Abstract
The surface-associated glycopeptides gp40, one of the most polymorphic Cryptosporidium antigens, and gp15, one of the most immunodominant Cryptosporidium antigens, are putative vaccine candidates because they mediate infection in vitro and induce immune responses in vivo. We evaluated antibody responses to these antigens before and after the first episode of symptomatic cryptosporidiosis in 51 children from a birth cohort study in an area in South India where Cryptosporidium is endemic and a major cause of parasitic diarrhea. IgG levels to gp15 and to homotypic and heterotypic gp40 antigens were measured in pre- and postdiarrheal sera by enzyme-linked immunosorbent assay (ELISA). There was a significant IgG response to gp15 (P < 0.001) following the first episode of cryptosporidial diarrhea. Using a general additive model, we determined the estimated time of the peak IgG response to gp15 to be 9.3 weeks (confidence interval, 5.2 to 13.4) following the diarrheal episode. In a subset of 30 children infected with Cryptosporidium hominis subtype Ia, there was a significant difference in IgG responses to homotypic C. hominis Ia and to heterotypic Cryptosporidium parvum II gp40 antigens (P = 0.035). However, there was also a significant correlation (P = 0.001) in the responses to both antigens in individual children, suggesting that while responses are in part subtype specific, there is significant cross-reactivity to both antigens. This is the first report of the characterization of immune responses to cryptosporidiosis in Indian children and the first study to investigate human immune responses to the polymorphic gp40 antigen. However, further studies are needed to determine whether immune responses to these antigens are protective against subsequent infections.
INTRODUCTION
Cryptosporidium spp. are frequent causes of infectious diarrhea in children in developing countries (reviewed in references 19, 23, and 27). In these countries, malnourished children are at greater risk of acquiring cryptosporidiosis, and in turn, the disease is more severe in malnourished than in well-nourished children (reviewed in references 19 and 23). Early childhood cryptosporidiosis in these areas may lead to worsening malnutrition and growth faltering as well as to physical and cognitive deficits (15, 16, 19, 24, 34). Treatment options for cryptosporidiosis are limited. Nitazoxanide, the only drug that has shown some efficacy in immunocompetent individuals (48), is not effective in immunocompromised patients (1) and has not been widely evaluated in children, particularly those who are malnourished, in developing countries. There is no vaccine available for the prevention of cryptosporidiosis.
Among Cryptosporidium species, C. parvum and C. hominis cause most human infections, with C. hominis predominating in developing countries (reviewed in reference 54). Cryptosporidiosis in children is widespread in India (4, 8, 29, 32, 36, 37) and is the major cause of parasitic diarrhea in children under the age of 5 in South India (3, 4). The most common species identified in Indian children is C. hominis (3, 18, 36).
Immune responses to Cryptosporidium are poorly understood, and the correlates of protective immunity are not known. While cell-mediated immunity is crucial for resistance to and resolution of infection, antibodies may play a role in preventing the parasite from attaching to and invading host cells during its invasive stages (reviewed in references 44 and 12). In adults who are naturally or experimentally infected with Cryptosporidium, antibody responses are associated with partial protection from subsequent challenge, and the presence of preexisting antibodies is associated with decreased severity and duration of infection (reviewed in references 12 and 44). However, it remains to be determined whether these responses are themselves protective or whether they are reflective of protective cellular responses (44). Regardless, knowledge of immune responses to putative protective antigens is essential in order to design immune-based preventive strategies.
Recent efforts to identify putative protective antigens have focused on surface-associated proteins that mediate attachment to and invasion of host cells by invasive stages of the parasite (reviewed in references 12 and 51). The best studied of these antigens are gp40 and gp15, proteolytic cleavage products of a major surface glycoprotein, gp40/15 (also called GP60 or S60), which we (14) and others (43, 49, 52) have cloned and characterized. gp15 (also known as Cp17 or S16) is the C-terminal cleavage product of gp40/15 and is one of the most immunodominant Cryptosporidium antigens identified to date. The presence of preexisting anti-gp15 antibodies is associated with protection from diarrhea in naturally or experimentally infected adults (reviewed in references 12 and 44). gp15 has also been shown to induce gamma interferon-mediated cellular responses in previously infected humans (40).
gp40, the N-terminal cleavage product of gp40/15, is a secreted, mucin-like glycoprotein that associates on the parasite surface with the glycosylphosphatidylinositol (GPI)-anchored gp15 glycopeptide (39) and mediates attachment to and subsequent invasion of host cells (14, 51). The gene encoding gp40/15 is one of the most polymorphic genes identified in Cryptosporidium spp. (reviewed in references 54 and 28). This high degree of polymorphism in gp40 is consistent with the possibility that it is a virulence determinant that is under selective host immune pressure. Because of the extensive polymorphisms, the gp40/15 locus is the most widely used for subtyping of clinical and environmental samples (54). Most of the polymorphisms are clustered in the hypervariable region of the gp40 part of the molecule, while gp15 is relatively conserved (31, 38, 50). Although gp40 has been shown to induce humoral and cell-mediated immune responses in mice (10, 47), it is not known whether this protein is immunogenic in infected humans or whether immune responses to it are species or subtype specific.
