Abstract
To construct a universal vaccine against mastitis induced by either Streptococcus agalactiae or Staphylococcus aureus, the B cell epitopes of the surface immunogenic protein (Sip) from S. agalactiae and clumping factor A (ClfA) from S. aureus were analyzed and predicted. sip-clfA, a novel chimeric B cell epitope-based gene, was obtained by overlap PCR, and then the recombinant Sip-ClfA (rSip-ClfA) was expressed and purified. rSip-ClfA and inactivated S. agalactiae and S. aureus were formulated into different vaccines with mineral oil as the adjuvant and evaluated in mouse models. The rSip-ClfA vaccination induced immunoglobulin G (IgG) titers higher than those seen in groups immunized with inactivated bacteria. Furthermore, the response to rSip-ClfA immunization was characterized as having a dominant IgG1 subtype, whereas both bacterial immunizations produced similar levels of IgG1 and IgG2a. The antiserum capacities for opsonizing adhesion and phagocytosis were significantly greater in the rSip-ClfA immunization group than in the killed-bacterium immunization groups (P < 0.05). The immunized lactating mice were challenged with either S. agalactiae or S. aureus via the intramammary route. At 24 h postinfection, the numbers of bacteria recovered from the mammary glands in the rSip-ClfA group were >5-fold lower than those in both inactivated-bacterium groups (P < 0.01). Histopathological examination of the mammary glands showed that rSip-ClfA immunization provided better protection of mammary gland tissue integrity against both S. agalactiae and S. aureus challenges. Thus, the recombinant protein rSip-ClfA would be a promising vaccine candidate against mastitis induced by either S. agalactiae or S. aureus.
INTRODUCTION
Streptococcus agalactiae and Staphylococcus aureus are the most common etiologic agents of contagious bovine mastitis, which results in the reduction of milk quantity and quality (31). It was estimated that S. agalactiae and S. aureus mastitis contributes to 10% of annual milk loss (15). Antibiotic treatment is the method most often used to fight mastitis. Because milk antibiotic residue affects food safety due to possible induction of drug resistance in bacteria, there is regulatory pressure to justify the use of antimicrobials to control mastitis in dairy cattle (12). Vaccines would be a logical and promising approach to prevent mastitis in food production animals (33). For bovine mastitis, a few conventional inactivated vaccines using killed bacteria or live attenuated bacteria are available (11, 18). However, to date, the reported efficacy of these approaches has been unsatisfactory (17). Problems have been due to the high number of mastitis pathogens and their heterogeneity, high production costs, and poor availability or poor efficiency (25). Immune boosting with epitope-based vaccines has been shown to be successful for various infectious diseases, such as Salmonella enterica serovar Enteritidis infection (32), Neisseria meningitidis infection (35), and tuberculosis (7). Moreover, the epitope vaccine can be easily delivered and is capable of stimulating effective immune responses while avoiding potentially hazardous and undesirable side effects (34). Currently, no vaccine against bovine mastitis containing one or two epitopes has been successfully developed.
The Sip protein from S. agalactiae and ClfA (clumping factor A) protein from S. aureus have both been suggested to be good vaccine candidates for S. agalactiae mastitis and S. aureus mastitis, respectively. On the one hand, Sip is a highly conserved protein for human group B streptococcal (GBS) serotypes and bovine S. agalactiae (27). The immunization of mice with purified recombinant Sip protein can induce cross-protective immunity against lethal infection by different GBS serotypes (3). Furthermore, Sip-specific antibodies can cross the placental barrier and confer protection against GBS diseases to newborn mice (20). On the other hand, ClfA is an important adhesin and a critical virulence factor in almost all S. aureus strains (24). DNA vaccination against S. aureus ClfA induces protective immunity against S. aureus-caused mastitis in both mice (5) and cows (23). In addition, the recombinant ClfA protein may provide protection against mouse S. aureus mastitis (10).
In the present study, we analyzed and predicted the B cell epitopes of Sip and ClfA. Two fragments containing B cell epitopes, one each from Sip and ClfA, were selected for the construction of a fusion gene and thereby production of a recombinant fusion protein named rSip-ClfA. The protective effect of rSip-ClfA against intramammary challenge with either S. agalactiae or S. aureus was then evaluated in mouse models.
MATERIALS AND METHODS
Strains and media.
S. agalactiae strain W34 and S. aureus strain J9 were isolated from local dairy cows having mastitis, and both were characterized and stored in our laboratory (19). The S. agalactiae strain W34 and S. aureus strain J9 were grown in tryptic soy broth (TSB) or agar (BD Difco, Sparks, MD). Escherichia coli was grown in Luria-Bertani (LB) broth or agar (Difco) at 37°C in the presence of 50 μg/ml kanamycin when necessary.
Construction of recombinant plasmids.
The sip gene without the signal peptide sequence was amplified using primers specific to the sequence published in GenBank (accession no. FJ808732) (Table 1). The reaction was programmed as indicated in Table 1. The PCR products were isolated from an agarose gel and cloned into the vector pET-30a(+) to obtain pET-30a-Sip. pET-28a-ClfA was constructed previously and stored in our laboratory (10).
Table 1.
