Abstract
Type 1 reaction (T1R) is a systemic inflammatory syndrome causing substantial morbidity in leprosy. T1R results from spontaneously enhanced cellular immunity in borderline types of leprosy, but there are no established laboratory markers for the reaction. Preliminary studies have identified elevated circulating CXC ligand 10 (CXCL10) during T1R. Correlation of CXCL10 with clinical T1R was studied in repeated serum specimens obtained before, during, and after T1R. CXCL10 gene expression was assessed in biopsy specimens taken before and during T1R, and sections were stained for the cytokine using monoclonal antibodies. Sequential serum specimens revealed elevation of circulating CXCL10 associated with episodes of T1R (P = 0.0001) but no evidence of an earlier, predictive change in the level of the chemokine. Reverse transcriptase (RT)-PCR revealed elevated expression of CXCL10 transcripts during T1R, but not in patients who did not have T1R. No significant correlation between CXCL10 and gamma interferon (IFN-γ) mRNA levels was observed. Immunohistochemical staining of the skin biopsy specimens suggested an overall increase in CXCL10 but did not identify a particular strongly staining population of leukocytes. Increased CXCL10 in lesions and serum is characteristic of T1R. CXCL10 measurement offers new possibilities for laboratory diagnosis and monitoring of T1R. Studies of the regulation of CXCL10 may provide insight into the mechanisms of T1R and identify potential new drug targets for treatment.
INTRODUCTION
Type 1 leprosy reaction (T1R) is a systemic inflammatory syndrome seen in borderline types of leprosy (borderline lepromatous [BL] and borderline tuberculoid [BT]) that interrupts the typically indolent course of the disease in many patients. T1R is a major cause of neuritis and other morbidities associated with leprosy (reviewed in reference 15). T1R appears to be a spontaneous enhancement of cellular immunity, widely regarded as a Th-1-dependent phenomenon, but the specific mechanism(s) underlying this clinical syndrome are unknown. The diagnosis of T1R is based on clinical criteria; even histologically, T1R may be difficult to differentiate from relapse or reactivation of the infection. No laboratory tests are currently available for diagnosis of T1R. Therefore, the identification of clinical markers for T1R and the development of new treatment strategies for the reaction have been identified as high-priority areas for research in leprosy.
More than 15 years ago, expression of the gene for CXC ligand 10 (CXCL10 [IP10]) was observed to be increased in tuberculoid skin lesions compared to lepromatous lesions (6). CXCL10 is a chemokine induced primarily by gamma interferon (IFN-γ) that plays a major role in inflammation (27). CXCL10 is produced constitutively by macrophages, as well as by stimulated T cells and keratinocytes, and it promotes chemotaxis of T cells to sites of tissue inflammation. Elevated levels of CXCL10 have been observed in tuberculosis (2) and tuberculosis-associated immune reconstitution inflammatory syndrome (12, 25) and in trypanosomiasis (5), as well as in multiple sclerosis and its relapses (19) and other neuroinflammatory conditions (10, 20).
The role of CXCL10 in leprosy had not been further examined until recently, as part of a cross-sectional study of reactions in leprosy (18). In that survey, significantly elevated plasma levels of CXCL10 were observed in association with T1R, but plasma levels of IFN-γ were not elevated in the same specimens. To further assess the association of the chemokine with T1R, patients who had been followed prospectively before, during, and after T1R were evaluated. Serum specimens and biopsy specimens from these sequential follow-up visits were assessed for CXCL10 protein levels and gene expression, respectively.
MATERIALS AND METHODS
Patients and specimens.
Patients and specimens for this study were obtained from three sources: the Blue Peter Research Center in Hyderabad, India; a multicenter study in India (ILEP Nerve Function Impairment in Reactions [INFIR]) (21), and the outpatient clinic of the National Hansen's Disease Programs (NHDP) in Baton Rouge, LA. All sera were stored at −70°C at the Blue Peter Research Center and were not thawed until they were assayed for CXCL10.
First, a cross-sectional study was done assessing CXCL10 levels in serum specimens obtained from patients with different types of leprosy and healthy controls in India. These consisted of 10 healthy controls (laboratory workers), 6 lepromatous leprosy (LL) patients and 10 LL patients with type 2 reactions (LL plus T2R), 10 BL patients, 10 BT patients, and 10 BT patients with type 1 reactions (BT plus T1R) (Table 1).
Table 1.