Previously, we investigated the clinical features and molecular and spatial epidemiology of cryptosporidiosis in a birth cohort of children in a semiurban slum community in South India (2, 3). The most common species identified in diarrheal stool samples from these children was C. hominis, with gp40/15 subtype Ia predominating. The overall goal of the present study was to assess serum antibody responses to gp15 and gp40 after the first episode of cryptosporidial diarrhea in the same cohort of children and to determine if antibody responses to gp40 are species and subtype specific.
(These data were presented, in part, at the 44th Annual Meeting of the Infectious Diseases Society of America, Toronto, Canada, October 2006.)
MATERIALS AND METHODS
Study subjects and samples.
Fifty-three children with 58 episodes of cryptosporidial diarrhea (defined as one or more episodes of diarrhea associated with the presence of Cryptosporidium spp. in the stool detected by microscopy) were enrolled in the study.
These children were part of a birth cohort of 452 children enrolled in a study on rotavirus infection (9, 22) and were monitored from birth until the age of 3 years. Briefly, the cohort was recruited from the semiurban slum areas of Ramnaickapalayam, Chinnallapuram, and Kaspa in Vellore, South India. An episode of diarrhea was defined as at least 1 day of diarrhea (three or more watery stools in a 24-h period) preceded and followed by 2 or more days without diarrhea. Stool samples were collected during all diarrheal episodes and fortnightly for surveillance. Serum was collected within 3 to 6 weeks of rotaviral diarrhea and at specific time points every 3 months during the first year and at 6-month intervals thereafter. Serum samples were stored in aliquots at −20°C prior to testing. The study was approved by the Institutional Review Board of Christian Medical College, and informed consent was obtained from the parents of the children enrolled in the study.
The average age (± the standard deviation [±SD]) of the children during their first episode of cryptosporidial diarrhea was 16.8 (±8.4) months. The clinical and sociodemographic characteristics of these children and the results of the molecular characterization of the Cryptosporidium spp. identified in their stools have been previously published (3).
Serum samples from 51 of the 53 children collected before (prediarrheal) and after (postdiarrheal) the first episodes of cryptosporidial diarrhea were available for testing in the current study.
ELISA.
Sequences encoding gp40 and gp15 from C. parvum subtype II (GCH1 isolate), obtained from Saul Tzipori, Tufts Cummings School of Veterinary Medicine, North Grafton, MA, gp40 from C. hominis subtype Ia (TU502 isolate), also obtained from S. Tzipori, and a control protein containing the His, thioredoxin, and S-tags alone (14) were cloned into the pET32Xa/LIC vector (Novagen). The recombinant (r) proteins were overexpressed in Escherichia coli and purified by metal affinity chromatography as described previously (14, 40). Levels of serum IgG against gp15 and gp40 were quantified by enzyme-linked immunosorbent assay (ELISA) as previously described (30), using recombinant forms of C. parvum subtype II gp15 (rCpgp15 II) (5a) and gp40 (rCpgp40 II) and C. hominis subtype Ia (rChgp40 Ia) as antigens. Briefly, 96-well microtiter plates (Nunc, Rochester, NY) were coated with recombinant test or control proteins at a concentration of 0.4 μg protein/well. Excess antigen was washed off with 20 mM sodium phosphate–150 mM sodium chloride (pH 7.2; phosphate-buffered saline [PBS]) containing 0.05% Tween 20, and nonspecific binding was blocked with 1% bovine serum albumin (BSA) in PBS. Wells were then incubated with serum diluted 1:100 in PBS with 1% BSA for 1 h at 37°C. After being washed three times, wells were incubated with 50 μl of alkaline phosphatase-conjugated goat-anti-human γ chain-specific IgG (Sigma, St. Louis, MO) diluted 1:5,000 in 0.25% BSA/PBS. After being washed, wells were incubated with 50 μl of substrate solution (100 mM Tris-HCl [pH 9.5], 100 mM NaCl, 5 mM MgCl2) containing p-nitrophenyl phosphate (1 mg/ml; Sigma, St. Louis, MO) at room temperature. The reaction stopped after 15 min, and absorbance was read at 405 nm (A405). The same positive- and negative-control sera (sera that were positive or negative by ELISA and Western blotting using C. parvum lysate as the antigen) were run on each plate to control for plate-to-plate variation. All samples were run in triplicate, and the mean A405 was determined. A405 values for the control protein containing only the fusion tags were subtracted from the A405 values for the patient sample. To adjust for interplate variability, values were normalized by dividing the A405 value of the sample by the A405 value of the positive control for that plate and multiplying by 100. The results were expressed as ELISA units (EU) (30).