Primer sequences, restriction enzymes and respective sites, and PCR amplification conditions
| Gene | Primera |
Restriction site | |
|---|---|---|---|
| Type | Sequence (5′-3′) | ||
| sip | Sense | CGCGGATCCCAAGAAACAGATACGACG | BamHI |
| Antisense | GTACGCGGCCGCTTATTTGTTAAATGATACG | NotI | |
| sip-clfA | Sense | CCCGGATCCGTTAAACCAACTCAGACGTCAGTCA | BamHI |
| Overlapping primer 1 | TATTTGTTTTATCGATTTGGAGCCGCCGCCGCCGGAGCCGCCGCCGCCAACTTCATTACCAAGTGCT | ||
| Overlapping primer 2 | GAGCACTTGGTAATGAAGTTGGCGGCGGCGGCTCCGGCGGCGGCGGCTCCCAAATCGATAAAACAAAT | ||
| Antisense | CGCAAGCTTCTCTGGAATTGGTTCAATTTCACCA | HindIII | |
The restriction sites are underlined. A 30-bp linker sequence of (G4S)2 flexible bridge (shown in boldface) was added to the overlapping primers for sip-clfA. The melting temperature for all primers was 56°C.
The B cell epitopes of Sip and ClfA were predicted using Protean (DNAStar, Madison, WI) and two online software programs (http://www.cbs.dtu.dk/services/BepiPred/ and http://imed.med.ucm.es/Tools/antigenic.html). Using comparative analysis of the prediction results, we selected the fragments of amino acid residues from V208 to V375 of Sip and from Q384 to E557 of ClfA containing B cell epitopes. An overlapping-primer method was performed to obtain a chimeric sip-clfA gene containing Sip V208 to V375, a (G4S)2 flexible bridge, and ClfA Q384 to E557 coding sequences (Table 1). The rSip-ClfA gene was cloned into the vector pET-30a(+) to obtain pET-30a-Sip-ClfA.
Expression and purification of recombinant protein.
E. coli strain BL21(DE3) cells harboring the recombinant plasmids pET-30a-Sip-ClfA, pET-30a-Sip, and pET-28a-ClfA were cultivated to the mid-log phase at 37°C when 0.8 mM isopropyl-β-d-thiogalactoside (IPTG) was added to the medium. After an additional 6-h induction period, the cells were harvested by centrifugation and resuspended in phosphate-buffered saline (PBS). The cells were disrupted by ultrasound, and the supernatant containing soluble recombinant proteins was collected. The proteins with a 6×His tag were purified with nickel-nitrilotriacetic acid (Ni-NTA) resin affinity chromatography (Qiagen), and protein purity was analyzed by SDS-PAGE.
Mouse immunization. (i) Vaccination schedule.
Overnight cultures of S. agalactiae strain W34 and S. aureus strain J9 were harvested and inactivated with formalin (0.1% final concentration). The growth after inactivation was tested by plating. The rSip-ClfA protein, two inactivated bacterial strains, and sterile PBS were emulsified 1:1.5 (vol/vol) in an oil adjuvant.
Forty-eight female specific-pathogen-free (SPF) BALB/c mice, 7 to 9 weeks old, were randomly allocated into six groups with eight mice in each group. Treatment and challenge descriptions for each group are shown in Table 2. One hundred microliters of the emulsion containing 40 μg of protein, 5 × 108 CFU inactivated S. agalactiae or S. aureus, or PBS was injected intraperitoneally. Immunization was performed three times at 2-week intervals. One week after each immunization, blood samples were collected for an immunological assay.
Table 2.
Vaccination protocol and challenge operation
| Group | Immunogena | Adjuvant | No. of mice | Challenge organismb |
|---|---|---|---|---|
| 1 | rSip-ClfA | Oil | 8 | S. agalactiae |
| 2 | Inactivated S. agalactiae | Oil | 8 | S. agalactiae |
| 3 | PBS | Oil | 8 | S. agalactiae |
| 4 | rSip-ClfA | Oil | 8 | S. aureus |
| 5 | Inactivated S. aureus | Oil | 8 | S. aureus |
| 6 | PBS | Oil | 8 | S. aureus |
Immunization was performed three times at 2-week intervals. The adjuvant was oil in each case, and eight mice were in each group.
The immunized lactating mice were anesthetized and challenged with 50 μl of S. agalactiae strain W34 or S. aureus strain J9 suspension, corresponding to 1 × 107 CFU/ml, through the fourth pair of teats.
(ii) Western blot assay.
The purified rSip-ClfA, recombinant Sip (rSip), and recombinant ClfA (rClfA) were separated by SDS-PAGE and electrophoretically transferred onto nitrocellulose membranes (Bio-Rad, Mississauga, ON, Canada). PBS with 3% (wt/vol) bovine serum albumin (BSA) was used to block the membranes. After several quick washes, the membranes were incubated for 1 h at room temperature with mouse antisera against rSip-ClfA (1:5,000 dilution). After extensive washing, the horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody was added, and the reaction was developed by 3,3′-diaminobenzidine (DAB)-H2O2.
(iii) Detection of specific antibodies with ELISA.
