Abstract
Myeloid-derived dendritic cells (DCs) generated from monocytes obtained from stage IIIB cervical cancer (CaCx IIIB) patients show dysfunctional maturation; thus, antitumor T cell functions are dysregulated. In an objective to optimize these dysregulated immune functions, the present study is focused on the ability of neem leaf glycoprotein (NLGP), a nontoxic preparation of the neem leaf, to induce optimum maturation of dendritic cells from CaCx IIIB patients. In vitro NLGP treatment of immature DCs (iDCs) obtained from CaCx IIIB patients results in upregulated expression of various cell surface markers (CD40, CD83, CD80, CD86, and HLA-ABC), which indicates DC maturation. Consequently, NLGP-matured DCs displayed balanced cytokine secretions, with type 1 bias and noteworthy functional properties. These DCs displayed substantial T cell allostimulatory capacity and promoted the generation of cytotoxic T lymphocytes (CTLs). Although NLGP-matured DCs derived from CaCx monocytes are generally subdued compared to those with a healthy monocyte origin, considerable revival of the suppressed DC-based immune functions is noted in vitro at a fairly advanced stage of CaCx, and thus, further exploration of ex vivo and in vivo DC-based vaccines is proposed. Moreover, the DC maturating efficacy of NLGP might be much more effective in the earlier stages of CaCx, where the extent of immune dysregulation is less and, thus, the scope of further investigation may be explored.
INTRODUCTION
Cervical cancer (CaCx) is the second most common type of tumor worldwide, and its incidence is disproportionately high (>80%) in the developing world (35). It remains an important health problem for women, especially in underserved and socioeconomically backward classes (29). Immunotherapy with mature monocyte-derived dendritic cells (DCs) pulsed with human papillomavirus type 16 or 18 (HPV16/18) E7 oncoprotein antigens is a promising approach in treating this disease (32). These alternate therapeutic approaches are aimed toward controlling late-stage detection and preventing recurrence (31), an arena where application of traditional therapeutic approaches like radical surgery or radiotherapy is difficult.
DCs are extremely potent antigen-presenting cells (APC) that function in vitro and in vivo to initiate T lymphocyte responses to antigens (14). The ability of DCs to stimulate T cells is attributed to their dichotomous nature as immature DCs (iDCs) and mature DCs (mDCs) (30). Immature DCs effectively capture antigens but lack full T cell stimulatory activity and are sensitive to the immunosuppressive effects of an immunoregulatory cytokine, interleukin-10 (IL-10). In contrast, mature DCs exhibit an enhanced level of antigen presentation, full T cell stimulatory activity, production of a type 1 cytokine, IL-12, and low production of a type 2 cytokine, IL-10 (36).
In the last few years, a multitude of diagnostic studies have shown a strong immunosuppressive state of the cervical epithelium, which is one major reason for the fragile health of cervical cancer patients (25). Such immune suppression usually manifests as defective DC maturation (27), increased frequencies of CD4+ CD25+ Foxp3+ T regulatory cells (12), altered cytokine production toward a type 2 bias (22), and a large amount of indoleamine 2,3-dioxygenase (IDO) (26) in the defensive niches of the host. As these events are tightly associated, the proper maturation of DCs would have an immense role in overcoming CaCx-associated immunosuppression.
A previous study of a nontoxic preparation of the neem leaf (16), a rich source of neem leaf glycoprotein (NLGP), demonstrated its ability to mature human myeloid (15)- and mouse bone marrow (34)-derived DCs. This NLGP may replace toxic lipopolysaccharide (LPS) or a cytokine cocktail as a maturating agent that promotes suppressor regulatory T cells (4). In an immunotherapeutic demand to overcome these limitations, the role of NLGP in rescuing DCs from a CaCx-associated immature state to a mature condition, thereby initiating optimum T cell functions, is investigated in the present study. These T cell activities are crucial for the clinical response of stage IIIB CaCx patients in conventional therapy. Obtained results demonstrated its overall potency in overcoming defective DC maturation in CaCx for the generation of optimum type 1 antitumor T cell responses. This knowledge will help in the planning and execution of in vivo and ex vivo DC modulation in stage IIIB CaCx patients during and after conventional treatment.
MATERIALS AND METHODS
Patient selection.
Twelve female patients aged 35 to 75 years with histopathologically confirmed CaCx and possessing Karnofsky scores between 70 and 90 (Table 1) were enrolled in the study, which was approved by the Institutional Ethical Committee. Peripheral venous heparinized blood samples were obtained from all patients before any surgical and/or radiotherapeutic treatment. They all were diagnosed with moderately differentiated squamous cell carcinoma at clinical stage IIIB. Female age-matched healthy donors participated as controls. Informed written consent was obtained from patients and healthy individuals.
Table 1.
Information on CaCx IIIB patients with moderately differentiated squamous cell carcinoma
| Patient no.b | Age (yr) | Socioeconomic status | Menopausal status | Maximum diam of tumor (cm) | Karnofsky score at time of sample collectiona |
|---|---|---|---|---|---|
| 1 | 62 | Low | Post | 4 | 80 |
| 2 | 48 | Middle | Post | 4 | 90 |
| 3 | 35 | Low | Pre | 6 | 90 |
| 4 | 65 | Low | Post | 3 | 90 |
| 5 | 40 | Low | Pre | 4 | 80 |
| 6 | 45 | Low | Pre | 4 | 90 |
| 7 | 51 | Low | Post | 15 | 90 |
| 8 | 45 | Low | Pre | 4 | 90 |
| 9 | 50 | Middle | Post | 4 | 90 |
| 10 | 56 | Low | Post | 4 | 90 |
| 11 | 50 | Middle | Post | 5 | 70 |
| 12 | 60 | Low | Post | 3 | 90 |
A measure of the patients overall physical health, judged by their level of activity.
All patients listed were HPV positive.