Statistical analysis.
Data were analyzed using Stata 10.1 for Windows (StataCorp, College Station, TX) and R 2.10.0 statistical software (http://www.r-project.org/). The differences in serum IgG levels pre- and postdiarrhea were compared using the paired t test, and log-fold changes in IgG levels postdiarrhea were compared to a null hypothesis of no change using the one-sample t test. The correlation between pre- and postdiarrheal serum IgG levels was calculated using Spearman's rank-order correlation coefficient.
Generalized additive model for analysis of serological response.
Assuming that the serum IgG response following a diarrheal episode was nonlinear and time dependent, we aligned pre- and postdiarrheal levels of anti-gp15 IgG with respect to the time of the first diarrheal episode (considering it to be at time 0). In order to obtain the timing of the peak IgG response to gp15, curve fitting was performed using the generalized additive model (53; R. Sarkar, submitted for publication) with cubic splines supported by 5 knots. The quality of the fit was determined based on adjusted R2 values. Modeling was performed with and without one pair of IgG EU values that had an unusual (reverse) pattern and resulted in a predicted curve and its confidence interval (CI). The uncertainty boundaries for the timing of the peak serum IgG response were then obtained by simulating the curve for the period of 4 to 20 weeks postdiarrhea, with a refined time increment of 0.01 week.
RESULTS
IgG response to gp15 before and after the first episode of cryptosporidial diarrhea.
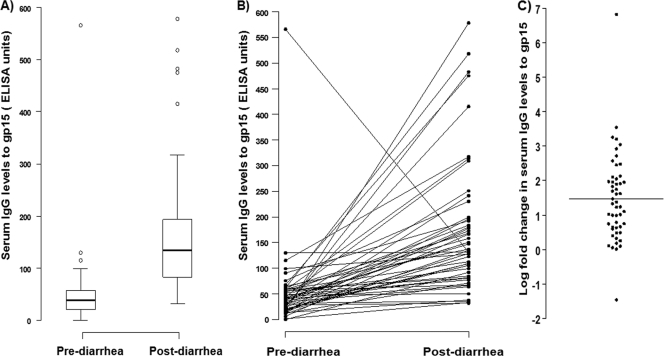
Serum anti-gp15 IgG levels assessed in 51 children before and after the first episode of cryptosporidial diarrhea showed a significant increase from the mean (±SD) prediarrheal level of 51.5 (±78.5) EU to the mean postdiarrheal level of 171.9 (±128.6) EU (P < 0.001) (Fig. 1 A). In these children, there was a mean 1.5 (±1.3)-log-fold increase in serum IgG levels after a diarrheal episode (P < 0.001) (Fig. 1C). There was no significant correlation between pre- and postdiarrheal IgG levels (Spearman's ρ = 0.154; P = 0.281) for each child (Fig. 1B), indicating that the response to the infection did not depend on the baseline values.
Fig. 1.
Serum IgG responses to gp15 in children following the first episode of cryptosporidial diarrhea (n = 51). (A) Box plots of serum IgG levels in pre- and postdiarrheal samples. (B) Pre- and postdiarrhea levels of IgG in response to gp15 in each child. (C) Log-fold increase in serum IgG levels following the first episode of diarrhea.
Time to peak serum IgG response to gp15.
When we plotted the data with respect to episode timing (Fig. 2 A), we observed that the time to the episode and the time since the episode varied, and increases in EU values were unevenly distributed with respect to the postepisode sample's timing. Analysis of the timing of serum sample collection revealed that sera were collected at a mean (±SD) of 8.6 (±6.9) weeks for prediarrheal sera and a mean of 8.6 (±5.2) weeks for postdiarrheal sera, indicating that the intervals between the diarrheal episode and both pre- and postsample collection were comparable (P = 0.985) (Fig. 2A). When the generalized additive model was applied to these data (Fig. 2B), the mean (confidence interval) predicted difference in anti-gp15 IgG levels between the pre- and postdiarrheal sera was 60.2 (±10.7) EU and the predicted maximum difference in anti-gp15 IgG levels between the pre- and postdiarrheal sera was 138.1 (±18.5) EU. The model predicted the time of peak antibody response to be 9.3 weeks (CI, 5.2 to 13.4) following a diarrheal episode. When the model was run without a pair of sera (outlier) with a reverse pattern, the results were not substantially affected (Fig. 2C). Here, the model predicted the time of peak antibody response to be 11 weeks (CI, 5.2 to 17.2). The predicted difference in anti-gp15 IgG levels between the pre- and postdiarrheal sera was 65.8 (±9.4) EU, and the predicted maximum difference in anti-gp15 IgG levels between the pre- and postdiarrheal sera was 148.5 (±16.8) EU.