The rSip-ClfA antibody titer was determined with an enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well polyvinylchloride plates (Falcon, Fisher Scientific, Montreal, Quebec, Canada) were coated overnight at 4°C with 0.1 μg of purified rSip-ClfA, rSip, or rClfA in 100 μl of 50 mM carbonate-bicarbonate buffer (pH 9.6). After blocking with a skim milk solution (5% [wt/vol]) for 1 h at 37°C, serum samples were added to the plates at the specified dilutions and incubated for 45 min at 37°C. HRP-conjugated goat anti-mouse IgG antibodies (Southern Biotech, Birmingham, AL) were added, and plates were incubated for 30 min at 37°C. A 3,3′,5,5′-tetramethylbenzidine (TMB)-H2O2 solution was used as the HRP substrate to develop the reaction. The enzyme reaction was stopped by adding 50 μl of hydrofluoric acid (HF). Three washes with PBS-0.05% Tween 20 were performed between each step. The optical density (OD) was read on a plate reader (BioTek Instruments, Winooski, VT) at a wavelength of 630 nm.
(iv) Detection of IgG1 and IgG2a subtypes.
An ELISA was performed to detect the IgG1 and IgG2a subtypes following the basic procedure described in the previous paragraph, except for the addition of specific components. Briefly, the wells were coated with 0.1 μg of purified rSip-ClfA or 3 × 105 to 5 × 105 CFU of inactivated S. agalactiae or S. aureus in 100 μl of 50 mM carbonate-bicarbonate buffer (pH 9.6). The corresponding antisera from rSip-ClfA-, S. agalactiae-, and S. aureus-immunized mice were overlaid. Finally, the secondary antibody was replaced by either mouse anti-IgG1-HRP (Southern) or mouse anti-IgG2a-HRP (Southern Biotech) at a dilution of 1:100 for the inactivated groups or 1:8,000 for the rSip-ClfA groups.
(v) Reactions of S. agalactiae and S. aureus with antisera.
The overall capability of IgG to bind to rSip-ClfA, S. agalactiae, and S. aureus after the third immunization was detected with an ELISA. The basic procedure was similar to that described above [“(iii) Detection of specific antibodies with ELISA”] except for the addition of specific components. Briefly, the antigens on 96-well flat-bottomed plates were replaced by either the killed S. agalactiae or the killed S. aureus. The antisera from immunized or mock-immunized animals were serially diluted 2-fold and overlaid onto the wells.
(vi) Neutrophil phagocytosis assay.
The phagocytosis assay was performed as previously described, with minor modifications (23, 26). Neutrophilic leukocytes from a healthy cow during early lactation were isolated by using methods described by Diarra et al. (8). The cells were resuspended in Dulbecco's modified Eagle's medium (DMEM) to a concentration of 9 × 106 cells/ml. S. agalactiae or S. aureus was incubated overnight at 37°C in TSB to reach an approximate concentration of 4.8 × 108 CFU/ml or 2.67 × 109 CFU/ml, respectively (determined by viability counts). Bacteria were washed twice with sterile PBS and collected by centrifugation. The bacteria resuspended in DMEM without serum or antibiotics were preincubated for 30 min at 37°C with sera from rSip-ClfA, inactivated-S. agalactiae or -S. aureus, or PBS negative-control groups after the third immunization at a bacterium/serum ratio of 100:1 (vol/vol). Preincubated bacteria (S. agalactiae or S. aureus [4 × 108 CFU) were added to the washed neutrophils at a 40:1 bacterium-to-neutrophil ratio. The coculture was then incubated at 37°C for 30 min with slow shaking to allow phagocytosis to proceed. Phagocytosis was stopped by the addition of 1 ml of cold sterile PBS. Extracellular bacteria were removed by four washes with PBS and centrifugation at 250 × g at 4°C for 5 min. Pellets were resuspended in DMEM (2 ml) and incubated at 37°C for 5 min. The cells were lysed by adding sterile distilled water after phagocytosis. The numbers of bacteria that were phagocytized by neutrophils were determined by dilution plating. The amounts of bacteria (CFU/ml) were calculated as the means of triplicate samples. Additionally, a HeLa cell control group was used to verify the efficiency of removing extracellular bacteria by washing.
Challenge of lactating mice.
One week before the third immunization, one male mouse and three female mice were cohabitated to ensure impregnation of the female mice. Four days after delivery, the female mice were anesthetized (6) and injected with 50 μl of S. agalactiae strain W34 or S. aureus strain J9 suspension, corresponding to 1 × 107 CFU/ml, through the fourth pair of teats (Table 2). After 24 h, the mice were euthanized, and their mammary glands were removed aseptically.
(i) Bacterial recovery from the mammary glands.
The glands were then placed in sterile PBS at a ratio of 1:5 (wt/vol) and homogenized by using a sterile Polytron homogenizer. The homogenate was clarified at 500 × g for 5 min, and the supernatant was diluted from 10−3 to 10−7. For each dilution, 100 μl of supernatant was spread on tryptic soy agar (TSA) plates for bacterial culture at 37°C overnight and colony counts.
(ii) Histological examination of the mammary glands.
The removed mammary glands were immediately fixed in 10% formalin-PBS (pH 7.4) for 24 h and then were embedded in paraffin wax. Thin sections (0.4 μm) were obtained and stained with hematoxylin and eosin (HE). Pathological histology was observed with light microscopy (Eclipse 80i; Nikon, Tokyo, Japan).
