after vaccination (2, 18, 19). The results of this study are consistent with previous findings, and we provide evidence that lymphocytes maintained immunological memory 1 year following vaccination with significantly increased overall lymphocyte proliferation (Fig. 3). While CD4+ and γδ T cells from Mono1-vaccinated animals proliferated when exposed to bacterial antigens, lymphocytes from control or Mono2-vaccinated animals lacked significant responses to antigen before challenge (Fig. 4A). Surprisingly, there was no statistical difference in CD4+ or γδ T cell proliferation among the three experimental groups following challenge (Fig. 4B). In contrast, CD8+ cells from Mono1-vaccinated animals exhibited immunological memory after challenge with a significant difference at 6 weeks p.i. (Fig. 4B). It should be noted that to maintain uniformity with previous studies (3, 4, 9, 18, 19), ex vivo lymphocyte stimulation assays used antigens prepared from the high-passage strain RZ33 rather than the low-passage challenge strain 203. Extended cultivation of L. borgpetersenii serovar Hardjo may alter outer membrane protein expression similarly to what has been reported for other pathogenic Leptospira species. Future studies may benefit from the use of low-passage bacteria to identify proteins important in inducing protective immunity.
Although NK cells have been primarily considered part of the innate immune response, recent studies show that NK cells have the capacity for immunological memory; this implies that they also have a role in acquired immunity (12, 20, 22, 23). The present study provides evidence that bovine NK cells possess the capacity for immunological memory in relation to leptospirosis; before infectious challenge, NK cells from Mono1-vaccinated animals responded to bacterial antigen by producing IFN-γ (Fig. 5). Following challenge, NK cells from all animal groups produced IFN-γ in response to bacterial antigen, and the percentage of IFN-γ-positive cells increased until termination of the study (Fig. 5). This finding suggests that NK cells help limit Leptospira infection and, if primed by vaccination, may help to resolve infection. By identifying specific antigens that stimulate NK, CD4+, CD8+, and γδ T cells, it may be possible to develop subunit vaccines that more rapidly clear L. borgpetersenii serovar Hardjo infection and better prevent disease transmission.
ACKNOWLEDGMENTS
We thank David Haake for his generous gift of anti-LipL32 sera, Richard Hornsby for exceptional technical support in all phases of this experiment, Aileen Hudspith, Deb Buffington, and Bruce Pesch for technical support with cell-based assays and flow cytometry, Ami Frank for technical assistance in PCR analysis of samples, Judith Stasko and Virginia Montgomery for histopathology support, and the animal caretakers at NADC.
Mention of trade names or commercial products in this article is solely for providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 2 February 2011.
REFERENCES
- 1. Alt D. P., Zuerner R. L., Bolin C. A. 2001. Evaluation of antibiotics for treatment of cattle infected with Leptospira borgpetersenii serovar Hardjo. J. Am. Vet. Med. Assoc. 219:636–639 [DOI] [PubMed] [Google Scholar]
- 2. Baldwin C. L., et al. 2002. Activation of bovine peripheral blood γδ T cells for cell division and IFN-γ production. Vet. Immunol. Immunopathol. 87:251–259 [DOI] [PubMed] [Google Scholar]
- 3. Blumerman S. L., Herzig C. T., Baldwin C. L. 2007. WC1+ γδ T cell memory population is induced by killed bacterial vaccine. Eur. J. Immunol. 37:1204–1216 [DOI] [PubMed] [Google Scholar]
- 4. Blumerman S. L., Herzig C. T., Wang F., Coussens P. M., Baldwin C. L. 2007. Comparison of gene expression by co-cultured WC1+ γδ and CD4+ αβ T cells exhibiting a recall response to bacterial antigen. Mol. Immunol. 44:2023–2035 [DOI] [PubMed] [Google Scholar]
- 5. Bolin C. A., Alt D. P. 2001. Use of a monovalent leptospiral vaccine to prevent renal colonization and urinary shedding in cattle exposed to Leptospira borgpetersenii serovar Hardjo. Am. J. Vet. Res. 62:995–1000 [DOI] [PubMed] [Google Scholar]
- 6. Bolin C. A., Cassells J. A., Zuerner R. L., Trueba G. 1991. Effect of vaccination with a monovalent Leptospira interrogans serovar Hardjo type hardjo-bovis vaccine on type hardjo-bovis infection of cattle. Am. J. Vet. Res. 52:1639–1643 [PubMed] [Google Scholar]