Healthy controls and patients without T1R evaluated for circulating CXCL10 at a single time point (cross sectional) and/or at monthly intervals for 6 months
| Cohorta | n | No. M/no. Fb | Age range (yr) | Leprosy type | Reaction | Notes |
|---|---|---|---|---|---|---|
| X | 10 | 7/3 | 23–42 | Healthy | NAc | |
| X | 6 | 5/1 | 19–55 | LL | None | |
| X | 10 | 8/2 | 20–55 | LL | T2R | |
| X | 10 | 8/2 | 15–50 | BL | None | |
| X, S | 10 | 8/2 | 15–50 | BL | T1R | 7 patients in both X and S |
| X | 10 | 6/4 | 20–50 | BT | None | |
| X, S | 10 | 7/3 | 20–50 | BT | T1R | 9 patients in both X and S |
X, cross sectional; S, 6-month intervals.
M, male; F, female.
NA, not applicable.
Next, sequential, repeated measures from individual patients were conducted on specimens from 40 patients who had been followed as part of the INFIR study, with clinical examination and serum collection at monthly intervals (21). Twenty patients with monthly specimens before, during, and after evidence of T1R (Table 2) were identified by the study group in collaboration with two investigators (I.N. and M.V.C.); similar repeated measures were performed on sera from 20 other patients who did not have a reaction.
Table 2.
Patients for whom CXCL10 levels were measured in serum specimens that had been obtained at monthly intervals before, during, and after T1R
| Patient no. | Sex | Age range (yr) | Leprosy type | Reaction | Severity of reaction |
|---|---|---|---|---|---|
| 1 | F | 55 | BT | T1R | Moderate |
| 2 | M | 30 | BT | T1R | Moderate |
| 3 | M | 50 | BT | T1R | Moderate |
| 4 | M | 18 | BT | T1R | Severe |
| 5 | M | 32 | BT | T1R | Severe |
| 6 | F | 25 | BT | T1R | Severe |
| 7 | M | 38 | BT | T1R | Severe |
| 8 | M | 22 | BT | T1R | Moderate |
| 9 | M | 22 | BT | T1R | NDa |
| 10 | F | 33 | BT | T1R | Moderate |
| 11 | M | 45 | BL | T1R | Severe |
| 12 | M | 18 | BL | T1R | Severe |
| 13 | F | 35 | BL | T1R | Severe, moderateb |
| 14 | M | 50 | BL | T1R | Severe |
| 15 | F | 24 | BL | T1R | Moderate |
| 16 | M | 55 | BL | T1R | Severe |
| 17 | F | 48 | BL | T1R | Moderate |
| 18 | M | 36 | BL | T1R | ND |
| 19 | F | 15 | BL | T1R | ND |
| 20 | M | 30 | BL | T1R | ND |
ND, not done.
Patient had 2 reactions.
A third group of 8 BL patients at the NHDP had been followed prospectively and had biopsies performed at diagnosis and at follow-up visits (Table 3). Five of these had T1R at the time of their second biopsy; one had received infliximab prior to the diagnosis of leprosy, and the clinical details were published previously (16). Biopsy specimens from 3 patients who did not have T1R at the time of either biopsy were examined as controls for the effect of multidrug treatment (MDT). Molecular and immunostaining studies were performed on portions of these biopsy specimens that had been snap-frozen and stored in liquid nitrogen.
Table 3.
Demographic and clinical data for patients biopsied before, during, and after T1R
| Patient no. | Sex | Age (yr) at 1st biopsy | Leprosy type | Interval between 1st and 2nd biopsies (mo) | Type 1 reaction |
Interval between 2nd and 3rd biopsies (mo) | ||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | ||||||
| 1 | F | 34 | BB | 12 | No | Yes | NDc | |
| 2 | F | 64 | BL | 12 | No | Yes | ND | |
| 3 | M | 50 | BL | 36 | No | Yes | No | 48 |
| 5a | M | 61 | BL | 1 | No | Yes | No | 22 |
| 6 | M | 46 | BL | 54 | No | Yes | ND | |
| 9b | M | 51 | BL | 42 | No | Yes | ND | |
| 4 | F | 44 | LL-BL | 12 | No | No | ND | |
| 7 | F | 63 | BL | 11 | No | No | ND | |
| 8 | M | 32 | BL | 13 | No | No | ND | |
Patient was treated with infliximab prior to diagnosis of leprosy; the agent was discontinued 1 month prior to the onset of T1R.
Immunostaining studies only.
ND, not done.
All patients in India and the United States were classified by clinical and histopathological criteria according to the Ridley-Jopling scale (13). The experimental protocols in both India and the United States were approved by the respective institutional review boards, and all patients gave written informed consent.