Statistics.
The arithmetic mean and standard error of the mean were calculated for each treatment. The data were analyzed by using an analysis of variance (ANOVA) with the SAS software package (SAS Institute, Cary, NC).
RESULTS
Construction and expression of the Sip-ClfA fusion gene.
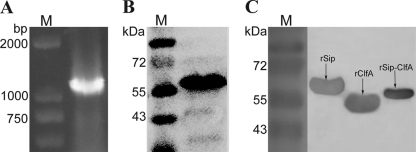
The fusion of epitope DNA fragments from sip and clfA genes was performed by overlap PCR (Fig. 1A), and the recombinant plasmid pET-30a-Sip-ClfA was confirmed by sequencing. The similarities were 99% for the Sip epitope and 99% for the ClfA epitope at the nucleic acid level and 100% at the amino acid level. The predicted mass of the resultant chimeric protein was confirmed by 12% SDS-PAGE (Fig. 1B). To determine that rSip-ClfA has an immunogenicity similar to those of the original proteins rSip and rClfA, Western blotting using the antiserum to rSip-ClfA was performed. The result showed that all three proteins, rSip, rClfA, and fusion protein rSip-ClfA, were able to react with antiserum against rSip-ClfA (Fig. 1C).
Fig. 1.
(A) Amplification of the fusion gene sip-clfA and its expression. PCR products analyzed by agarose gel electrophoresis. M, DL2000 DNA marker. (B) The purified rSip-ClfA analyzed by 12% SDS-PAGE. M, reference proteins with the molecular mass labeled on the left. (C) Western blot assay of rSip-ClfA proteins. M, prestained reference proteins with the molecular mass labeled on the left. Purified recombinant proteins rSip, rClfA, and rSip-ClfA were separated by a 12% SDS-PAGE gel, and the mouse antiserum to rSip-ClfA after the third immunization was used as a primary antibody. After incubation with anti-mouse IgG-HRP, the bands were developed by adding DAB-H2O2 solution.
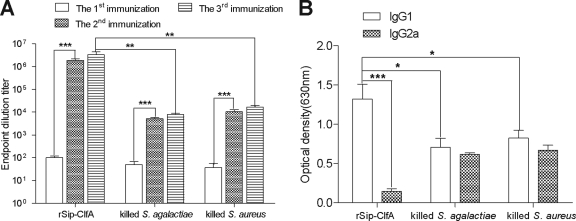
Comparison of antibody responses induced by rSip-ClfA and inactivated bacteria. (i) IgG titer detection.
The ELISA titers of serum IgG against rSip-ClfA and inactivated S. agalactiae and S. aureus for the immunized mice were compared (Fig. 2A). After immunization, all mice produced specific antibodies in the sera against the inoculated antigens. The antibody titers against rSip-ClfA and inactivated S. agalactiae and S. aureus reached the highest levels after the third immunization. Among the three immunized groups, rSip-ClfA stimulated the highest titer, 1:108.5, and there was a significant difference (P < 0.01) in antibody titers between the rSip-ClfA-immunized group and the inactivated-S. agalactiae- and -S. aureus-immunized groups. With respect to immunization times, there was no significant difference between the third and second immunizations, but there was a significant difference between the first and second immunizations. This result indicates that one boost after priming was sufficient to induce high titers of antibodies for these three antigens (Fig. 2A).
Fig. 2.
IgG antibody titers (A) and serum IgG1 and IgG2a levels (B) were determined by ELISA. The mice were immunized three times at 2-week intervals, and sera were collected at 1-week intervals after each immunization. Results are shown as means ± standard deviations (SD). *, P < 0.05 between the indicated groups; **, P < 0.01 between the indicated groups; ***, P < 0.001 between the indicated groups.
(ii) IgG subtype detection.
The IgG1 and IgG2a titers in the sera were detected after the third immunization (Fig. 2B). There were significant differences between IgG1 and IgG2a titers for rSip-ClfA immunization, and the IgG1 subtype was dominant. Although the IgG1 titers were slightly higher than the IgG2a titers, there was no significant difference between these two IgG subtypes in the inactivated-bacterium groups. A comparison of the IgG1 and IgG2a titers of different groups revealed that the IgG1 level induced by rSip-ClfA immunization was significantly higher than that induced by bacterial immunization (P < 0.05), whereas the IgG2a level induced by rSip-ClfA immunization was lower than that induced by bacterial immunization (P < 0.001).
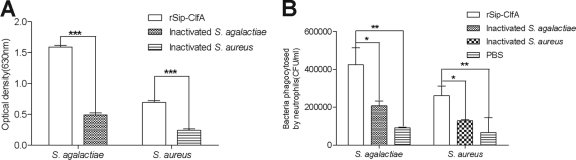
Antibody bioactivity test. (i) Reaction of antiserum with S. agalactiae or S. aureus.
Antisera from each group after the third immunization were pooled for this test. The antisera against rSip-ClfA reacted more strongly with either S. agalactiae or S. aureus than the antisera against both inactivated bacteria (P < 0.01) (Fig. 3A). Additionally, this reaction was dose dependent. With an increase in serum dilution, the capacity of antisera to bind to S. agalactiae or S. aureus decreased (data not shown).