Reagents and antibodies.
Recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) and recombinant human IL-4 (rhIL-4) were obtained from BD Pharmingen (San Diego, CA). Fluorescence-labeled anti-human CD1a monoclonal antibodies were procured from BD Pharmingen (San Diego, CA). Fluorescence-labeled anti-human CD14, CD40, CD80, CD86, CD83, and HLA-ABC were obtained from eBioscience (San Diego, CA). Anti-rabbit IgG-horseradish peroxidase (HRP) was procured from Sigma (St. Louis, MO). OptEIA kits for cytokine estimation (IL-12, IL-10, and gamma interferon [IFN-γ]) and a Cytofix/Cytoperm kit were obtained from BD Pharmingen (San Diego, CA). A lactate dehydrogenase (LDH) release assay kit for cytotoxicity detection was obtained from Roche Diagnostics, Mannheim, Germany. Lymphocyte separation media (LSM) were purchased from MP Biomedicals (Solon, OH).
NLGP.
Extract obtained from neem (Azadirachta indica) leaves was prepared by using the method described earlier (6, 11). Mature leaves of the same size (on average, length of 6.5 cm, width of 2.2 cm, and surface area of 15.7 mm3) and color (dark green) (indicative of same age), taken from a standard source (leaves were collected from an identified tree in the investigator's area; comparable results were also obtained with leaves of other sources, as tested in a tumor growth restriction assay [6] and IFN-γ release assay [8], as described previously), were shed dried and pulverized. The leaf powder was soaked overnight in phosphate-buffered saline (PBS) prepared in endotoxin-free water, pH 7.4, and supernatant was collected by centrifugation at 1,500 rpm. The neem leaf preparation (NLP) was then extensively dialyzed against PBS, pH 7.4, and concentrated by a Centricon membrane filter (Millipore Corporation, Bedford, MA), with a 10-kDa molecular mass cutoff. The endotoxin content of the freshly prepared NLP was determined by Limulus amebocyte lysate (LAL) testing, as per the manufacturer's instructions (Salesworth, Bangalore, India). The glycoprotein present in this NLP (NLGP) was isolated and characterized by the method described previously (5, 13). Endotoxin contamination of NLGP was checked by a polymyxin B assay, as described previously (22). The endotoxin content of all the batches of NLGP was found to be less than 6 pg/ml, as reported previously (6). The protein concentration of the purified preparation containing NLGP was measured by Lowry's method using Folin's phenol reagent (21). The average protein concentration of the obtained preparation was 0.77 μg/mg of dry neem leaf powder. The purity of NLGP was confirmed by high-pressure liquid chromatography (HPLC) before use (data not shown), and a NLGP concentration of 1.5 μg/ml was used in most of the experiments (15).
Tumor cell lines.
Cervical cancer (HeLa) and lymphoma (U937) cell lines originally obtained from the National Center for Cell Sciences, Pune, India, were maintained in minimal essential medium (MEM; Life Technologies, NY) with 10% fetal bovine serum (FBS), penicillin (50 units/ml), and streptomycin (50 μg/ml) at 37°C with a supply of 5% CO2.
Generation and maturation of DCs.
Myeloid DCs were generated from the adherent fraction of peripheral blood mononuclear cells (PBMC) by the method described earlier (15). In brief, adherent fractions were cultured in 6-well plates (1.0 × 106 cells/ml) with fresh complete medium supplemented with 1,000 U/ml rhGM-CSF and 500 U/ml rhIL-4 for 5 days and then cultured for an additional 3 days with either LPS (1 μg/ml) or NLGP (1.5 μg/ml). Every 2 days, the media, including the supplements, were refreshed. After 8 days of culture, mDCs were harvested and extensively washed before use. Following 6 days or 8 days of DC culture, supernatants (stored at −80°C) were used for the estimation of IL-12 and IL-10.
Flow cytometric analysis of immune cellular markers.
Flow cytometric analysis of surface phenotypic markers for immature DCs (iDCs) and mature DCs (mDCs) was performed after the cells were labeled with different anti-human fluorescence-labeled antibodies (20 μl each; CD1a, CD14, CD80, CD86, CD83, CD40, and HLA-ABC) for 30 min, as per the manufacturer's recommendation. After being labeled, the cells were washed in FACS buffer (PBS, pH 7.4, with 1% FBS) and fixed in 1% paraformaldehyde in PBS, and cytometry was performed using Cell Quest software on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Suitable negative isotype controls were used to rule out the background fluorescence. The data were generated by cytofluorometric analyses of 10,000 events. The percentage of each positive population and mean fluorescence intensities (MFI) were determined using quadrant statistics.
Preparation of HeLa cell extract as an antigen source and its pulsation in DCs.
HeLa cells harvested from confluent culture were stirred in cold PBS for 2 h and then centrifuged at 12,000 × g for 1 h. Supernatant containing tumor-associated antigen extracted from HeLa cells was collected and stored at −20°C. The protein concentration of the extract was determined by the Bradford assay by following the manufacturer's instructions. For antigen pulsing, iDCs on day 6 were incubated with HeLa extract for 48 h with the simultaneous addition of the maturating agent (NLGP and the control maturating agent LPS) at 37°C. The DCs expressing tumor-associated antigen on their surfaces (as monitored by flow cytometry) were then collected, the number of cells was counted, and their morphology was studied microscopically and processed for subsequent analysis.
Cytokine detection assays.
To quantify cytokines, supernatants from iDC and mDC culture were harvested, and cell-free supernatant was stored. Secretion of IL-12p70 and IL-10 was assessed with enzyme-linked immunosorbent assay (ELISA) kits, as per the manufacturer's instructions, and the optical density at 450 nm was measured using a microplate reader (BioTek, United Kingdom).
Purification of T cells.