Type 1 reactions were diagnosed based on typical clinical manifestations of erythema and induration of preexisting skin lesions, usually accompanied by acute neuritis or by one or more systemic symptoms, including fever and malaise (23). In three patients in the Indian cohort (patients 12, 16, and 17) (see Fig. 2B), the diagnosis of T1R was made based on histological criteria alone (7), e.g., tissue edema and the presence of giant cells, without clinical diagnosis of T1R. Since the basic diagnostic definition of T1R for this study was dependent on clinical evidence of T1R, results from these three patients were excluded from some analyses. The severity of T1R was graded by clinical criteria in the INFIR patients (24). Patients with T1R were treated with corticosteroids at the time the reaction was diagnosed, immediately after blood had been drawn. The dosage was usually tapered monthly by 10 mg/day.
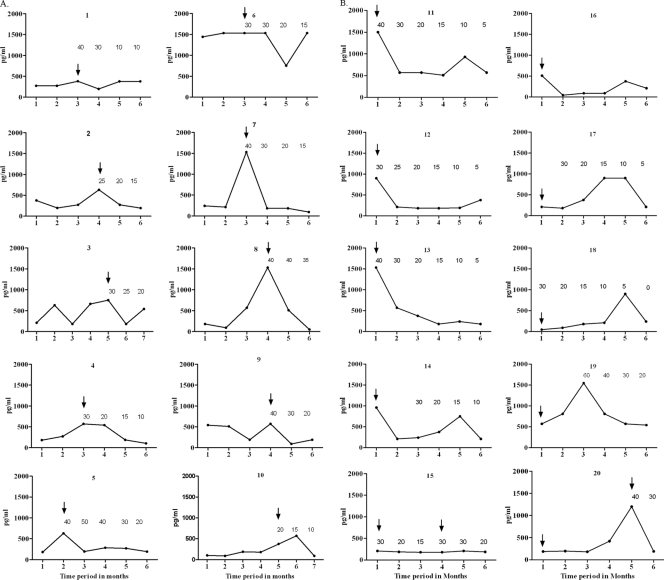
Fig. 2.
Circulating CXCL10 in repeated samples from leprosy patients who developed T1R. Each graph depicts the measurements made from serum samples obtained at 1 month intervals in borderline tuberculoid (A, no. 1 to 10) and borderline lepromatous (B, no. 11 to 20) patients. All patients with clinical T1R received standard multidrug treatment starting at the first visit. The arrows indicate the visits at which T1R was diagnosed. The number above each time point indicates the dose of prednisone started on that day, tapered monthly as indicated; in some cases, corticosteroids were not used initially but were started 1 to 2 months after the initial clinical diagnosis of T1R. Each serum specimen was obtained before corticosteroid treatment was initiated. CXCL10 was significantly elevated during T1R in the 10 BT patients (A) (P = 0.0006) and the 10 BL patients (B) (P = 0.0001). In patients 12, 16, and 17 (B), T1R was diagnosed by histopathological criteria only; when they were excluded from the analysis, the association of T1R with CXCL10 remained significant (P < 0.05).
ELISA.
Selected serum specimens were thawed at the time of assay and diluted 1:3 with the calibrator diluent RD5K (R&D Systems, Minneapolis, MN). CXCL10 concentrations for each specimen were determined in duplicate using enzyme-linked immunosorbent assay (ELISA) reagents from R&D Systems, according to the manufacturer's instructions, and the optical density at 450 nm (OD450) (with a reference correction at 540 nm) was determined in an ELISA reader (Bio-Rad Microplate reader model 550). The concentrations in each sample were determined from a standard curve of serial dilutions of known concentrations prepared on each plate.
RNA extraction and amplification.
Total RNA was extracted from individual samples (approximately 20 mg of tissue) using the RNAgents Total RNA isolation system (Promega, Madison, WI) according to the manufacturer's instructions. Following extraction, RNA was DNase treated using the DNA-free kit (Ambion, Inc., Austin, TX), and the concentration and purity of RNA preparations were determined using a NanoDrop-1000 spectrophotometer (Thermo Fisher Scientific, Pittsburgh, PA). RNA samples were amplified using the MessageAmp aRNA Amplification Kit (Ambion, Inc.) according to the manufacturer's protocols. Possible traces of genomic DNA in the amplified RNA were removed using the DNA-free kit.
Reverse transcription of RNA.
RNA was converted to cDNA using the Advantage cDNA Polymerase Mix and Advantage RT-for-PCR Kit (BD Bio-Sciences, San Jose, CA) according to the manufacturer's recommendations with oligo(dT) primer. Controls for DNA contamination consisted of total RNA incubated with the reverse transcription reagents, excluding reverse transcriptase (RT).