Fig. 3.
(A) We compared the capacities for antibody binding to bacteria among the groups immunized with rSip-ClfA, inactivated S. agalactiae, and inactivated S. aureus. The plates were coated with either inactivated S. agalactiae or S. aureus. Serum from the third immunization diluted to 1:800 was used. (B) In vitro phagocytosis of bacteria after opsonization. Bacteria were preincubated with sera from rSip-ClfA, inactivated vaccines, or control groups. Bacteria were then added to the neutrophils and incubated for 30 min. The numbers of bacteria phagocytized by neutrophils were determined by dilution plating. Results are shown as means ± SD. *, P < 0.05 between the indicated groups; **, P < 0.01 between the indicated groups; ***, P < 0.001 between the indicated groups.
(ii) Opsonophagocytosis assay.
After preincubation of S. agalactiae or S. aureus with pools of antiserum from the rSip-ClfA, inactivated-S. agalactiae or -S. aureus, or PBS negative-control groups, ingestion of bacteria by bovine neutrophils was detected (Fig. 3B). The number of S. agalactiae bacteria opsonized by the antiserum to rSip-ClfA that were ingested by bovine neutrophils was 4.24 × 105 ± 6.30 × 104 CFU/ml, which was significantly higher than the numbers corresponding to the antiserum to the inactivated-S. agalactiae group (2.07 × 105 ± 1.80 × 104 CFU/ml) (P < 0.05) and the PBS group (9.15 × 104 ± 2.50 × 103 CFU/ml) (P < 0.05). Similarly, the number of S. aureus bacteria opsonized with antiserum to rSip-ClfA that were ingested by bovine neutrophils was 2.60 × 105 ± 3.30 × 104 CFU/ml, which was significantly higher than the numbers corresponding to the antiserum to the inactivated-S. aureus group (1.32 × 105 ± 3.72 × 104 CFU/ml) (P < 0.05) and the PBS group (1.07 × 105 ± 1.45 × 104 CFU/ml) (P < 0.05). These results indicate that the antibodies induced by rSip-ClfA immunization had the strongest opsonic activities.
Additionally, few bacteria were obtained from the lysed HeLa cells, indicating that the extracellular bacteria were washed off efficiently.
Protective immunity detection. (i) Bacterial recovery from the mammary glands.
The lactating mice immunized with rSip-ClfA, inactivated S. agalactiae, inactivated S. aureus, and PBS were challenged with either S. agalactiae or S. aureus. After 24 h, the bacterial loads in the homogenized tissues were measured (Table 3). Compared with the PBS-immunized group, the recombinant-protein-immunized groups showed a reduction in the number of recovered bacteria. The amounts of S. agalactiae in the mammary glands of mice immunized with rSip-ClfA and inactivated S. agalactiae were reduced by 2.926 and 0.537 logarithms, respectively, representing a 5.4-fold difference between the two immunization groups. The amounts of S. aureus in the mammary glands of mice immunized with rSip-ClfA and inactivated S. aureus were decreased by 2.878 and 0.564 logarithms, respectively, representing a 5.1-fold difference between the two immunization groups. These findings demonstrate that rSip-ClfA immunization generates significantly better protection against both S. agalactiae and S. aureus challenge than do inactivated bacteria. Furthermore, the inactivated-bacterium immunization could produce only partial protection against the homologous bacterial challenge.
Table 3.
Recovery of bacteria from the mammary glands in the rSip-ClfA, inactivated-bacterium, and PBS groups
| Group | Immunogen/challengea | Bacteria in mammary glands [log10 (CFU)/ml] | Reduction of bacteria [log10 (CFU)/ml]b | Significance (P) relative to control |
|---|---|---|---|---|
| 1 | rSip-ClfA/S. agalactiae | 5.269 ± 0.748 | 2.926 | <0.0001 |
| 2 | Inactivated S. agalactiae/S. agalactiae | 7.658 ± 0.503 | 0.537 | 0.0163 |
| 3 | PBS/S. agalactiae | 8.195 ± 0.175 | ||
| 4 | rSip-ClfA/S. aureus | 6.413 ± 0.539 | 2.878 | <0.0001 |
| 5 | Inactivated S. aureus/S. aureus | 8.727 ± 0.814 | 0.564 | 0.1269 |
| 6 | PBS/S. aureus | 9.291 ± 0.800 |
The immunized mice were challenged with 5 × 105 CFU of S. agalactiae or S. aureus per gland.
Reduction of the number of bacteria in mammary gland [log10 (CFU)/ml] is a measure of protective immunity and here is relative to the value for mice immunized with PBS.
(ii) Histopathological examination of the mammary glands.