CD8+ T cells were purified using a magnetic assisted cell sorter (MACS), according to the manufacturer's instructions (Miltenyi Biotec, GmbH). In brief, isolated PBMC were labeled with a biotin-antibody cocktail, followed by incubation with actin-biotin microbeads. The cell suspension was then loaded on a MACS column and allowed to pass through. The effluent containing the cell population enriched with the CD8+ T cell fraction was collected. The purity of cells was checked by flow cytometry after being labeled with fluorescent-conjugated anti-CD8 antibody, and cell preparations with >90% purity were used for the experiment.
Generation of CTLs and intracellular secretion of IFN-γ.
Cytotoxic T lymphocytes (CTLs) were generated after coculturing autologous T cells with DCs pulsed with HeLa extract for 48 h and simultaneously matured with NLGP. After fixation and permeabilization using Cytofix/Cytoperm solutions, CTLs were stained for intracellular secretion of IFN-γ using corresponding fluorescence-conjugated antibody. Suitable negative-isotype controls were used to rule out the background fluorescence. Cells were analyzed with FACSCalibur using CellQuest software. The data were generated by cytofluorometric analysis of 10,000 events. The percentage of each positive population and MFI were determined using quadrant statistics.
Allogeneic T cell proliferation assay.
MACS-purified T cells were used for allogeneic T cell proliferation assays. For proliferation, mature DCs irradiated (3,000 rad) and pulsed with HeLa extract were cocultured with allogeneic T cells at different DC/T cell ratios. Proliferation was checked after 96 h by a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay as described previously (6).
In vitro cytotoxicity assay.
The cytotoxicity of CTLs against HeLa and U937 cells was tested by LDH release assay using a cytotoxicity detection kit. In brief, tumor cells (1 × 104 cells) were plated overnight as targets in 96-well cell culture plates. CTLs (1 × 105) generated from CaCx patients were added in triplicate in each well as effectors and cocultured overnight. Cell-free supernatant was used to measure the level of released LDH, using following formula: % cytotoxicity = [(lysis from effector-target mixture − lysis from effector only − spontaneous lysis)/(maximum lysis − spontaneous lysis)] × 100.
Statistical analysis.
All results represent the average values obtained from six separate in vitro experiments. In the ELISA, LDH release assay, etc., a value represents the mean result from six individual observations and is presented as the mean ± standard deviation (SD). Statistical significance was established by using INSTAT3 software.
RESULTS
CaCx IIIB-associated altered DC phenotypes are partially corrected by NLGP.
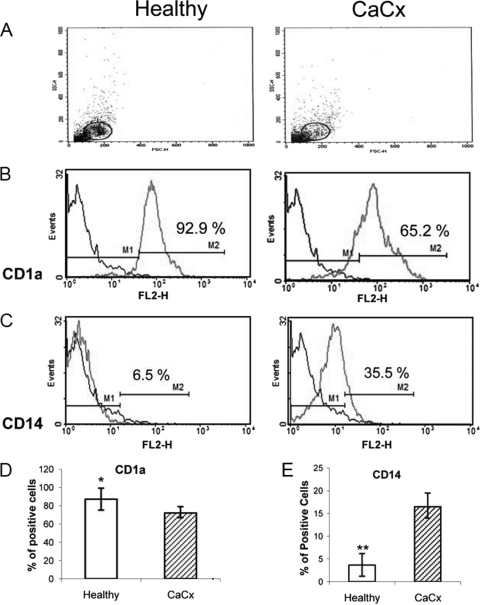
Unhealthy immature DCs (iDCs) were noticed frequently in our study with stage IIIB CaCx (CaCx IIIB) patients after in vitro differentiation of iDCs from monocytes with GM-CSF and IL-4, in comparison to those obtained from age-matched healthy controls (Fig. 1). The number of CD1a-expressing cells is comparatively less in iDCs (n = 6) from CaCx patients than in those from healthy donors. As differentiation of monocytes into iDCs is accompanied by the acquisition of CD1a, these observations are indicative of the dysregulated functioning of monocytes in stage IIIB CaCx patients. Monocyte differentiation into DCs resulted in the loss of the CD14 marker. However, in CaCx DCs, incomplete loss of the CD14 marker was noted, in comparison with the loss of that marker in healthy DCs, denoting dysregulation in DC differentiation in CaCx patients (Fig. 1). Based on our earlier observations on the ability of NLGP for unique immunomodulation (9, 10) and for induction of DC maturation (15), here we attempted to study the potential of NLGP to rectify the undesired phenotypic changes of iDCs, related to DC activation, maturation, and functions in CaCx IIIB patients.
Fig. 1.
Comparative status of GM-CSF- and IL-4-differentiated iDCs from myeloid cells obtained from CaCx patients and healthy individuals. Monocytes were isolated from blood specimens obtained from CaCx patients (n = 6) and healthy individuals (n = 6) and differentiated with GM-CSF plus IL-4 for 5 days. The statuses of CD1a and CD14 in iDCs before maturation between CaCx patients and healthy individuals were compared. (A) Forward scatter (FSC)/side scatter (SSC) dot plot of DCs; (B, D) acquisition of CD1a phenotypes, depicted by a representative figure (B) and a bar diagram showing the percentage of positive cells (D); (C, E) loss of CD14 phenotypes, depicted by a representative figure (C) and a bar diagram showing the percentage of positive cells (E). *, P < 0.01 (CD1a cells in comparison to healthy cells); **, P < 0.001 (CD14 cells in comparison to healthy cells).