Real-time PCR.
The levels of human CXCL-10 and IFN-γ mRNAs were determined by real-time RT-PCR using TaqMan technologies in skin biopsy specimens of patients (i) prior to, (ii) during, and (iii) in some cases months after TIR resolution. Primers and probes were purchased commercially (TaqMan Gene Expression Assays [CXCL10 assay identifier, Hs00171042_m1, and IFN-γ assay identifier, Hs 99999041_m1[), and assays were performed according to the manufacturer's recommendations (Applied Biosystems, Foster City. CA) using a 7300 Real Time PCR System (Applied Biosystems). The data were obtained using the standard-curve method with 10-fold dilutions of human cDNA (Sigma-Aldrich, St. Louis, MO) to generate the curve for each assay, and all samples were normalized using 18S rRNA values for template variability. The relative fold differences of each of the gene transcripts were obtained by dividing the normalized values from biopsy 2 (during T1R) or biopsy 3 (after T1R) for each patient by that of biopsy 1 (prior to T1R) for the same patient.
Immunostaining.
Formalin-fixed, paraffin-embedded biopsy specimens were rehydrated, and antigen retrieval was performed in 10 mM citrate buffer, pH 6.0, with a BioCare Medical Decloaking Chamber. Endogenous peroxidase was quenched with hydrogen peroxide, and nonspecific binding was blocked with normal donkey serum prior to the addition of primary antibodies.
Tissue sections were stained by an indirect immunoperoxidase method using goat anti-human CXCL10 (R&D Systems, Inc.; 1:40) or rabbit anti-human CXCR3 (Sigma) (1:75) for 60 min, followed by biotinylated donkey anti-goat IgG or goat anti-rabbit IgG (both 1:40; Jackson ImmunoResearch Laboratories, Inc.) for 30 min. Peroxidase-conjugated avidin-biotin complexes (Vectastain Elite ABC Reagent kit) were applied for 30 min. All incubations were followed by three washes with phosphate-buffered saline isotonic buffer, pH 7.4. Color was developed by adding peroxidase substrate and 3-amino-9-ethylcarbazole (Vector Laboratories), following the manufacturer's instructions.
Images were captured at ×10 magnification for a Zeiss Axioplan microscope with a Microfire camera and merged using Adobe Photoshop. Inflamed areas were manually outlined digitally, and the areas were calculated using ImagePro software. Positively stained cells were counted manually at ×200 magnification, and the number of strongly stained cells per 100,000 μm2 was calculated.
Statistical analysis of data.
For measurements of serum CXCL10, statistical significance between groups at a single time point was tested using the Mann-Whitney 2-tailed test. The SAS statistics package GLM procedure (14) was used to analyze data as a repeated-measures design in a split-plot arrangement of treatments. This design allows more powerful tests with a smaller sample size because of the better control of error in blocking on subjects, with each subject serving as his/her own control, so that intersubject variation is excluded from the residual error (11). When overall analysis of variance indicated significance, post hoc pairwise comparisons were conducted with Tukey's test for main effects and the t test of least-squares means for interaction effects. Spearman correlation coefficients were calculated. The Wilcoxon matched-pairs signed-rank test was used to assess the statistical significance of cell counts in paired biopsy specimens in immunostaining studies. All comparisons were considered significant at a P value of <0.05.
RESULTS
CXCL10 protein levels in serum.
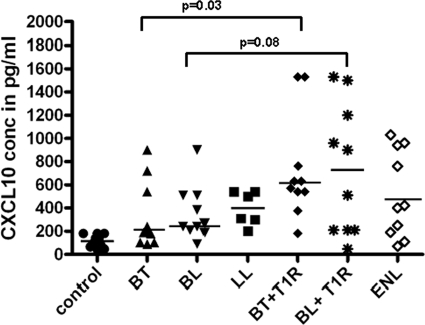
A cross-sectional comparison of CXCL10 values in patients with T1R, T2R, or nonreactional leprosy and healthy controls revealed significantly greater median levels of CXCL10 in patients with T1R than in any other group (P ≤ 0.004) (Fig. 1). Among BT patients, significantly higher median levels of CXCL10 were observed in sera from patients with T1R (727 pg/ml) than in sera from patients without reaction (327 pg/ml) (P = 0.031). Among BL patients, higher median levels were also observed in patients with T1R (728 pg/ml) than in patients without reaction (353 pg/ml), but the statistical significance of the difference had a P value of >0.05 (P = 0.081). Median CXCL10 levels in LL patients with type 2 reactions were not significantly elevated (513 pg/ml) compared to those of LL patients who did not have T2R (398 pg/ml) (P = 0.9). Median CXCL10 levels were higher in all leprosy patient groups than in healthy controls (P ≤ 0.01).