Fig. 2.
Generalized additive model to estimate time to peak serum IgG levels in response to gp15 following the first episode of cryptosporidial diarrhea (n = 51). (A) Times of pre- and postdiarrhea serum collection in relation to first diarrheal episode (week 0). The dashed line indicates week 0. (B and C) Generalized additive model demonstrating the peak antibody response in the weeks following the diarrheal episode with the outlier (B) and without the outlier (C). The dashed vertical lines indicate the lower and upper confidence interval for the predicted time of peak response; the solid vertical line indicates the predicted time of peak responses; the dashed plot lines indicate the lower and upper confidence interval for the predicted maximum difference in antibody level; the solid plot line indicates the predicted maximum difference in antibody level.
IgG response to gp40 before and after the first episode of diarrhea in C. hominis subtype Ia-infected children.
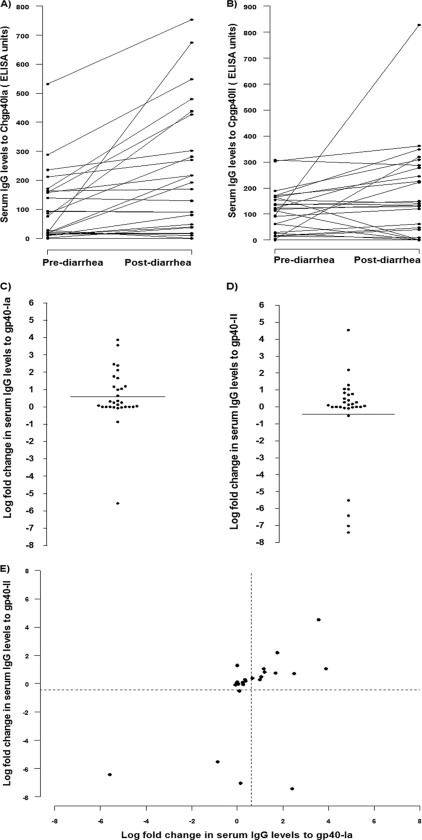
In our previous study on the molecular characterization of Cryptosporidium species and subtypes infecting the children in the cohort, we found that most children were infected with C. hominis and that the predominant gp40/15 subtype was Ia (3). In order to determine if antibody responses to the polymorphic gp40 antigen were subtype specific or cross-reactive between antigens, we compared IgG levels in response to the homotypic gp40 antigen from the infecting species and subtype (C. hominis gp40 Ia) with those in response to the next-most-common heterotypic gp40 antigen (C. parvum gp40 IIa) in all 30 children who were infected with C. hominis subtype Ia. Comparison of pre- and postdiarrheal IgG levels for each child showed that there was a significant correlation between IgG levels before and after diarrhea in response to the homotypic antigen (Spearman's ρ = 0.568; P < 0.001) as well as to the heterotypic antigen (Spearman's ρ = 0.678; P < 0.001) (Fig. 3 A and B). While there was a 0.6 (±1.6)-log-fold increase in mean (±SD) IgG levels in response to the homotypic antigen following the episode of diarrhea, there was a 0.4 (±2.7)-log decrease in mean (±SD) IgG levels in response to the heterotypic antigen, and this difference in response was statistically significant (P = 0.035) (Fig. 3C and D). However, comparison of the log-fold change in IgG levels for each individual child showed that there was a significant correlation in the levels of antibody against both (Spearman's ρ = 0.598; P < 0.001) (Fig. 3E). These results suggest that while serum IgG responses to gp40 are in part subtype specific, there is cross-reactivity between homotypic and heterotypic antigens.
Fig. 3.
Serum IgG responses to homotypic and heterotypic gp40 in children infected with C. hominis subtype Ia (n = 30). (A and B) Pre- and postdiarrhea serum IgG levels in response to Chgp40Ia (A) and Cpgp40II (B) in each child. (C and D) Log-fold increase in IgG levels in response to Chgp40Ia (C) and Cpgp40II (D). (E) Correlation of log-fold changes in serum IgG levels in response to Chgp40Ia and Cpgp40II IgG in each child infected with C. hominis subtype Ia. The dashed vertical line and the dashed horizontal line indicate the mean log fold change in serum IgG levels to Chgp40 Ia and II, respectively.