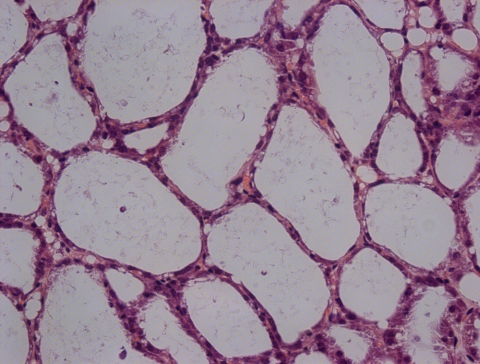
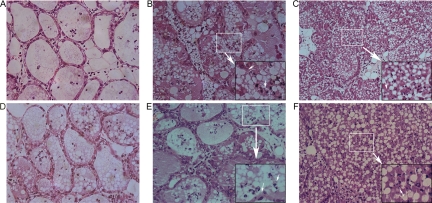
Histopathological alterations of the mouse mammary glands in different groups were observed. The mammary acinar structure of mice that received the rSip-ClfA immunization was similar to the normal structure (Fig. 4), with only a few polymorphonuclear leukocytes (PMN) infiltrating into the acini. Compared with those of the rSip-ClfA group (Fig. 5A and D), the mammary glandular cavities of mice from the inactivated S. agalactiae-immunized group (Fig. 5B) or S. aureus-immunized group (Fig. 5E) were partially fused, with mild infiltration of PMN in the acini. Between the two bacterium-immunized groups, the acinar structure of the S. agalactiae-immunized group was more severely damaged than that of the S. aureus-immunized group (Fig. 5B and E). In contrast, the structure of the mammary gland was completely destroyed in the PBS group. A large number of inflammatory cells and necrotic cell debris infiltrated into the alveoli in both the S. agalactiae (Fig. 5C) and S. aureus (Fig. 5F) challenge groups. These results demonstrate that rSip-ClfA immunization produced the best protection against both S. agalactiae and S. aureus challenges. The two killed-bacterium vaccines yielded partial protection against homologous challenge; however, S. aureus immunization provided better protection than did S. agalactiae against homologous challenge.
Fig. 4.
Microscopic image of a mammary gland from a mouse in the negative-control group (magnification, ×400).
Fig. 5.
Microscopic images of the mammary gland in the rSip-ClfA groups (A and D), the inactivated-S. agalactiae group (B), the inactivated-S. aureus group (E), and the PBS control groups (C and F). All of the groups corresponding to panels A, B, and C were challenged with S. agalactiae, whereas all of the groups corresponding to panels D, E, and F were challenged with S. aureus. Female BALB/c mice were immunized with rSip-ClfA or inactivated bacteria just before pregnancy. Four days after delivery, the lactating mice were challenged with S. agalactiae or S. aureus in the right and left fourth teats of the mammary abdominal glands. Twenty-four hours later, mammary gland histology was assessed. The bigger arrows signify magnification from the white panes to the black panes. The smaller arrows show the destruction of glandular cavities or the necrosis of neutrophils (magnification, 0 ×400).
DISCUSSION
Several attempts to produce mastitis vaccines using inactivated whole cells or virulence factors have been made recently, but the results have been unsatisfactory (12). Immunoprevention and immunotherapy targeting epitopes provide a new approach in the control of mastitis. Epitope-based vaccines can focus immune responses to biologically active epitopes. For example, a mutiepitope-based vaccine which combined several B cell epitopes and T cell epitopes elicited high-titer neutralizing antibodies and cell-mediated immune responses against Japanese encephalitis virus (JEV) (34). More importantly, epitope-based vaccines have increased the opportunity to rationally engineer epitopes for increased potency and breadth (30). B cell epitopes, which are recognized and bound by the B cell receptor, are essential for the induction of protective antibody responses (34). Therefore, in this study, a chimeric B cell epitope-based vaccine was proposed as a possible candidate vaccine with the remarkable ability to induce protection against mastitis in mice.
B cell epitopes have been classified into continuous and discontinuous epitopes (2). Strictly speaking, all determinants are discontinuous to some extent (1). Therefore, it is very difficult to find ideal epitopes with current biological tests. However, bioinformatic screening is an efficient way to predict epitopes in both the study of basic immunology and the development of novel vaccines. In the present study, Protean and two online software programs were used to predict the B cell epitopes of Sip and ClfA proteins. Parameters such as hydrophilicity (16), accessibility (9), antigenicity (13), flexibility (14), and secondary structures were used to comprehensively analyze the epitopes. The results showed that the fragments from V208 to V375 of Sip and Q384 to E557 of ClfA received the highest scores (provided by the software), and they were therefore determined as the discontinuous-epitope fragments.
By using overlap PCR, the rSip-ClfA coding sequence was successfully constructed, and the recombinant protein rSip-ClfA was expressed in E. coli. Combined with mineral oil adjuvant, which was previously demonstrated to be the most effective adjuvant in a protein-based vaccine (33), the recombinant protein rSip-ClfA and inactivated S. agalactiae and S. aureus were formulated into three vaccines. In mice, the antibody induced by rSip-ClfA could be equally recognized by rSip-ClfA itself and intact rSip or rClfA as determined by Western blot assay (Fig. 1C). These results demonstrate that the fused epitope protein rSip-ClfA retains the native conformations and antigenicities of rSip and rClfA. In addition, the antibody to rSip-ClfA could be recognized by both S. agalactiae and S. aureus (Fig. 3A); however, the bacterium-specific antibody titers were lower than the recombinant-protein-specific antibody titers. Therefore, this fusion protein can replace the original two proteins to induce immunity in vivo.