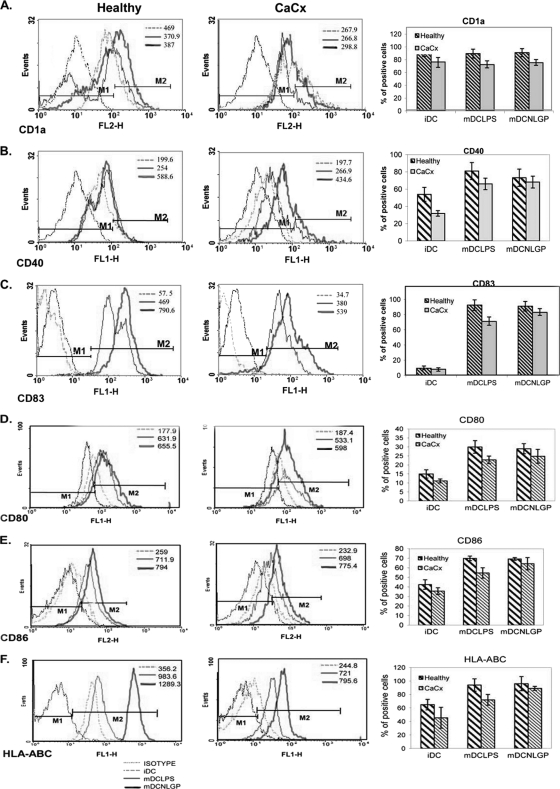
To meet this objective, we have subjected iDCs (from CaCx IIIB patients) to undergo maturation in the presence of NLGP (1.5 μg/ml) on day 6 of culture, keeping LPS as a control (n = 6 in each case). Following stimulation (48 h), we analyzed the DC phenotypes by flow cytometry. Observation indicates the remarkable ability of NLGP to restore DC phenotypes in cancer patients, which is comparable to that of bacterial LPS. NLGP promoted the upregulation of CD1a (Fig. 2 A), a protein that mediates the presentation of primarily lipid and glycolipid antigens of self or microbial origin to T cells (28).
Fig. 2.
Expression of costimulatory and maturation markers on dendritic cells (DCs) obtained from CaCx patients and healthy individuals. Human myeloid cells from CaCx patient (n = 6) and healthy control (n = 6) samples were differentiated with GM-CSF/IL-4 and matured with either LPS to DCs (mDCLPS) or NLGP to DCs (mDCNLGP). Expression of CD1a (A), CD40 (B), CD83 (C), CD80 (D), CD86 (E), and HLA-ABC (F) was studied using various fluorescence-labeled markers as indicated and analyzed by flow cytometry using Cell Quest and WINMD1 software. An appropriate isotype-matched antibody was used as a negative control. The percentage of positive cells was monitored by flow cytometric analysis after gating of DC-rich zones from FSC/SSC plots. A representative figure from each case is presented. Bar diagrams show the average expression levels as the mean percentages of positive cells ± SDs. The values indicated on the histograms are the mean fluorescence intensities (MFI) of the positive cells in the gated population. The results are representative of six similar experiments. Levels of significance are as follows. (A) For CD1a, iDCs versus NLGP DCs from healthy individuals, P < 0.05; iDCs from healthy individuals versus iDCs from CaCx patients, P = 0.0047. (B) For CD40, iDCs versus NLGP/LPS DCs from healthy individuals, P < 0.001; iDCs versus NLGP/LPS DCs from CaCx patients, P < 0.005; iDCs from healthy individuals versus iDCs from CaCx patients, P = 0.002. (C) For CD83, iDCs versus NLGP/LPS DCs from healthy individuals and CaCx patients, P < 0.001; iDCs versus LPS DCs from CaCx patients, P < 0.01; iDCs from healthy individuals versus iDCs from CaCx patients, P = 0.0072. (D) For CD80, iDCs versus NLGP/LPS DCs from healthy individuals, P < 0.001; iDCs versus NLGP/LPS DCs from CaCx patients, P < 0.001. (E) For CD86, iDCs versus NLGP/LPS DCs from healthy individuals, P < 0.001. (F) For HLA-ABC, iDCs versus NLGP/LPS DCs from healthy individuals, P < 0.01; iDCs versus NLGP DCs from CaCx patients, P = 0.0015; iDCs from healthy individuals versus iDCs from CaCx patients, P = 0.03.
Antigen transport to the cell surface coincides with increased expression of costimulatory molecules, such as B7-1/CD80, B7-2/CD86, and major histocompatibility complexes (19), and as such, their expression is increased in NLGP-cultured DCs, including both healthy and CaCx DCs. CD40-CD40L interaction plays a critical role in DC-T cell cross talk (24) and NLGP-upregulated CD40 expression on CaCx DC surfaces (Fig. 2B). As reported previously, CD40L expression was also increased by NLGP on T cells (9). Along with these changes, upregulation of maturation-specific marker CD83 (3) was also noted in CaCx IIIB DCs after stimulation with NLGP (Fig. 2C), confirming the augmentation of the maturation process by NLGP. NLGP upregulated several markers on mDCs to a significant extent (like CD80, CD86, and HLA-ABC) (Fig. 2D to F), as reflected in the percentage of positive cells and in the level of MFI (expression per cell). However, a parallel comparison to healthy controls revealed that NLGP-matured DCs generated from monocytes obtained from healthy females displayed much higher proportions of cells expressing these surface markers than NLGP-matured DCs from CaCx patients (Fig. 2A to F).
CaCx IIIB-associated dysregulated cytokine production from DCs is modulated by NLGP.