The postdiarrheal levels of IgG in response to C. hominis gp40 Ia and C. parvum gp40 II in children infected with C. hominis subtype Ia showed greater individual variability than those in response to the gp15 antigen. This finding, along with the fact that there were fewer data points (51 children in the study versus 30 children infected with C. hominis subtype Ia), resulted in wider confidence intervals for the time of peak response as determined by the general additive model (data not shown), so no meaningful conclusions about the timing of the response could be drawn.
DISCUSSION
Although Cryptosporidium spp. are well known to be a major cause of parasitic diarrhea in children and HIV-infected adults in India (5, 6, 20, 45), this is the first study to investigate immune responses to this parasite in India. In our study, the birth cohort design and the collection of stool samples from all diarrheal episodes enabled us to identify the first episode of symptomatic cryptosporidiosis in children in the cohort and to investigate the immune responses to putative protective antigens resulting from this episode. We found a strong antibody response to the immunodominant gp15 antigen (35, 42), which was predicted to peak ∼9 weeks after the episode, and showed for the first time that the polymorphic gp40 antigen induces humoral immune responses in infected humans and that these responses, while in part subtype specific, are cross-reactive between homotypic and heterotypic antigens.
Previous studies have documented an immune response to gp15 in symptomatic infections in adults and children (33, 42, 46) (Allison et al., submitted) and in urban populations following waterborne outbreaks of cryptosporidiosis (21, 33). The data from our study in a semiurban slum in a developing country where cryptosporidiosis is endemic confirms and extends these findings. We found a highly significant serum IgG response to this immunodominant antigen in children following the first episode of cryptosporidial diarrhea. In our study, although most children were infected with C. hominis, we used recombinant gp15 derived from C. parvum as the antigen in the ELISAs, since gp15 is relatively conserved between the two species. In a previous study on antibody responses to gp15 in Bangladeshi children with diarrhea, we found a highly significant correlation between serum antibody responses to recombinant gp15 derived from both species even though most children were infected with C. hominis (Allison et al., submitted). Preidis et al. found that C. hominis but not C. parvum gp15 induced gamma interferon-mediated cellular immune responses in adult volunteers with serological evidence of prior Cryptosporidium infections (40). However, the infecting Cryptosporidium species in their study was not known.
In a novel approach, we applied the generalized additive model to these data and calculated the predicted time to the peak antibody response after an episode of diarrhea. Other investigators have used this temporal modeling approach to study the impact of vaccination on the incidence of hepatitis B in infants (25), the emergence of fluoroquinolone resistance (11), the time of HIV infection in hemophiliacs (7), etc., but to our knowledge this is the first time it has been applied to data to model antibody responses over time. Validating this statistical approach, the predicted prediarrheal anti-gp15 IgG levels and the mean rise in IgG levels were similar to the mean IgG level measured by ELISA. Application of these statistical tools to serological data can provide an approach to understanding the dynamics of humoral immune responses over time. In other studies, seroconversion following an episode of diarrhea has been used as a more sensitive marker of infection than direct detection of a Cryptosporidium sp. in stool samples (41) and as a method to evaluate interventional tools to improve water quality (17).
The predominance of a single species (C. hominis) and gp40/15 subtype (Ia) in the study area (3) facilitated a natural experiment on species- and subtype-specific responses to the polymorphic gp40 antigen from both major Cryptosporidium species in these children. The mucin-like gp40 antigen mediates infection in vitro (13) and may thus serve as a target for preventive or interventional modalities. This antigen contains both conserved and polymorphic domains. The N-terminal polyserine domain is conserved (except for the number of serine residues) (14, 49, 52) among all species and subtypes identified thus far and is predicted to be heavily O glycosylated (H. Ward, unpublished data). The region that is C terminal to the polyserine domain is highly polymorphic among all known species and subtypes and contains only a single conserved serine residue (Ward, unpublished). If gp40 is to be considered a putative vaccine candidate, it is essential to determine whether immune responses to this antigen are species and subtype specific or cross-reactive among different polymorphic forms and whether these responses are directed at glycan, peptide, or glycopeptide epitopes.
The findings of our study indicate that antibody responses to nonglycosylated peptide epitopes of gp40 do occur, since the antigens used for ELISA were E. coli-derived recombinant proteins that are not glycosylated. The significant fold increase in serum IgG levels in response to both heterotypic and homotypic gp40 antigens and the significant correlation between responses to both antigens following the first episode of symptomatic cryptosporidiosis in these children suggest that there is cross-reactivity between the antigens, most likely to peptide epitopes in the conserved polyserine domain. However, the finding that the fold increase in IgG levels was significantly greater in response to the homotypic C. hominis subtype Ia gp40 antigen than to the heterotypic C. parvum subtype IIa antigen in children infected with C. hominis subtype Ia suggests that subtype-specific responses may also occur, most likely to peptide epitopes in the hypervariable domain.