Fig. 1.
Circulating CXCL10 in leprosy patients and healthy controls. Each point represents one sample from a different individual. The groups were healthy controls, borderline tuberculoid without reaction (BT), borderline lepromatous without reaction (BL), polar lepromatous without reaction (LL), BT with reaction (BT plus RR), BL with reaction (BL plus RR), and LL patients with T2R (erythema nodosum leprosum). For patients with reaction (BT plus RR, BL plus RR, and T2R), the sample was taken at the time of reaction, prior to treatment. For LL, n = 6; for all other groups, n = 10. The horizontal lines indicate median values. The median level of CXCL10 in LL patients with type 2 reaction was not significantly elevated (P = 0.9).
Repeated measures of CXCL10 in individual patients (Fig. 2A and B) revealed a greater degree of association between T1R and elevated CXCL10 than was observed in the cross-sectional analysis. Comparing specimens from individuals over time, serum CXCL10 was significantly elevated during T1R in BL (P = 0.0006) and in BT (P = 0.0001) patients. In general, BT patients showed a more consistent association of CXCL10 elevation with histopathological and clinical evidence of T1R. In three BL patients (numbers 12, 16, and 17) (Fig. 2B), T1R was diagnosed histopathologically but not clinically; when these three patients were omitted from the analysis, the association of CXCL10 with T1R remained significant (P < 0.05). No consistent, predictive change in CXCL10 levels was observed in specimens obtained 1 month prior to episodes of clinical T1R.
CXCL10 and severity of T1R.
In the 17 patients with clinical signs of T1R (10 BT and 7 BL) (Fig. 2), the severity of the reaction was assessed as moderate or severe using clinical criteria described previously (23). However, no correlation was observed between the levels of CXCL10 and these assessments of the severity of T1R (P = 0.85).
Effects of corticosteroids.
All leprosy patients (n = 17) who had clinical signs of moderate to severe T1R were treated with prednisone, as indicated in Fig. 2. Prednisone was usually started at a dose of 40 mg/day and reduced by 10 mg/day at monthly intervals. Following initiation of corticosteroid treatment, CXCL10 serum levels dropped in several, but not all, cases (Fig. 2A and B). In some patients, the levels rose initially and then declined. No significant correlation could be established between the corticosteroid dose and decline in CXCL10 (data not shown).
CXCL10 and IFN-γ mRNA levels in biopsy specimens.
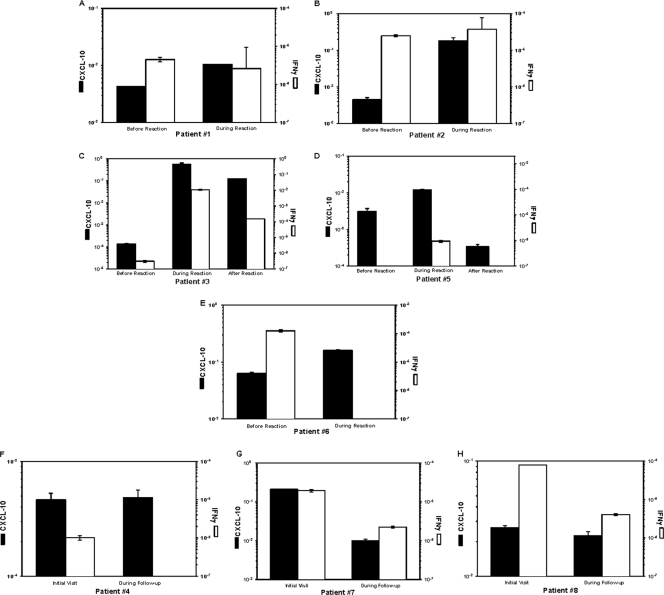
CXCL10 mRNA was detected in all biopsy specimens from leprosy patients tested. However, median levels were 16-fold higher (P < 0.02) in biopsy specimens from patients with T1R than in biopsy specimens from patients without T1R (0.16 versus 0.01, respectively) (Fig. 3). CXCL10 mRNA levels ranged from 2.5-fold to >52-fold higher in biopsy specimens taken during T1R than in biopsy specimens from the same individuals prior to the reaction (Fig. 3A to E). This contrasts with results in patients who were treated with the standard MDT regimen who did not develop T1R, for whom no increase in CXCL10 mRNA levels was observed in the 2nd biopsy (Fig. 3F to H). When a 3rd biopsy specimen was taken from two patients after T1R had resolved (and more than 1 month after discontinuation of corticosteroid treatment), a decline in CXCL10 mRNA levels was observed relative to the level observed during the reaction in each individual (Fig. 3C and D).