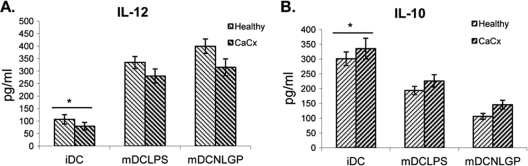
The soluble cytokine profile secreted by matured DCs varies with the existing microenvironment and the degree of differentiation and maturation (7). Antigens that prime DCs to secrete IL-12 will typically induce type 1 differentiation (17), whereas antigens that inhibit IL-12 production (e.g., IL-10) will promote type 2 differentiation (18). Type 1 polarization of DCs is required for optimum antitumor function, and as such, we measured the status of IL-12 and IL-10 in cell-free culture supernatant from matured DCs. CD40-CD40L interaction enhances IL-12 production from macrophages and DCs with NLGP (11, 15). Here we noted an increase in IL-12 secretion with maturation (Fig. 3A) that supports our earlier observation that NLGP upregulates expression of CD40 on mature DCs. IL-10 secretion was also measured, and the level was significantly lower upon maturation with NLGP (Fig. 3B), supporting our earlier observations on the ability of this molecule to drive the type 1 response (10, 23). The secretory statuses of these two cytokines from NLGP-matured DCs were comparable to those from DCs matured with LPS. Healthy controls displayed higher levels of secretion of IL-12 and much lower levels of secretion of IL-10, mimicking a more optimally matured DC microenvironment with NLGP (Fig. 3A and B).
Fig. 3.
Type 1 polarization by NLGP-matured DCs. Immature DCs generated from monocytes obtained from CaCx patients (n = 6) and healthy individuals (n = 6) were matured in vitro with NLGP/LPS, and the secretory statuses of type 1 cytokine IL-12 (A) and type 2 cytokine IL-10 (B) were assessed in culture supernatants by ELISA. The mean ± SD is presented in each case. *, P < 0.001 (for iDCs versus NLGP/LPS DCs from healthy individuals and for iDCs versus NLGP/LPS DCs from CaCx patients).
Dysregulated IFN-γ secretion from T cells under the influence of CaCx DCs is corrected by NLGP.
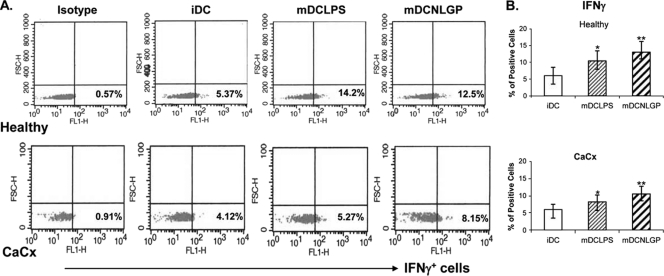
IFN-γ is the hallmark type 1 cytokine (17). Interaction between matured DCs and T cells promotes IFN-γ secretion from T cells and induces type 1 differentiation by upregulating the transcription factor T-bet (2). Our earlier observations implicated NLGP as a potential inducer of IFN-γ from T (9) and NK (11) cells. Hence, we studied the ability of NLGP-matured DCs to induce IFN-γ secretion from T cells upon stimulation with HeLa cell extract (source of the CaCx-associated antigen). Immature DCs on day 6 of culture were pulsed with an extract of HeLa cells at a concentration of 1.5 μg/ml and simultaneously matured with NLGP for 48 h. On day 8, matured DCs were then cocultured with purified T cells from an autologous T cell culture at a ratio of 1:10 for another 48 h. IFN-γ expression in the CTLs against HeLa antigen was then studied by flow cytometry after intracellular staining. NLGP-matured DCs significantly upregulated the expression of IFN-γ in CTLs over that of the controls (Fig. 4).
Fig. 4.
Secretion of IFN-γ from autologous T cells induced by NLGP-matured DCs. MACS-purified autologous CD8+ T cells obtained from CaCx patients and healthy individuals (n = 6 in each case) were cocultured with iDCs, mDCs-LPS and mDCs-NLGP at ratios of 10:1 for 48 h. Intracellular release of IFN-γ in T cells, cultured under various conditions, was measured using fluorescence-labeled anti-IFN-γ antibody and analyzed by flow cytometry using Cell Quest software. An appropriate isotype-matched antibody was used as a negative control. The percentage of positive cells was monitored by flow cytometric analysis after gating of the lymphocyte-rich zone. (A) A representative dot plot from each case is shown. (B) The bar diagram shows the average expression as the percentage of positive cells.*, P < 0.05 (iDCs versus LPS DCs from healthy individuals/CaCx patients); **, P < 0.01 (iDCs versus NLGP DCs from healthy individuals/CaCx patients).
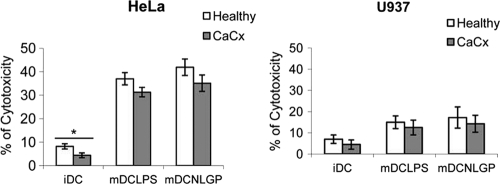
CaCx DC-influenced defective T cell-mediated antigen-specific cytotoxicity is rectified by NLGP.
Next, we assessed the functional ability of DCs matured with NLGP. To meet this objective, immature DCs (n = 6) were pulsed with an extract of HeLa cells and simultaneously matured with NLGP as stated above. Matured DCs was then cocultured with T cells (autologous) in a mixed lymphocyte reaction (MLR) for 48 h at a ratio of 1:10 for the generation of CTLs against HeLa antigen. CTLs thus generated were used to lyse target cells, including HeLa (antigen-positive) and U937 (antigen-negative) cells, and lysis was quantitated by a cytotoxicity assay. We observed higher levels of cytotoxicity in HeLa cells by using NLGP-matured DC-generated CTLs (Fig. 5), without much of a lytic effect on antigen-negative U937 cells. This observation supports the fact that improvement in maturation correlates with the betterment of functional activity of DCs, in terms of the induction of antigen-specific lysis. Immature and unpulsed mature DCs, used as negative controls, did not lead to the generation of CTLs, as reflected in low cytotoxicity values. Healthy controls showed comparatively better cytotoxicity levels than CaCx patients.
Fig. 5.