In conclusion, we took advantage of samples and data from an existing birth cohort study and incorporated molecular, immunological, and statistical techniques to study antibody responses to the immunodominant gp15 and polymorphic gp40 antigens following the first episode of symptomatic cryptosporidiosis among children in a developing country where Cryptosporidium spp. are endemic. Both gp15 and gp40 are surface-associated glycopeptides that are actively involved in the process of sporozoite attachment to and invasion of host cells and that induce antibody and cell-mediated immune responses and are therefore attractive vaccine candidates (10, 26, 51). This study is an initial step in determining whether either or both of these antigens could be targeted for vaccine development.
However, there are a number of limitations to our study. In addition to the small number of subjects, we were limited to investigation of systemic antibody responses to peptide epitopes of gp15 and gp40 following the first episode of symptomatic cryptosporidiosis. Since this retrospective study was designed for analysis of the correlates of immunity to rotavirus infection, there was no screening for asymptomatic cryptosporidial infections, and therefore the sera were collected at various time points in relation to the diarrheal episode associated with cryptosporidiosis and so could not be temporally correlated to a specific episode of cryptosporidial infection. In addition, we were not able to investigate cell-mediated responses or to determine whether humoral or cellular immune responses are directed at glycan or glycopeptide epitopes of both antigens or at conserved or polymorphic domains of gp40 and, most importantly, whether immune responses to either or both of these antigens induce protective immunity. Currently, our efforts are directed at investigating these possibilities in a prospective, longitudinal study of systemic and mucosal cellular and humoral immune responses to peptide and glycopeptide forms of these antigens expressed in Toxoplasma gondii (39) during symptomatic and asymptomatic cryptosporidiosis in a birth cohort of children in this community.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants FIC R03 TW2711, NIAID R01 AI52786, and R01 AI072222. S.S.R.A. and R.S. were supported by FIC training grant D43 TW007392. G.A. was supported by NIAID training grant T32 AI07389.
None of the authors has any financial interest in any commercial company represented in this study nor any other potential conflict of interest.
Footnotes
Published ahead of print on 2 February 2011.
REFERENCES
- 1. Abubakar I., Aliyu S. H., Arumugam C., Hunter P. R., Usman N. K. 2007. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst. Rev. 2007:CD004932 [DOI] [PubMed] [Google Scholar]
- 2. Ajjampur S., et al. 2010. Symptomatic and asymptomatic Cryptosporidium infections in children in a semi-urban slum community in southern India. Am. J. Trop. Med. Hyg. 83:1110–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ajjampur S. S., et al. 2007. Molecular and spatial epidemiology of cryptosporidiosis in children in a semiurban community in South India. J. Clin. Microbiol. 45:915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ajjampur S. S., et al. 2010. Multisite study of cryptosporidiosis in children with diarrhea in India. J. Clin. Microbiol. 48:2075–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ajjampur S. S., Sankaran P., Kang G. 2008. Cryptosporidium species in HIV-infected individuals in India: an overview. Natl. Med. J. India 21:178–184 [PubMed] [Google Scholar]
- 5a. Allison G., et al. Antibody responses to the immunodominant Cryptosporidium gp15 antigen and gp15 polymorphisms in a case control study of cryptosporidiosis in children in Bangladesh. Am. J. Trop. Med. Hyg., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anand L., Dhanachand C., Brajachand N. 1998. Prevalence and epidemiologic characteristics of opportunistic and non-opportunistic intestinal parasitic infections in HIV positive patients in Manipur. J. Commun. Dis. 30:19–22 [PubMed] [Google Scholar]
- 7. Bacchetti P., Quale C. 2002. Generalized additive models with interval-censored data and time-varying covariates: application to human immunodeficiency virus infection in hemophiliacs. Biometrics 58:443–447 [DOI] [PubMed] [Google Scholar]
- 8. Ballal M., Shivananda P. G. 2002. Rotavirus and enteric pathogens in infantile diarrhoea in Manipal, South India. Indian J. Pediatr. 69:393–396 [DOI] [PubMed] [Google Scholar]