Fig. 3.
Expression levels of CXCL10 and IFN-γ genes in skin biopsy specimens from leprosy patients. (A to H) Real-time quantitative RT-PCR was performed on cDNA obtained from sequential skin biopsy specimens from individual leprosy patients who were placed on MDT, none of whom had T1R at the time of the first biopsy. Five patients (A to E) had clinical symptoms of T1R at the time of the second biopsy; two of these (C and D) had a third biopsy after the reaction had resolved. Three patients (F to H) had no clinical symptoms of T1R at the time of the initial or second biopsy. Data were obtained using the standard-curve method and were normalized using 18S rRNA values, repeated three times for all determinations. The results are presented as the mean ± standard deviation.
During T1R some patients had elevated levels of transcripts of both CXCL10 and IFN-γ mRNAs (Fig. 3). However, in this small sample, no significant correlation was observed between the intralesional levels of CXCL10 and IFN-γ mRNA.
Immunostaining for CXCL10 and CXCR3.
When biopsy specimens taken before and during T1R were compared, no consistent pattern of increase or decrease in the number of leukocytes staining for either the cytokine or its receptor was identified in the lesions (data not shown). In the epidermis, focal increases in immunostaining for CXCL10 were also noted in some biopsy specimens, but no trend was detected and no attempt was made to quantify CXCL10 staining in the epidermis. Similarly, some of the epithelial cells of sweat glands, sebaceous glands, and hair follicles stained strongly for CXCR3 in different biopsy specimens, but no consistent changes were observed with respect to T1R, and the observations were not assessed quantitatively.
DISCUSSION
The findings in this study indicate a strong association between the elevation of circulating CXCL10 and the occurrence of T1R in leprosy, demonstrated in sequential monthly serum specimens. These results also confirm preliminary observations of an association between T1R and elevated circulating CXCL10 in BT patients (18) and extend this finding to BL patients. In addition, a significant elevation of CXCL10 mRNA levels in skin biopsy tissue of patients was observed during T1R compared to biopsy specimens from the same patients prior to the reaction. The availability of sequential serum and biopsy samples enabled a view of within-subject distributions across time and a comparison of values at individual time points with or without T1R.
The strong association between elevated CXCL10 and clinical T1R suggests that this chemokine may be useful as a laboratory marker to aid in the diagnosis of T1R. This, in itself, is an important advance, since currently there are no laboratory markers for T1R. Even in biopsy specimens of lesions there are no pathognomonic morphological features of T1R, and the information obtained from biopsy specimens does not provide conclusive evidence of this reaction in a large percentage of cases (7). However, these data do not demonstrate that elevation of circulating CXCL10 can be used as a predictor of T1R; additional studies are necessary to evaluate the possibility. Further, although the association between elevated CXCL10 and T1R is strong, not every elevation of CXCL10 was associated with T1R (e.g., patients 3, 6, and 9). Without further information we cannot determine whether such elevations of T1R might represent “subclinical T1R” or might have some other biological significance.
The severity of T1R in the Indian cohort was assessed using the scale used in the INFIR study (24), but no correlation was observed between CXCL10 levels and the degree of clinical severity of T1R. The sample size in this study was small, however, and the possibility that CXCL10 may be a useful laboratory test to assess severity of T1R deserves additional evaluation.
Patients had different baseline serum levels of CXCL10, and the characteristic finding was an increase in serum CXCL10 at the time of reaction compared to the same patient at other times without reaction. Distributions of CXCL10 levels overlapped substantially in different patient groups, however. With this small sample size it was not possible to ascertain a cutoff level that was diagnostic for T1R, so the sensitivity and specificity of serum CXCL10 for the diagnosis of T1R could not be established.
No statistically significant correlation between corticosteroid dose and decline in CXCL10 could be established. This raises the possibility that CXCL10 levels, or other underlying immunological activities involved in T1R, may not be directly affected by corticosteroids, even though corticosteroids clearly reduce the inflammation associated with the reaction.