Because the mouse model for S. aureus-induced mastitis was successfully used to evaluate S. aureus mastitis in our laboratory and S. agalactiae mastitis in another laboratory (4, 12, 26), the current study used the same model to evaluate protective immunity elicited by rSip-ClfA. After intramammary challenge of lactating mice with either S. agalactiae or S. aureus, the PBS-mock-vaccinated group developed apparent mastitis as evidenced by the complete disruption of the mammary gland structure and abundant infiltration of neutrophils in alveoli, which are characteristics of acute inflammation. During inflammation, neutrophils experience necrosis, and necrotic cells release potentially toxic and immunogenic intracellular substances into the tissues (22, 28). This would contribute to the destruction of mammary gland structures in this study.
Fortunately, rSip-ClfA immunization developed the best protection against both bacterial challenges, as evaluated by mammary gland morphology (Fig. 4). The bacterial recovery results following bacterial challenge confirmed the above conclusion. The number of recovered S. agalactiae bacteria from the mammary glands in the rSip-ClfA immunization group was 5.4-fold lower than that in the inactivated-S. agalactiae immunization group. Additionally, the number of recovered S. aureus bacteria from the mammary glands in the rSip-ClfA immunization group was 5.1-fold lower than that in the inactivated-S. aureus immunization group.
To explain the mechanism underlying protective immunity developed by the rSip-ClfA vaccine, the antibody titers and their bioactivities were tested. First, rSip-ClfA induced serum IgG production significantly more effectively than either killed S. agalactiae or killed S. aureus, as indicated by the different IgG titers in the sera (Fig. 2A). Second, the binding of rSip-ClfA-induced antibodies to both bacteria was significantly better than that of the bacterium-stimulated antibodies (Fig. 3A). Additionally, rSip-ClfA immunization altered the balance of IgG1 and IgG2a and induced a serum level of IgG1 significantly higher than that of IgG2a. IgG2a levels in the rSip-ClfA group were even lower than were those in the groups immunized with killed S. agalactiae and S. aureus. For whole-bacterium immunization, serum IgG1 and IgG2a levels did not show significant differences. As IgG1 was previously demonstrated to be the most efficient opsonization isotype for neutrophils (21, 29), we tested the antibody opsonization ability against both bacteria. These findings confirmed that the antiserum from the rSip-ClfA-vaccinated mice was more effective in promoting ingestion of the bacteria by bovine neutrophilic leukocytes than were those from the inactivated-bacterium groups or the PBS control groups. Therefore, the higher titers of total IgG and the dominant IgG1 subtype might be responsible for the superior protection conferred by rSip-ClfA immunization compared with immunization with the inactivated S. agalactiae or S. aureus.
In conclusion, mouse vaccination with the fusion protein rSip-ClfA, which is composed of epitope fragments of S. agalactiae Sip and S. aureus ClfA, induced specific and significant protective immunity against both S. agalactiae and S. aureus. Therefore, it is a promising candidate for the construction of novel and universal vaccines against bovine mastitis induced by either S. agalactiae or S. aureus. Moreover, the present study also provides useful information for the further development of a multiepitope mastitis vaccine.
ACKNOWLEDGMENTS
This work was supported by the National “Eleventh Five-Years” Science and Technology Support Program of China-Key Special Project for Dairy Industry (grant no. 2006BAD04A05), the National Agricultural Public Benefit Research Foundation (grant no. 200803018), the Program for Changjiang Scholars and Innovative Research Team of the University of China (PCSIRT) (grant no. IRT0726), and the Fundamental Research Funds for the Central Universities (grant no. 2010QC002).
Footnotes
Published ahead of print on 20 April 2011.