- 9. Banerjee I., et al. 2007. Neonatal infection with G10P[11] rotavirus did not confer protection against subsequent rotavirus infection in a community cohort in Vellore, South India. J. Infect. Dis. 195:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benitez A. J., McNair N., Mead J. R. 2009. Oral immunization with attenuated Salmonella enterica serovar Typhimurium encoding Cryptosporidium parvum Cp23 and Cp40 antigens induces a specific immune response in mice. Clin. Vaccine Immunol. 16:1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berger P., et al. 2004. Generalized additive model demonstrates fluoroquinolone use/resistance relationships for Staphylococcus aureus. Eur. J. Epidemiol. 19:453–460 [DOI] [PubMed] [Google Scholar]
- 12. Borad A., Ward H. 2010. Human immune responses in cryptosporidiosis. Future Microbiol. 5:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cevallos A. M., et al. 2000. Mediation of Cryptosporidium parvum infection in vitro by mucin-like glycoproteins defined by a neutralizing monoclonal antibody. Infect. Immun. 68:5167–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cevallos A. M., et al. 2000. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect. Immun. 68:4108–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Checkley W., et al. 1998. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am. J. Epidemiol. 148:497–506 [DOI] [PubMed] [Google Scholar]
- 16. Checkley W., et al. 1997. Asymptomatic and symptomatic cryptosporidiosis: their acute effect on weight gain in Peruvian children. Am. J. Epidemiol. 145:156–163 [DOI] [PubMed] [Google Scholar]
- 17. Crump J. A., et al. 2007. Comparing serologic response against enteric pathogens with reported diarrhea to assess the impact of improved household drinking water quality. Am. J. Trop. Med. Hyg. 77:136–141 [PubMed] [Google Scholar]
- 18. Das P., et al. 2006. Molecular characterization of Cryptosporidium spp. from children in Kolkata, India. J. Clin. Microbiol. 44:4246–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dillingham R. A., Lima A. A., Guerrant R. L. 2002. Cryptosporidiosis: epidemiology and impact. Microbes Infect. 4:1059–1066 [DOI] [PubMed] [Google Scholar]
- 20. Dwivedi K. K., et al. 2007. Enteric opportunistic parasites among HIV infected individuals: associated risk factors and immune status. Jpn. J. Infect. Dis. 60:76–81 [PubMed] [Google Scholar]
- 21. Frost F. J., et al. 1998. A two-year follow-up survey of antibody to Cryptosporidium in Jackson County, Oregon following an outbreak of waterborne disease. Epidemiol. Infect. 121:213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gladstone B. P., et al. 2010. Burden of illness in the first 3 years of life in an Indian slum. J. Trop. Pediatr. 56:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Griffiths J. K. 1998. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv. Parasitol. 40:37–85 [DOI] [PubMed] [Google Scholar]
- 24. Guerrant D. I., et al. 1999. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am. J. Trop. Med. Hyg. 61:707–713 [DOI] [PubMed] [Google Scholar]
- 25. Hens N., et al. 2008. Estimating the impact of vaccination using age-time-dependent incidence rates of hepatitis B. Epidemiol. Infect. 136:341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hong-Xuan H., et al. 2005. Expression of the recombinant fusion protein CP15-23 of Cryptosporidium parvum and its protective test. J. Nanosci. Nanotechnol. 5:1292–1296 [DOI] [PubMed] [Google Scholar]
- 27. Huang D. B., Chappell C., Okhuysen P. C. 2004. Cryptosporidiosis in children. Semin. Pediatr. Infect. Dis. 15:253–259 [DOI] [PubMed] [Google Scholar]
- 28. Jex A. R., Gasser R. B. 2010. Genetic richness and diversity in Cryptosporidium hominis and C. parvum reveals major knowledge gaps and a need for the application of “next generation” technologies—research review. Biotechnol. Adv. 28:17–26 [DOI] [PubMed] [Google Scholar]
- 29. Kaur R., Rawat D., Kakkar M., Uppal B., Sharma V. K. 2002. Intestinal parasites in children with diarrhea in Delhi, India. Southeast Asian J. Trop. Med. Public Health 33:725–729 [PubMed] [Google Scholar]
- 30. Khan W. A., et al. 2004. Cryptosporidiosis among Bangladeshi children with diarrhea: a prospective, matched, case-control study of clinical features, epidemiology and systemic antibody responses. Am. J. Trop. Med. Hyg. 71:412–419 [PubMed] [Google Scholar]