Induction of tumor cell cytotoxicity by antigen-primed T cells. MACS-purified T cells obtained from CaCx patients (n = 6) and healthy individuals (n = 6) were cocultured with irradiated iDCs and mDCs for 48 h. mDCs-LPS and mDCs-NLGP were pulsed with HeLa cell extract before use in coculture. CTLs generated under these conditions were used to lyse antigen-positive HeLa cells and antigen-negative U937 cells after coculturing at an effector/target (E/T) ratio of 1:10. Bar diagrams represent the mean cytotoxicity values ± SDs obtained from six individual experiments. *, P < 0.001 (iDCs versus NLGP/LPS DCs from healthy individuals/CaCx patients).
NLGP-matured DCs accelerate allogeneic T cell proliferation.
CaCx and healthy DCs matured with NLGP (n = 6) were tested for their ability to stimulate T cells in an allogeneic MLR. Coculturing them with allogeneic T cells (at a ratio of 1:10) stimulated better proliferation of these cells than that of immature DCs, used as a control (Fig. 6). This result demonstrates that DCs matured with NLGP are functional and that the activity levels of these mDCs in the allogeneic MLR correlates closely with their high surface expression levels of CD40, CD83, CD80, and CD86 immunoregulatory proteins. NLGP-matured DCs obtained from healthy controls were consistently better stimulators of allogeneic T cells than DCs obtained from the CaCx patients.
Fig. 6.
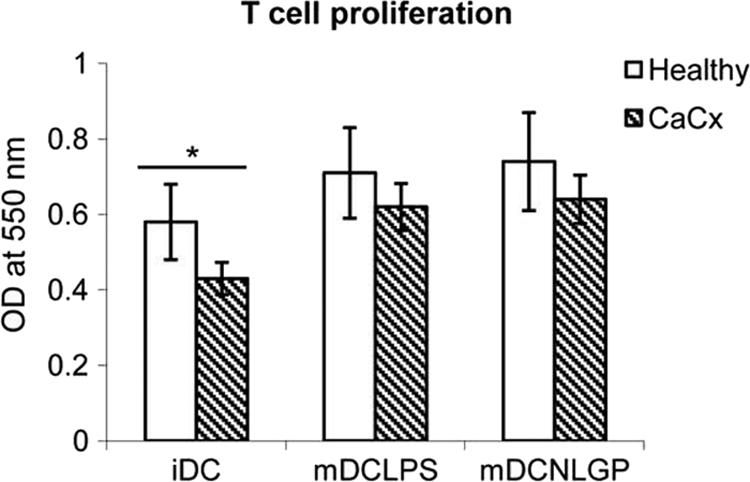
Induction of allogeneic T cell proliferation after antigen presentation by DCs. Allogeneic CD8+ T cells obtained from CaCx patients and healthy individuals (n = 6 in each case) were purified by MACS analysis and cocultured with iDCs, mDCs-LPS, and mDCs-NLGP pulsed with HeLa extract at a ratio of 10:1 for 96 h. Proliferation was checked by the MTT assay. The bar diagram represents the averages from six separate experiments. *, P < 0.001 (iDCs versus NLGP/LPS DCs from healthy individuals/CaCx patients).
DISCUSSION
A mechanism of immune evasion by cancer is the disruption of dendritic cell maturation (1). One way to affect their maturation is to hinder their differentiation from CD14+ monocytes. Monocytes isolated from CaCx IIIB patients display substantial cellular irregularities, which manifest as weak migratory and adhesive capacities and poor differentiating abilities in comparison to those of healthy controls (20). DCs generated from these monocytes exhibit early but dysfunctional maturation (37). The present study was undertaken to investigate the response of monocytes isolated from CaCx IIIB patients, their differentiation into iDCs by treatment with GM-CSF plus IL-4, and their further maturation into mDCs upon in vitro stimulation with NLGP. The substantial potency of NLGP in overcoming immune dysfunction in cancer by exerting its modulatory effect on various interrelated arms of the immunity (5, 6, 8–11, 13, 15) results in NLGP emerging as a promising drug worthy of further exploration. We have reported recently that NLGP induced the DC maturation of healthy human monocyte precursors (15) and mouse bone marrow cells (34). The present study points out that CaCx-associated monocytes display a subnormal differentiating ability into immature DCs, as indicated by comparatively low expression of CD1a and high expression of CD14 upon stimulation with GM-CSF and IL-4, in comparison to that of healthy controls. As such, the ability of NLGP to rectify this dysregulated state was assessed by their efficacy to generate mature DCs from iDCs prepared from poorly functioning CaCx monocytes. DC maturation is accompanied by temporal changes in the expression pattern of various cell surface and intracellular markers. NLGP-matured DCs displayed a significant upregulation of surface determinants, like CD40, CD83, CD80, CD86, and HLA-ABC, compared to that of the iDCs (GM-CSF and IL-4 treatment only). However, the maturating ability of NLGP is always better in the case of DCs generated from healthy monocytes than those generated from CaCx monocytes. CD14 expression in matured DCs was also checked in a few samples from each case. Its expression was completely absent in healthy DCs after maturation, but little expression was detected in CaCx DCs, further denoting the presence of remnants of undifferentiated phenotypes originated from patient monocytes. This is true for both NLGP- and LPS-matured DCs (data not shown). Still, partial restoration of antigen-presenting ability in CaCx DCs may help to restore T cell functions in immunosuppressed patients at an advanced disease stage.
As in our previous studies (15), we have used LPS as a standard positive control. NLGP is superior to LPS in several aspects. First, LPS is toxic and, thus, cannot be recommended in human cancer systems for DC maturation. However, neem and its derivative NLGP can be used in humans, as it is completely nontoxic (16). Second, in both the phenotypic and functional profiles, NLGP-matured DCs from CaCx patients show better results than LPS-matured DCs, as shown. NLGP appears to be superior in reviving the functional differentiation of monocytes from CaCx patients than LPS. The fact should be considered here that the efficacy of any immunomodulator or drug is dependent on the stage of the cancer, with the later stages supposedly being more refractile to immunomodulation than the earlier stages. Stage IIIB is a fairly advanced stage of cervical carcinoma, distinguished by the spread to the pelvic wall, blockage of the flow of urine to the bladder, and progressive immune deformities. Whether this is what that hinders NLGP and LPS in restoring the fully functional normal matured DC phenotype is a subject matter that remains to be elucidated by study of CaCx patients in earlier stages of disease at the time of diagnosis.