In sequential biopsy specimens from individual patients, it is clear that CXCL10 gene expression is increased during T1R, as it was observed in 5/5 such cases but not in any of the 3 patients who did not have T1R. Increased IFN-γ gene expression was also observed in 3/5 T1R specimens, but it was not consistently overexpressed in the biopsy specimens with abundant CXCL10 transcripts. This finding agrees with previous reports in which increased expression of IFN-γ has frequently, but not consistently, been associated with T1R (9, 22, 26). Similar elevation of CXCL10 without increased IFN-γ has also recently been reported in tuberculosis (1). In contrast, some individuals with tuberculosis are unable to produce CXCL10 even when they produce normal levels of IFN-γ (4). Additional studies are necessary to determine the relevance of this finding to the production of CXCL10 during T1R in borderline leprosy.
Since IFN-γ is the most potent inducer of CXCL10, it is possible that IFN-γ transcripts are generated early in the reaction and then quickly decline, while CXCL10 expression is delayed and remains elevated for a longer time. However, because there are no clear clinical or laboratory criteria to determine the time of onset of T1R, it is not possible to test this hypothesis rigorously in clinical studies at this time. No increase in CXCL10 transcripts was observed in follow-up biopsies from patients on MDT who did not have a reaction, indicating that neither treatment itself nor resolution of lesions leads to an increase in CXCL10 gene expression in cutaneous lesions.
The inconsistent and diffuse immunostaining of T1R dermal infiltrates for both CXCL10 and its receptor, CXCR3, suggest that these are not satisfactory immunohistochemical markers for T1R. The diffuse immunostaining for CXCL10 appears to be a detection issue, but no clear evidence is available to satisfactorily explain this. However, similar diffuse staining has also been observed in biopsy specimens of other infectious lesions (M. V. Chaduvula and I. Nath, unpublished data). The staining of epidermal cells in biopsy specimens of leprosy lesions, using a polyclonal antibody to CXCR3, contrasts with previous findings using monoclonal antibodies, in which no CXCR3 staining was observed in keratinocytes, skin appendages, endothelial cells, or Langerhans cells in normal skin (3). The use of these different antibodies may be responsible for the differences in results. However, keratinocytes may play important immunological roles in T1R that could include production of CXCL10 and its receptor. In addition, receptors on these epithelial cells may act as scavengers for CXCL10 in the tissue, as has been described for CXCR3 expression on normal salivary gland ductal epithelial cells (17). The evidence thus far, however, does not indicate that CXCL10 or CXCR3 is useful as an immunohistochemical marker for T1R in skin biopsies.
Increased production of CXCL10 is a well-documented consequence of infection with other mycobacteria (2, 5, 8), and it is notable that circulating levels of the cytokine were also increased in all leprosy patients without reaction compared to healthy controls in this study. However, the more important finding here is that significant increases in CXCL10 are associated with T1R, further delineating the role of cellular immunity in T1R. The mechanisms involved in the triggering of this enhanced reactivity remain unclear. Although available data do not indicate that it is a predictive marker, CXCL10 appears to offer a useful laboratory marker to aid in the diagnosis of T1R and in further clinical studies of T1R. Recognition of its involvement in this reaction may aid in studies aimed at an understanding of the immunological and inflammatory mechanisms involved in the reaction itself.
ACKNOWLEDGMENTS
This work was supported by grants from American Leprosy Missions, with additional financial assistance from the Department of Science and Technology (DST), Government of India. Funding for the INFIR study was provided by The Leprosy Mission, Naini and Faisabad Hospitals, Follereux Luxembourg, and Lepra.
We are indebted to the INFIR project for access to their specimen collection and database and to Sujai Suneetha and Peter Nicholls for assistance in specimen selection. Excellent technical assistance was received from Ahmed at BPRC and from Greg McCormick, Tana Pittman, and Angelina Deming at NHDP.
We declare no conflicts of interest.
Footnotes
Published ahead of print on 20 April 2011.