- 31. Leav B. A., et al. 2002. Analysis of sequence diversity at the highly polymorphic Cpgp40/15 locus among Cryptosporidium isolates from human immunodeficiency virus-infected children in South Africa. Infect. Immun. 70:3881–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahajan M., Mathur M., Talwar V. 1992. Cryptosporidiosis in east Delhi children. J. Commun. Dis. 24:133–137 [PubMed] [Google Scholar]
- 33. McDonald A. C., et al. 2001. Cryptosporidium parvum-specific antibody responses among children residing in Milwaukee during the 1993 waterborne outbreak. J. Infect. Dis. 183:1373–1379 [DOI] [PubMed] [Google Scholar]
- 34. Mølbak K., et al. 1997. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, west Africa. Am. J. Clin. Nutr. 65:149–152 [DOI] [PubMed] [Google Scholar]
- 35. Moss D. M., et al. 1998. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J. Infect. Dis. 178:827–833 [DOI] [PubMed] [Google Scholar]
- 36. Nagamani K., Pavuluri P. R., Gyaneshwari M., Prasanthi K., Rao M. I. S., Saxena N. K. 2007. Molecular characterisation of Cryptosporidium: an emerging parasite. Indian J. Med. Microbiol. 25:133–136 [DOI] [PubMed] [Google Scholar]
- 37. Nath G., Shukla B. N., Reddy D. C., Sanyal S. C. 1993. A community study on the aetiology of childhood diarrhoea with special reference to Campylobacter jejuni in a semiurban slum of Varanasi, India. J. Diarrhoeal Dis. Res. 11:165–168 [PubMed] [Google Scholar]
- 38. O'Connor R. M., Thorpe C. M., Cevallos A. M., Ward H. D. 2002. Expression of the highly polymorphic Cryptosporidium parvum Cpgp40/15 gene in genotype I and II isolates. Mol. Biochem. Parasitol. 119:203–215 [DOI] [PubMed] [Google Scholar]
- 39. O'Connor R. M., Wanyiri J. W., Cevallos A. M., Priest J. W., Ward H. D. 2007. Cryptosporidium parvum glycoprotein gp40 localizes to the sporozoite surface by association with gp15. Mol. Biochem. Parasitol. 156:80–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Preidis G. A., et al. 2007. Seropositive human subjects produce interferon gamma after stimulation with recombinant Cryptosporidium hominis gp15. Am. J. Trop. Med. Hyg. 77:583–585 [PubMed] [Google Scholar]
- 41. Priest J. W., et al. 2005. Changes in serum immunoglobulin G levels as a marker for Cryptosporidium sp. infection in Peruvian children. J. Clin. Microbiol. 43:5298–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Priest J. W., et al. 2006. Longitudinal analysis of Cryptosporidium species-specific immunoglobulin G antibody responses in Peruvian children. Clin. Vaccine Immunol. 13:123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Priest J. W., Kwon J. P., Arrowood M. J., Lammie P. J. 2000. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol. Biochem. Parasitol. 106:261–271 [DOI] [PubMed] [Google Scholar]
- 44. Riggs M. W. 2002. Recent advances in cryptosporidiosis: the immune response. Microbes Infect. 4:1067–1080 [DOI] [PubMed] [Google Scholar]
- 45. Sadraei J., Rizvi M. A., Baveja U. K. 2005. Diarrhea, CD4+ cell counts and opportunistic protozoa in Indian HIV-infected patients. Parasitol. Res. 97:270–273 [DOI] [PubMed] [Google Scholar]
- 46. Sandhu S. K., et al. 2006. The natural history of antibody responses to Cryptosporidium parasites in men at high risk of HIV infection. J. Infect. Dis. 194:1428–1437 [DOI] [PubMed] [Google Scholar]
- 47. Singh I., Theodos C., Tzipori S. 2005. Recombinant proteins of Cryptosporidium parvum induce proliferation of mesenteric lymph node cells in infected mice. Infect. Immun. 73:5245–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith H. V., Corcoran G. D. 2004. New drugs and treatment for cryptosporidiosis. Curr. Opin. Infect. Dis. 17:557–564 [DOI] [PubMed] [Google Scholar]
- 49. Strong W. B., Gut J., Nelson R. G. 2000. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 68:4117–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Strong W. B., Nelson R. G. 2000. Preliminary profile of the Cryptosporidium parvum genome: an expressed sequence tag and genome survey sequence analysis. Mol. Biochem. Parasitol. 107:1–32 [DOI] [PubMed] [Google Scholar]
- 51. Wanyiri J., Ward H. 2006. Molecular basis of Cryptosporidium-host cell interactions: recent advances and future prospects. Future Microbiol. 1:201–208 [DOI] [PubMed] [Google Scholar]
- 52. Winter G., Gooley A. A., Williams K. L., Slade M. B. 2000. Characterization of a major sporozoite surface glycoprotein of Cryptosporidum parvum. Funct. Integr. Genomics 1:207–217 [DOI] [PubMed] [Google Scholar]
- 53. Wood S. N. 2006. Generalized additive models: an introduction with R. Chapman and Hall/CRC Press, Boca Raton, FL [Google Scholar]
- 54. Xiao L. 2010. Molecular epidemiology of cryptosporidiosis: an update. Exp. Parasitol. 124:80–89 [DOI] [PubMed] [Google Scholar]