A strong induction of a type 1 immune microenvironment is a recurring theme in NLGP-mediated immune modulation (10). Therefore, we wanted to validate the same in the context of NLGP-induced DC maturation. Supernatants obtained from mature DC cultures, in the case of both healthy and CaCx IIIB patients, were assayed for the secretion of IL-12p70, a signature type 1 cytokine, and IL-10, a representative from the type 2 cytokine family. Both NLGP and LPS significantly promoted IL-12 secretion, with a concomitant decrease in IL-10 secretion. However, type 1 commitment was stronger in the case of healthy controls, as noted previously, reinforcing the partial potency of NLGP and LPS in promoting a full-bodied maturation of DCs obtained from CaCx IIIB patients.
Lastly, we performed a few MLR studies to delineate the actual functional state of the mature DCs in CaCx. Our earlier observations suggest the ability of NLGP to enhance antigen uptake and presentation by macrophages and DCs (15, 33). The present report suggested that NLGP-matured DCs pulsed with HeLa cell extract enhanced the proliferation and cytotoxic ability of T cells, strengthening our previous findings and supporting NLGP's suitability in therapeutic vaccine preparations. In another MLR study, substantial enhancement of the intracellular content of IFN-γ was noticed in T cells upon coculturing them with matured DCs that were pulsed with HeLa cell lysate and matured with NLGP. This observation is consistent with our earlier observation, showing that NLGP-matured DCs are active in reducing the IL-4 release from T cells (15). We also reported that during vaccination with carcinoembryonic antigen, NLGP reduces the release of IL-4 (from T cells) and IL-10 (from monocytes and DCs) and enhances the release of IFN-γ (from T cells) and IL-12 (from monocytes and DCs) (33, 34). The capacity of NLGP-matured DCs to prime the production of IFN-γ from T cells and IL-12 secretion from DCs may be partly attributed to the high level of surface expression of CD83/CD86 and/or CD40 in DCs under NLGP stimulation (15) and NLGP-mediated upregulation of the counterpart molecules on the T cell surface, i.e., CD28 and CD40L (9, 10), which coordinates the formation of an effective antitumor immune milieu. At the same time, NLGP downregulates IDO in DCs (data not shown), thus strengthening effective T effector functions by inhibiting regulatory T cell suppressive activities.
Immunomodulatory agents that offer opportunities to reduce treatment-related toxicity of anticancer therapy without diminution of efficacy are a subject of intense investigation. None of the available agents satisfy the criteria for such an ideal immunomodulation. This has stimulated research for discovering natural resources with immunomodulatory activities. Previous studies on head and neck squamous cell carcinoma patients demonstrated the unique immunomodulatory potency of NLGP (9, 13). Current findings in the context of NLGP-induced DC maturation of CaCx IIIB patients add to the unique immunomodulatory repertoire of NLGP and qualify this vaccine approach as a potent candidate to be regarded as a supportive therapy to the conventional treatment regime. Accordingly, NLGP-modulated DC vaccines in in vivo and ex vivo translational settings, along with radiotherapy, may be proposed. At this point, it is of interest to know the gateway of the signal transduction cascade initiated by NLGP. To get a preliminary answer to this question, fluorescein isothiocyanate (FITC)-coupled NLGP was reacted with DCs. NLGP-FITC binds nicely with DC surfaces, as determined by flow cytometric and immunofluorescence studies (data not shown). This observation suggests that DCs may possess some receptors for NLGP. Exploration of its relationship with Toll-like receptors (TLRs) is our future research interest.
ACKNOWLEDGMENT
We thank Subrata Laskar for his help in the characterization of NLGP.
Footnotes
Published ahead of print on 9 February 2011.
REFERENCES
- 1. Almand B., et al. 2000. Clinical significance of defective dendritic cell differentiation in cancer. Clin. Cancer Res. 6:1755–1766 [PubMed] [Google Scholar]
- 2. Aune T. M., Collins P. L., Chang S. 2009. Epigenetics and T helper 1 differentiation. Immunology 126:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banchereau J., et al. 2009. Harnessing human dendritic cell subsets to design novel vaccines. Ann. N. Y. Acad. Sci. 1174:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banerjee D. K., Dhodapkar M. V., Matayeva E., Steinman R. M., Dhodapkar K. M. 2006. Expansion of FOXP3 high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood 108:2655–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baral R. N., Sarkar K., Mandal-Ghosh I., Bose A. 2010. Relevance of neem leaf glycoprotein as a new vaccine adjuvant for cancer immunotherapy, p. 21–45 In Gupta V. K. (ed.), Research in bioactive natural products. Studium Press LLC, Houston, TX [Google Scholar]
- 6. Baral R. N., Chattopadhyay U. 2004. Neem (Azadirachta indica) leaf mediated immune activation causes prophylactic growth inhibition of murine Ehrlich carcinoma and B16 melanoma. Int. Immunopharmacol. 4:355–366 [DOI] [PubMed] [Google Scholar]
- 7. Blanco P., Palucka A. K., Pascual V., Banchereau J. 2008. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 19:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bose A., Haque E., Baral R. 2007. Neem leaf preparation induces apoptosis of tumor cells by releasing cytotoxic cytokines from human peripheral blood mononuclear cells. Phytother. Res. 21:914–920 [DOI] [PubMed] [Google Scholar]