REFERENCES
- 1. Barlow D. J., Edwards M. S., Thornton J. M. 1986. Continuous and discontinuous protein antigenic determinants. Nature 322:747–748 [DOI] [PubMed] [Google Scholar]
- 2. Benjamin D. C., et al. 1984. The antigenic structure of proteins: a reappraisal. Annu. Rev. Immunol. 2:67–101 [DOI] [PubMed] [Google Scholar]
- 3. Brodeur B. R., et al. 2000. Identification of group B streptococcal Sip protein, which elicits cross-protective immunity. Infect. Immun. 68:5610–5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brouillette E., Malouin F. 2005. The pathogenesis and control of Staphylococcus aureus-induced mastitis: study models in the mouse. Microbes Infect. 7:560–568 [DOI] [PubMed] [Google Scholar]
- 5. Brouillette E., et al. 2002. DNA immunization against the clumping factor A (ClfA) of Staphylococcus aureus. Vaccine 20:2348–2357 [DOI] [PubMed] [Google Scholar]
- 6. Castagliuolo I., et al. 2006. Mucosal genetic immunization against four adhesins protects against Staphylococcus aureus-induced mastitis in mice. Vaccine 24:4393–4402 [DOI] [PubMed] [Google Scholar]
- 7. De Groot A. S., et al. 2005. Developing an epitope-driven tuberculosis (TB) vaccine. Vaccine 23:2121–2131 [DOI] [PubMed] [Google Scholar]
- 8. Diarra M. S., et al. 2003. Lactoferrin against Staphylococcus aureus mastitis. Lactoferrin alone or in combination with penicillin G on bovine polymorphonuclear function and mammary epithelial cells colonisation by Staphylococcus aureus. Vet. Immunol. Immunopathol. 95:33–42 [DOI] [PubMed] [Google Scholar]
- 9. Emini E. A., Hughes J. V., Perlow D. S., Boger J. 1985. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 55:836–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong R., et al. 2010. Evaluation of clumping factor A binding region A in a subunit vaccine against Staphylococcus aureus-induced mastitis in mice. Clin. Vaccine Immunol. 17:1746–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hogan J. S., Smith K. L., Todhunter D. A., Schoenberger P. S. 1992. Field trial to determine efficacy of an Escherichia coli J5 mastitis vaccine. J. Dairy Sci. 75:78–84 [DOI] [PubMed] [Google Scholar]
- 12. Hu C., Gong R., Guo A., Chen H. 2010. Protective effect of ligand-binding domain of fibronectin-binding protein on mastitis induced by Staphylococcus aureus in mice. Vaccine 28:4038–4044 [DOI] [PubMed] [Google Scholar]
- 13. Jameson B. A., Wolf H. 1988. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput. Appl. Biosci. 4:181–186 [DOI] [PubMed] [Google Scholar]
- 14. Karplus P. A., Schultz G. E. 1985. Prediction of chain flexibility in proteins. Immunology 72:212–213 [Google Scholar]
- 15. Keefe G. P. 1997. Streptococcus agalactiae mastitis: a review. Can. Vet. J. 38:429–437 [PMC free article] [PubMed] [Google Scholar]
- 16. Kyte J., Doolittle R. F. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105–132 [DOI] [PubMed] [Google Scholar]
- 17. Lee J. W., et al. 2005. Effect of a trivalent vaccine against Staphylococcus aureus mastitis lymphocyte subpopulations, antibody production, and neutrophil phagocytosis. Can. J. Vet. Res. 69:11–18 [PMC free article] [PubMed] [Google Scholar]
- 18. Leitner G., et al. 2003. Development of a Staphylococcus aureus vaccine against mastitis in dairy cows. II. Field trial. Vet. Immunol. Immunopathol. 93:153–158 [DOI] [PubMed] [Google Scholar]
- 19. Liu Z., et al. 2007. Isolation, identification and analysis of drug resistance of bovine mastitis-causing pathogens from Hubei province. China Dairy Cattle 7:35–37 [Google Scholar]
- 20. Martin D., et al. 2002. Protection from group B streptococcal infection in neonatal mice by maternal immunization with recombinant Sip protein. Infect. Immun. 70:4897–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maurer M., von Stebut E. 2004. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 36:1882–1886 [DOI] [PubMed] [Google Scholar]
- 22. Miles K., et al. 2009. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J. Immunol. 183:2122–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nour El-Din A. N., Shkreta L., Talbot B. G., Diarra M. S., Lacasse P. 2006. DNA immunization of dairy cows with the clumping factor A of Staphylococcus aureus. Vaccine 24:1997–2006 [DOI] [PubMed] [Google Scholar]
- 24. Peacock S. J., et al. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987–4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pyörälä S. 2002. New strategies to prevent mastitis. Reprod. Domest. Anim. 37:211–216 [DOI] [PubMed] [Google Scholar]
- 26. Rennermalm A., et al. 2001. Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine 19:3376–3383 [DOI] [PubMed] [Google Scholar]
- 27. Rioux S., et al. 2001. Localization of surface immunogenic protein on group B Streptococcus. Infect. Immun. 69:5162–5165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rydell-Törmänen K., Uller L., Erjefält J. S. 2006. Direct evidence of secondary necrosis of neutrophils during intense lung inflammation. Eur. Respir. J. 28:268–274 [DOI] [PubMed] [Google Scholar]
- 29. Schlageter A. M., Kozel T. R. 1990. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect. Immun. 58:1914–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sette A., Fikes J. 2003. Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Curr. Opin. Immunol. 15:461–470 [DOI] [PubMed] [Google Scholar]
- 31. Shkreta L., Talbot B. G., Diarra M. S., Lacasse P. 2004. Immune responses to a DNA/protein vaccination strategy against Staphylococcus aureus induced mastitis in dairy cows. Vaccine 23:114–126 [DOI] [PubMed] [Google Scholar]
- 32. Strindelius L., Degling Wikingsson L., Sjöholm I. 2002. Extracellular antigens from Salmonella enteritidis induce effective immune response in mice after oral vaccination. Infect. Immun. 70:1434–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talbot B. G., Lacasse P. 2005. Progress in the development of mastitis vaccines. Livestock Prod. Sci. 98:101–113 [Google Scholar]
- 34. Wei J. C., et al. 2010. Design and evaluation of a multi-epitope peptide against Japanese encephalitis virus infection in BALB/c mice. Biochem. Biophys. Res. Commun. 396:787–792 [DOI] [PubMed] [Google Scholar]
- 35. Zhu D., et al. 2008. A DNA fusion vaccine induces bactericidal antibodies to a peptide epitope from the PorA porin of Neisseria meningitidis. Infect. Immun. 76:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]