- 7. Bolin C. A., Thiermann A. B., Handsaker A. L., Foley J. W. 1989. Effect of vaccination with a pentavalent leptospiral vaccine on Leptospira interrogans serovar Hardjo type hardjo-bovis infection of pregnant cattle. Am. J. Vet. Res. 50:161–165 [PubMed] [Google Scholar]
- 8. Bolin C. A., Zuerner R. L., Trueba G. 1989. Effect of vaccination with a pentavalent leptospiral vaccine containing Leptospira interrogans serovar Hardjo type hardjo-bovis on type hardjo-bovis infection of cattle. Am. J. Vet. Res. 50:2004–2008 [PubMed] [Google Scholar]
- 9. Brown R. A., et al. 2003. Comparison of three different leptospiral vaccines for induction of a type 1 immune response to Leptospira borgpetersenii serovar Hardjo. Vaccine 21:4448–4458 [DOI] [PubMed] [Google Scholar]
- 10. Bulach D. M., et al. 2006. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc. Natl. Acad. Sci. U. S. A. 103:14560–14565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cole J. R., Jr., Sulzer C. R., Pursell A. R. 1973. Improved microtechnique for the leptospiral microscopic agglutination test. Appl. Microbiol. 25:976–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper M. A., et al. 2009. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. U. S. A. 106:1915–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellinghausen H. C., Jr., McCullough W. G. 1965. Nutrition of Leptospira Pomona and growth of 13 other serotypes: fractionation of oleic albumin complex and a medium of bovine albumin and polysorbate 80. Am. J. Vet. Res. 26:45–51 [PubMed] [Google Scholar]
- 14. Faine S., Adler B., Bolin C., Perolat P. 1999. Leptospira and leptospirosis. MediSci, Melbourne, Australia [Google Scholar]
- 15. Johnson R. C., Harris V. G. 1967. Differentiation of pathogenic and saprophytic leptospires. I. Growth at low temperatures. J. Bacteriol. 94:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Littell R., Milliken G., Stroup W., Wolfinger R., Schabenberger O. 2006. SAS for mixed models, 2nd ed. SAS Press, Cary, NC [Google Scholar]
- 17. Matsunaga J., Werneid K., Zuerner R. L., Frank A., Haake D. A. 2006. LipL46 is a novel surface-exposed lipoprotein expressed during leptospiral dissemination in the mammalian host. Microbiology 152:3777–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naiman B. M., Alt D., Bolin C. A., Zuerner R., Baldwin C. L. 2001. Protective killed Leptospira borgpetersenii vaccine induces potent Th1 immunity comprising responses by CD4 and γδ T lymphocytes. Infect. Immun. 69:7550–7558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naiman B. M., et al. 2002. Evaluation of type 1 immune response in naive and vaccinated animals following challenge with Leptospira borgpetersenii serovar Hardjo: involvement of WC1+ γδ and CD4 T cells. Infect. Immun. 70:6147–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Leary J. G., Goodarzi M., Drayton D. L., von Andrian U. H. 2006. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7:507–516 [DOI] [PubMed] [Google Scholar]
- 21. Olsen S. C., Boyle S. M., Schurig G. G., Sriranganathan N. N. 2009. Immune responses and protection against experimental challenge after vaccination of bison with Brucella abortus strain RB51 or RB51 overexpressing superoxide dismutase and glycosyltransferase genes. Clin. Vaccine Immunol. 16:535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun J. C., Beilke J. N., Lanier L. L. 2009. Adaptive immune features of natural killer cells. Nature 457:557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun J. C., Lanier L. L. 2009. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity? Eur. J. Immunol. 39:2059–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wagenaar J., Zuerner R. L., Alt D., Bolin C. A. 2000. Comparison of polymerase chain reaction assays with bacteriologic culture, immunofluorescence, and nucleic acid hybridization for detection of Leptospira borgpetersenii serovar Hardjo in urine of cattle. Am. J. Vet. Res. 61:316–320 [DOI] [PubMed] [Google Scholar]
- 25. Zuerner R. L. 2005. Laboratory maintenance of pathogenic Leptospira. Curr. Protoc. Microbiol. 12E:1–13 [DOI] [PubMed] [Google Scholar]
- 26. Zuerner R. L., Alt D., Bolin C. A. 1995. IS1533-based PCR assay for identification of Leptospira interrogans sensu lato serovars. J. Clin. Microbiol. 33:3284–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]