REFERENCES
- 1. Berry M. P., et al. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466:973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Djoba Siawaya J. F., Ruhwald M., Eugen-Olsen J., Walzl G. 2007. Correlates for disease progression and prognosis during concurrent HIV/TB infection. Int. J. Infect. Dis. 11:289–299 [DOI] [PubMed] [Google Scholar]
- 3. Garcia-López M. A., et al. 2001. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab. Invest. 81:409–418 [DOI] [PubMed] [Google Scholar]
- 4. Goletti D., et al. 2010. IFN-gamma, but not IP-10, MCP-2 or IL-2 response to RD1 selected peptides associates to active tuberculosis. J. Infect. 61:133–143 [DOI] [PubMed] [Google Scholar]
- 5. Hainard A., et al. 2009. A Combined CXCL10, CXCL8 and H-FABP panel for the staging of human. African trypanosomiasis patients. PLoS Negl. Trop. Dis. 3:e459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan G., Luster A. D., Hancock G., Cohn Z. A. 1987. The expression of a γ-interferon-induced protein (IP-10) in delayed immune responses in human skin. J. Exp. Med. 166:1098–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lockwood D. N., et al. 2008. The histological diagnosis of leprosy type 1 reactions: identification of key variables and an analysis of the process of histological diagnosis. J. Clin. Pathol. 61:595–600 [DOI] [PubMed] [Google Scholar]
- 8. Méndez-Samperio P., Perez A., Rivera L. 2009. Mycobacterium bovis Bacillus Calmette-Guerin (BCG)-induced activation of PI3K/Akt and NF-kB signaling pathways regulates expression of CXCL10 in epithelial cells. Cell Immunol. 256:12–18 [DOI] [PubMed] [Google Scholar]
- 9. Moraes M. O., et al. 1999. Cytokine mRNA expression in leprosy: a possible role for interferon-gamma and interleukin-12 in reactions (RR and ENL). Scand. J. Immunol. 50:541–549 [DOI] [PubMed] [Google Scholar]
- 10. Müller M., Carter S., Hofer M. J., Campbell I. L. 2010. The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity—a tale of conflict and conundrum. Neuropathol. Appl. Neurobiol. 36:368–387 [DOI] [PubMed] [Google Scholar]
- 11. Neter J., Wasserman W. 1974. Applied linear statistical models; regression, analysis of variance, and experimental designs. Richard D. Irwin, Inc., Homewood, IL [Google Scholar]
- 12. Oliver B. G., et al. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J. Infect. Dis. 202:1728–1737 [DOI] [PubMed] [Google Scholar]
- 13. Ridley D. S., Jopling W. H. 1966. Classification of leprosy according to immunity—a five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255–273 [PubMed] [Google Scholar]
- 14. SAS 1999. SAS/STAT user's guide, version 8. SAS Institute Inc., Cary, NC [Google Scholar]
- 15. Scollard D. M., et al. 2006. The continuing challenges of leprosy. Clin. Microbiol. Rev. 19:338–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scollard D. M., Joyce M. P., Gillis T. P. 2006. Development of leprosy and type 1 leprosy reactions after treatment with infliximab: a report of 2 cases. Clin. Infect. Dis. 43:e19–e22 [DOI] [PubMed] [Google Scholar]
- 17. Sfriso P., et al. 2006. Epithelial CXCR3-B regulates chemokines bioavailability in normal, but not in Sjogren's syndrome, salivary glands. J. Immunol. 176:2581–2589 [DOI] [PubMed] [Google Scholar]
- 18. Stefani M. M., et al. 2009. Potential plasma markers of type 1 and type 2 leprosy reactions: a preliminary report. BMC Infect. Dis. 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Szczuciński A., Losy J. CCL5, CXCL10 and CXCL11 chemokines in patients with active and stable relapsing-remitting multiple sclerosis. Neuroimmunomodulation 18:67–72 [DOI] [PubMed] [Google Scholar]
- 20. Tanuma N., Sakuma H., Sasaki A., Matsumoto Y. 2006. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 112:195–204 [DOI] [PubMed] [Google Scholar]
- 21. van Brakel W. H., et al. 2005. The INFIR Cohort Study: investigating prediction, detection and pathogenesis of neuropathy and reactions in leprosy. Methods and baseline results of a cohort of multibacillary leprosy patients in north India. Lepr. Rev. 76:14–34 [PubMed] [Google Scholar]
- 22. Verhagen C. E., et al. 1997. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T cell reactivity in lesional skin: a follow-study. J. Immunol. 159:4474–4483 [PubMed] [Google Scholar]
- 23. Walker S. L., Lockwood D. N. 2008. Leprosy type 1 (reversal) reactions and their management. Lepr. Rev. 79:372–386 [PubMed] [Google Scholar]
- 24. Walker S. L., et al. 2008. Development and validation of a severity scale for leprosy type 1 reactions. PLoS Negl. Trop. Dis. 2:e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whittaker E., Gordon A., Kampmann B. 2008. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFNgamma? PLoS One 3:e3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamamura M., et al. 1992. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149:1470–1475 [PubMed] [Google Scholar]
- 27. Yoshie O., Imai T., Nomiyama H. 2001. Chemokines in immunity. Adv. Immunol. 78:57–110 [DOI] [PubMed] [Google Scholar]