- 9. Bose A., et al. 2009. Neem leaf glycoprotein induces perforin mediated tumor cell killing by T and NK cells through differential regulation of IFNγ signaling. J. Immunother. 32:42–53 [DOI] [PubMed] [Google Scholar]
- 10. Bose A., et al. 2009. Neem leaf glycoprotein directs T-bet associated Th1 type immune commitment. Hum. Immunol. 70:6–15 [DOI] [PubMed] [Google Scholar]
- 11. Bose A., Baral R. N. 2007. NK cellular cytotoxicity of tumor cells initiated by neem leaf preparation is associated with CD40-CD40L mediated endogenous production of IL-12. Hum. Immunol. 68:823–831 [DOI] [PubMed] [Google Scholar]
- 12. Corthay A. 2009. How do regulatory T cells work? Scand. J. Immunol. 70:326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chakraborty K., Bose A., Pal S., Chattopadhyay U., Baral R. 2008. Neem leaf glycoprotein restores the impaired chemotactic activity of peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients by maintaining CXCR3/CXCL10 balance. Int. Immunopharmacol. 8:330–340 [DOI] [PubMed] [Google Scholar]
- 14. Chapuis F., et al. 1997. Differentiation of human dendritic cells from monocytes in vitro. Eur. J. Immunol. 27:431–441 [DOI] [PubMed] [Google Scholar]
- 15. Goswami S., et al. 2010. Neem leaf glycoprotein matures myeloid derived dendritic cells and optimizes anti-tumor T cell functions. Vaccine 28:1241–1252 [DOI] [PubMed] [Google Scholar]
- 16. Haque E., Mandal I., Pal S., Baral R. 2006. Prophylactic dose of neem (Azadirachta indica) leaf preparation restricting murine tumor growth is nontoxic, hematostimulatory and immunostimulatory. Immunopharmacol. Immunotoxicol. 28:33–50 [DOI] [PubMed] [Google Scholar]
- 17. Heufler C., et al. 1996. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur. J. Immunol. 26:659–668 [DOI] [PubMed] [Google Scholar]
- 18. Hirata N., Yanagawa Y., Iwabuchi K., Onoé K. 2009. Selective regulation of interleukin-10 production via Janus kinase pathway in murine conventional dendritic cells. Cell. Immunol. 258:9–17 [DOI] [PubMed] [Google Scholar]
- 19. Kurtz J., Raval F., Vallot C., Der J., Sykes M. 2009. CTLA-4 on alloreactive CD4 T cells interacts with recipient CD80/86 to promote tolerance. Blood 113:3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang S., et al. 2003. Impaired monocyte function in cancer patients: restoration with a cyclooxygenase-2 inhibitor. FASEB J. 17:286–288 [DOI] [PubMed] [Google Scholar]
- 21. Lowry O. H., Rosenbrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 22. Lucey D. R., Clerici M., Shearer G. M. 1996. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 9:532–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mandal-Ghosh I., Chattopadhyay U., Baral R. N. 2007. Neem leaf preparation enhances Th1 type immune response and anti-tumor immunity against breast tumor associated antigen. Cancer Immun. 7:8–17 [PMC free article] [PubMed] [Google Scholar]
- 24. Murugaiyan G., Agrawal R., Mishra G. C., Mitra D., Saha B. 2007. Differential CD40/CD40L expression results in counteracting antitumor immune responses. J. Immunol. 178:2047–2055 [DOI] [PubMed] [Google Scholar]
- 25. Narayan S., et al. 2009. Epithelial expression of human papillomavirus type 16 E7 protein results in peripheral CD8 T-cell suppression mediated by CD4+ CD25+ T cells. Eur. J. Immunol. 39:481–490 [DOI] [PubMed] [Google Scholar]
- 26. Nelson B. H. 2009. IDO and outcomes in ovarian cancer. Gynecol. Oncol. 115:179–180 [DOI] [PubMed] [Google Scholar]
- 27. Patel S., Chiplunkar S. 2009. Host immune responses to cervical cancer. Curr. Opin. Obstet. Gynecol. 21:54–59 [DOI] [PubMed] [Google Scholar]
- 28. Pedersen A. W., et al. 2009. Phenotypic and functional markers for 1alpha,25-dihydroxyvitamin D(3)-modified regulatory dendritic cells. Clin. Exp. Immunol. 157:48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiao Y. L. 2010. Perspective of cervical cancer prevention and control in developing countries and areas. Ai Zheng 29:1–3 [DOI] [PubMed] [Google Scholar]
- 30. Rescigno M., Di S. A. 2009. Dendritic cells in intestinal homeostasis and disease. J. Clin. Invest. 119:2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santin A. D., et al. 2005. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr. Pharm. Des. 11:3485–3500 [DOI] [PubMed] [Google Scholar]
- 32. Santin A. D., et al. 2008. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J. Virol. 82:1968–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sarkar K., et al. 2008. Neem leaf glycoprotein helps to generate carcinoembryonic antigen specific anti-tumor immune responses utilizing macrophage-mediated antigen presentation. Vaccine 26:4352–4362 [DOI] [PubMed] [Google Scholar]
- 34. Sarkar K., et al. 2010. Neem leaf glycoprotein enhances carcinoembryonic antigen presentation of dendritic cells to T and B cells for induction of anti-tumor immunity by allowing generation of immune effector/memory response. Int. Immunopharmacol. 10:865–874 [DOI] [PubMed] [Google Scholar]
- 35. Scarinci I. C., et al. 2010. Cervical cancer prevention: new tools and old barriers. Cancer 116:2531–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schäkel K. 2009. Dendritic cells—why can they help and hurt us. Exp. Dermatol. 18:264–273 [DOI] [PubMed] [Google Scholar]
- 37. Tas M. P., Simons P. J., Balm F. J., Drexhage H. A. 1993. Depressed monocyte polarization and clustering of dendritic cells in patients with head and neck cancer: in vitro restoration of this immunosuppression by thymic hormones. Cancer Immunol. Immunother. 36:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]